Daniel Scharfstein
advertisement

Do Trauma Centers
Save Lives?
A Statistical Solution
Daniel O. Scharfstein
Collaborators
•
•
•
•
Brian Egleston
Ciprian Crainiceanu
Zhiqiang Tan
Tom Louis
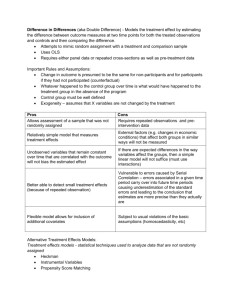
Issues
• Outcome Dependent Sampling
• Missing Data
• Confounding
– Direct Adjustment
– Propensity Score Weighting
• Propensity Model Selection
• Weight Trimming
• Clustering
Big Picture
Counterfactual Population: Y(1),X
Counterfactual Population: Y(0),X
Counterfactual Sample
Counterfactual Sample
Population: (Y,X,T)
Sample
Sub-Sample
Population: (Y,X,T)
Sample
Sub-Sample
TC
NTC
N
10970 4039
% Dying
8.0%
5.9%
TC
NTC
N
3044
1999
% Dying
27.8% 11.9%
Sample Weights
• Reciprocal of the conditional probability of
being included in the sub-sample given
–
–
–
–
–
ISS
AIS
Age
Dead/Alive at Sample Ascertainment
Dead/Alive at 3 Months post injury
• Weights depend on outcome - they can’t be
ignored.
Missing Data
Socio-demographic
Pre-Hospital Injury Severity
Hospital Injury Severity
Age (0%)
SBP/Shock (38.1%)
AIS (0%)
Gender (0%)
GCS Motor (30.0%)
NISS (0%)
Race (0%)
Paralytics (8.6%)
Lowest SRR (0%)
Insurance (1.2%)
Intubation (6.0%)
APS (0%)
EMS Level/
Mode of Transport (19.0%)
SBP/Shock (1.0%)
MOI (2.0%)
Pupils (5.7%)
Outcome
Death (0%)
GCS Motor (2.4%)
Midline Shift (1.8%)
Co-morbidities
Open Skull Fracture (0%)
Obesity (4.6%)
Flail Chest (0%)
Coagulopathy (4.6%)
Heart Rate (0.9%)
Charlson (4.6%)
Paralysis (1.1%)
Long Bone
Fracture/Amputation (0%)
Multiple Imputation
• For proper MI, we fill in the missing data by randomly
drawing from the posterior predictive distribution of
the missing data given the observed data.
• To reflect the uncertainty in these imputed values, we
create multiple imputed datasets.
• An estimate (and variance) of the effect of trauma
center is computed for each completed data.
• The results are combined to obtain an overall
estimate.
• The overall variance is the sum of the within
imputation variance and the between imputation
variance.
Multiple Imputation
• To draw from the posterior predictive distribution, a
model for the joint distribution of the variables and a
prior distribution on the model parameters must be
specified.
• Joe Schafer’s software
• UM’s ISR software - IVEWARE
– Specifies a sequence of full conditionals, which is
not, generally, compatible with a joint distribution.
• WINBUGS - Crainiceanu and Egleston
– Specifies a sequence of conditional models, which
is compatible with a joint distribution
Selection Bias
TC
NTC
Age < 55
79%
53%
Male
73%
57%
White, Non-Hispanic
56%
72%
Hispanic
18%
13%
Non-white, Non-Hispanic
26%
16%
0
77%
58%
1
14%
17%
2
5%
10%
3 or more
5%
16%
Race
Charlson
Selection Bias
TC
NTC
Blunt - Motor Vehicle
53%
32%
Blunt - Fall
20%
53%
Blunt - Other
10%
10%
Penetrating - Firearm
12%
4%
Penetrating - Other
5%
2%
9%
5%
6
74%
90%
4-5
8%
4%
2-3
1%
1%
1 - Not Chemically Paralyzed
5%
3%
Chemically Paralyzed
12%
2%
Mechanism of Injury
Pupils - Abnormal
GCS Motor Score
Selection Bias
TC
NTC
<16
24%
52%
16-24
16%
56%
25-34
29%
15%
>34
18%
9%
<=3
8%
73%
4
27%
20%
5-6
15%
7%
ALS - Intubated
12%
3%
ALS - Not Intubated
69%
41%
BLS
11%
35%
Not Transported by EMS
8%
22%
NISS
Max AIS
EMS Level/Intubation
Notation
• T denotes treatment received (0/1)
• X denotes measured covariates
• Y(1) denotes the outcome a subject would
have under trauma care.
• Y(0) denotes the outcome a subject would
have under non-trauma care.
• Only one of these is observed, namely
Y=Y(T), the outcome of the subject under the
care actually received.
• Observed Data: (Y,T,X)
Causal Estimand
Selection Bias
• We worked with scientific experts to define all
possible “pre-treatment” variables which are
associated with treatment and mortality.
• We had extensive discussions about
unmeasured confounders.
• Within levels of the measured variables, we
assumed that treatment was randomized.
• T is independent of {Y(0),Y(1)} given X
Example (Hernan et al., 2000)
Direct Adjustment
Direct Adjustment
Direct Adjustment
Direct Adjustment
Direct Adjustment
Direct Adjustment
Counterfactual Population: Y(1),X
Counterfactual Population: Y(0),X
Counterfactual Sample
Counterfactual Sample
Population: (Y,X,T)
Sample
Sub-Sample
Propensity Score Weighting
Y(1) Counterfactual Population
Y(0) Counterfactual Population
Why does this work?
Propensity Model Selection
• Select a propensity score model such
that the distribution of X is comparable
in the two counterfactual populations
(Tan, 2004).
