lecture 13 - what is entropy
advertisement

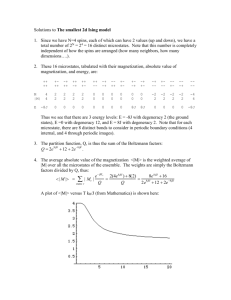
Announcements 9/26/12 Prayer Exam 1 starts Saturday morning, goes until Thursday evening On Friday at the start of class I will talk a bit about what to expect for the exam Exam review session: Today, 5 pm, room C295 Come with probems for me to discuss! (HW, optional HW, old exams, etc.) Pearls Before Swine From warmup Extra time on? a. “What is entropy” handout Other comments? a. What is the best thing for us to do to prepare for the test we have next week? b. Will there be another review by a TA or is today's the only one? From warmup Why does heat flow from hot to cold when the process of energy exchange between two objects is "random". (How can you get directed motion of heat, when energy is being exchanged both ways?!) a. There is more energy on the hot side than the cold side which means that although it is random, there are more possibilities for heat to flow from the hot to cold than from cold to hot. There is also heat being exchanged from cold to hot but there is a lot less of it so the heat generally flows from hot to cold. Microstates vs Macrostates Reminder a. Left microstate: part of the “royal flush” macrostate b. Right microstate: part of the “garbage” macrostate c. The most common macrostates are those with the most microstates p.413a Marble Example 50 red, 50 green in a bag. Draw 4. a. Microstates? b. Macrostates? “most disordered” most entropy About HW 13 Hint: same as DS for a “free expansion” HW 13, cont. (Various questions about micro- and macrostates follow.) HW 13, cont. HW 13-4: my plot From warmup When two systems A and B can exchange energy, the entropy of system A *always* decreases when system A gives energy to system B. If that's so, why would energy ever spontaneously flow from system A to system B? (It often will. When? Why?) a. Because system B is at a lower temperature and the increase in entropy of system B will be greater than the decrease in entropy of system A. Clicker question: Which of the following is the equation that relates the # microstates (W) to entropy (S)? a. S = CV W b. S = kB eW c. S = kB lnW d. S = tan(W) e. S = tan-1(W) Dice You roll two dice. What are the microstates? (1,1),(1,2),(1,3),(1,4),(1,5),(1,6),(2,1),(2,2),… How many microstates are there? How does that compare to the number of microstates for rolling one die? How many microstates if we roll 3 dice? What are the macrostates for 2 dice? (sum of numbers) What is the most likely macrostate? MANY Dice You roll 1023 dice with your left hand. a.How many microstates are there? You roll 1023 dice with your right hand. a.How many microstates are there? How many microstates are there in the COMBINED system? Isn’t this ridiculous? Solution: Use logarithms S = some constant ln(#microstates) [units of J/K] a. Much more manageable numbers. b. Combining two systems: Stot = C ln(#microstates1 #microstates2) = C ln(#microstates1) + C ln(#microstates2) = S1 + S2 c. 2nd Law: System in macrostate with most microstates System in macrostate with largest S System and Reservoir System: E1 Large reservoir: E2 Etot = E1 + E2 (const. volume so no work) 0 = dE1 + dE2 Want to maximize S: take dS/dE1, set = 0 dS d S1 S2 dE1 dE1 dS1 dS 2 dE1 dE1 dS1 dS 2 dE1 dE2 dS1 dS 2 dE1 dE2 From warmup In the "What is entropy?" handout, what was significant about the equation dS1/dE1 = dS2/dE2? a. It provide a value that is the same for both systems and relates to the inverse of the temperature: (J/K)/J = 1/K. Temperature dS/dE is the same for two systems in thermal contact! Temperature is also the same for two systems in thermal contact! a. dS/dE has units of 1/K, so… Compare to dS 1 dE T dQ dS T We are assuming no work, so dE=dQ (First Law) This “works” if the constant is chosen properly: S = kB ln(#microstates) Small system with 2 possible energies: E1A vs E1B (1 microstate each) Probability of system 1 being in state A vs state B? P1A ~ (#microstates of system 1 having energy E1A) (#microstates of system 2 having energy E2A = E – E1A) Let #microstates of E1A = 1 for now. Same thing for state 1B… P1A # microstates of 2 for case A P1B # microstates of 2 for case B e S2 A S e 2B kB kB Math… P1A # microstates of 2 for case A e S2 A S P1B # microstates of 2 for case B e 2 B kB kB S 2 A S 2 ( E E1 A ) “of”, not “times” dS 2 S2 ( E ) E1 A dE E1 A S2 ( E ) T (same with S 2 B ) E1 A / k BT P1A e Result: E /k T P1B e 1B B The Boltzmann Factor Pbeing in state with energy E ~ e E / k BT “Boltzmann Factor” Prob is proportional to BF, but not equal Must normalize: Prob = BF/(sum of all BFs) Worked Problem: Suppose an atom has only two available energy levels, which are separated by 210-23 J. If the temperature is 1.5 K, what is the probability the atom is in the lower state? Maxwell-Boltzmann Velocity Distribution E = ½mv2 What’s probability of having speed 5 vs speed 10? P1A e E1 A / kBT E /k T P1B e 1B B v 2 1 mv 2 / k BT e 2 1 mv A2 / k BT 2 2 e P1 A v A 1 P1B mvB 2 / k BT 2 2 vB e Multiplicities (Number of states with speed v) ~ v2 Maxwell-Boltzmann Velocity Distribution The result: P(speed v) v 2 1 mv 2 / k BT e 2 1 2 mv / k BT 2 2 v e 1 2 mv / k BT 2 2 v e dv 0 Exactly the equation given for the velocity distribution in your textbook! (after you do the integral, e.g. with Mathematica)