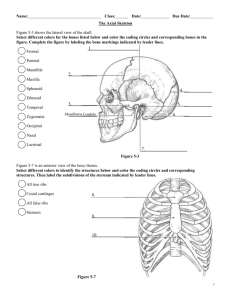
SEXUAL DIMORPHISM IN THE 12 THORACIC VERTEBRA AND ITS
advertisement

SEXUAL DIMORPHISM IN THE 12TH THORACIC VERTEBRA AND ITS POTENTIAL FOR SEX ESTIMATION OF HUMAN SKELETAL REMAINS A Thesis by Meghan Dawn Voisin Honours B.A., Wilfrid Laurier University, 2009 Submitted to the Department of Anthropology and the faculty of the Graduate School of Wichita State University in partial fulfillment of the requirements for the degree of Master of Arts May 2011 © Copyright 2011 by Meghan Dawn Voisin Note: All data used and analyzed in the current manuscript were used with the permission of Dr. Peer H. Moore-Jansen. None of this data or their derivatives may be published elsewhere without the written permission of Meghan Voisin and Dr. Moore-Jansen. All Rights Reserved SEXUAL DIMORPHISM IN THE 12TH THORACIC VERTEBRA AND ITS POTENTIAL FOR SEX ESTIMATION OF HUMAN SKELETAL REMAINS The following faculty members have examined the final copy of this thesis for form and content, and recommend that it be accepted in partial fulfillment of the requirement for the degree of Master of Arts with a major in Anthropology. ____________________________________ Peer H. Moore-Jansen, Committee Chair ____________________________________ Robert Lawless, Committee Member ____________________________________ David T. Hughes, Committee Member ____________________________________ Mary Liz Jameson, Committee Member iii DEDICATION To my parents Michael & Susan Voisin and my fiancé Christopher Kiss Your unconditional love and support has made me into who I am today. iv The whole of a human being is merely a vertebra… ~ Lorenz Oken (1779-1851) v ACKNOWLEDGMENTS I would like to acknowledge and thank numerous individuals for their time, support and assistance in the preparation and successful completion of this thesis. Firstly, I would like to thank Dr. Peer Moore-Jansen, who served as my advisor for the course of my Masters degree as well as the committee chair for my thesis committee. I am and will be eternally grateful for all of the guidance, support and opportunities that he has so generously offered me throughout my time here at Wichita State University. Not only have I gained considerable practical and teaching experiences and skills, but I have also developed and honed my knowledge and understanding of human skeletal biology as a result of participating in and being assigned an abundance of projects that forever kept me occupied and challenged. Thank you, Dr. Moore-Jansen, for your infinite knowledge, support and guidance; the timely completion and success of this thesis could not have been possible without you. I would also like to thank the members of my thesis committee: Dr. Robert Lawless, Dr. David Hughes, and Dr. Mary Liz Jameson. Your efforts, insight and keen editing skills have greatly contributed to the final draft of this thesis. I have also been fortunate to receive funding and financial support which has also greatly offset travel expenditures and influenced the timely completely of this thesis. The Nancy J. Berner Fund through the Wichita State University Department of Anthropology as well as the Moore-Jansen scholarship assisted in funding the research expenses during the data collection portion of my thesis. As a result of the research that went into this thesis, I have had the tremendous experience of traveling to Johannesburg, South Africa and Cleveland, Ohio, U.S.A., in order to use the collections housed at each of these locations. I would like to thank the faculty and vi curator of the Raymond A. Dart Collection of Human Skeletons at the University of Witwatersrand in Johannesburg, South Africa, particularly Brendon Billings and Dr. Jason Hemingway, for access to the collection as well as your assistance, insight and motivation. I would also like to thank Lyman Jellema of the Hamann-Todd Human Osteological Collection at the Cleveland Museum of Natural History for access to the collection as well as your knowledge and insight. This experience would not have been the same had it not been for my fellow biological anthropology graduate students, and I would like to thank them for their motivation, assistance and friendship. Shannon Arney and Janeal Godrey had assisted me with the testing of the reliability of my measurement protocol, which I truly appreciate. I would also like to extend a special thanks to Shannon Arney, Ivy Davis, and James Simmerman…your friendship, reassurance, motivation, and assistance (not to mention company during far too many late night study sessions) has made this academic experience a truly memorable one. I would like to thank my parents, Susan and Michael Voisin, and my sisters, Lauren and Allison Voisin. Your continued support and belief in me has enabled me to meet my goals in all of my academic pursuits. I also wish to thank my soon-to-be family for providing me with the exceptional opportunity to travel to South African and conduct a portion of the research for this thesis. Your hospitality will never be forgotten. Finally, I wish to thank my fiancé, Christopher Kiss, who not only allowed me to venture off to Kansas for two years to pursue a Masters Degree, but provided me with the unconditional love, support, and motivation that made this thesis possible. Please know that your sacrifices do not go unrecognized; I owe my dreams to you. vii ABSTRACT The purpose of this study is to determine the presence/degree of sexual dimorphism of the 12th thoracic vertebra through a quantitative analysis and to further examine its potential and reliability in the sex estimation of human skeletal remains. This study also explores the agerelated changes of human skeletal remains and how these affect morphological variation conducive to sex estimation. In order to assess this, the 12th thoracic vertebrae, femur and sacrum of 168 mature skeletal remains (94 males and 74 females) from the Raymond Dart Collection in Johannesburg, South Africa and 407 (205 males and 202 females) mature skeletal remains from the Hamann-Todd Collection in Cleveland, Ohio were analyzed. Only individuals whose group affiliation was designated as “South African Black” from the Raymond Dart Collection and “African American” from the Hamann-Todd Collection were measured. This was done to permit the examination of geographical variance within and between the two samples. The morphology of the 12th thoracic vertebra was examined by means of univariate and multivariate analyses to better assess each effect. These analyses resulted in relatively high correct classifications of males and females in all samples, with mean measurement values being larger in males in all measurements. While age-related changes have little effect on the high reliability of sex estimation in the African American sample, age-related changes decreases the reliability of sex estimation in the South African sample. Overall, this study reveals that the 12th thoracic vertebra has potential for use in sex estimation as a result of the skeletal morphological variation between males and females both documented in the Raymond A. Dart Collection of Human Skeletons and the Hamann-Todd Osteological Collection. viii TABLE OF CONTENTS Chapter I. Page INTRODUCTION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1 Context in the Field of Anthropology and Biological Anthropology . . . . . . . . . . . . . .1 Statement of Purpose . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2 Research in Skeletal Morphological Variation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4 II. BACKGROUND . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6 Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6 The Vertebral Column: An Anatomical Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . .6 The 12th Thoracic Vertebra: An Anatomical Overview . . . . . . . . . . . . . . . 8 The Sacrum: An Anatomical Overview . . . . . . . . . . . . . . . . . . . . . . . . . . 11 Muscles Associated with the Vertebral Column . . . . . . . . . . . . . . . . . . . .13 The Lower Appendage. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .18 The Femur: An Anatomical Overview . . . . . . . . . . . . . . . . . . . . . . . . . . .18 Muscles Associated with the Femur . . . . . . . . . . . . . . . . . . . . . . . . . . . . .19 Research on the Skeletal Morphology of the Vertebral Column . . . . . . . . . . . . . . . . 22 Research on the Skeletal Morphology of the 12th Thoracic Vertebra . . . .23 Sexual Dimorphism . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24 Sexual Dimorphism in the Human Skeleton . . . . . . . . . . . . . . . . . . . . . . .24 Sexual Dimorphism in the Vertebral Column . . . . . . . . . . . . . . . . . . . . . 25 Sexual Dimorphism in the Sacrum . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .27 Sexual Dimorphism in the Femur . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .29 Age-Related Changes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29 Growth and Development in the Human Skeleton . . . . . . . . . . . . . . . . . .29 Degenerative Age-Related Changes in the Human Skeleton . . . . . . . . . .30 Growth and Development in the Vertebral Column . . . . . . . . . . . . . . . . .31 Degenerative Age-Related Changes in the Vertebral Column . . . . . . . . .33 Temporal Variation and Secular Effects in Biocultural Contexts . . . . . . . . . . . . . . . 35 III. MATERIALS AND METHODS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .38 Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .38 Collection History . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .39 The Raymond A. Dart Collection of Human Skeletons . . . . . . . . . . . . . .39 The Hamann-Todd Osteological Collection . . . . . . . . . . . . . . . . . . . . . . .40 Comparison of Raymond Dart and Hamann-Todd Collections . . . . . . . . 42 Study Samples . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .43 The Raymond A. Dart Collection of Human Skeletons Sample . . . . . . . 44 The Hamann-Todd Osteological Collection Sample . . . . . . . . . . . . . . . . 45 Measurement Protocol . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 45 Traditional Measurements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 47 ix TABLE OF CONTENTS (continued) Chapter Page Non-Traditional Measurements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 48 Statistical Procedures and Analysis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 48 Intra-Observer Error . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 49 IV. RESULTS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .50 Summary Statistics . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 50 Estimation of Sex . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 56 Sex Estimation in the South African Black Sample . . . . . . . . . . . . . . . . .56 Sex Estimation in the African American Sample . . . . . . . . . . . . . . . . . . .61 Sex Estimation in the Pooled Sample . . . . . . . . . . . . . . . . . . . . . . . . . . . .65 Effects of Age-Related Change . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 69 Effects of Age in the 12th Thoracic Vertebra in South African Black Samples . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .69 Effects of Age in the 12th Thoracic Vertebra in African American Samples . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 74 Effects of Age in the Femur in South African Black Samples . . . . . . . . .78 Effects of Age in the Femur in African American Samples . . . . . . . . . . .81 V. DISCUSSION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 85 Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .85 Sex Estimation in the 12th Thoracic Vertebra . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .87 Sex Estimation in the South African Black Sample . . . . . . . . . . . . . . . . .88 Sex Estimation in the African American Sample . . . . . . . . . . . . . . . . . . .91 Sex Estimation in the Pooled Sample . . . . . . . . . . . . . . . . . . . . . . . . . . . .93 Effects of Age in Sex Estimation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 97 Effects of Age in the South African Black Sample . . . . . . . . . . . . . . . . . 98 Effects of Age in the African American Sample . . . . . . . . . . . . . . . . . . 100 Effects of Group Affiliation in Sex Estimation . . . . . . . . . . . . . . . . . . . . . . . . . . . . 102 Summary . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .103 Sexual Dimorphism in the 12th Thoracic Vertebra . . . . . . . . . . . . . . . . .103 Potential of the 12th Thoracic Vertebra in Sex Estimation . . . . . . . . . . .104 Effects of Age on the 12th Thoracic Vertebra . . . . . . . . . . . . . . . . . . . . .105 BIBLIOGRAPHY . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .106 APPENDICES . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 114 A. Data Collection Forms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .115 B. Measurement Protocol . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .117 C. Univariate Summary Statistics . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 123 x LIST OF TABLES Table Page 1. Vertebral Summary Statistics – male sample . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .51 2. Vertebral Summary Statistics – female sample . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 51 3. Vertebral Summary Statistics – South African Black male sample . . . . . . . . . . . . . . . . . . . .52 4. Vertebral Summary Statistics – African American male sample . . . . . . . . . . . . . . . . . . . . . .52 5. Vertebral Summary Statistics – South African Black female sample . . . . . . . . . . . . . . . . . . 53 6. Vertebral Summary Statistics – African American female sample . . . . . . . . . . . . . . . . . . . . 53 7. Femoral Summary Statistics – male sample . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .54 8. Femoral Summary Statistics – female sample . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 54 9. Femoral Summary Statistics – South African Black male sample . . . . . . . . . . . . . . . . . . . . .54 10. Femoral Summary Statistics – African American male sample . . . . . . . . . . . . . . . . . . . . . . .55 11. Femoral Summary Statistics – South African Black female sample . . . . . . . . . . . . . . . . . . . 55 12. Femoral Summary Statistics – African American female sample . . . . . . . . . . . . . . . . . . . . . 55 13. Stepwise models for South African Black sample . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 57 14. Classification rates for step 1 model for the South African Black sample . . . . . . . . . . . . . . .57 15. Classification rates for step 2 model for the South African Black sample . . . . . . . . . . . . . . .58 16. Classification rates for step 3 model for the South African Black sample . . . . . . . . . . . . . . .59 17. Classification rates for step 4 model for the South African Black sample . . . . . . . . . . . . . . .59 18. Classification rates for step 5 model for the South African Black sample . . . . . . . . . . . . . . .60 19. Classification rates for step 6 model for the South African Black sample . . . . . . . . . . . . . . .61 20. Stepwise models for African American sample . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 62 21. Classification rates for step 1 model for the African American sample . . . . . . . . . . . . . . . . .62 xi LIST OF TABLES (continued) Table Page 22. Classification rates for step 2 model for the African American sample . . . . . . . . . . . . . . . . .63 23. Classification rates for step 3 model for the African American sample . . . . . . . . . . . . . . . . .63 24. Classification rates for step 4 model for the African American sample . . . . . . . . . . . . . . . . .64 25. Classification rates for step 5 model for the African American sample . . . . . . . . . . . . . . . . .64 26. Stepwise models for Pooled sample . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .65 27. Classification rates for step 1 model for the Pooled sample . . . . . . . . . . . . . . . . . . . . . . . . . .66 28. Classification rates for step 2 model for the Pooled sample . . . . . . . . . . . . . . . . . . . . . . . . . .67 29. Classification rates for step 3 model for the Pooled sample . . . . . . . . . . . . . . . . . . . . . . . . . .67 30. Classification rates for step 4 model for the Pooled sample . . . . . . . . . . . . . . . . . . . . . . . . . .68 31. Classification rates for step 5 model for the Pooled sample . . . . . . . . . . . . . . . . . . . . . . . . . .68 32. Classification rates for step 6 model for the Pooled sample . . . . . . . . . . . . . . . . . . . . . . . . . .69 33. Stepwise models for vertebral measurements of young South African Black sample . . . . . .70 34. Classification rates for step 1 model for the young South African Black sample . . . . . . . . . 70 35. Classification rates for step 2 model for the young South African Black sample . . . . . . . . . 71 36. Classification rates for step 3 model for the young South African Black sample . . . . . . . . . 71 37. Stepwise models for vertebral measurements of old South African Black sample . . . . . . . . 72 38. Classification rates for step 1 model for the old South African Black sample . . . . . . . . . . . .72 39. Classification rates for step 2 model for the old South African Black sample . . . . . . . . . . . .73 40. Classification rates for step 3 model for the old South African Black sample . . . . . . . . . . . .74 41. Stepwise models for vertebral measurements of young African American sample . . . . . . . .75 42. Classification rates for step 1 model for the young African American sample . . . . . . . . . . . 75 xii LIST OF TABLES (continued) Table Page 43. Classification rates for step 2 model for the young African American sample . . . . . . . . . . . 75 44. Classification rates for step 3 model for the young African American sample . . . . . . . . . . . 76 45. Stepwise models for vertebral measurements of Old African American sample . . . . . . . . . .76 46. Classification rates for step 1 model for the Old African American sample . . . . . . . . . . . . . 77 47. Classification rates for step 2 model for the Old African American sample . . . . . . . . . . . . . 77 48. Classification rates for step 3 model for the Old African American sample . . . . . . . . . . . . . 78 49. Stepwise models for femoral measurements of young South African sample . . . . . . . . . . . .78 50. Classification rates for step 1 model for the young South African Black sample . . . . . . . . . 79 51. Classification rates for step 2 model for the young South African Black sample . . . . . . . . . 79 52. Stepwise models for femoral measurements of old South African Black sample . . . . . . . . . 80 53. Classification rates for step 1 model for the old South African Black sample . . . . . . . . . . . .80 54. Classification rates for step 2 model for the old South African Black sample . . . . . . . . . . . .81 55. Stepwise models for femoral measurements of young African American sample . . . . . . . . .81 56. Classification rates for step 1 model for the young African American sample . . . . . . . . . . . 82 57. Classification rates for step 2 model for the young African American sample . . . . . . . . . . . 82 58. Stepwise models for femoral measurements of old African American sample . . . . . . . . . . . 83 59. Classification rates for step 1 model for the old African American sample . . . . . . . . . . . . . .83 60. Classification rates for step 2 model for the old African American sample . . . . . . . . . . . . . .84 61. Dart Collection Vertebral Measurements – Total Sample . . . . . . . . . . . . . . . . . . . . . . . . . . 123 62. Dart Collection Vertebral Measurements – Male Sample . . . . . . . . . . . . . . . . . . . . . . . . . . 123 63. Dart Collection Vertebral Measurements – Female Sample . . . . . . . . . . . . . . . . . . . . . . . . .123 xiii LIST OF TABLES (continued) Table Page 64. Dart Collection Femoral Measurements – Total Sample . . . . . . . . . . . . . . . . . . . . . . . . . . . 124 65. Dart Collection Femoral Measurements – Male Sample . . . . . . . . . . . . . . . . . . . . . . . . . . . 124 66. Dart Collection Femoral Measurements – Female Sample . . . . . . . . . . . . . . . . . . . . . . . . . 124 67. Dart Collection Vertebral Measurements with Ages – Total Sample . . . . . . . . . . . . . . . . . .124 68. Dart Collection Vertebral Measurements with Ages – Male Sample . . . . . . . . . . . . . . . . . .125 69. Dart Collection Vertebral Measurements with Ages – Female Sample . . . . . . . . . . . . . . . .125 70. Dart Collection Femoral Measurements with Ages – Total Sample . . . . . . . . . . . . . . . . . . 126 71. Dart Collection Femoral Measurements with Ages – Male Sample . . . . . . . . . . . . . . . . . . .126 72. Dart Collection Femoral Measurements with Ages – Female Sample . . . . . . . . . . . . . . . . .126 73. Hamann-Todd Collection Vertebral Measurements – Total Sample . . . . . . . . . . . . . . . . . . 126 74. Hamann-Todd Collection Vertebral Measurements – Male Sample . . . . . . . . . . . . . . . . . . 127 75. Hamann-Todd Collection Vertebral Measurements – Female Sample . . . . . . . . . . . . . . . . 127 76. Hamann-Todd Collection Femoral Measurements – Total Sample . . . . . . . . . . . . . . . . . . .127 77. Hamann-Todd Collection Femoral Measurements – Male Sample . . . . . . . . . . . . . . . . . . . 127 78. Hamann-Todd Collection Femoral Measurements – Female Sample . . . . . . . . . . . . . . . . . 127 79. Hamann-Todd Collection Vertebral Measurements with Ages – Total Sample . . . . . . . . . 128 80. Hamann-Todd Collection Vertebral Measurements with Ages – Male Sample . . . . . . . . . .128 81. Hamann-Todd Collection Vertebral Measurements with Ages – Female Sample . . . . . . . .129 82. Hamann-Todd Collection Femoral Measurements with Ages – Total Sample . . . . . . . . . . 129 83. Hamann-Todd Collection Femoral Measurements with Ages – Male Sample . . . . . . . . . . 129 84. Hamann-Todd Collection Femoral Measurements with Ages – Female Sample . . . . . . . . .129 xiv LIST OF FIGURES Figure Page 1. The Vertebral Column from Anterior, Lateral, and Posterior Views (after Netter 1989) . . . . 7 2. Superior View and Lateral View of a Thoracic Vertebra (after Aiello and Dean 1990) . . . . 10 3. The Posterior and Anterior Views of the Sacrum (after Jacob et al. 1978) . . . . . . . . . . . . . . 11 4. The Superficial Muscles of the Back (after Jacob et al. 1978) . . . . . . . . . . . . . . . . . . . . . . . .14 5. The Intermediate Muscles of the Back (after Jacob et al. 1978) . . . . . . . . . . . . . . . . . . . . . . 16 6. The Anterior and Posterior Views of the Femur (after Aiello and Dean 1990) . . . . . . . . . . .19 7. The Anterior Compartment of the Leg (after Jacob et al.1978) . . . . . . . . . . . . . . . . . . . . . . .20 8. The Medial Compartment of the Leg (after Jacob et al. 1978) . . . . . . . . . . . . . . . . . . . . . . . .20 9. The Posterior Compartment of the Leg (after Jacob et al. 1978) . . . . . . . . . . . . . . . . . . . . . . 20 xv LIST OF PLATES Plate Page 1. Anterior view of a thoracic vertebra . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8 2. Lateral view of a thoracic vertebra . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8 3. Anterior view of the 12th thoracic vertebra . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8 4. Lateral view of the 12th thoracic vertebra . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8 5. Anterior view of a lumbar vertebra . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8 6. Lateral view of a lumbar vertebra . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8 xvi CHAPTER 1 INTRODUCTION Context in the Field of Anthropology and Biological Anthropology The study of sexual dimorphism in the morphology of skeletal remains is beneficial to multiple areas of biological anthropology, including but not limited to studies of human variation and the sex estimation of skeletal remains in past and present human populations. The estimation of sex is a crucial variable in both of these instances, and not only allows for biological reconstructions of past populations to be conducted, but also the identification of unknown human remains through the generation of biological profiles. A variety of skeletal elements are currently used in the estimation of sex of skeletal remains. Most commonly these elements include the pelvic girdle, the skull, as well as the femur. In instances where only few bones are recovered or where the most commonly used elements for sex estimation are damaged or fragmentary, it is crucial to understand the potential of other elements as indicators of sex. Anthropologists working with human skeletal materials must frequently identify the sex of an individual based on fragmented and few elements. In this case, the degree of sexual dimorphism of elements not typically used in sex estimation studies would be useful. The 12th thoracic vertebra is easily identifiable in a disarticulated skeleton as a result of its unique morphology. Its place as a transitional vertebra results in the morphological characteristics of both thoracic and lumbar vertebrae. Thus, the 12 th thoracic vertebra can be identified consistently which is a difficult task in the majority of vertebral elements. Unlike other skeletal elements that are frequently and reliably used in the estimation of sex, the vertebral column is comprised of dense cancelleous bone which assists in the maintenance of its structural 1 integrity throughout taphonomic processes. Thus, there is an increased probability that vertebral elements will be intact and usable for biological profiling purposes if the skeletal elements that are more commonly used, such as the fragile composition of the cranium, are absent or damaged. As the most reliable estimation of factors contributing to the biological profile of an unknown individual is through the use of a complete, or nearly complete, set of human skeletal remains, the biological anthropologist is rarely confronted with this situation, and therefore, must be familiar with a variety of means by which to assess sex from fragmentary and unconventional skeletal elements. Statement of Purpose This study aims to identify and illustrate the presence and degree of sexual dimorphism in the 12th thoracic vertebra through a quantitative analysis and to further examine its potential in the sex estimation of human skeletal remains. Specifically, this research addresses how reliably the sex of human skeletal remains can be estimated using measurements of the 12 th thoracic vertebra. Also, this study explores age-related changes of human skeletal remains and if and how they affect morphological variation conducive to sex estimation. Essential to the study is the hypothesis that measurements of the 12th thoracic vertebra will exhibit size and shape differences between males and females. Indeed, it is suggested that of the measurements obtained of the 12th thoracic vertebra there will be differences exhibited in particular or all measurements. In addition to the measurements of the 12th thoracic vertebra, various dimensions of the sacrum and the femur are also examined. Since the sacrum has a positive correlation with the sex of an individual (Moore-Jansen and Plochocki 1999) and the femur’s use as a reliable 2 indicator of the stature of an individual (Krogman and İşcan 1986), both of these elements are measured in order to determine the potential and reliability associated with the estimation of sex of unidentified human skeletal remains. This study has pertinent and beneficial applications to the subfields of biological and archaeological anthropology. Not only would this study assist in the sex estimation of unknown human skeletal remains, which has forensic, archaeological and paleoanthropological implications, but also in exploring and increasing the capacity for the understanding of morphological variation in human populations. Through the use of the measurements of the 12th thoracic vertebra, sacrum and femur, this study explores the morphological variability between and within males and females of South African and American Black samples. While prior research has been conducted on living populations with the use of three-dimensional computer tomographic image reconstructions of the 12th-thoracic vertebra (Sheng-Bo Yu et al. 2008) and radiographs (Taylor and Twomey 1984), the present study relies on the measurements obtained using skeletal material. By assessing the presence and degree of variability in Black males and females in two samples, Raymond A. Dart Collection of Human Skeletons and the Hamann-Todd Osteological Collection, the present study not only explores the variability present between the sexes, but also between and within the two geographically distinct samples. This not only facilitates the estimation of sex through the use of the 12th thoracic vertebra, but also assesses the degree to which geographical variation impacts sex estimation. Along with the potential of the 12th thoracic vertebra in the estimation of sex in human skeletal remains, this study also explores the effects of age-related changes, geographical variation, as well as secular effects that play a role in the reliability of creating a biological profile. 3 Research in Skeletal Morphological Variation To assess and estimate the sex of unidentified human skeletal remains, a combination of traditional qualitative and quantitative methods of analysis are usually employed to known reliable skeletal elements – namely the cranium, pelvic girdle, and long bones, if they are present and usable. Although previous research has been conducted on the morphological variations between males and females of the 12th thoracic vertebra (Sheng-Bo Yu et al. 2008; Taylor and Twomey 1984), these studies were performed through the utilization of various technologies, such as radiographs and computer tomography as previously mentioned, and were not performed directly on human skeletal material. Also, previous research had been conducted on single population and group affiliations (Sheng-Bo Yu et al. 2008), none of which addressed the morphological variation between populations and how this affects the morphological variation within and between the sexes. Since the components of a biological profile of an individual all require an examination of skeletal morphology and the variation inherent within that morphology, the biological anthropologist must bear in mind the influences each of these may have on the reliability of each individual component of biological profiles. There is has been a considerable amount of research done on the effects of aging on the human skeleton and how this may influence the reliability of the creation of biological profiles (Cardoso and Ríos 2011; Scheuer and Black 2000). In order to account for any age-related changes in the measurements of the samples used in the present study, the sample was divided into two age groups to account for any differences in morphology that may have been influenced by aging processes. Further, to account for any discrepancies of the size and shape morphology of the 12 th thoracic vertebra, two measurements of the femur were taken to act as a control against the stature and size of the individual. 