The changes in that occur during can be
advertisement
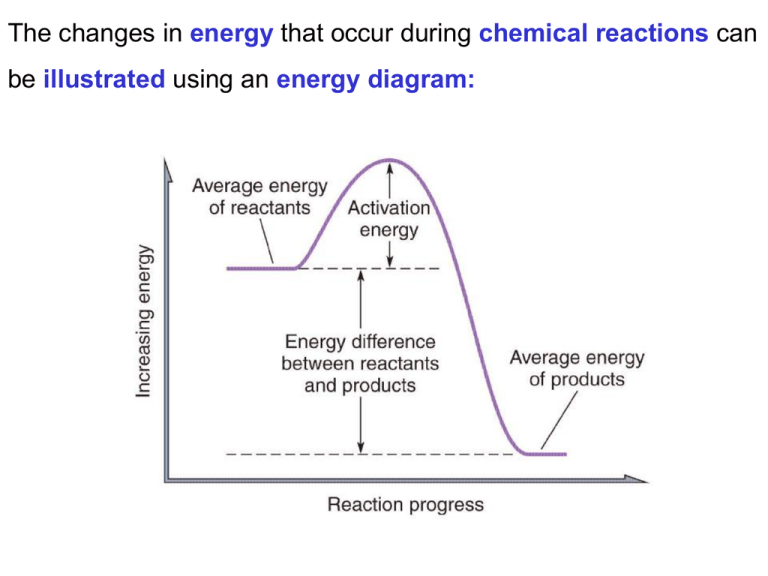
The changes in energy that occur during chemical reactions can be illustrated using an energy diagram: Many reactions take place in solution: e.g. 2AgNO3(aq) + Na2CO3(aq) Ag2CO3(s) + 2NaNO3(aq) The co-efficients in equations allow us to determine the relative amounts of products and reactants. # moles of Ag2CO3 = ½(# moles of AgNO3) # moles of Na2CO3 = ½(# moles of AgNO3) # moles of NaNO3 = # moles of AgNO3 2AgNO3(aq) + Na2CO3(aq) Ag2CO3(s) + 2NaNO3(aq) In the case of this reaction all reagents are in the form of aqueous solutions. If we combine two solutions of known volume of the reagents how could we calculate the amount of product produced? If we know the molarity of the reagent solutions we can use the relationship between molarity and volume to determine the number of moles of each reagent. M = n/V n=MxV We can then determine the yield of product as we would for any other stoichiometry calculation. Would anyone like to do an example? Luckily I thought that might be the case so I prepared a slide! Consider the following reaction: 2AgNO3(aq) + Na2CO3(aq) Ag2CO3(s) + 2NaNO3(aq) If I combined 20 mL of 0.20 molL-1 AgNO3(aq) solution with 10mL of 0.20 molL-1 Na2CO3(aq) what mass of Ag2CO3 would be produced? # of moles of AgNO3 = M x V = 0.2 molL-1 x 20 mL x (1 x10-3 LmL-1) = 0.004 moles of AgNO3 # moles of Ag2CO3 = ½(# moles of AgNO3) # moles of Ag2CO3 = ½ x 0.004 moles If the limiting reagent is AgNO3 0.002 moles of Ag2CO3 will be produced. # of moles of Na2CO3 = M x V = 0.2 molL-1 x 10 mL x (1 x10-3 LmL-1) = 0.0020 moles of Na2CO3 # moles of Ag2CO3 = # moles of Na2CO3 # moles of Ag2CO3 = 0.0020 moles If the limiting reagent is Na2CO3 0.0020 moles of Ag2CO3 will be produced. Conclude that we produce 0.0020 moles of Ag2CO3 in this reaction. However question asked for what mass. Mass = n x MW Mass of Ag2CO3 = 0.002mol x (2x107.9 + 12.01 + 3x16.00)gmol-1 Mass of Ag2CO3 = 0.55 grams The reaction 20 mL of 0.20 molL-1 AgNO3(aq) solution with 10mL of 0.20 molL-1 Na2CO3(aq) would produce 0.55 g of Ag2CO3. Pure water conducts electricity poorly. Addition of a solute can affect the ability of water to conduct electricity. • solutes that result in aqueous solutions that conduct electricity well are called strong electrolytes. • solutes that result in aqueous solutions that conduct electricity poorly are called weak electrolytes. • solutes that result in aqueous solutions that do not conduct electricity are called nonelectrolytes. The ability of a solute to conduct electricity increases with the extent to which it dissociates into ions. Soluble ionic compounds tend to completely dissociate into ions and are strong electrolytes: NaCl(aq) Na+(aq) + Cl-(aq) Polar covalent compounds dissociate depending on how polar the bonds they contain are: HCl(aq) H+(aq)+ Cl-(aq) Non-polar covalent compounds are nonelectrolytes. Lets do some practice problems now. Some properties of solutions do not depend on the chemical nature of the solute but only on the concentration of the solute. •These kinds of properties are called colligative properties We will discuss three colligative properties: • Boiling point elevation • Freezing point depression • Osmotic pressure The vapor pressure above a solution is lower than that above the pure solvent. This has some interesting effects: • The boiling point of a solution is higher than the pure solvent (boiling point elevation). • The freezing point of a solution is lower than the pure solvent (freezing point depression). The change in boiling point and freezing point can be calculated using the following similar equations: Δ tf =nKfM Δtb = nKbM Where: Δtb = change in boiling poing Δtf = change in freezing point n = number of moles of solute particles put in to solution when 1 mole of solute is dissolved. Kb = a constant characteristic of the solvent Kf = a constant characteristic of the solvent M = molarity What is the boiling point of a 0.1 molL-1 solution of MgCl2? Δtb = nKbM Δtb = ? n = 3 (2 x Cl- and 1 x Mg2+) Kb = 0.52 oC/M (from Table 7.6) M = 0.1 molL-1 Δtb = 3 x 0.52 x 0.1 = 0.156 oC Boiling point = 100oC + 0.156oC = 100.2oC When solutions having different concentrations of solute are separated by a semipermeable membrane, solvent flows through the membrane from the less concentrated solution into the more concentrated solution this is called osmosis. This will ultimately result in unequal