Cleveland State University, October 31, 2005
advertisement

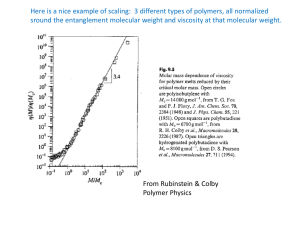
Putting a speed gun on macromolecules: what can we learn from how fast they go, and can we do something useful with that information? Monday, October 31 Cleveland State University National Science Foundation Generic Talk Outline • Thank hosts • Tell joke, story or limerick • Explain what we’re trying to do • Explain what we actually did • Today, that will lead naturally to applied things • Thank accomplices This is what I mainly came to say! There once was a theorist from France who wondered how molecules dance. “They’re like snakes,” he observed, “As they follow a curve, the large ones can hardly advance.” D ~ M -2 P. G. de Gennes Nobel Prize Physics Tons per mole! Diffusion P.G. de Gennes Scaling Concepts in Polymer Physics Cornell University Press, 1979 When does the speed of polymers (and stuff dispersed in them) matter? • • • • • How fast can it dissolve? How fast can we process it? How long until the additives ooze out? How long does it take to weld polymers together? How fast do chain termination steps occur during polymeriztion? • How fast will phase separation destroy the polymer? • Will an image on film (remember film?) stay sharp? • Speed Viscosity DLS for Molecular Rheology of Complex Fluids: Prospects & Problems Studied a lot Barely studied + + + Wide-ranging autocorrelators > 10 decades of time in one measurement! – – – Contrast stinks: everything scatters, esp. in aqueous systems or most supercritical fluids, where refractive index matching cannot hide the matrix. Translational Diffusion Leads to Intensity Fluctuations Intensity t Rotational Diffusion Between Polarizers Leads to Intensity Fluctuations Looking into the laser, vertically polarized Polarizer Crystalline inclusion Analyzer dim dim bright Dynamic Light Scattering LASER Uv = q2Dtrans V LASER V H Hv = q2Dtrans + 6Drot q 4n sin / 2 o Uv Geometry (Polarized) Hv Geometry (Depolarized) DLS can be used for sizing if viscosity is known, for viscosity if size is known Large, slow molecules Small, fast molecules Is t DLS diffusion coefficient, inversely proportional to size. Stokes-Einstein Law kT Rh 6πηo Dtrans Dtransh = constant Also Droth = constant Correlation Functions etc. Where: G() ~ cMP(qRg) = q2D q2kT/(6hRh) Rh = XRg g (t ) G ( ) exp( t )d g(t) ILT G() log10t log10D q2D 1/2 c MAP CALIBRATE log10M M Strategy •Find polymer that should (???) “entangle” Dextran •Find polymer that should not “entangle” Ficoll •Find a rodlike probe that is visible in DDLS TMV •Measure its diffusion in solutions of each polymer separately •Random coil •Polysaccharide •Invisible in HvDLS •Highly-branched •Polysaccharide •Invisible in HvDLS •Rigid rod •Virus •Visible in HvDLS BARELY As expected, viscosity rises with c BothViscosity 11 Dextran 670,000 Ficoll 420,000 10 9 8 hsp/c /dL-g -1 7 6 5 4 3 2 1 0 0 5 10 15 20 c/g-dL 25 -1 30 35 40 DIY farming--keeping the “A” in LSU A&M Seedlings Sick Plants And close-up of mosaic pattern. TMV Characterization Sedimentation, Electron Microscopy and DLS •Most TMV is intact. •Some TMV is fragmented –(weaker, faster mode in CONTIN) •Intact TMV is easy to identify –(stronger, slower mode in CONTIN) All measurements made at low TMV concentrations—no self-entanglement nL 0 1 3 6 5 -8 Dr /s Translation Dt /10 cm s -1 500 400 Rotation 3 300 2 Experiments are in dilute regime. 