4 Morphological variation in the human skeleton is also largely attributed to group affiliation, or the geographical location and ethnic background of an individual or group (Giles 1966). This study assesses the morphological variation within a sample and between two geographically different samples in order to assess how group-affiliated skeletal variation affects the reliability of sex estimation. The impact of group affiliation is reduced in this study by only using specimens documented socially as of a Black group affiliation. By collecting data from a South African Black sample and an American Black sample, the effects of geographical variation can be analyzed. Finally, the samples included in this study only contain individuals that were born in select years (with a preference of a ten year birth range when possible) to account for potential morphological variation that may be the result of non-biological change. The following chapters consist of the background, materials and methods, results, and discussion. The second chapter is that of the background and will provide an overview of the anatomy and associated muscles of the skeletal elements that are used in this study, address the concept of sexual dimorphism, and explore relevant past research conducted on the skeletal elements used in this study. A general overview of age-related changes in the human skeleton and the vertebral column will also be provided, and the concepts of temporal variation and the effects of secular change will be addressed and explored. The third chapter consists of the materials and methods portion. It is comprised of a brief history of both collections used in this study, the composition of study samples, the development of the measurement protocol, an overview of the measurements employed, and the means of analysis and intra-observer errors will also be contained in this chapter. The fourth chapter is comprised of a report of the results of all statistical analyses conducted for this study, while the fifth and final chapter explores and discusses these results in association with the purpose of this study. 5 CHAPTER 2 BACKGROUND Introduction In order to successfully account for any distortions in the results of this study, the biases and influences involved in the estimation of sex of human skeletal remains are discussed. This chapter consists of an overview of the skeletal anatomy, musculature associated with and affecting the skeletal elements in question, as well as the effects of skeletal morphological variation associated with sexual dimorphism of the human skeleton and the specific elements of the 12th thoracic vertebrae, sacrum and femur. The patterns of growth and development as well as the degenerative effects of aging and other age-related changes are examined in terms of the morphological effects on the skeletal elements of this study. Finally, a conspectus of other relevant research conducted on the vertebral column and femur, and the morphological variation associated with temporal variation and secular change are discussed. The Vertebral Column: An Anatomical Overview The vertebral column is comprised of four segments: cervical vertebrae, thoracic vertebrae, lumbar vertebrae, and sacral vertebrae (Figure 1). The cervical vertebrae are positioned most superiorly, with the first cervical vertebra (also commonly referred to as atlas) articulating with the occipital bone at the base of the skull. This portion of the vertebral column entails seven vertebrae which are located in the neck region and terminate with the seventh cervical vertebra at the base of the neck. The vertebral column then transitions into the thoracic vertebrae which are composed of twelve vertebrae. These vertebrae were named after their anatomical region, the thorax, and each of these vertebrae contain the morphological features of 6 costal facets, or areas where a rib articulates with a vertebra. As a result of this, the twelve vertebrae in this portion of the vertebral column are congruent with the number of ribs in the human skeleton. The portion of the vertebral column that is located in the lower back region are the lumbar vertebrae, which consist of five vertebrae. Lastly, the sacral vertebrae range from four to six in number and fuse (complete fusion normally occurs by around 25 years of age, but can deviate slightly from this due to human variation) together to form the sacrum (White et al. 2011). The vertebral column as a whole serves several purposes. Firstly, it is the “back bone” of the skeleton and as such, it not only functions to hold the skeleton erect, but serves as an anchor for several crucial muscle groups, which allow for the majority of human movement, from locomotion to sitting upright to carrying items (Aiello and Dean 1990). Also, the vertebral column houses and acts as a protective barrier to the spinal cord, as well as numerous cranial nerves and the vertebral arteries. Figure 1. The Vertebral Column from Anterior, Lateral, and Posterior Views. After Netter 1989:150. 7 The 12th Thoracic Vertebra: An Anatomical Overview The 12th thoracic vertebra is unique in comparison to other thoracic vertebrae as it contains morphological characteristics not seen in the other eleven vertebrae in this portion of the vertebral column. These unique morphological characteristics are the result of this vertebra being a “transitional vertebra”, meaning that it is the last vertebra in the thoracic vertebral segment and positioned between the 11th thoracic vertebra above and the 1st lumbar vertebra below (Schwartz 2007). As a result of this, the 12th thoracic vertebra contains a combination of morphological characteristics, of which specific characteristics can typically only be found within their respective vertebral segment (Plates 1-6). Plate 1. Anterior view of a thoracic vertebra Plate 2. Lateral view of a thoracic vertebra Plate 3. Anterior view of the 12th thoracic vertebra Plate 5. Anterior view of a lumbar vertebra Plate 4. Lateral view of the 12th thoracic vertebra Plate 6. Lateral view of a lumbar vertebra 8 There are several morphological characteristics that are specific only to thoracic vertebrae (Figure 2) as a result of the region that these vertebrae are located in and its associated functions. Since these vertebrae are located in the thorax region, it is only these vertebrae that are associated with the ribs. Each rib articulates in two places on each of the thoracic vertebrae: the lateral faces of the centum or vertebral body, and the transverse processes. The location where the rib articulates with the thoracic vertebra can be visually observed and identified as a result of the feature formed to accommodate this articulation. These features are referred to as costal facets, where costal refers to the features origins, the ribs, and facet being the classification type of the feature (Matshes et al. 2005). Other morphological characteristics specific to the thoracic vertebral segment are the shape and orientation of the superior and inferior articular facets. In this case, both the superior and inferior articular surfaces are flat and parallel to each other. The superior articular surfaces face dorsally, while the inferior articular surfaces face anteriorly. With the shape and orientation of the articular facets being the same on all of the thoracic vertebrae, this allows for the flat and parallel superior articular facets of one thoracic vertebra to fit against, or articulate with the flat and parallel inferior articular facets of the thoracic vertebra above and vice versa (Cox and Mays 2000). This differs from the lumbar vertebral portion of the vertebral column which follows the thoracic vertebrae. In the lumbar vertebrae, the superior articular facets are curved, facing both dorsally and medially, whereas the inferior articular facets are also curved and can be observed when viewing the vertebrae laterally. In this case, the dorsal facing and medially oriented superior articular facets of one lumbar vertebra allow for the articulation of the laterally oriented inferior articular facets of the lumbar vertebra immediately superior to it (White et al. 2011). The 12th thoracic vertebra is unique in terms of its articular facet orientation. The superior half of the 12th thoracic vertebra exhibits 9 typical thoracic vertebral characteristics, with the superior articular facets being flat, parallel and oriented dorsally. The inferior half of the vertebra, however, exhibits lumbar characteristics, with the inferior articular facets being curved laterally (Matshes et al. 2005). This allows for the 12th thoracic vertebra to articulate with the 11th thoracic vertebra superiorly and the 1st lumber vertebra inferiorly. The 12th thoracic vertebra also exhibits the thoracic characteristic of a costal facet for the articulation with the 12th and final rib (Aiello and Dean 1990). Finally, the morphology of the spinous process is intermediate to those typically portrayed by the thoracic and lumbar vertebral columns, with it being more horizontal than the inferiorly oriented thoracic spinous processes, but is not yet at the same horizontal plane as the typical lumbar vertebrae (White et al. 2011). It is the result of this combination of thoracic and lumbar traits that classifies the 12th thoracic vertebra as a transitional and “lumbarized” vertebra, making it unique and easily discernable while amongst other vertebrae (Matshes et al. 2005; Cox and Mays 2000). Figure 2. Superior View (left) and Lateral View (right) of a Thoracic Vertebra. After Aiello and Dean 1990:276. 10 The Sacrum The sacrum comprises the final segment of the vertebral column (Figure 3). It is located in the pelvic region, and the sacrum together with the right and left os coxae compose the pelvis girdle. Even though the sacrum is no longer located in the back region like the other vertebrae, it is still classified as a portion of the vertebral column as it carries out the same functions; it houses and provides a protective barrier for the spinal cord, and allows for various blood vessels and nerves to transport through this area (Cox and Mays 2000; White et al. 2011). It is in the sacrum that the spinal cord terminates, with the cauda equina and filum terminale, or the most distal portion of the spinal cord, anchoring to the distal portion of the sacrum (Netter 1989; Carter 2000a&b). In this case, the sacrum also functions to secure the spinal cord. Figure 3. The Posterior (left) and Anterior (right) Views of the Sacrum. After Jacob et al. 1978:114. In a mature adult, the sacrum is visibly different from the preceding vertebral elements. Even though the sacrum is comprised of several vertebrae, these individual vertebrae fuse throughout one’s childhood, with complete or nearly complete fusion occurring in early 11 adulthood – approximately 25 years of age, with females typically being slightly earlier than that of males (Belcastro et al. 2008). As a result of this, there are 4-6 (5 is most typical) sacral vertebrae, however, they are fused together to form a single bone in a skeletally mature individual (Schwartz 2007). While having similar morphological features to those of the other vertebral segments, the sacrum also has features that are unique to this skeletal element. Each vertebral element in the sacrum has a centrum, or vertebral body, and a neural arch composed of a right and left pedicle and a spinous process. These vertebrae also have the features of the superior and inferior articular surfaces, which not only allow the vertebrae to articulate with the element above and below it, but it is also a point of fusion (Cox and Mays 2000). All of the morphological characteristics mentioned above are the same as those exhibited in regular vertebrae. Apart from these, the most superior portion of the sacrum is referred to as the promontory which is the portion of the sacrum that articulates with the fifth lumbar vertebra above. Also, whereas in the upper three segments of the vertebral column the vertebrae ascend in size, with the first few cervical vertebrae being the smallest overall and the following vertebrae progressively increasing in size, with the largest vertebrae being the last few vertebrae in the lumber vertebral segment, this typical pattern of vertebral size increase is reversed in the sacrum (Matshes et al. 2005). In this case, the first sacral vertebra is the largest and the following vertebrae decrease in size until the smallest vertebrae, which is the one most distal. Since the largest sacral vertebrae are located at the proximal portion of the sacrum, the lateral portions of the bone (known as the transverse processes in other vertebrae) are also significantly larger in this area. It is the result of this that the alae, or wings of the sacrum, are formed which is the area that articulates with the right and left os coxae; an articulation referred to as the sacroiliac (SI) joint which enables the formation of 12 the pelvic girdle (Aiello and Dean 1990; White et al. 2011). The fusion of the sacral vertebrae also allows for the formation of foramen, or natural forming holes that allows arteries, veins and nerves to run through and provide nutrients to the bone, on either side of the vertebral bodies. In an anatomically typical sacrum that has 5 vertebrae there will be four foramena on either side of the sacrum which allows for the passage of the four sacral nerves, which together compose the nerve network of the sacral plexus (Carter 2000a&b; Matshes et al. 2005). The sacrum ends at the inferior surface of the fifth, or most inferior sacral vertebra where it articulates with the first coccygeal vertebra, which is the most superior portion of the coccyx and is more commonly referred to as the “tailbone”. Muscles Associated with the Vertebral Column There are a number of muscles that originate and insert on aspects of the vertebral column in order to support, contract, flex and rotate the back region, as well as to allow for the movements associated with everyday life, such as turning the head and walking. The muscles of the back region that are associated with the vertebral column are classified by layers: the superficial layer (Figure 4), or the muscles closest to the surface of the back, the deep layer, or the muscles in closest proximity to the vertebral column, and the intermediate layer which are those muscles sandwiched between the deep and superficial muscle layers (Carter 2000a&b). 