amounts of liquid on each side of the membrane. A pressure will be exerted hindering the movement of solvent across the membrane. Eventually the pressure will prevent the movement of solvent across the membrane. This is called the osmotic pressure and is given by equation: π= nMRT Where: π = osmotic pressure n = number of moles of solute particles put in to solution when 1 mole of solute is dissolved. M = molarity (molL-1) R = universal gas constant T = temperature (Kelvin) A process very similar to osmosis is dialysis. In dialysis a membrane is used that allows the smaller molecules to cross but do not the larger ones. Dialysis is used extensively for purifying solutions of large biomolecules. e.g. proteins A commonly known use of dialysis is in purifying the blood of people whose kidneys do not function correctly. If we now replace the outer solvent we can repeat the process until we have the desired purity in the dialysis bag. We have all observed how some processes tend to spontaneously occur while others don’t. e.g. Objects roll downhill but not up. Our rooms effortlessly become disorganized but don’t spontaneous tidy themselves up. We age rather than grow younger. It would be very useful to predict what processes will occur and what ones won’t? We have already classified chemical processes as either being endothermic (heat in) or exothermic (heat out) processes. HINT: if you are having trouble remembering what these things mean think about the words entrance and exit. Heat is only one form of energy, others include light, sound, kinetic energy etc. In a more general sense, taking all forms of energy into account, we can classify reactions as being exergonic (energy out) or endogonic (energy in). When a chemical reaction occurs there will be a change in the order of the system as well as energy. e.g. The system could become more organized, such as liquid water freezing to become ice. H2O(l) H2O(s) The system could become less organized such as a reaction where from one mole of reactants forms several moles of products are formed. 2HgO(s) 2Hg(l) + O2(g) The degree of order or randomness in a system is called the entropy. Spontaneous reactions tend to release energy and have an increase in entropy. If these conditions don’t exist then there must be a larger change in the other parameter to off set the effect of the other in order for the process to be spontaneous. When looking at a reaction there are only several possible ways in which the energy and entropy can change that allow us to predict the spontaneity of the process. 1. If the reaction has no change in energy or gains energy from the surroundings. It will only be spontaneous if there is an increase in entropy. 2. A process will always be spontaneous if the reaction releases energy to the surroundings and is accompanied by an increase in entropy. 3. A process in which the entropy decreases will only be spontaneous if accompanied by a release of energy. Spontaneous reactions tend to release energy and have an increase in entropy. 1. If there is no change in energy or a gain of energy from the surroundings. Process will only be spontaneous if there is an increase in entropy. Can anyone think of a spontaneous process that falls into this case? H2O(s) + energy H2O(l) Spontaneous reactions tend to release energy and have an increase in entropy. 2. A process will always be spontaneous if the reaction releases energy to the surroundings and is accompanied by an increase in entropy. Can anyone think of a spontaneous process that falls into this case? NaOH(s) Na+(aq) + OH-(aq) + heat Spontaneous reactions tend to release energy and have an increase in entropy. 3. A process in which the entropy decreases will only be spontaneous if accompanied by a release of energy. Can anyone think of a spontaneous process that falls into this case? H2O(g) H2O(l) + heat A reaction mechanism describes the pathway or process by which a reaction occurs. This is different from a reaction rate we discussed previously which just tells you how quickly the reaction occurs. e.g. Comparing the difference between reaction rate and mechanism is like comparing how long it takes to complete a journey and the directions to a location. e.g. “ it takes 4.5 hours to fly to Chicago and Chicago lies to the east of Seattle.” Some aspects of reaction mechanisms are common to most reactions: 1. The reactant particles must collide (come into contact) Can anyone think of an exception to this rule ? Decomposition reactions have only one reagent: AB+C The second aspect of reaction mechanisms that is common to most reactions is: 2. The reactant particles must collide with a certain minimum amount of energy When a reaction occurs old bonds are broken and often new ones form. This typically requires an initial input of energy called the activation energy. Once the activation energy is overcome the process proceeds spontaneously.The energy of the reactants is made up of two components: 1. The kinetic energy of the molecules. 