3 TMV overlap (1/L ) 1 200 0 0.0 0.5 1.0 1.5 2.0 c/mg-mL -1 2.5 3.0 2 -1 4 Matrix is invisible TMV + Dextran 215 s acquisition 1.3 g (2) 1.2 1.1 1.0 Dextran >6000 s acquisition 0.9 1E-6 1E-5 1E-4 1E-3 0.01 0.1 1 10 100 Hv correlation functions for 14.5% dextran and 28% ficoll with and without added 0.5 mg/mL TMV t/s g(2) 1.4 TMV + Ficoll 600s aquisition The dilute TMV easily “outscatters” either matrix 1.2 Ficoll >6000 s acquisition 1.0 1E-6 1E-5 1E-4 1E-3 0.01 t/s 0.1 1 10 100 Hey, it works! 4000 3500 /s -1 3000 Hv TMV / Buffer 2500 2000 Uv TMV / Buffer 1500 1000 Hv TMV / Dextran / Buffer 500 0 0 1 2 3 2 10 4 -2 q /10 cm 5 I didn’t think—I experimented. ---Wilhelm Conrad Roentgen Early results—very slight errors 350 6 /10-8cm2 s-1 300 200 trans 150 100 5 4 3 2 D rot D / s-1 250 50 1 0 0 0 2 4 6 8 10 wt% dextran rotation 12 14 16 0 2 4 6 8 10 12 14 16 wt% dextran translation Macromolecules 1997,30, 4920-6. Stokes-Einstein Plots: if SE works, these would be flat. Instead, apparent deviations in different directions for Drot and Dtrans 2 4 6 8 10 12 14 16 1.5 -1 4 -9 hDt /10 g-cm-s hDr /g-cm -s -1 0 1.0 -2 2 0.5 0 0.0 0 2 4 6 8 wt% Dextran 10 12 14 16 At the sudden transition: L/xc.m. ~ 13 and L/x ~ 120 x Dextran overlap 5 10 15 20 9 8 80 6 60 4 40 2 20 h/cP Dr/Dt /10 cm -2 0 xcm L 0 0 0 5 10 15 20 wt % dextran Macromolecules 1997,30, 4920-6. 350 300 rotation 250 D / s-1 200 rot We believed that the transition represented topological constraints. 150 100 It was suggested that more systems be studied. 50 0 0 2 4 6 8 10 BEGIN FICOLL 12 14 16 18 20 22 24 26 28 30 wt% ficoll trans D When we did Ficoll, many more points were added! /10-8cm2 s-1 6 translation 5 4 3 2 1 0 0 2 4 6 8 10 12 14 16 18 wt% ficoll 20 22 24 26 28 30 Huh? Drot still diving in Ficoll? 3.5 0.8 2.5 0.6 -1 rotation 0.5 2.0 0.4 1.5 0.3 1.0 0.2 0.0 0.0 0 5 10 15 20 wt% ficoll 25 30 -1 0.1 -1 0.5 -9 hDrot /g-cm -s -1 0.7 hDtrans /10 g-cm -s 3.0 translation Uh-oh, maybe we should think now. The chiral dextran and ficoll alter polarization slightly before and after the scattering center. With a strongly depolarizing probe, this would not matter, but… rTMV = IHv/IUv ~ 0.003 While matrix scattering is minimal, polarized scattering from TMV itself leaks through a “twisted” Hv setup. Most damaging at low angles Mixing in Polarized TMV Light Uv light from misalign True Hv light 6Drot q2 Drot too low q2 6Drot q2 Even at the highest concentrations, only a few degrees out of alignment. 300 Optical Rotation / arc-minutes 250 Dextran Ficoll 200 150 100 50 0 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 wt % Slight, but important, improvement. NewFicollRatio_PR 350 Right way Wrong way 300 Drot / s -1 250 200 150 100 50 0 0 5 10 15 20 wt% ficoll 25 30 35 Improved Drot/Dtrans Ratio Plots NewDexConcStudy_PR 8 7 7 6 6 Drot/Dtrans/ 10 cm -2 -2 8 5 5 9 9 Drot/Dtrans/ 10 cm NewFic 4 3 2 1 4 3 2 1 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 wt% dextran 0 0 5 10 15 20 25 wt% ficoll 30 35 40 Improved Stokes-Einstein Plots Black = TMV Translation Blue = TMV Rotation NewFicollRatio_PR NewDexConcStudy_PR 5.0 5.0 0.8 4.5 2.0 1.5 0.5 0.6 3.0 2.5 0.4 2.0 1.5 0.2 1.0 0.5 0.0 0 2 4 6 8 10 wt% dextran 12 14 0.0 16 0.0 0.0 0 5 10 15 20 wt% ficoll 25 30 35 -2 1.0 -2 0.2 3.5 -9 0.4 -9 2.5 -1 3.0 hDrot/g-cm s 0.6 hDtrans/ 10 g-cm-s 3.5 -1 4.0 hDtrans/10 g-cm-s -1 -1 4.0 hDrot/g-cm s 0.8 4.5 Hydrodynamic Ratio—Effect of Matrix M at High Matrix Concentration DextranMWStudy_PR 9 8 -2 5 Drot/Dtrans/10 cm 6 9 7 4 3 2 1 0 0 2 4 6 8 10 12 5 14 16 dextran MW/ 10 daltons 18 