13 Figure 4. The Superficial Muscles of the Back. After Jacob et al. 1978:19. The superficial layer of muscles associated with the vertebral column (Figure 4) consists of two large muscles: the trapezius and the latissimus dorsi. The trapezius is a large trapezoidalshaped muscle located in the neck to mid-back which functions to stabilize and retract the scapula, as well as enabling the upward rotation and abduction of the arms (McMinn and Hutchings 1977). While the trapezius inserts on portions of the clavicle and scapula, this muscle originates from various features of the occipital bone of the cranium, as well as the spinous processes from the last, or seventh, cervical vertebra down to the twelfth thoracic vertebra. The 14 latissimus dorsi, a large broad muscle in the mid to lower back, has several functions, including: stabilization of the shoulder, adduction, medial rotation, flexion, and extension of the arms. Not unlike the trapezius, the latissimus dorsi inserts on a bone not associated with the vertebral column, the humerus, but originates from the os coxae, ribs and the spinous processes of the seventh thoracic vertebra, all of the lumbar vertebrae, as well as the first sacral vertebra (Carter 2000a&b; Hiatt and Gartner 1987). There are seven muscles which comprise the intermediate layer of muscles (Figure 5): the rhomboideus major, rhomboideus minor, levator scapulae, serratus posterior superior, serratus posterior inferior, and the two splenius muscles. Both, the rhomboideus major and rhomboideus minor function together to retract the scapula by elevating the medial border of the scapula (Carter 2000a&b; Tortora 1983). The rhomboideus minor is smaller and lies just superior to the rhomboideus major, but both muscles lie directly underneath of the trapezius. Both rhomboids insert on the scapula, but the rhomboideus major originates from the spinous processes of the second to fifth thoracic vertebrae, whereas the rhomboideus minor originates from the spinous processes of the seventh cervical vertebra and first thoracic vertebra, as well as a feature on the occipital bone of the cranium (Steele and Bramblett 1988; Netter 1989). The levator scapulae muscle also lies underneath of the trapezius and functions to elevate the scapula from its medial angle. Like the rhomboids, the levator scapulae inserts on the scapula, but originates from the transverse processes of the first through to the fourth cervical vertebrae. Together, the serratus posterior superior and serratus posterior inferior muscles function to expand and increase the capacity of the thorax, which allows for deep breathing (Carter 2000a&b; McMinn and Hutchings 1977). This occurs as the serratus posterior superior elevates the upper ribs while the serratus posterior inferior depresses the lower ribs, thus increasing the volume of the thorax 15 region and providing for an increased quantity of air during periods of deep inhalation. The serratus posterior superior originates from the spinous processes of the seventh cervical to the third thoracic vertebrae and the occipital bone, inserts on the second to fifth ribs and lies underneath the rhomboids in the upper back area (Netter 1989). The serratus posterior inferior muscle originates from the eleventh thoracic to the second lumbar vertebrae, inserts on the ninth through to the twelfth rib and lies underneath the latissimus dorsi in the lower back. Figure 5. The Intermediate Muscles of the Back. After Jacob et al. 1978:20. Finally, there are two splenius muscles which lie underneath the trapezius in the neck region, the splenius capitis and splenius cervicis. Together these muscles function to extend the head as well as the lateral rotation and flexion of the neck which enables the head to turn to the side 16 (Tortora 1983). Both splenius muscles originate from the occipital bone as well as the spinous processes of the seventh cervical through to the sixth thoracic vertebrae. The splenius capitis, however, inserts onto the occipital bone and the mastoid process of the temporal bone, while the splenius cervicis inserts onto the transverse processes of the first to the third cervical vertebrae (Carter 2000a&b; Hiatt and Gartner 1987). Lastly, the deep layer of muscles associated with the vertebral column are referred to as intrinsic muscles of the back and are comprised of two major groups of muscles: the three muscles classified as the erector spinae muscles, and the three muscles classified as the transversospinalis muscles. As the name implies, the erector spinae muscles function to hold the vertebral column upright or extended, and consist of (from most medially located to most laterally located): the spinalis, longissimus and iliocostalis muscles (Carter 2000a&b). All three of the erector spinae muscles have the similar points of origination of the lumbar vertebrae, ilium, and the sacrum. Their points of insertion, however, are slightly different with the spinalis inserting onto the spinous processes of the vertebra, the longissimus inserting onto the ribs, mastoid process and transverse processes of the cervical vertebrae, and the iliocostalis inserting onto the ribs and the fourth through sixth cervical vertebrae (Hiatt and Gartner 1987). The transversospinalis muscles consist of three muscles located in layers one atop of the other. The most superficial of the transversospinalis muscles is the semispinalis muscles which allow the back to bend laterally and control the degree of flexion in the back. The intermediate layer is the multifidus muscles which function in the lumbar region to maintain lumbar lordosis by extending the spine (Tortora 1983). The last and deepest layer contains the rotatores muscles which allow for the rotating of the back in the thoracic region. All three of these layers of muscles originate on the spinous processes and insert on the transverse processes of the vertebrae, but in a slightly 17 different arrangement which enables each to perform their particular function. The semispinalis muscles insert on the transverse processes of every fifth vertebrae, the multifidus muscles on the transverse processes of every third vertebrae, and the rotatores on the transverse processes of every vertebrae (Carter 2000a&b; Hiatt and Gartner 1987). The Lower Appendage The Femur: An Anatomical Overview The femur (Figure 6) is the bone in your upper leg, often referred to as the “thigh bone” and is the largest bone in the human body. The femur is part of the appendicular skeleton and is classified as a long bone. Long bones occur in the appendicular skeleton and contain two epiphyses or areas where a growth plate occurs in younger individuals until they are skeletally mature. These epiphyses are located at a proximal end of a bone (usually where the “head” of the bone occurs) and at the distal portion of the bone. In between the two epiphyses lies the diaphysis, or shaft of the bone (Cox and Mays 2000). In the case of the femur, the proximal epiphyseal end of the bone contains the femoral head, which fits into the acetabulum of the os coxa to form the ball-and-socket joint commonly referred to as the hip. This portion of the bone also contains two features – the greater and lesser trochanters, which are attachment sites for several quadriceps and hamstring muscles (Netter 1989). The distal epiphyseal end of the femur forms the top portion of “the knee”. It is here that the femur articulates with the proximal end of the tibia, which together forms the hinge joint known as the knee. There is a concave circular feature present at the distal end of the femur which identifies the articulation location of the 18 patella, or the “knee cap”, which is a heart-shaped sesamoid bone (White et al. 2011). The femur is an integral bone of the human skeleton, as its morphology enables a striding, habitual bipedal gait, it supports the body when upright and bears and distributes the majority of weight through the legs when upright (Aiello and Dean 1990). Figure 6. The Anterior (left) and Posterior (Right) Views of the Femur. After Aiello and Dean 1990:401. Muscles Associated with the Femur There is an amplitude of muscles associated with the femur that all contribute to the actions of flexion, extension, abduction, adduction, and medial and lateral rotation of the thigh and leg. The muscles of the thigh are categorized based on location, with them being divided into the muscles of the anterior (Figure 7) and medial thigh (Figure 8) as well as those of the posterior thigh (Figure 9). The anterior and medial thigh are further subdivided into two compartments: the anterior compartment and the medial compartment, both of which are 19 anatomically divided by fascia, or a segment of connective tissue; while the posterior thigh is regarded as only one compartment (Carter 2000a&b). Figures 7 – 9. The Anterior Compartment (Fig. 7, left), the Medial Compartment (Fig. 8, center), and the Posterior Compartment (Fig. 9, right). After Jacob et al. 1978:188-190. The anterior compartment of the anterior and medial thigh consists of the quadriceps femoris muscle group and the sartorius muscle. The quadriceps femoris muscle group consists of four muscles: the rectus femoris, vastus lateralis, vastus medialis, and the vastus intermedialis (McMinn and Hutchings 1977). All four muscles insert via the patellar tendon to the patella and all muscles, with the exception of the rectus femoris which originates from the ilium, originate along the diaphysis of the femur. The quadriceps femoris muscles work together to allow for the flexion and extension of the leg. The sartorius muscle, also contained within the anterior 20 compartment, crosses over the quadriceps femoris muscles from its lateral origin on the ilium to its medial insertion on the tibia. As a result of its origin and insertion locations, the sartorius crosses over both the hip and knee joints which allow this muscle to carry out the actions of the flexion of the thigh and the leg (McMinn and Hutchings 1977; Carter 2000a&b). The second compartment of the anterior and medial thigh is the medial compartment, which consists of six muscles: the gracilis, pectineus, adductor longus, adductor brevis, adductor magnus, and the obturator externus. All of these muscles function to medially rotate the hip and adduct the thigh and leg, originating from the os coxae and inserting either on the femur or the tibia. There are, however, two muscles in this compartment which also enable other functions. In addition to being an adductor, the gracilis muscle, being the only muscle in the medial compartment to cross both the hip and knee joints, also allows for the flexion of the leg. Additionally, the obturator externus muscle also acts as a stabilizer for the hip joint and enables the lateral rotation of the thigh (Carter 2000a&b). The posterior thigh is a single compartment that consists of the hamstring muscles, gastrocnemius, popliteus, and plantaris muscles. There are three muscles that comprise the hamstrings: the semitendinosus, semimembranosus, and the biceps femoris. All of these muscles have a common origin, being the ischium and all enable the actions of the extension of the thigh and flexion of the leg. Both, the semitendinosus and semimembranosus insert onto the tibia, while the biceps femoris inserts onto the fibula. The gastrocnemius, plantaris and popliteus muscles all originate from the distal portion of the femur, but have different insertion locations and functions (Netter 1989). The gastrocnemius (commonly referred to as the “calf” muscle) inserts on the calcaneus, which is the bone that makes up the heel of the foot, and assists in the flexion of the leg and plantar flexion of the foot. The plantaris muscle inserts on the calcaneus 21 via the calcaneus tendon and is an unnecessary muscle as it has no real function, and as a result is often harvested for tendon repairs or transplants if needed. Finally, the popliteus muscle inserts on the posterior surface of the tibia and performs the actions of the flexion of the leg and the medial rotation of the tibia (Carter 2000a&b). Research on the Skeletal Morphology of the Vertebral Column Previous research has demonstrated that elements of the vertebral column yield morphological variation, particularly variation due to sex and age differences. Taylor and Twomey (1984) examined 166 lateral radiographs taken of juvenile and adolescent individuals of both sexes, which were then measured for height and transverse diameter for the 12 th thoracic vertebra through to the 3rd lumbar vertebra. Their study revealed that female vertebrae were overall smaller than males, with this difference being marginal at a young age and increasing throughout adolescence. It has also been shown that the vertebral bone densities, dimensions and cross-sectional areas of the first through third lumbar vertebrae exhibit differences between males and females. These differences begin in childhood, increase throughout growth and development processes, and reach the greatest disparity upon sexual and skeletal maturity (Gilsanz et al. 1994b; 1997). When studying the effects of age as is reflected in the changes of the vertebral body of the lumbar vertebrae, Allbrook (1956) found that the height of the vertebral bodies shows an increase in the measurements with age. This increase is also greater in males than in females. Age-related changes are also observed in the increased transverse breadths of the vertebral bodies. It has been suggested that this increase in the transverse breadth of the vertebrae is not simply degeneration due to aging, but also bears a functional significance as this broadening 22 would provide stability in the face of cancelleous bone loss (Ericksen 1974; 1976). It has also been suggested that the osteophytic growths, which increase the breadth of the vertebral bodies, grow in response to the increase in stress placed on the vertebrae as aging renders the intervertebral discs less adequate to absorb this stress. As a result, this broadening of the vertebral bodies are a compensation mechanism to alleviate some stress via a larger and broader support base (Hadley 1964). The aforementioned research all demonstrates the value of aspects of the vertebral column for use in the estimation of sex as a result of the inherent morphological variation. Research on the Skeletal Morphology of the 12th Thoracic Vertebra There has been considerable progress made with quantitative studies involving the degree of sexual dimorphism as well as the potential of non-standard elements and their use for sex estimation. One such study was carried out by Sheng-Bo Yu et al. (2008) and involved 33 measurements which were applied to three-dimensional models of 12th thoracic vertebra of 102 Korean individuals. Using discriminant function analysis, they determined that 3 measurements of the 12th thoracic vertebra provided a 90% accuracy of sex within the Korean sample. This study is useful in acknowledging the potential for sex estimation using this element, however, Sheng-Bo Yu et al. stipulates that the accuracy in this study may only be reflective of a Korean population, since no other populations were used. Therefore, other geographically variant samples must be incorporated into studies to also assess the effects of group affiliation and geographical variation on 12th thoracic vertebral morphology. As mentioned above, Taylor and Twomey (1984) demonstrated that the maximum length and height of the 12 th thoracic vertebral 23 body in children revealed a disparity between males and females with males being overall larger and this trend only being increased throughout adolescence. While both of these studies exemplify sexual dimorphism and the potential for the estimation of sex in the 12th thoracic vertebra, they utilized radiographs and computed tomography scans rather than utilizing and testing on a human skeletal collection sample as is the case in the present study. Sexual Dimorphism Sexual Dimorphism in the Human Skeleton While the human skeleton as a whole has a relatively small degree of sexual dimorphism in terms of size differences between the sexes in comparison to other non-human primates, there are various skeletal elements with comparatively significant differences between males and females as a result of the differences in function, muscular development and general size and shape morphological variations. Essentially, sexual dimorphism is the size and shape differences between males and females. While all mammals exhibit varying degrees and patterns of sexual dimorphism, the pattern associated with modern humans is that males tend to be larger than females. These differences stem primarily from functional differences between the sexes and overall