1. (10 pts) If a compound with the molecular formula... H NBr
advertisement
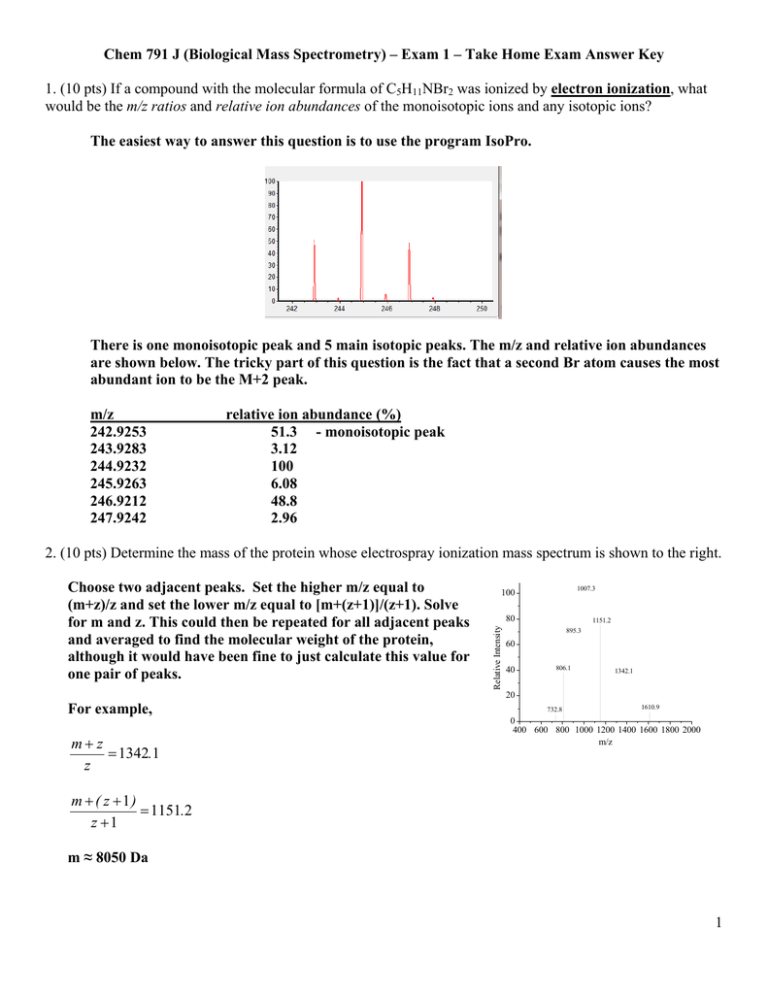
Chem 791 J (Biological Mass Spectrometry) – Exam 1 – Take Home Exam Answer Key 1. (10 pts) If a compound with the molecular formula of C5H11NBr2 was ionized by electron ionization, what would be the m/z ratios and relative ion abundances of the monoisotopic ions and any isotopic ions? The easiest way to answer this question is to use the program IsoPro. There is one monoisotopic peak and 5 main isotopic peaks. The m/z and relative ion abundances are shown below. The tricky part of this question is the fact that a second Br atom causes the most abundant ion to be the M+2 peak. m/z 242.9253 243.9283 244.9232 245.9263 246.9212 247.9242 relative ion abundance (%) 51.3 - monoisotopic peak 3.12 100 6.08 48.8 2.96 2. (10 pts) Determine the mass of the protein whose electrospray ionization mass spectrum is shown to the right. For example, m z 1342.1 z 1007.3 100 80 Relative Intensity Choose two adjacent peaks. Set the higher m/z equal to (m+z)/z and set the lower m/z equal to [m+(z+1)]/(z+1). Solve for m and z. This could then be repeated for all adjacent peaks and averaged to find the molecular weight of the protein, although it would have been fine to just calculate this value for one pair of peaks. 1151.2 895.3 60 40 806.1 1342.1 20 732.8 1610.9 0 400 600 800 1000 1200 1400 1600 1800 2000 m/z m ( z 1) 1151.2 z 1 m ≈ 8050 Da 1 3. (10 pts) Explain why singly-charged protein ions are predominantly formed during MALDI, whereas multiply-charged ions are typically formed during electrospray ionization. Multiply-charged ions are formed in electrospray because the application of the voltage to the spraying needle results in the formation of droplets that have an excess charge. Ions emerge from these charged droplets, and this excess of charge allows multiply-charged ions to emerge. In MALDI, no such voltage is applied to produce species with excess charge. Instead, in MALDI the laser provides enough energy to enable charge separation, but there are an equal number of positive and negative ions produced. Analyte ions emerge with a net charge as a result of this charge separation and eventual proton transfer with the matrix. The lack of excess charge and the short time scale available for proton transfer means that analyte ions are typically produced with only a single charge. 4. (6 pts) You have a sample with one of the following peptides that have the amino acid sequences of VACHETQ and VACHETK. The (M+H)+ ion of the VACHETQ peptide has a monoisotopic m/z of 787.340. The (M+H)+ ion of the VACHETK peptide has a monoisotopic m/z of 787.377. What mass accuracy (in ppm) would be needed to identify which peptide you have? minimum mass accuracy = (Δm / m) x 106 = ([787.377 – 787.340]/[787.377]) x 106 = 47 ppm A mass accuracy just below 47 ppm would be necessary to confirm the identity. A mass accuracy of exactly 47 ppm would still lead to an ambiguous result because one could still measure an ion that is exactly between the m/z of the two peptides, and one would still be unsure about the identity. 5. (10 pts) You have detected a new antibacterial compound that has a m/z ratio of 386.231. From a friend, who knows a lot about mass spectrometry, you learn that a mass accuracy of 1 mmu (millimass unit) would be sufficient to narrow down the number of possible molecular formulas for this compound to 1. Approximately, what is minimum mass resolving power you will need to be sure that the compound of interest is not contaminated by an interfering ion so that you can make your 1 mmu mass accuracy measurement with confidence? The ion of interest would need to be resolved from a potential interfering ion that is 0.001 Da (or greater) away. The approximate minimum resolving power needed would be about 386,000 (m/Δm 386.231 = 386/0.001). Δm = 0.001 potential interfering ion 6. (15 pts) The three methods used to improve resolution in time-of-flight mass analyzers are (i) orthogonal acceleration, (ii) delayed extraction, and (iii) a reflectron. Considering these three methods, explain which of these methods can be combined together in the same instrument. Briefly justify your answers. Orthogonal acceleration and delayed extraction cannot be combined