Weight Trimming
• The propensity score weighted
estimator can be sensitive to individuals
with large PS weights.
• When the weights are highly skewed,
the variance of the estimator can be
large.
• We trim the weights to minimize MSE.
Clustering
• Assumed a working independence
correlation structure.
• Fixed up standard errors using the
sandwich variance technique.
Results
Sample
Counterfactual
Populations
TC
NTC
TC
NTC
Age < 55
79%
53%
72%
73%
Male
73%
57%
69%
67%
White, Non-Hispanic
56%
72%
60%
58%
Hispanic
18%
13%
16%
17%
Non-white, Non-Hispanic
26%
16%
24%
25%
0
77%
58%
72%
73%
1
14%
17%
14%
13%
2
5%
10%
6%
6%
3 or more
5%
16%
8%
8%
Race
Charlson
Results
Sample
Counterfactual
Populations
TC
NTC
TC
NTC
Blunt - Motor Vehicle
53%
32%
48%
50%
Blunt - Fall
20%
53%
28%
27%
Blunt - Other
10%
10%
10%
9%
Penetrating - Firearm
12%
4%
10%
10%
Penetrating - Other
5%
2%
4%
4%
9%
5%
8%
9%
6
74%
90%
78%
77%
4-5
8%
4%
7%
6%
2-3
1%
1%
1%
1%
1 - Not Chemically Paralyzed
5%
3%
4%
4%
Chemically Paralyzed
12%
2%
10%
11%
Mechanism of Injury
Pupils - Abnormal
GCS Motor Score
Results
Sample
Counterfactual
Populations
TC
NTC
TC
NTC
<16
24%
52%
30%
30%
16-24
16%
56%
7%
7%
25-34
29%
15%
26%
24%
>34
18%
9%
16%
19%
<=3
8%
73%
61%
60%
4
27%
20%
26%
26%
5-6
15%
7%
13%
14%
ALS - Intubated
12%
3%
10%
10%
ALS - Not Intubated
69%
41%
61%
61%
BLS
11%
35%
17%
17%
Not Transported by EMS
8%
22%
12%
12%
NISS
Max AIS
EMS Level/Intubation
Results
30 Days
90 Days
365 Days
% Dying in TC
7.6%
8.7%
10.4%
% Dying in NTC
10.0%
11.4%
13.8%
RR
0.76 (0.58,1.00)
0.77 (0.60,0.98)
0.75 (0.60,0.95)
Total NSCOT Population
Case Fatality Ratios
Adjusted for Differences in Casemix
15
Adjusted
Relative Risk:
.75
.60
.95
10
5
0
In
30 days 90 days 365 days
Hospital
TCs
NTCs
Results
MAXAIS <=3
30 Days
90 Days
365 Days
% Dying in TC
7.6%
8.7%
10.4%
% Dying in NTC
10.0%
11.4%
13.8%
RR
0.76 (0.58,1.00)
0.77 (0.60,0.98)
0.75 (0.60,0.95)
% Dying in TC
1.5%
1.5%
1.8%
% Dying in NTC
1.1%
1.2%
2.9%
RR
1.39 (0.68,2.84)
1.33 (0.68,2.60)
0.63 (0.32,1.22)
AGE<55
AGE>=55
Results
MAXAIS = 4
30 Days
90 Days
365 Days
% Dying in TC
6.0%
6.7%
7.4%
% Dying in NTC
9.4%
11.1%
13.2%
RR
0.64 (0.44,0.93)
0.60 (0.41,0.89)
0.56 (0.38,0.82)
% Dying in TC
14.0%
17.2%
23.9%
% Dying in NTC
15.1%
23.0%
27.4%
RR
0.92 (0.54,1.57)
0.75 (0.51,1.11)
0.87 (0.58,1.32)
AGE<55
AGE>=55
Results
MAXAIS = 5,6
30 Days
90 Days
365 Days
% Dying in TC
25.1%
26.1%
26.3%
% Dying in NTC
38.5%
38.5%
38.5%
RR
0.65 (0.45,0.94)
0.68 (0.47,0.98)
0.68 (0.47,0.99)
% Dying in TC
44.6%
50.2%
51.5%
% Dying in NTC
61.6%
63.7%
63.7%
RR
0.72 (0.45,1.17)
0.79 (0.51,1.21)
0.81 (0.53,1.23)
AGE<55
AGE>=55
Relative Risks by Age and
Severity
Age of Patient
Severity
Moderate (AIS 3)
Serious (AIS 4)
Severe (AIS 5-6)
< 55
>=55
0.32
0.631.22
0.74
1.081.57
0.38
0.560.82
0.58
0.871.32
0.47
0.680.99
0.53
0.811.23
Potential Lives Saved
Nationwide H-CUP Hospital Discharge Data
360,293 adults
who meet NSCOT inclusion criteria
45% Treated in NTCs
162,132
22,374 Deaths
If Treated in NTCs
16,862 Deaths
If Treated in TCs
5,512 Each Year
Conservative Estimate
• Study non-trauma centers were
limited to those treating at least 25
major trauma patients each year;
most non-trauma centers are
smaller
• 17 of the study non-trauma centers
had a designated trauma team and
8 had a trauma director
Conclusions . . . to date
• The results demonstrate the benefits
of trauma center care and argue
strongly for continued efforts at
regionalization
• At the same time, they highlight the
difficulty in improving outcomes for the
geriatric trauma patient
Biostatistician’s Dream
• More efficient estimation (Tan,Wang)
• Functional outcomes in the presence of death
(Egleston)
• Sensitivity Analysis (Egleston)
• Instrumental variable analysis (Cohen, Louis,
Crainiceanu)
• Imputation (Crainiceanu, Egleston)