Continuations Checklist
advertisement

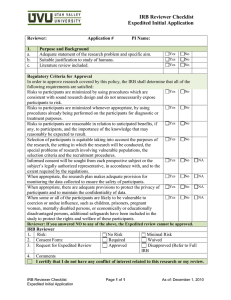
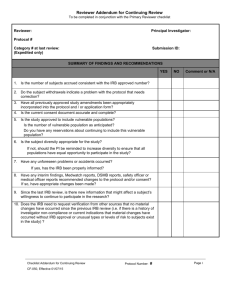
University of Virginia IRB for Health Sciences Research Continuation Reviewer’s Checklist: Full Board IRB-HSR# PI: Reviewer: Meeting Date: Study Status: Actively Accruing Subjects Closed to Accrual (subjects currently in treatment) Instructions: COMPLETION OF THIS ENTIRE FORM IS REQIRED TO DOCUMENT REVIEW. Review Notes: Page 1 of 5 Version: 05-25-16 University of Virginia IRB for Health Sciences Research Continuation Reviewer’s Checklist: Full Board Review the Status Form, Event and AE reports. Have any of the following occurred Yes No NA since the last IRB continuation approval? a. Adverse events, untoward events, or outcomes experienced by participants b. Unanticipated problems involving risks to participants or others c. Participant withdrawals and the reasons for withdrawals d. Complaints about the research e. Amendments or modifications f. Hold Notifications (FDA) g. Audits (FDA, PAM) h. Major Protocol violations or deviations i. Change in FDA labeling or withdrawal from marketing of drug or device If you answered YES to any item above, do any negatively affect the risk/benefit ratio so that the study should be modified or stopped? YES NO Were any of the following submitted since the last IRB continuation approval? a. b. c. d. e. Yes No NA Interim Findings Recent Relevant Literature DSMB Reports Any Relevant multi-center reports Adverse event reports, protocol deviations, unanticipated problems If you answered YES to any item above, do you feel the information affects any of the following such that the study should be modified or stopped? YES NO Equipoise of study (still not sure which intervention is better than the other) Risks associated with the research (increase or decrease) Risk-benefit analysis Alternative to participation or Participant’s willingness to continue participating in the research. Page 2 of 5 Version: 05-25-16 University of Virginia IRB for Health Sciences Research Full Board Continuation Reviewer’s Checklist Additional considerations for continuing review: 1. Have requirements stated in the protocol designed to provide additional protection to subjects and monitor the safety of the protocol been implemented? (e.g. DSMB oversight, consent observation, advocate for subject who is ward of state, two parent signatures, surrogate consent etc.This information may be found in the new protocol receipt or initial approval event. ) If yes, specify which ones have been implemented: Comment: 2. Does the consent form contain the most accurate, up to date information? If NO, describe how the consent form(s) should be revised”: 3. Has the study team exceeded the number of subject they were approved to enroll? Yes No If yes, explain: Does this protocol have the potential for benefit? Yes No Is this protocol sponsored by a commercial sponsor? Yes No If both questions above are answered NO- answer the question below: 4. Do you have any concerns regarding the ability of the study team to meet enrollment goals? Comment: Note to staff : If YES, Enter the following in main comment field: The IRB had enrollment concerns with (enter year) continuation. Enter the following on the assurance form: The board expressed concerns regarding enrollment with the review of this continuation. Enrollment will be reviewed again at your next continuation review Page 3 of 5 Version: 05-25-16 Yes No NA University of Virginia IRB for Health Sciences Research Full Board Continuation Reviewer’s Checklist Check the applicable boxes below and complete the text boxes, as necessary. In order to approve this continuation, the IRB shall determine that all of the following requirements are satisfied. Under Federal Regulations, the IRB is responsible for a continuing review of research at intervals appropriate to the degree of risk, but at least once each year. [45CFR 46.109(e)]. Does the protocol continue to meet the following approval criteria? Yes No NA 1. Risks to participants continue to be minimized by using procedures which are consistent with sound research design and do not unnecessarily expose participants to risk. 2. Risks to participants continue to be minimized by using procedures, whenever appropriate, that are already being performed on the participants for diagnostic or treatment procedures. 3. Risks to participants continue to be reasonable in relation to anticipated benefits, if any, to participants, and the importance of the knowledge that may reasonably be expected to result. 4. Selection of subjects remains equitable taking into account the purposes of the research, the setting in which the research will be conducted, the special problems of research involving vulnerable populations, the selection criteria and the recruitment process. 5. Informed consent continues to be sought from each prospective subject or the subject’s legally authorized representative, in accordance with, and to the extent required by the regulations. 6. When appropriate, the research plan continues to make adequate provision for monitoring the data collected to ensure the safety of participants. 7. When appropriate, there are continued adequate provisions to protect the privacy of participants and to maintain the confidentiality of data. 8. When some or all of the participants are likely to be vulnerable to coercion or undue influence, such as children, prisoners, pregnant women, mentally disabled persons, or economically or educationally disadvantaged persons, additional safeguards continue to be included in the study to protect the rights and welfare of these participants. Closed to enrollment REVIEWER: If you select NO to any of the above, the continuing review cannot be approved. Summarize any other major concerns: [e.g. Study design will not yield useful information; compensation not acceptable; ethical issues; consenting process issues; inadequate monitoring; specific inappropriate inclusion/exclusion criteria] Page 4 of 5 Version: 05-25-16 University of Virginia IRB for Health Sciences Research Full Board Continuation Reviewer’s Checklist Documentation of Reviewer Recommendation Approve Protocol Continuation as submitted for one year Approve Protocol Continuation for period of less than 1 year (Designate time frame: 3 months/ 6 months/ other) Approve Continuation Pending Minor Modifications and review by IRB-HSR Staff and Chair or designee Changes include (bullet): PI will need to resubmit revised protocol and revised consent (if applicable), final approval will be given by IRB-HSR Chair or Designee after modifications have been made. Examples of items which are considered minor: grammatical, spelling errors, template issues, providing investigator specific wording to insert. List required modifications: Withhold approval pending major modifications Additional information is needed from the PI in order to approve continuation. The PI will then need to re-submit the revised protocol and revised consent (if applicable) at a future IRB-HSR meeting and may be asked to attend. The study team will be granted 60 days to complete the request and submit to the IRB-HSR for review. The protocol approval will not expire during this time period. The protocol will be reviewed at a subsequent full board meeting. List major concerns: _____________________________________________ ____________ Reviewer Name Date Page 5 of 5 Version: 05-25-16