robusticity. Females tend to be more gracile, with less prominent muscle attachment sites and a broader pelvic girdle to accommodate for the function of rearing children. Males tend to be larger overall, with larger and more prominent muscle attachment sites, larger and more robust skeletal elements and a larger mean stature. This is speculated to be the result of the male’s innate function to protect and ensure the survivability and reproductive success of a 24 population (France 1988). Sexual dimorphism, if present, is usually minor in children with the disparity between males and females increasing throughout adolescence, and the greatest difference between the sexes being once sexual and skeletal maturity is reached (Gilsanz et al. 1994b; 1997). Sexual dimorphism in the human skeleton can be found in varying degrees in the majority of bones with the most common morphological variation due to the disparity of size in males and females. Males tend to be more muscular overall than females, and if certain muscles are consistently in use in males, for example, but not in females, this would increase the distance between the differences in morphology between the sexes (France 1988; McCarter 2006). The more pronounced muscularity in males is reflected on skeletal elements via the muscle attachment sites; increased muscularity and robustness in males of a population presents larger and more prominent muscle attachment sites than females of the same population (France 1988). Sexual Dimorphism in the Vertebral Column Gilsanz et al. (1994b) conducted research concerning the sexual dimorphism present in various aspects of the first through third lumbar vertebrae. Vertebral bone densities, dimensions and cross-sectional areas were obtained via computed tomography scans to assess the differences between the sexes in individuals between the ages of four and twenty that were categorized in groups based on chronological age, bone age, weight, and height. The results of Gilsanz’s research showed that all variables were greater in males with minor differences appearing in childhood and increasing throughout adolescence, with the greatest disparity between males and females being once sexual maturity was reached (Gilsanz 1994b). This study revealed no differences in the heights of the vertebral bodies at any age between the sexes (Gilsanz et al. 25 1994b; 1997). Taylor and Twomey (1984) also discovered that sexual dimorphism was apparent in a child’s thoracic and vertebral column. Through the utilization of radiographs, it was demonstrated that the vertebral bodies of females in the sagittal plane were more slender than males of the same age, weight and height from eight years of age. This difference between the sexes only increased with age (Taylor and Twomey 1984). Through the utilization of computed tomography scans of a living Korean population, the sexual dimorphism of the 12th thoracic vertebra has also been shown to have statistically significant results (Sheng-Bo Yu et al. 2008). Studies (Marino 1995; Wescott 1999) have also demonstrated the reliability in estimating the sex of an individual through sexually dimorphic quantitative measurements of atlas, or the first cervical vertebra. Since the first cervical vertebra articulates with the occipital condyles of the cranium, a load-bearing region, it was hypothesized that the function of the first cervical vertebra would result in morphological differences in the sexes of a population. These findings were applied by Marino (1995) to a test sample of the Terry Collection with the sex estimation accuracy falling between 75-85%. Wescott (1999) also demonstrated that the second cervical vertebra, as well as the first, could estimate the sex of an individual to a similar reliability as using any single traditionally used skeletal element. A study of the sexual dimorphism of the vertebral neural canal concluded that there are differences in the dimensions of the vertebral neural canal between males and females and that these differences could largely be attributed to functional reasons (Clark 1988). Since the vertebral neural canal tends to be significantly larger in males in the transverse dimension, females have a larger vertebral neural canal in the anterioposterior dimension, which is beneficial in the case of pregnancy. During the period of pregnancy, females experience extra compressive forces on the vertebral column, especially that of the lumbar vertebrae which accounts for the 26 lumbar lordosis during this time (Porter et al. 1980). An increased anterioposterior dimension of the vertebral neural canal can potentially counter this force by providing protection for the spinal cord throughout this time of excessive compression (Clark 1988; Porter et al. 1980) Sexual Dimorphism in the Sacrum There have been numerous studies linked to the reliability of sex estimation through the quantitative assessments of sexual dimorphic traits of the sacrum (Moore-Jansen and Plochocki 1999; Plochocki 2010; Flander 1978). With the sacrum being designated as a portion of the pelvic girdle, which is a known reliable and frequently used means of estimating sex, the sacrum’s location and function does imply that it may also have potential in sex estimation. In order for the pelvic inlet of females to be enlarged for the accommodation of child rearing, it has been proposed that female sacra exhibit a lesser degree of sacral curvature which would be useful in sex estimation (Plochocki 2010). Recent research into the sexual dimorphism of the sacrum has focused on the subtense or degree of curvature at various points along the anterior surface, as well as the traditional anterior lengths and breadths measurements of the sacrum. Flander (1978) carried out research on the statistical significance of sexually dimorphic traits in the sacrum in a Black sample. It was deduced that the means of the measurements were significantly separated between males and females, with the most significant measurements being that of the sacral curvature with males having a significantly greater curvature mean than females. In the study, the measurements of the anterior length and breadth of the sacrum did not prove to be statistically significant. This study also noted that while the degree of curvature was found to be statistically significant in the Black sample, previous research has suggested that 27 Black populations have a tendency for a greater degree of curvature and that this may not be applicable or as statistically significant in other group affiliations (Flander 1978). Additional studies have supported this, with the depth of the sacral curvature being larger in males than in females and the maximum curvature depth consistently being at the articulation between the second and third sacral vertebrae in both sexes (Moore-Jansen and Plochocki 1999; Plochocki 2010; Trotter 1926). Also, Flander (1978) found that one of the most significantly sexual dimorphic traits to be the anterior straight breadth of the sacrum at the first sacral vertebra. The reliability of the maximum sacral breadth as sexually dimorphic is somewhat controversial, with some researchers finding it an accurate representation of sex, while others claim that the measurement is unusable in sex estimation (Benazzi et al. 2009). Research suggests that the sacrum not only exhibits sexually dimorphic patterns, but that the accuracy of sex estimation differs in each sex, with this accuracy also being influenced by morphological variation associated with group affiliation. It has been demonstrated that Black females are the most positively correlated with sexually dimorphic measurements of the sacrum, with the accuracy in a study conducted by Moore-Jansen and Plochocki (1999) being in the 96th percentile. While still highly reliable, the accuracy of sex estimation in the White female sample of this study was 88%. The male samples of both group affiliations, however, were not proven to be reliable, with 58% of Black males and 53% of White males being correctly classified (Moore-Jansen and Plochocki 1999). 28 Sexual Dimorphism in the Femur There has been significant research conducted on the correlations of the femur and the sex of the individual. With the femur being the largest and strongest bone in the human body, it can withstand most taphonomic processes and is consistently shown to be a reliability indicator of sex even when in a fragmentary form. The maximum and bicondylar lengths, circumference of the diaphysis, maximum vertical and anterioposterior diameters of the head all have proven to be highly reliable in the estimation of sex as males tend to be significantly larger than females in all of the aforementioned measurements (Krogman and İşcan 1986). Since the femur is the site of the origins and insertions of several major muscle groupings as previously discussed, the morphology of muscle attachment sites have also been used as a successful indicator of sex. As it is the longest bone in the human body, the femur distinguishes itself by a positive correlation with the stature of an individual. Males tend to be larger than females and correspondingly, males also tend to be taller in stature (Krogman and İşcan 1986). For the purposes of this study, measurements of the femur are included in order to account for the size variation due to the stature of the individual, and also as a control and a means to assess the reliability of sexual dimorphism in the 12th thoracic vertebra. Age-Related Changes Growth and Development in the Human Skeleton As can be observed with the naked eye, humans grow from birth, incrementally gaining size and stature until they are skeletally mature adults. This growth is accompanied by the 29 growth of the skeleton as well as morphological variation in the individual bones as they mature to their adult form. Being familiar with the growth and development patterns of human skeletal remains is crucial as it is reflective of the age of the individual. Degenerative Age-Related Changes in the Human Skeleton Shortly after humans reach skeletal maturity, at approximately thirty years of age, the human body begins to gradually degenerate. This degeneration manifests itself in several forms throughout the human body, affecting the morphological variation of the skeleton and the composition of lean muscle mass in particular (McCarter 2006). While aging is a natural biological process, it occurs as a result of the decrease in the maintenance and reproduction of body cells after peak reproductive potential has been achieved (Crews 1993). Overall, females tend to encounter the degenerative effects of aging, in terms of skeletal as well as muscular decline, at a less gradual rate than males as a result of the hormonal fluctuations that occur with the onset of menopause (Twomey et al. 1983; Wise 2006). One of the most common conditions associated with the aging of the human skeleton is that of degenerative arthritis. This form of arthritis is the result of progressive degeneration of the hyaline cartilage, which is located on the articular surfaces of joints (Ortner and Putschar 1981). The joint most commonly affected by degenerative arthritis in the human skeleton is that of the knee, followed by the other major joints of the hip, shoulder and elbow, all of which are those that withstand the majority of weight, tension and strain from everyday activities throughout one’s life (Heine 1926; Ortner and Putschar 1981). Degenerative changes of skeletal material can occur in several forms, not always being a loss of bone as the term “degeneration” 30 implies. Inflammation as a result of the reduction or friction of cartilage on the surfaces of bone can result in osteoblastic (bony growths or deposits) processes, such as eburnation or osteophytic lipping. Age-related degenerative changes can also present itself in the form of osteoclastic processes (bone loss), such as pitting on the articular surfaces of bones. While this study focuses on the manifestation of age-related changes in the human skeleton, as a biological anthropologist the effects of sarcopenia must also be considered when assessing morphological variation in the human skeleton. Sarcopenia is the decline in muscle mass and muscle function associated with the aging of an individual (McCarter 2006). Since the skeletal and muscle systems of the body work together to allow for movement, stability and the protection and nutrient supply of internal organs, the natural muscle degeneration of an elderly individual must be considered when assessing age-related changes through skeletal morphological variation. Increases in age are also reflective in increases of human variation in the form of skeletal morphological variation, as not all age-related changes and degenerative processes act upon all individuals in similar patterns, as some patterns are specific to geographical, sociocultural and environmental factors (Crews 1993). Growth and Development in the Vertebral Column The vertebral column grows in response to the functional demands of the human body and undergoes a large transformation in the first year of one’s life. In this case, an infants’ vertebral column has a primary curvature, or a gentle single curve while in utero, as there are no external factors or needs that act on the vertebral column at this time. A few months after birth, the infant develops normal curvature of the vertebral column in the cervical region, which 31 corresponds with the need for an infant to be able to support their weight while sitting or holding themselves upright and to support the weight of their heads on their own. Finally, nearing the first year of life, a second curve forms in the lumbar region of the vertebrae in order for balance and proper weight distribution during walking (Carter 2000a; Taylor 1975). There are three primary ossification centers of the vertebrae: one on either side of the vertebral body, separating it from the neural arch, and one along the sagittal plane at the spinous process. The neural arches begin to fuse within the last few months of the first year of life, and this fusion first occurs in the upper lumbar and low thoracic vertebrae, with the cervical vertebrae fusing later, in the first few months of the second year, and the last to fuse being the most inferior lumbar vertebrae at up to six years of age. The ossification centers between the vertebral bodies and neural arches commence fusion a little later, typically between two and five years of age (Scheuer and Black 2000). For the growth patterns of the thoracic vertebral column specifically, the transverse processes are the first epiphyses to commence fusion, followed by the annular epiphysis and then the spinous process at approximately eleven, fourteen and fifteen years, respectively (Cardoso and Ríos 2011). Through a study of the epiphyseal fusion growth patterns in the presacral vertebrae of children, Cardoso and Ríos (2011) concluded that there are no statistically significant sex differences in vertebral fusion, however, in several epiphyses there is a trend towards slightly earlier fusion in females. The sacrum fuses from twenty-one primary ossification centers with fusion commencing at the neural arches between two and five years of age, with the vertebral bodies fusing up to six years of age. The primary ossification centers around the laminae begin fusing between seven and fifteen years of age (Scheuer and Black 2000). The secondary ossification centers of the vertebral body epiphyseal plates, spinous processes and transverse processes commence fusion 32 between ten and twenty years of age (Belcastro et al. 