20 Effect of Dextran Molecular Weight— High Dextran Concentration (~ 15%) TMV Translation TMV Rotation DextranMWStudy_PR DextranMWStudy_PR 100 10 -9 Drot / s -1 2 Dtrans/ 10 cm s -1 -0.62 ± 0.04 -0.72 ± 0.01 1 0.1 10000 100000 1000000 Dextran MW 1E7 10 1 10000 100000 1000000 Dextran MW 1E7 Summary: Depolarized DLS = new opportunities in nanometer-scale rheology. Randy Cush David Neau Ding Shih Holly Ricks Jonathan Strange Amanda Brown Zimei Bu Grigor Bantchev Zuhal & Savas Kucukyavuz--METU Seth Fraden—Brandeis Nancy Thompson—Chapel Hill I cannot tell you the coolest part of this, but postdoc Grigor Bantchev found a trick that is definitely a treat! “Too much dancing and not nearly enough prancing!” Can probe diffusion actually do something? C. Montgomery Burns, “The Simpsons” Matrix Fluorescence Photobleaching Recovery for Macromolecular Characterization Garrett Doucet, Rongjuan Cong, David Neau, Others Louisiana State University Funding: NSF, NIH, Dow Blue input light Green Detected Light Fluorescent Sample Fluorescence & Photobleaching Blue input light Green Detected Light Slowly Recovers Fluorescent Sample With Fluorescence Hole in Middle Recovery of Fluorescence Modulation FPR Device Lanni & Ware, Rev. Sci. Instrum. 1982 SCOPE 5-10% bleach depth IF PA c X TA/PVD * PMT * D S * DM OBJ M RR * M ARGON ION LASER AOM * = computer link Cue The Movie Dextran Diffusion in Hydroxypropylcellulose, a probe diffusion study: the more HPC, the more nonlinearity in semilog plots. Hmmm…. Bu & Russo, Macromolecules, 27, 1187 (1994) Can FPR be used for MWD characterization? Questions bearing on this • Need: are new analytical methods needed in a GPC/AFFF multidetector world? • Ease of labeling the analyte? • How hard to calibrate? • Worth the price of setup? • Miniaturization? GPC •Solvent flow carries molecules from left to right; big ones come out first while small ones get caught in the pores. •Non-size mechanisms of separation complicate regular GPC, are much less of a problem for multidetector methods, but they correspondingly more complicated. They were young when GPC was. Small Subset of GPC Spare Parts To say nothing of unions, adapters, ferrules, tubing (low pressure and high pressure), filters and their internal parts, frits, degassers, injector spare parts, solvent inlet manifold parts, columns, pre-columns, pressure transducers, sapphire plunger, and on it goes… Other SEC Deficiencies • • • • • • • 0.05 M salt at 11 am, 0.1 M phosphate pH 6.5 at 1 pm? 45oC at 8 am and 80oC at noon? Non-size exclusion mechanisms: binding. Big, bulky and slow (typically 30 minutes/sample). Temperature/harsh solvents no fun. You learn nothing fundamental by calibrating. For straight GPC, what you measure is not what you calibrated. Good for qualitative work, otherwise problematic. Must we separate ‘em to size ‘em? Your local constabulary probably doesn’t think so. Atlanta, GA I-85N at Shallowford Rd. A Saturday at 4 pm Molecular Weight Distribution by DLS/Inverse Laplace Transform--B.Chu, C. Wu, &c. Where: G() ~ cMP(qRg) = q2D q2kT/(6hRh) Rh = XRg g (t ) G ( ) exp( t )d g(t) ILT G() log10t log10D q2D 1/2 c MAP CALIBRATE log10M M Hot Ben Chu / Chi Wu Example Macromolecules, 21, 397-402 (1988) MWD of PTFE Special solvents at ~330oC Problems: •Only “works” because MWD is broad •Poor resolution. •Low M part goofy. •Some assumptions required. Matrix Diffusion/Inverse Laplace Transformation Goal: Increase magnitude of —this will improve resolution. Difficult in