Announcements 1/26/11
advertisement

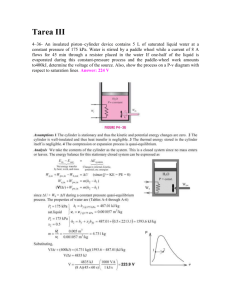
Announcements 1/26/11 Prayer Please do this “Quick Writing” assignment while you’re waiting for class to start: Ralph is confused because he knows that when you compress gases, they tend to heat up (think of a bicycle pump nozzle getting hotter as you force the gas from the pump to the tire). So, how are “isothermal” processes possible? How can you compress a gas without its temperature increasing? Demo Constant volume change, aka “alcohol rocket” Thought question How will the temperature of the gas change during this process from A to B? a. Increase b. Decrease c. First increase, then decrease d. First decrease, then increase e. Stay the same A 2.0 1.8 1.6 Pressure (atm) 1.4 1.2 1.0 B 0.8 0.6 0.4 0.2 0.0 0.0 0.2 0.4 0.6 0.8 1.0 1.2 3 Volume (m ) 1.4 1.6 1.8 2.0 Reading quiz What is “CV”? a. heat capacity b. mass-pacity c. molar heat capacity d. molar heat capacity, but only for constant volume changes e. your “curriculum vitae”, a detailed resumé Thought question Which will be larger, the molar heat capacity for constant volume changes or the molar heat capacity for constant pressure changes? (Hint: Think of the First Law.) a. constant volume b. constant pressure c. they are the same d. it depends on the temperature CV and CP Constant volume change (monatomic): W=0 Eint = Qadded (3/2)nRT = Qadded Compare to definition of C: Qadded = nCVT CV = (3/2)R (monatomic) Constant pressure change a. What’s different? b. result: CP = (5/2)R (monatomic) What would be different for gases with more degrees of freedom? Reading quiz (graded) What does gamma equal in the equation for an adiabatic process: PV constant a. CP + CV b. CP - CV c. CV - CP d. CV / CP e. CP / CV Isothermal vs Adiabatic Isothermal: PV constant Adiabatic: PV constant steeper curves for adiabatic Thought question How much do you think the temperature of the air in this room would change by if I compressed it adiabatically by a factor of 10? (Vf = V0/10) a. less than 0.1 degree C b. about 0.1 degrees C c. about 1 degree C d. about 10 degrees C e. more than 10 degrees C Demo/Video Demo: freeze spray Video: adiabatic expansion Demo: adiabatic cotton burner Derivation of PV (for Monatomic) Eint = Qadded + Won (3/2) nRT = - PdV (3/2) nRdT = -PdV (3/2) nR d(PV/nR) = -PdV (3/2) (PdV + VdP) = -PdV What’s different if diatomic? (3/2) VdP = -(5/2) PdV dP/P = -(5/3) dV/V lnP = (-5/3)lnV + constant lnP = ln(V-5/3) + constant P = constant V-5/3 (it’s a different constant) P V5/3 = constant Thought question Which of the curves on the PV diagram below is most likely to represent an isothermal compression, followed by an adiabatic expansion back to the initial volume? a. b. c. d. e. Thought questions What would be the molar specific heat for an adiabatic process? (Hint: think of Q = nCT.) a. CV b. CV + R c. CV + 2R d. CV - R e. none of the above What would be the molar specific heat for an isothermal process? (Same hint.) a. CV b. CV + R c. CV + 2R d. CV - R e. none of the above Water/steam “saturation curve”: ideal gas? 30000 bk = actual data for water/steam Pressure (kPa) 25000 20000 All Gas 15000 10000 5000 All Liquid 0 0.00 adding heat energy, T=325C Part Liquid, Part Gas 0.01 0.02 0.03 0.04 0.05 3 Volume, divided by mass (m /kg) 0.06 1 atm = 101 kPa Water/steam “saturation curve”: ideal gas? 30000 bk = actual data for water/steam rd = ideal gas, T=400 bl = ideal gas, T=500 gn = ideal gas, T=600 Pressure (kPa) 25000 20000 All Gas 15000 10000 5000 All Liquid 0 0.00 adding heat energy, T=325C Part Liquid, Part Gas 0.01 0.02 0.03 0.04 0.05 3 Volume, divided by mass (m /kg) 0.06 1 atm = 101 kPa Water/steam “saturation curve”: ideal gas? 30000 25000 Pressure (kPa) "critical point" bk = actual data for water/steam rd = ideal gas, T=400 bl = ideal gas, T=500 gn = ideal gas, T=600 20000 All Gas 15000 10000 5000 All Liquid 0 0.00 adding heat energy, T=325C Part Liquid, Part Gas 0.01 0.02 0.03 0.04 0.05 3 Volume, divided by mass (m /kg) 0.06 1 atm = 101 kPa Water/steam “saturation curve”: ideal gas? 30000 ???? 25000 Pressure (kPa) "critical point" bk = actual data for water/steam rd = ideal gas, T=400 bl = ideal gas, T=500 gn = ideal gas, T=600 20000 All Gas 15000 10000 5000 All Liquid 0 0.00 adding heat energy, T=325C Part Liquid, Part Gas 0.01 0.02 0.03 0.04 0.05 3 Volume, divided by mass (m /kg) 0.06 1 atm = 101 kPa