together to further improve resolution. Orthogonal acceleration improves resolution by “turning” the ion beam so 2 that the t ion spreead in spacee and time iss no longer in the direcction of the d detector. Deelayed extra action impro oves resoluttion by acceelerating thee ions after they have spread out in n the direcction of the detector d im mmediately after a ionizattion. If delayyed extractiion is used, subsequentt ortho ogonal accelleration would negate the t advantaage obtained d by delayed d extraction n. Orth hogonal acceeleration an nd a reflectrron can be ccombined toogether to fu urther imprrove resollution. The reflectron r im mproves ressolution by correcting any spread in the kinettic energies of a given g m/z, regardless r of when this spread arisses. Delay yed extractiion and a reeflectron can n be combin ned togetheer to furtherr improve reesolution. The reflectron r im mproves ressolution by correcting aany spread in the kinettic energies of a given m/z regardless r of o when it arrises. Any correction in n the spread d of ion kineetic energiess not achieeved by dela ayed extracttion will be corrected b by the reflecctron, thereby further iimproving resollution. 7. (15 pts) Using g the equatio ons for ax and d qx below, will w an with a m/z of 10000 be successsfully transm mitted throuugh a quadru upole mass analyzer a thatt is operated under the foollowing connditions DC voltaage: 1200 V frequency y = 1.0 MHzz (recall thatt Ω = 2πf) 8 8zeU 4zeV ax = qx = 2 2 mr0 Ω mr02Ω2 RF voltage:: 7200 V r0 = 1 cm Whaat about an io on at m/z 500, will it be transmitted through a quuadrupole m mass analyzerr that is operrated under the aabove condittions? for m/z 1000 1 ax = 0.2345 0 qx = 0.7036 (tthe key to geetting thesee values corrrect is to reccognize thatt ax and qx aare unit-lesss, so the units that you use for th he different values mustt all cancel in the end; also you neeed to use kg g fo or mass and d m for dista ance) d so m/z 1000 paass through h the quadru upole these ax and qx values fall in the stability diagram, for m/z 500 5 ax = 0.469 0 qx = 1.407 these ax and qx values do not fa all in the sta ability diagrram, so m/z 500 does noot pass through the quadrup pole m analyzeers that we taalked about iin class, givee one exampple of a methhod that 8. (12 pts) Consiidering the mass imprroves resoluttion without sacrificing sensitivity. s Briefly B explaain how the rresolution iss improved in this methhod and why y sensitivity is not lost. 3 There arre at least th hree possibiilities here. The reflectron r im mproves ressolution witthout sacrifi ficing sensitiivity by refllecting all ioons. It correects spreadss in ion kineetic energiess, while alsoo increasingg the path length of the time-offlightt analyzer. It I does thesee things witthout losingg ions. Addiing helium to t a quadru upole ion tra ap improvess resolution without saccrificing sen nsitivity. It does this by giving the ions a more con nsistent startting point b before their ejection froom the ion trap to the detecctor. This minimizes m the spread off ions in timee, thereby im mproving rresolution, whilee also impro oving the effficiency of ion i ejection by collapsin ng the ion ccloud to a sm maller radiu us, enabling g fewer ions to be lost. Using g resonancee ejection in n a quadrupole ion trap p improves rresolution w without sacrrificing sensiitivity. It does this by moving m the io ons closer in n space to oon another, so that ionss of the samee m/z are ejeected closerr in time. It does d this wiithout losingg ion signall. 9. (12 pts) Consiider the plot to the right that shows the t relationshhip betw ween resolutiion (or resolv ving power) and m/z forr an FTICR aand an Orbiitrap. Briefly y explain (i)) why the ressolving poweer decreasess for bothh mass analyzzers as m/z ratio r increases and (ii) why w the resollving poweer drops morre quickly att higher m/z ratios in an FTICR. (i) Resolving power decreases at a higher m//z values beecause ion oscilllation frequ uencies decrrease at high her m/z valu ues. Recall th hat maximu um resolving g power is ft/2 ft kly at higherr m/z valuess in an FTIC CR becausee the (ii) Resolving powerr decreases more quick m/z)-1 in an ICR I but onlly (m/z)-0.5 in n an Orbitrrap. frequenccy is proporrtional to (m 4