DLS because matrix log10D Solution: 1/2 D D Matrix: log10M Stretching scatters, except special cases. Difficult anyway: dust-free matrix not fun! Still nothing you can do about visibility of small scatterers DOSY not much better Replace DLS with FPR. Selectivity supplied by dye. Matrix = same polymer as analyzed, except no label. No compatibility problems. G() ~ c (sidechain labeling) G() ~ n (end-labeling) Sample Arbitrary Amplitude The Plan to Measure M Using FPR Collect Data Using FPR 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0.0 -0.1 -0.2 Convert to Molar Mass by Mapping onto Calibration Plot -3 10 -2 10 -1 /s -1 10 0 10 Analyze Using ILT 5 C(t) 4 3 2 1 0 0 200 400 t/s 600 800 1000 Arbitrary Amplitude 6 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0.0 -0.1 -0.2 10 3 10 4 M / Da 10 5 Labeling is Often Easy Pullulan H O H O OH O O OH OH OH O O OH O O OH n OH OH OH OH OH OH OH OH n Dextran M = 2 Million Da as the matrix at different concentrations in 5 mM NaN3 solution O O OH COOH Pullulans of different M labeled with 5-DTAF as probes Cl N N NH N Cl 5-(4,6-dichlorotriazinyl)amino fluorescein Matrix FPR for Pullulan (a polysaccharide) 1 10 5 10 4 M -7 Dapp / 10 cm s 2 -1 10 0.1 NaN3(aq) solution ( = 0.537 ± 0.035) 5% Matrix solution ( = 0.822 ± 0.018) 10% Matrix solution ( = 0.907 ± 0.038) 15% Matrix solution ( = 0.922 ± 0.037) 0.01 4 10 10 5 0.1 1 10 -7 M Probe Diffusion: Polymer physics 2 Dapp / 10 cm s -1 Calibration: polymer analysis GPC vs. FPR for a Nontrivial Case 1.0 12 0.9 10 0.7 Amplitude/Arbitrary Relative Concentration 0.8 0.6 0.5 0.4 0.3 8 6 4 2 0.2 0.1 0 0.0 4 10 M / g mol -1 5 10 PL Aquagel 40A & 50A 10 4 M 10 5 User-chosen CONTIN 25% Matrix only ~1 20,000 & 70,000 Dextran How Good COULD it Be? Simulation of FPR Results for = 2 (Most Desirable Situation) 6 5 y = -0.4998x + 1.1518 log D 0 -2 0 -4 -6 log M 4 2 4 y = -2.0009x + 2.3045 3 2 4 6 8 2 -8 -10 -12 log M 1 0 -10 -8 -6 -4 log D -2 0 What could we separate from 10K, according to = 2 simulations? Shazamm! 10000 MDetected 100000 Sim ula ted M 20000 40000 57000 80000 113000 160000 Using an HPC Matrix Pullulan, 8% HPC Solution, M=12,200 and 48,000 1.0 FArbitrary Units 0.8 CONTIN Exponential Exponential 0.6 0.4 0.2 0.0 1000 10000 100000 M 1000000 Indicates targeted M. MFPR Conclusions We are entitled to expect something better than GPC. For some situations, MFPR could really work. What is good about GPC (straight GPC) is the simple concept; Matrix FPR keeps that—just replaces Ve with D. We haven’t yet addressed two questions --Is it worth setting this up? --Miniaturization/Automation? Macromolecules for The Demented and methods for their study Help from Keunok Yu, Jirun Sun, Bethany Lyles, George Newkome and LSU’s Alz-Hammer’s Research Team Krispy Kreme Donut Day, September 2003 Supported by National Institutes of Health-AG, NSF-DMR and NSF-IGERT • • • • How Alzheimer’s happens Attempts to prevent or reverse it Characterization challenges Alzheimer’s model systems with materials implications Positron emission tomography Age: 20 -- 80 Normal -- 80 AD Postmortem Coronal Sections Normal Alzheimer’s PET images courtesy of the Alzheimer's Disease Education and Referral Center/National Institute on Aging; Postmortem images courtesy of Edward C. Klatt, Florida State University College of Medicine APP = Amyloid Precursor Protein http://www.bmb.leeds.ac.uk/staff/nmh/amy.html APP = the larger, lighter pink one •Transmembrane protein •Normal function not known •Educated guesses May help stem cells develop identity Or help relocate cells to final location May “mature” cells into structural type May protect brain cells from injury Synaptic action Copper homeostasis •Anyway, you need it. •Normal “clipping” of APP by a “secretase” enzyme (in red, and also assumed to be a transmembrane protein) is shown. •There are several secretases, also associated proteins, and they seem to mutate easily: there is a genetic link. •It is not exactly clear why things go awry with advanced age. Amyloid hypothesis: fibrils or protofibrils cause cell death, possibly as the body’s own defenses tries to clear such “foreign” matter. Peter Lansbury Group http://focus.hms.harvard.edu/1998/June4_1998/neuro.html Competing hypothesis: channel formation disrupts Ca+2 metabolism Two FPR Contrast Decay Modes are Often Observed: Fast = small; Slow = large. Contrast / Arbitrary 1 pH 2.7 pH 6.9 pH 11 0.1 0.01 1E-3 1 10 100 t/s 1000 Doing More Experiments Faster with Less Precious Amyloid: Dialysis FPR Pump Exchange Fluid Cover slip Sample PTFE spacer Dialysis membrane O-ring Reversing Amyloid Aggregation…by pH FPR Study: Reversibility of -Amyloid Aggregation 100M 5-CF--Amyloid1-40+ -Amyloid1-40 pH 11 dialysis against 50mM PB pH 7.4 -6 D/10 cm s 2 -1 1E-6 1E-7 dialysis against 50mM PB pH 2.7 1E-8 0 200 400 600 800 1000 1200 1400 1600 1800 Time/min Diffusion from in situ FPR of 5-carboxyfluorescein-A1-40 (25% mixed with unlabeled 75% A1-40) starting at pH 11, then alternately dialyzed between 50 mM phosphate (pH 2.7) and 50 mM phosphate (pH 7.4). Probe diffusion works at fundamental and practical levels. Happy Halloween! M = 10,000 and 20,000 Examples of Separation Results from Simulation Data 2.0 FArbitrary Units 1.5 CONTIN 2 Exponential 1.0 0.5 0.0 1000 10000 M = 10,000 and 160,000 100000 M 2.0 M = 10,000 and 57,000 CONTIN 2 Exponential 1.5 FArbitrary Units 1.5 FArbitrary Units 2.0 CONTIN 2 Exponential 1.0 0.5 1.0 0.0 1000 0.5 10000 100000 1000000 M 0.0 1000 10000 M 100000 Indicates targeted M. Matrix FPR Chromatogram Pullulan, 5%w/w Dextran Matrix, 50/50 mix of 380K and 11.8K 45 40 Sure this is easy. Also easy for GPC. But not for DLS or DOSY! CONTIN Analysis Exponential Analysis Exponential Analysis 35 FArbitrary Units 30 25 20 15 10 5 0 1000 10000 100000 M 1000000 Indicates targeted M. Making the M vs. D calibration is fast & easy } 6 fractions from analytical scale GPC Enough for 100’s of FPR runs in ½ hour Mw/Mn’s as now as good as anionically polymerized, patchy standards. Cong, Turksen & Russo Macromolecules 37(12), 4731-4735 (2004) “Cleanup on Aisle 1” Millipore Centricon Device Pre-poured gel filtration columns are also very useful. Analytical scale GPC itself is a great way to clean up unreacted dye. Millipore Centricon -http://www.millipore.com/userguides.nsf/docs/p99259 Why is the cup half empty? Rg ~ M (0.158 ± 0.002) Rg ~ M R g / nm 10 5 10 Matrix Dextran FD500s FD70 FD40 FD150 1 6 M (0.410 ± 0.005) 10 Half empty, continued 4 M / g mol 10 -1 5 10 1.0 0.9 0.8 0.7 10 D/D 10 Rh / nm x / nm 0 0.6 0.5 0.4 0.3 0.2 0.1 0.0 1 1 0.0 0.1 0.2 0.3 0.4 0.5 0.6 Rh / x 0.1 w dextran (●), and pullulan probes (○). Pullulan (destran similar) 0.7 No wonder the cup is half empty— no plateau modulus! 100 G' / Pa-s 10 1 0.1 0.01 1 10 / Hz 100 Correlations—suggests soft-sphere like behavior from branching of matrix. 50 -5 Scattering / 10 Arbitrary Units 45 40 35 30 25 20 15 10 5 0 0.0 0.1 0.2 0.3 0.4 0.5 2 0.6 -2 q / nm 0.7 0.8 0.9 1.0