1957). As mentioned earlier, the sacral vertebrae fuse together, however all sacral vertebrae remain open until puberty and fuse from the most inferior sacral vertebrae upwards to the most superior, which usually remains open until around twenty-five years of age. In a study conducted by McKern and Stewart (1957), age estimations were performed on a sample from interpreting the growth and development patterns of the sacrum. In this case, it was concluded that an individual is estimated to be less than twenty years of age if any vertebral bodies have failed to fuse. An individual is estimated to be less than twenty-three years of age if all but the space between the first and second sacral vertebrae has fused, with the age of complete fusion of all sacral vertebrae estimated between twenty-three up to thirty-two years of age (McKern and Stewart 1957). As a result of the growth patterns mentioned above, the vertebral column as a whole is usually considered completely fused by around twenty-five years of age in females and up to thirty years of age in males (McKern and Stewart 1957; Scheuer and Black 2000). Degenerative Age-Related Changes in the Vertebral Column While the vertebral column is composed of dense cancelleous bone to meet the external requirements and consistent weight distributed throughout it, these skeletal elements, not unlike any other bone in the human skeleton, begin to degenerate shortly after complete skeletal maturity has been obtained. In fact, the most stress projected onto the vertebral column occurs during our habitual form of locomotion – bipedalism. While in the upright position, the weight of the human body is absorbed and distributed down the spinal column to the lower limbs to walk efficiently through the use of a striding gait. 33 In between each vertebra there is a cartilaginous disk which functions to cushion the weight and reduce friction in between each vertebra. However, over time these cartilaginous discs begin to degenerate, contributing to the degenerative conditions associated with old age, such as osteoarthritis (Novak and Šlaus 2011). As the cartilaginous discs degenerate with age in the thoracic and lumbar vertebrae specifically, the load that is absorbed and distributed down the vertebral column when in the upright position undergoes a shift. Rather than the anterior portion of the vertebral bodies bearing the compressive load, as it does throughout childhood and into adolescence, the load is shifted more posteriorly to the neural arch (Brown et al. 2008). This transition affects both males and females equally, and places a tremendous amount of stress on the apophyseal joints that are located inferiorly and superiorly to the base of the spinous process at the most posterior portion of the neural arch. This leads to a considerable amount of spinal compression, with the tenth to eleventh thoracic and second to fifth lumbar vertebrae being the most affected by this apophyseal joint degeneration (Derevenski 2000). Apophyseal joint degeneration commences at thirty years of age, regardless of whether observable characteristics of this are present or not. Throughout aging and as the degeneration becomes more pronounced, apophyseal joint degeneration can result in subchondral bone reactions sparked by cartilage loss and compression in the vertebral column and can also result in mild to extreme cases of synovitis, which is the inflammation of the synovial membranes between the joints (Derevenski 2000). While the present study is assessing the effects of age on the skeletal morphological variation in the 12 th thoracic vertebra, any specimens with severe pathological conditions such as those listed above, were excluded from the sample as the integrity and measurements of the bone would be compromised. 34 Temporal Variation and Secular Effects in Biocultural Contexts Not all skeletal variation is the result of senescence and other biological-based morphological variations, but that of secular variation. Secular variation is a form of nongenetic, biological change that can act on the skeletal morphology of a population over time, but is not rooted in the purely biological expressions of morphological variations, such as the standard patterns of growth and development previously discussed. Rather, secular variation can alter the morphology of skeletal elements as a result of plastic and microevolution responses to sociocultural and environmental factors that largely affects successive generations (MooreJansen 1989). Secular variation affects the phenotypic expression rather than the genetic composition of individual, and morphological variation attributed to secular variation is most commonly the result of the physical environment, malnutrition, disease, status, sociocultural practices and forms of lifestyle (Johnston and Zimmer 1989). Previous studies have demonstrated that the greatest impact of environmental influences resulting in the secular variation of the human skeletal framework occurs in infancy and early childhood between the ages of six months and three years of age. Also, the human body is also susceptible to plastic change during puberty until skeletal maturity is reached (McCullough and McCullough 1984). An indicator of secular change in a population, and one that has been frequently addressed is that of stature and the change in mean statures in a population. Jantz and Jantz’s (1999) research on the variation in long bone length, which contributes to the overall stature of an individual, as evidence of secular change between the 19th to late 20th century in the United States reveals discrepancies in the driving forces behind secular influences on skeletal material. While the rates of disease and nutrition in a population’s environment typically causes disruption in typical growth and development patterns, this study demonstrates that perhaps the 35 leading cause of secular change in the United States is that of status. In this study, the most positive correlations between causality variables and secular effects in adult stature were found to be the effects of low income distribution resulting in lower standards of nutrition, lower birth weights and maternal pregnancy weights (Jantz and Jantz 1999). Another study that focused on the secular change exhibited in the stature of children in a Japanese population found differences in the mean statures prior to and following World War Two. In this case, children six years of age and twelve years of age formed the two study groups with the mean statures recorded between 1934-1937, prior to the Second World War. Mean statures were also compiled following the War, between 1951-1958 for children of the same age groups, which revealed a slight increase in stature (between 1 and 4 centimeters) in these age groups. It is speculated that this secular trend is the result of the influx of Western goods and food sources and increased levels of nutrition following the War (Kimura 1984). It has been argued that while secular change can play a large role in the skeletal morphology of a population, not all individuals in that population will be affected to the same degree. In a study conducted by Wolanski and Kasprzak (1976), males and females of the same population responded differently to the same environmental stresses. It was concluded that males are the most effected by environmental changes, with females being more resistant. This, however, may be selected for, as a higher resistance in the females of a population will help maintain the reproductive fitness of a population (Jantz and Jantz 1999; Stinson 1985; Wolanski and Kasprzak 1976). In the case of the present study, the samples from each collection consisted of specimens born within a ten year range in order to account for and minimize the effects of secular variation. Through employing this selection criterion, the morphological variation present in the elements 36 studied can be attributed to sexual dimorphism and not be confused with differences between the sexes that may have been plastic responses to environmental and sociocultural factors. 37 CHAPTER 3 MATERIALS AND METHODS Introduction Two human osteological collections, the Raymond A. Dart Collection of Human Skeletons and the Hamann-Todd Human Osteological Collection, were utilized for the present study. Both of these collections are comprised of a significant quantity of human skeletal remains, with the Raymond A. Dart Collection being the largest and most used and referenced human skeletal collection in Africa, and the Hamann-Todd Collection being one of the largest and most referenced collections in the United States. These collections have both been used extensively by anthropologists, anatomists, other researchers, and students alike and specimens of both collections have been used as a means to assess and create standards of measurements for the estimation of aging, sexing, stature, and group affiliation of individuals. Both of these collections were selected specifically for this sample as a result of their sample composition. With the present study assessing the morphological variability in the 12 th thoracic vertebra between two geographical varieties of a group affiliation, as well as between two age groups, the collections utilized must have a large enough sample to accommodate the particular selection criteria. The first collection used was that of the Raymond A. Dart collection as a result of the collection composition being primarily that of South African Blacks between the ages of 20-70 years. In order to minimize the effects of temporal variation and secular change, the ten year birth year period of 1910-1919 was selected for this collection, as it had an abundance of skeletally mature South African Black specimens born within this time frame. The Hamann-Todd Osteological Collection was then selected as this collection provided a large sample of skeletally mature African American specimens. The approximate birth years of the 38 specimens in this sample were also close to that of the South African Black sample, which allows for a good comparative study. Collection History The Raymond A. Dart Collection of Human Skeletons The Raymond A. Dart Collection of Human Skeletons is located in the Department of Anatomical Sciences at the University of the Witwatersrand in Johannesburg, South Africa. This collection was created in 1921 after Raymond Dart had travelled to the United States and Canada and was greatly influenced by the value of the Terry and Hamann-Todd human skeletal collections that had recently emerged there (Hunt and Albanese 2005; Tobias 1985). The collection was begun shortly after beginning his appointment as the Chair of the anatomy department at this institution, and currently houses over 2,600 complete human skeletons. The human skeletal remains accessioned into the collection were derived from cadaver origins under the provision of the South African Human Tissues Act and were provided to aid in the advancement of medical and biological research, as teaching specimens, and for other beneficial educational purposes. Initially, the majority of cadavers received by Dart were those of unclaimed remains that had demised in hospitals in the Gauteng provincial region, and this was the prevalent form of donation until 1958 which marked the dawn of a bequeathment program for individual and family donation to the collection upon death (Dayal et al. 2009; Tobias 1985). The collection was entitled “The Raymond A. Dart Collection of Human Skeletons” by Dart’s successor and previous student, Doctor Tobias V. Phillips in 1959 (Dayal et al. 2009; 39 Hunt and Albanese 2005). Tobias added to this collection through the accession of human skeletal material that would allow for this collection to have a more equal representation of both sexes as well as the various populations residing in South Africa (Dayal et al. 2009). While this collection contained an extraordinary amount of human skeletal remains, databases were in disarray and no electronic form was in existence for some period of time. It wasn’t until the 1980s that an electronic database of all human skeletal remains and associated information, such as sex, age at death, date of birth, etcetera was compiled. This greatly supplemented the advantageous use of this collection (Dayal et al. 2009). Of the 2,600 human skeletal remains in this collection, the majority consists of South African males. The collection is divided primarily into the group affiliations of South African Africans (72%), South African Whites (18%), with the remaining 10% constituting a variety of minority populations within South Africa. Also, of the 2,600 skeletons, approximately 71% of this are males with the remaining 29% being females. The population demographics of this collection is said to be representative, in terms of population affinity, of the current population in South Africa (Dayal et al. 2009). The Hamann-Todd Osteological Collection The Hamann-Todd Osteological Collection is located in the Cleveland Museum of Natural History in Cleveland, Ohio. While created at the Western Reserve Medical School (now Case Western Reserve University) by Doctor Carl August Hamann in 1893, Hamann was succeeded in 1912 by Doctor T. Wingate Todd after being appointed Dean of the medical school. To date, there are approximately 3,100 complete human skeletal remains, born between 1825 and 40 the mid-20th century, of cadaver-derived origins in this collection, all with age, sex, group affiliation, height, weight, occupation, pathological conditions, cause of death, anthropometric measurements, and even source of donation when possible (de la Cova 2011; Hunt and Albanese 2005; Kelley and El-Najjar 1980). The majority of cadavers were inspected before and after their autopsies by Todd, in order to compile and report any pathological and somatological conditions that were observed and that could affect skeletal measurements and deductions. During this process, all abnormalities and preexisting conditions, including any evidence of dietary deficiencies, disease, lesions, osteoclastic or osteoblastic bone abnormalities, etcetera, were carefully interpreted and recorded (Kelley and El-Najjar 1980). Being as the majority of specimens housed in the Hamann-Todd Osteological Collection were born before the “Prebiotic Era”, or prior to the creation and distribution of antibiotics, the cause of death in the majority of specimens in this collection was due to systemic or other infections. Of these diseases and illnesses, the disease which affected a large percentage of the population at this time, and is also reflected in the specimens, is tuberculosis. Tuberculosis is the reported cause of death in approximately 20% of the specimens in the Hamann-Todd Collection. This chronic infectious disease can present itself if varying forms and degrees, from latent to hyperacute, and can affect the composition and integrity of skeletal elements in the same manner. When lesions are present in the human skeleton, they have a tendency to affect the lower thoracic and lumbar vertebrae in the form of pitting, decalcification, and vertebral collapse. Although there is a large variance in the physical manifestation of tuberculosis on the human skeleton due to the variables of host resistance, sex, genetic predisposition and virulence of the disease, studies have shown that Blacks have an increased susceptibility over those of those group affiliations (Kelley and El-Najjar 1980). While tuberculosis is not only known to have 41 osteoclastic effects on the skeletal structures of an individual, but also can result in the collapse of the vertebral