SCREENING LOG Protocol Title: IRB-HSR Protocol #:
advertisement
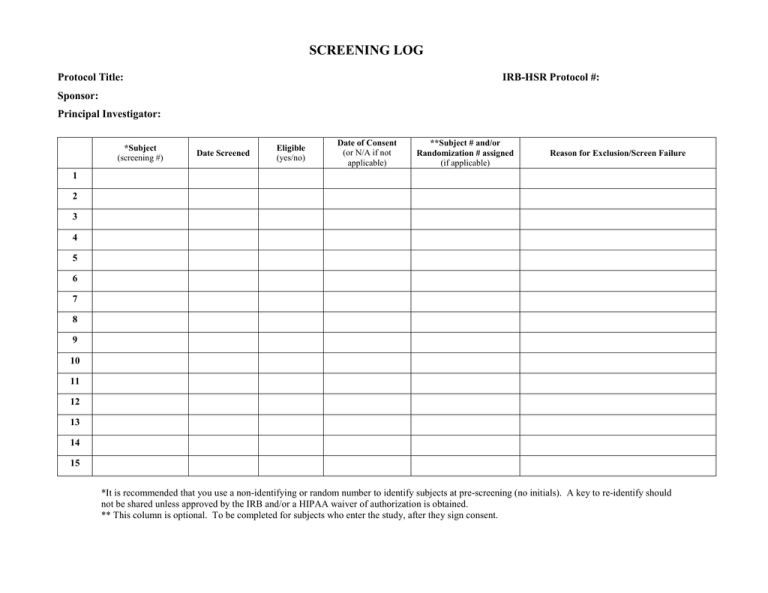
SCREENING LOG Protocol Title: IRB-HSR Protocol #: Sponsor: Principal Investigator: *Subject (screening #) Date Screened Eligible (yes/no) Date of Consent (or N/A if not applicable) **Subject # and/or Randomization # assigned (if applicable) Reason for Exclusion/Screen Failure 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 *It is recommended that you use a non-identifying or random number to identify subjects at pre-screening (no initials). A key to re-identify should not be shared unless approved by the IRB and/or a HIPAA waiver of authorization is obtained. ** This column is optional. To be completed for subjects who enter the study, after they sign consent.


![Lesson Study Project Informed Consent for Students 2011-12 [TEMPLATE]](http://s2.studylib.net/store/data/011897429_1-e9cd20ac12fa907a0c9dbbb5866bfc98-300x300.png)