2.The internal energy of the reactant molecules. The kinetic energy of the reactants describes how fast the reactant molecules are moving. This will be greater for gases and liquids than solids and increases with temperature. The internal energy describes how the atoms are moving within a molecule. The internal energy of a molecule increases with temperature. For many reactions there is a minimum temperature below which the reaction does not occur. The third aspect of reaction mechanisms that is common to most reactions is: 3. The reactant particles must collide with a certain orientation For polyatomic species the orientation of the reactants may be significant. e.g. AB + CD → AC + BD In the above A must be near C and B must be near D. Reactant molecules that are gases and liquids have a greater ability to orient themselves favourably than reactants that are solids and have molecules held in fixed positions. Good luck in the final !! Keep watching the website for updates The energy of products from an exothermic process are lower in energy than the reactants. The energy of products from an endothermic process are higher in energy than the reactants. The reaction rate of all reactions are affected by: 1. The nature of the reactants 2. The concentration of the reactants 3. The temperature of the reactants 4. The presence of catalysts Important chemical properties of the reagents that can affect the reaction rate include: The type of bonds the reactants contain The charge of the reactants The state of the reactants Reactions that involve the breaking and making of covalent bonds tend to proceed slowly than those involving ionic species. Other properties of the type of bonds present in the reactants that are can affect the reaction rate include: Shape (VSEPR) How many bonds Bond polarity (strength of bond) Bond order (number of electrons in bond) Reactions where the reactants are oppositely charged ions will have a larger number of collisions between reagents that lead to products than reactants involving reagents with no charge or the same charge. If everything other than the charge is equal which of the following would you expect to have the greatest rate? A+ + B- AB A+ + A+ A2 2+ A + B AB By increasing the temperature of the reagents we increase the proportion of reagents that have sufficient energy to overcome the activation energy. A good rule of thumb is that for every 100C rise in temperature the reaction rate doubles. As we have mentioned before for a reaction to occur the reacting particles must collide. As we increase the concentration of the reagents the likelihood of an effective collision increases and therefore the reaction rate. For solids reactions typically take place at the surface of the reactant. By finely dividing solids we can increase the surface area and the rate at which they react. Catalysts are substances that change the rate of a reaction without being consumed. Catalysts that slow the rate of a reaction are known as inhibitors. Catalysts that are in the same state as the reagents and distributed as individual molecules or ions are known as homogeneous catalysts. Many catalysts are solids (often with large surface areas) while the reactants are gases or liquids, these are known as heterogeneous catalysts. E.g. catalytic convertors in cars. Catalysts work by providing an alternative, faster, pathway (or mechanism) from the reactants to the products. This alternative mechanism may have a lower activation energy (EA). More reactants are likely to have sufficient energy to overcome EA and so the reaction proceeds quicker. Solid catalysts can accelerate reactions by binding one of the reactants in a desirable orientation. Furthermore, molecules that are not moving are easier targets for collisions. In this diagram the catalyst holds the reactant in an orientation such that the yellow atoms are in a favourable position in order for them to react.