bodies (Kelley and El-Najjar 1980); no specimens that exhibited pathological conditions that could skew or disable a measurement on any of the three elements used in this study were used. The proportion of Black specimens within this collection is said to be representative of The Great Migration of Southern African Americans following the American Civil War. During this time, large populations of African ancestry migrated from the enslavement of the southern regions of the United States to more densely populated cities in the north in order to seek new beginnings, employment and freedom. Two cities that saw an influx in African American populations during this time is that of St. Louis, Missouri and Cleveland, Ohio which are evident in the osteological collection of both, the Terry Collection and the Hamann-Todd Collection. Also, census data in both of these locations during this time reveals that African Americans had a higher prevalence of biological stress, disease, higher death rates and the highest frequency of tuberculosis, as is evident in the demographics of both of the aforementioned collections (de la Cova 2011). Comparison of Raymond Dart and Hamann-Todd Collections The Raymond A. Dart Collection of Human Skeletons and the Hamann-Todd Osteological Collections were assembled as approximately the same time and are largely composed of individuals of low socioeconomic standing. The obvious difference between the two is the differences in geographic populations in which both collections consist of. Both of these collections were employed for this very reason. By assessing the sexual dimorphism and 42 potential for the estimation of sex in each collection, the data sets can then be compared to assess for the variation present in the pooled sample that can be attributed to geographical or population variation. In this case, the measurements of the 12th thoracic vertebra will not only be assessed for skeletal variation due to sexual dimorphism, but for the skeletal variation that exists between South African Blacks and American Blacks. By studying the effects of population variation and its effects on the reliability of the estimation of sex, one is able to infer the how applicable this study will be in other group affiliations that have not yet been researched. Study Samples In order to account for apparent biases inherent in a skeletal population, such as representativeness of the samples (Ubelaker 1978), a protocol of measurements (refer to Appendix B) was developed and if any known conditions that would affect the integrity and reliability of a measurement were observable on a specimen, they were expelled from the sample used. Both, the protocol and selection criteria for this study will be further discussed in the following sections. Data was collected from two samples, both of which consisted of a relatively large sample and an equal distribution of males, females and of both age groups (skeletally mature to 40 years of age, and above 40 years of age). With each of these variables equally represented in both samples, the skeletal morphological variation resulting from sexual dimorphism, age-related changes, stature, group affiliation, and temporal or secular variation can be accounted for and the reliability of the results of the analysis of the measurements can be assessed. 43 The Raymond A. Dart Collection of Human Skeletons Sample Measurements were collected from a sample of 168 specimens of known age, sex and group affiliation in the Raymond A. Dart Collection of Human Skeletons. The specimens in this sample consisted of 94 males, 56 of which were 40 years of age and under, and 38 being over 40 years of age. The remaining 74 specimens were female, with the younger adult and older adult subgroups consisting of 38 and 36 specimens, respectively. All of these specimens were born within a ten year range, between the years of 1910 and 1919 in order to account for and minimize the effects of secular variation as this study relies on assessing sexual dimorphism resulting from a combination of senescence and biological differences. This specific ten year period was selected for multiple reasons. Firstly, since the samples of this study focuses on the group affiliation of Blacks, specifically South African Black, in this case, limiting the external variation of the specimens is integral to the success of this study. This ten year range limits external influence as it precedes the period of European colonialism and the influx of Western goods and dietary sources. This period also precedes Apartheid in South Africa and the major political transformations and migration movements out of South Africa that occurred during this time (Tobias 1985). Finally, the range of 1910-1919 contains a large proportion of specimens in the Raymond Dart Collection, which allows for a more regimented selection criterion to be enforced as there is the option of replacing more less desirable specimens (being those that exhibit a form of pathological condition or damage) for a more desirable ones. 44 The Hamann-Todd Osteological Collection Sample Measurements were collected from a sample of 407 specimens of known age, sex and group affiliation in the Hamann-Todd Osteological Collection. The specimens in this sample consisted of 205 males, 105 of which were 40 years of age and under, and 100 being over 40 years of age. The remaining 202 specimens were female, with the younger adult and older adult subgroups consisting of 100 and 102 specimens, respectively. With American Blacks being less frequent in this collection, only the male specimens in the younger adult subgroup able to remain within a ten year birth date to account for secular change. The older adult male subgroup was increased to those born within a twelve year period and the younger adult female subgroup was increased to a fifteen year period. Having a constricted range for years of birth was not possible in the older adult female subgroup; however, as all skeletally mature American Black females in the Hamann-Todd Collection had to be used in order to meet the quota of 100 specimens. Measurement Protocol The protocol for this study was developed in the Biological Anthropology Laboratory in Wichita State University, Wichita, Kansas through using specimens from a combination of collections. To test the potential for quantifying sexual dimorphism in the 12th thoracic vertebra and its preliminary use in the sex estimation of skeletal remains, a case study using a sample of the WSU Biological Anthropology Laboratory skeletal collections was performed. For this study, 9 of the measurements listed in the protocol (refer to Appendix B) for the 12th thoracic vertebra were taken from 11 skeletal remains of known age, sex and group affiliation. These measurements were then analyzed using univariate analysis, which revealed a positive 45 correlation between the measurement values and the sex of the individual of particular measurements. The protocol was developed as a result of this case study, with three additional measurements of the 12th thoracic vertebrae included, as well as measurements of the femur. As a result, the protocol is comprised of 17 traditional and non-traditional measurements which were specifically selected for or designed to assess the degree of sexual dimorphism in the measurements, if it is present in all measurements, and to analyze the degree of reliability of each measurement in estimating the sex of an individual. Although this study focuses on the measurements of the 12th thoracic vertebra, additional measurements from the sacrum and the femur of each individual were also performed. Since the sacrum is a known sexually dimorphic element, 3 measurements were taken with the purpose of correlating them with the measurements of the vertebrae to ensure the degree of sexual dimorphism in the 12th thoracic vertebrae. However, due to the high variance associated with the sacral measurements in all samples, the femur was used as a control for sex instead. The femur is an element with a correlation to the living stature and sex of an individual and is also used in this study to discern possible discrepancies between the measurements, the degree of sexual dimorphism and the overall size of the individual. Discrepancies in the values of the 12 th thoracic vertebrae may be due to the stature of the individual, and a femoral standard may be able to account for these differences as a result of its known correlation to living stature size of an individual. All measurements were performed with standard osteometric instruments, including: an osteometric board, Mitutoyo sliding calipers, and a GPM coordinate caliper. All measurements performed with calipers were recorded in millimetres to the nearest tenth, with the osteometric board measurement recorded in centimetres (to correspond with stature estimation protocols) to 46 the nearest tenth. All measurements were initially entered on Data Collection Forms (Appendix A) and were then later entered electronically into Excel while screening for potential errors. Traditional Measurements A total of 9 traditional measurements were employed during this study. There were four 12th thoracic traditional vertebral measurements taken using sliding calipers. These measurements include the maximum sagittal length of the vertebra and maximum length of the vertebral foramen at the sagittal plane, which were adopted from Wescott’s (1999) study and previously Marino (1995). The remaining 2 traditional measurements of the 12th thoracic vertebra were adopted after Sheng-Bo Yu et al.’s (2008) study and consist of the maximum width of the transverse processes, as well as the maximum breadth of the inferior articular surfaces. Three traditional measurements of the sacrum were also performed. Two measurements were performed with sliding calipers and consisted of the maximum height of the sacrum and the maximum breadth of the sacrum, both from the anterior surface (Flander 1978; Moore-Jansen and Plochocki 1999; Plochocki 2010; Trotter 1926). The remaining measurement, the degree of curvature (subtense), was conducted with coordinate calipers (Moore-Jansen and Plochocki 1999; Plochocki 2010; Trotter 1926). While these measurements have been recorded for all samples, they were omitted in the current study as the analysis of univariate statistics revealed a high variance, making it apparent that these measurements would be an unreliable means of sex estimation. 47 Finally, two traditional measurements of the femur were used. The bicondylar length of the femur was taken using an osteometric board, and the maximum vertical diameter of the femoral head was performed using sliding calipers (Krogman and İşcan 1986; Moore-Jansen et al. 1994). Non-Traditional Measurements There were 8 non-traditional measurements of the 12th thoracic vertebra that were designed and implemented specifically for the present study. All non-traditional measurements were performed using sliding calipers and consisted of the following measurements: maximum height of vertebral body, length of vertebral body at sagittal plane, maximum width of vertebral body, maximum width of vertebral foramen, maximum breadth of superior articular surfaces, length of vertebral body and vertebral foramen, maximum width of pedicles, and the length of the spinous process. These measurements were created as a result of their correlation to muscle attachment sites, compression force areas, or indication of a potential variation by other researchers. Statistical Procedures and Analysis In order to assess the effects of age and the potential for the estimation of sex after all of the data had been collected, statistical procedures were used to analyze all measurements individually, as well as in multivariate contexts. For the univariate statistical analysis, each measurement of the 12th thoracic vertebra has been analyzed separately, with the means, standard deviations, variance and ranges being calculated and analyzed for each. In this case, each 48 measurement was assessed for its degree of sexual dimorphism and its potential in sex estimation. Multivariate analyses of variance were then performed using the measurements of the 12th thoracic vertebra as well as the femur, in order to discern the effects of sex, age and possible limits of the skeletal collection. Multivariate analyses were performed through the utilization of Statistical Analysis Software (SAS). A PROC STEPWISE with a MAXR option (SAS Statistical Institute Inc. 1985; 1999) was used in order to identify optimal discrimination models to apply to the calibration and test samples to discern the reliability of a combination of measurements through correct classifications. A PROC DISRIM was performed to calculate correct classification of males and females for each of these samples (SAS Statistical Institute Inc. 1985; 1999). The calibration sample was used initially to derive models and determine the efficiency of the correct classification of each sex. These models were then applied to the test samples to calculate the correct classifications of each sex on independent samples. Intra-Observer Error Another aspect that could affect the reliability of the use of the 12 th thoracic vertebra as an indicator of sex, is the replicability potential of the measurements, or the degree to which the measurements could be performed reliability and consistently. In order to assess this, the replicative ability of the measurements, or the intra-observer error was measured. All twelve measurements of the 12th thoracic vertebra were performed twice on a case study sample, using the WSU BAL skeletal collections. This revealed insignificant differences between the two instances of measurements. Additionally, several individuals performed all twelve measurements of a 12th thoracic vertebra by following the protocol (Appendix B). These sets of measurements had an insignificant difference between the measurers (<3.00%), which is well within the acceptable error rate range (Droessler 1981). 49 CHAPTER 4 RESULTS Summary Statistics Through the analysis of the summary statistics, several observations regarding the morphological variation of the 12th thoracic vertebra within and between sexes, age groups and geographical samples can be discerned. The measurements of the 12 th thoracic vertebra and the femur reveal differences and similarities in size and shape morphology among the samples. All results in the summary statistics are recorded in millimeters and are rounded to the nearest tenth of a millimeter. Univariate summary statistics for all samples are located in the appendix (Appendix C). The summary statistics of the means for the 12th thoracic vertebrae measurements from the male samples in both collections (Table 1) reveal an increase in size in the African American sample as opposed to the South African Black sample. The maximum sagittal length (MSVL), maximum width of the vertebral body (MWVB), maximum width of the superior articular facets (MWSA), length of the vertebral body and foramen (LVBF), width of the pedicles (MWIP) and length of the spinous process (MLSP) reveal the greatest differences in the size of these features between the two samples. The mean measurements of the 12th thoracic vertebrae in the South African Black and African American female samples (Table 2) reveal a similar trend. The mean measurements in the African American female sample are all slightly larger than that of the South African Black female sample, with only the maximum sagittal length (MSVL) of the 12th thoracic vertebra exhibiting a rather large difference between both samples. 