Haemophilus influenza , type B combined with Meningococcal Conjugate, serotypes C/Y (Hib-MenCY) Vaccine Protocol for At-risk Children Age 2 Months through 18 Months (Word)
advertisement

Haemophilus influenza, type B combined with Meningococcal
Conjugate, serotypes C/Y (Hib-MenCY) Vaccine Protocol
for At-risk Children Age 2 Months through 18 Months
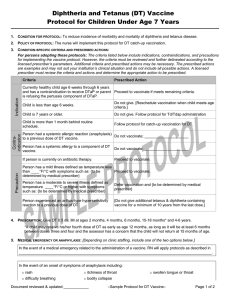
1. CONDITION FOR PROTOCOL: To reduce incidence of morbidity and mortality of Neisseria meningitidis disease due to types
C and Y and of Haemophilus influenza, type b in at risk persons ages 2 through 18 months.
2. POLICY OF PROTOCOL: The nurse will implement this Hib-MenCY protocol for at-risk persons as prescribed.
Precautions
Contraindications
Indications
3. CONDITION-SPECIFIC CRITERIA AND PRESCRIBED ACTIONS:
Attention persons adopting these protocols: The criteria below list indications, contraindications, and precautions that are
necessary to implement the vaccine protocol. The prescribed actions include examples shown in [ ] but may not suit your
institution’s clinical situation and may not include all possible actions. A licensed prescriber must review the criteria and
actions and determine the appropriate action to be prescribed. (Delete this paragraph before version is signed.)
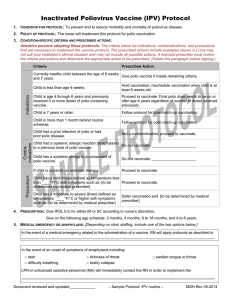
Criteria
Prescribed Action
Currently healthy person age 2 through 18 months
(minimum age 6 weeks) at increased risk of
meningococcal disease due to complement component
deficiency or due to anatomic or functional asplenia.
Initiate or continue the Hib-MenCY vaccination series if
meets remaining criteria.
Currently healthy person age 2 through 18 months
(minimum age 6 weeks) who is at risk during a
community outbreak attributable to serotype C or Y.
Initiate or continue the Hib-MenCY vaccination series if
meets remaining criteria.
Currently healthy infant that will be traveling to an
endemic or hyper endemic area of meningococcal
disease.
Do not give Hib-MenCY. Depending on product
available and age indications follow the respective
protocol.
Child is younger than age 6 weeks.
Do not give. [Reschedule vaccination when child meets
age criteria.]
Person is 19 months or older with risk indication for
meningococcal disease.
Do not give Hib-MenCY. Depending on product
available follow the respective protocol.
Child had a life-threatening allergic reaction
(anaphylaxis) to a previous dose of Hib-MenCY vaccine.
Do not vaccinate; _____________________
Child has a life-threatening allergic reaction (anaphylaxis)
Do not vaccinate; _____________________
to a component of Hib-MenCY vaccine.
Person is currently on antibiotic therapy.
Proceed to vaccinate.
Person has a mild illness defined as temperature less
than ____°F/°C with symptoms such as: {to be
determined by medical prescriber}
Proceed to vaccinate.
Person has a moderate to severe illness defined as
temperature ____°F/°C or higher with symptoms such
as: {to be determined by medical prescriber}
Defer vaccination and {to be determined by medical
prescriber}
Person has a history of Guillain-Barré syndrome
following a previous dose of tetanus-containing
vaccination.
[Do not vaccinate; _____________________]
[Do not vaccinate; refer to primary care physician to
determine and discuss risk and benefit of Hib-Men CY
vaccination.]
4. PRESCRIPTION: Give Hib-MenCY (MenHibrix) 0.5 mL, IM.
o
o
Give the series at the following ages: 2 months (as early as 6 weeks), 4 months, 6 months, & 12-15 months.
May give at the same time as other routinely scheduled vaccines.
o
Hib-MenCY satisfies routine vaccination for Haemophilus influenza, type b (Hib).
Document reviewed and updated:____________
– Sample protocol: Hib-MenCY –
MDH 07-2014
5. MEDICAL EMERGENCY OR ANAPHYLAXIS: [Depending on clinic staffing, include one of the two options below.]
In the event of a medical emergency related to the administration of a vaccine. RN will apply protocols as described in
____________________________________________________________________________________________.
In the event of an onset of symptoms of anaphylaxis including:
o
rash
o
itchiness of throat
o
difficulty breathing
o
bodily collapse
o
swollen tongue or throat
LPN or unlicensed assistive personnel (MA) will immediately contact the RN in order to implement the
____________________________________________________________________________________________.
6. QUESTIONS OR CONCERNS:
In the event of questions or concerns, call Dr. ____________________________at _____________________________.
This protocol shall remain in effect for all patients of ______________________________until rescinded or until
_____________________________________.
Name of prescriber: _______________________________________________________________________________
Signature: ________________________________________________________________________________________
Date: ___________________________
Document reviewed and updated:____________
– Sample protocol: Hib-MenCY –
MDH 07-2014