50 Code MSVL MHVB SLVB MWVB MLVF MWVF MWTP MWSA LVBF MWIP MLSP MWIA Table 1. Vertebral Summary Statistics – male sample South African Black n = 94 Measurement Mean St. Dev. Maximum Sagittal Length of Vertebra 72.1 4.2 Maximum Height of Vertebral Body 23.0 1.8 Vertebral Body Length at Sagittal Plane 27.8 3.8 Maximum Width of Vertebral Body 39.5 2.9 Maximum Length of Vertebral Foramen 16.6 1.7 Maximum Width of Vertebral Foramen 18.2 1.9 Maximum Width of Transverse Processes 49.9 7.0 Maximum Width Superior Articular Surfaces 35.8 3.4 Length Vertebral Body and Foramen 43.8 3.6 Maximum Width of Pedicles 32.7 2.6 Maximum Length of Spinous Process 31.4 3.4 Maximum Width Inferior Articular Surfaces 28.1 3.4 African American n = 205 Mean. St. Dev. 77.4 4.6 23.2 1.9 29.5 2.4 42.4 2.9 17.4 1.6 19.6 2.1 51.7 6.4 38.1 4.0 46.2 2.9 34.8 3.1 33.6 3.8 29.9 3.8 Code MSVL MHVB SLVB MWVB MLVF MWVF MWTP MWSA LVBF MWIP MLSP MWIA Table 2. Vertebral Summary Statistics – female sample South African Black n = 74 Measurement Mean St. Dev. Maximum Sagittal Length of Vertebra 68.0 5.4 Maximum Height of Vertebral Body 21.9 1.6 Vertebral Body Length at Sagittal Plane 24.9 2.1 Maximum Width of Vertebral Body 36.7 2.8 Maximum Length of Vertebral Foramen 17.1 1.6 Maximum Width of Vertebral Foramen 18.0 2.0 Maximum Width of Transverse Processes 45.4 7.4 Maximum Width Superior Articular Surfaces 33.9 4.3 Length Vertebral Body and Foramen 40.8 2.9 Maximum Width of Pedicles 30.2 2.6 Maximum Length of Spinous Process 29.8 3.6 Maximum Width Inferior Articular Surfaces 27.4 3.2 African American n = 202 Mean. St. Dev. 70.3 3.9 22.5 1.5 25.5 2.2 37.4 2.8 17.4 1.6 19.0 1.6 46.2 4.1 34.8 3.2 42.4 2.5 31.7 2.8 30.7 3.3 27.7 3.2 The summary statistics for the differences in the measurements of the 12 th thoracic vertebrae in the South African Black (Table 3) and African American male samples (Table 4) reveals slight differences between the two age groups. The majority of the mean measurements show a trend for the slight enlargement of this element as age increases, with the most significant being the maximum sagittal length of the vertebra in both cases. The length (MLVF) and width of the vertebral foramen (MWVF) and width of the pedicles (MWIP) in South African Black 51 males, and the length of the vertebral foramen (MLVF) and width of the superior articular surfaces (MWSA) in African American males exhibit the opposite, by slightly reducing in size from the younger adult to the older adult age group. Code MSVL MHVB SLVB MWVB MLVF MWVF MWTP MWSA LVBF MWIP MLSP MWIA Table 3. Vertebral Summary Statistics – South African Black male sample ≤ 40 years of age > 40 years of age n = 56 n = 38 Measurement Mean St. Dev. Mean. St. Dev. Maximum Sagittal Length of Vertebra 71.6 4.1 72.8 4.4 Maximum Height of Vertebral Body 23.0 1.9 23.1 1.6 Vertebral Body Length at Sagittal Plane 27.8 4.6 27.9 2.1 Maximum Width of Vertebral Body 39.0 2.8 40.3 2.9 Maximum Length of Vertebral Foramen 16.9 1.5 16.1 1.9 Maximum Width of Vertebral Foramen 18.3 1.8 18.2 2.1 Maximum Width of Transverse Processes 49.5 6.9 50.4 7.3 Maximum Width Superior Articular Surfaces 35.5 3.3 36.1 3.5 Length Vertebral Body and Foramen 43.6 2.4 44.1 4.8 Maximum Width of Pedicles 32.8 2.5 32.6 2.7 Maximum Length of Spinous Process 31.0 3.2 32.0 3.6 Maximum Width Inferior Articular Surfaces 27.8 3.2 28.6 3.6 Code MSVL MHVB SLVB MWVB MLVF MWVF MWTP MWSA LVBF MWIP MLSP MWIA Table 4. Vertebral summary statistics – African American male sample ≤ 40 years of age > 40 years of age n = 105 n = 100 Measurement Mean St. Dev. Mean. St. Dev. Maximum Sagittal Length of Vertebra 76.5 4.3 78.3 4.6 Maximum Height of Vertebral Body 23.2 2.0 23.3 1.9 Vertebral Body Length at Sagittal Plane 29.2 2.3 29.9 2.5 Maximum Width of Vertebral Body 41.7 2.6 43.2 3.1 Maximum Length of Vertebral Foramen 17.4 1.5 17.3 1.7 Maximum Width of Vertebral Foramen 19.5 2.2 19.7 2.1 Maximum Width of Transverse Processes 51.5 6.4 52.0 6.4 Maximum Width Superior Articular Surfaces 38.1 4.2 38.0 3.9 Length Vertebral Body and Foramen 45.8 2.6 46.7 3.1 Maximum Width of Pedicles 34.2 3.0 35.3 3.1 Maximum Length of Spinous Process 33.1 3.6 34.2 3.8 Maximum Width Inferior Articular Surfaces 29.6 3.5 30.2 4.0 A comparison of the mean measurements between the two age groups in the South African Black (Table 5) and African American female samples (Table 6) reveals a similar trend. The majority of the measurement means exhibit an increase from the younger to older adult age 52 groups in both samples. Like the South African Black male sample, the length (MLVF) and width of the vertebral foramen (MWVF) and width of the pedicles (MWIP) in the South African Black female sample reduces in size in the older age group. This is the case in the height of the body (MHVB), length of the vertebral foramen (MLVF) and width of the inferior articular surfaces (MWIA) in the African American female sample. The maximum sagittal length of the vertebra (MSVL) increases the largest of the measurements in both cases. Code MSVL MHVB SLVB MWVB MLVF MWVF MWTP MWSA LVBF MWIP MLSP MWIA Table 5. Vertebral summary statistics – South African Black female sample ≤ 40 years of age > 40 years of age n = 38 n = 36 Measurement Mean St. Dev. Mean. St. Dev. Maximum Sagittal Length of Vertebra 66.7 4.0 69.4 6.4 Maximum Height of Vertebral Body 21.9 1.7 22.0 1.4 Vertebral Body Length at Sagittal Plane 24.0 1.8 25.8 2.0 Maximum Width of Vertebral Body 35.8 2.8 37.6 2.4 Maximum Length of Vertebral Foramen 17.3 1.5 16.8 1.8 Maximum Width of Vertebral Foramen 17.9 1.9 18.1 2.1 Maximum Width of Transverse Processes 44.3 6.3 46.5 8.3 Maximum Width Superior Articular Surfaces 33.5 5.0 34.4 3.4 Length Vertebral Body and Foramen 39.6 2.6 42.0 2.8 Maximum Width of Pedicles 29.7 2.7 30.8 2.4 Maximum Length of Spinous Process 29.2 3.7 30.4 3.5 Maximum Width Inferior Articular Surfaces 27.0 3.5 27.9 2.8 Code MSVL MHVB SLVB MWVB MLVF MWVF MWTP MWSA LVBF MWIP MLSP MWIA Table 6. Vertebral summary statistics – African American female sample ≤ 40 years of age > 40 years of age n = 100 n = 102 Measurement Mean St. Dev. Mean. St. Dev. Maximum Sagittal Length of Vertebra 69.3 3.7 71.4 3.9 Maximum Height of Vertebral Body 22.7 1.5 22.4 1.6 Vertebral Body Length at Sagittal Plane 24.9 1.8 26.2 2.3 Maximum Width of Vertebral Body 36.5 2.7 38.3 2.7 Maximum Length of Vertebral Foramen 17.6 1.5 17.2 1.5 Maximum Width of Vertebral Foramen 18.9 1.7 19.2 1.5 Maximum Width of Transverse Processes 45.7 4.4 46.8 3.9 Maximum Width Superior Articular Surfaces 34.5 3.3 35.1 3.1 Length Vertebral Body and Foramen 42.0 2.3 42.8 2.6 Maximum Width of Pedicles 31.2 2.8 32.2 2.8 Maximum Length of Spinous Process 30.3 3.4 31.1 3.2 Maximum Width Inferior Articular Surfaces 27.9 3.2 27.5 3.2 53 Measurements of the femur in the South African Black and African American male samples (Table 7) and female samples (Table 8) reveals differences between the sexes and the collections. In both sexes, the African American samples exhibit larger means in both of the measurements of the femur. The South African Black and African American male samples also exhibit larger mean measurements than the female samples in both measurements of the femur. Code FBL FHVD Table 7. Femoral summary statistics – male sample South African Black n = 94 Measurement Mean St. Dev. Bicondylar Length of Femur 45.0 2.4 Vertical Diameter of Femoral Head 45.2 2.5 African American n = 205 Mean. St. Dev. 47.2 2.5 48.0 2.5 Code FBL FHVD Table 8. Femoral summary statistics – female sample South African Black n = 74 Measurement Mean St. Dev. Bicondylar Length of Femur 41.6 2.0 Vertical Diameter of Femoral Head 40.1 2.1 African American n = 202 Mean. St. Dev. 43.6 2.3 42.1 2.4 Not unlike the summary statistics of the 12 th thoracic vertebra, the mean measurements of the femur also reveal a trend towards a slight enlargement of the measurements from the younger adult age group to the older adult age group. Mean values of both measurements of the femur in the South African Black male samples (Table 9) show a slight increase in the older adult age group as opposed to the younger adult age group. Code FBL FHVD Table 9. Femoral summary statistics – South African Black male sample ≤ 40 years of age > 40 years of age n = 56 n = 38 Measurement Mean St. Dev. Mean. St. Dev. Bicondylar Length of Femur 45.0 2.5 45.2 2.2 Vertical Diameter of Femoral Head 44.9 2.3 45.6 2.7 54 The mean values of the femoral measurements of the African American male samples (Table 10) only expresses this trend in one of the measurements. There is a slight increase in the mean value of the vertical diameter of the femoral head (FHVD) from the younger to the older adult age group. The mean value of the bicondylar length of the femur (FBL) reduces slightly in the older age group of this sample. Code FBL FHVD Table 10. Femoral summary statistics – African American male sample ≤ 40 years of age > 40 years of age n = 105 n = 100 Measurement Mean St. Dev. Mean. St. Dev. Bicondylar Length of Femur 47.3 2.5 47.2 2.6 Vertical Diameter of Femoral Head 47.9 2.7 48.1 2.3 The summary statistics of the femoral measurements of the South African Black female sample (Table 11) and the African American female sample (Table 12) reveal a trend similar to that exhibited in all previously examined elements. The mean values of both of the measurements of the femur increase slightly from the younger adult age group to the older adult age group in both, the South African Black and the African American female samples. Code FBL FHVD Table 11. Femoral summary statistics – South African Black female sample ≤ 40 years of age > 40 years of age n = 38 n = 36 Measurement Mean St. Dev. Mean. St. Dev. Bicondylar Length of Femur 41.4 1.8 41.9 2.2 Vertical Diameter of Femoral Head 39.5 1.8 40.7 2.3 Code FBL FHVD Table 12. Femoral summary statistics – African American female sample ≤ 40 years of age > 40 years of age n = 100 n = 102 Measurement Mean St. Dev. Mean. St. Dev. Bicondylar Length of Femur 43.5 2.2 43.6 2.4 Vertical Diameter of Femoral Head 41.6 2.2 42.6 2.4 The analysis of the summary statistics of the 12th thoracic vertebra and femur demonstrates the similarities and differences within and between sex groups, age groups, as well 55 as both collections, or geographical samples, employed in this study. The mean values of the females of both collections are more similar to each other than either is to their respective male sample, and vice-versa. Also, the South African Black samples are more similar to each other than they are to the African American samples. The mean values of the measurements of both the 12th thoracic vertebra and the femur are larger in the male samples and the African American samples than in the female and South African Black samples. The male samples also exhibit a greater degree of difference in comparing collections and age groups than do the female samples. Estimation of Sex Sex Estimation in the South African Black Sample A combination of PROC STEPWISE and PROC DISCRIM procedures were performed to illustrate the potential and reliability of sex estimation through the use of specific measurements of the 12th thoracic vertebra. A PROC STEPWISE procedure with the MAXR option was applied to the measurements of the 12th thoracic vertebra in the South African Black sample (Table 13). This produced six models with an R-squared value that increases significantly with every additional model. All six models have an overall model probability of <.0001, with each measurement having a partial probability of <.05. The four-variable model of step six was the last one generated to be used, as the R-squared value did not increase significantly beyond 0.3973 and several of the individual measurements in each model exceeded a <.05 probability, reducing the reliability of the measurement. The best single-variable model in the South African Black sample is that of the maximum width of the pedicles (MWIP) (Table 56 14). This model has an R-squared value of 0.1883 and an F-value of 22.74. In terms of the estimation of sex, this model correctly classifies 69.49% of males and 68.33% of females with an overall correct classification of 68.91% in the calibration sample. In the test sample, this model correctly classified 67.65% of males and 64.29% of females, for an overall correct classification of 66.67% in the test sample. Table 13. Stepwise models for South African Black sample Variables R2 Model F Maximum Width of Pedicles (V10) 0.1883 22.74 Partial F 22.74 P <.0001 2 Vertebral Body Length at Sagittal Plane (V3) Maximum Width of Pedicles (V10) 0.2743 18.33 11.49 13.03 0.0010 0.0005 3 Maximum Height of Vertebral Body (V2) Vertebral Body Length at Sagittal Plane (V3) 0.2872 19.54 15.02 17.14 0.0002 <.0001 4 Maximum Height of Vertebral Body (V2) Vertebral Body Length at Sagittal Plane (V3) Maximum Width Superior Articular Surfaces (V8) 0.3618 18.14 15.51 11.57 11.22 0.0002 0.0010 0.0012 5 Maximum Height of Vertebral Body (V2) Vertebral Body Length at Sagittal Plane (V3) Maximum Length of Vertebral Foramen (V5) Maximum Width Superior Articular Surfaces (V8) 0.3888 15.11 17.79 9.72 4.20 12.00 <.0001 0.0024 0.0432 0.0008 6 Maximum Height of Vertebral Body (V2) Maximum Length of Vertebral Foramen (V5) Maximum Width Superior Articular Surfaces (V8) Length Vertebral Body and Foramen (V9) 0.3973 15.65 13.77 12.35 9.75 11.19 0.0003 0.0007 0.0024 0.0012 Step 1 All measurements are significant at α=.05 (P=<.0001) Table 14. Classification rates for step 1 model for the South African Black sample Calibration Sample Test Sample Male Female Male Female Male 41/59 18/59 Male 23/34 11/34 69.49% 30.51% 67.65% 32.35% Female Total Correct 19/60 31.67% 41/60 68.33% Female 82/119 68.91% Total Correct 57 5/14 35.71% 9/14 64.29% 32/48 66.67% The two-variable model (Table 15) estimates sex through the combined measurements of the vertebral body length at the sagittal plane (SLVB) and the maximum with of the pedicles (MWIP). This model is associated with an increased R-squared value of 0.2743 and an F-value of 18.33. This model correctly classifies 75.00% of males and 83.33% of females, with an overall correct classification of 79.31% in the South African Black calibration sample. When applied to the test sample, this model correctly classifies 76.47% of males and 78.57% of females, for an overall correct classification of 77.08%. Table 15. Classification rates for step 2 model for the South African Black sample Calibration Sample Test Sample Male Female Male Female Male 42/56 14/56 Male 26/34 8/34 75.00% 25.00% 76.47% 23.53% Female Total Correct 10/60 16.67% 50/60 83.33% Female 92/116 79.31% Total Correct 3/14 21.43% 11/14 78.57% 37/48 77.08% The best two-variable model (Table 16) still uses the vertebral body length at the sagittal plane measurement (SLVB), as in the previous model, in addition to the maximum height of the vertebral body (MHVB) instead of the maximum width of the pedicles (MWIP). This model has an associated R-squared value of 0.2872 and an F-value of 19.54. When applied to the South African Black calibration sample, this model correctly classifies 77.19% of males and 76.67% of females for an overall correct sample classification of 76.92%. When applied to the test sample, this model correctly classifies 67.65% of males and 85.71% of females for an overall correct test sample classification of 72.92%. 58 Table 16. Classification rates for step 3 model for the South African Black sample Calibration Sample Test Sample Male Female Male Female Male 44/57 13/57 Male 23/34 11/34 77.19% 22.81% 67.65% 32.35% Female Total Correct 14/60 23.33% 46/60 76.67% Female 90/117 76.92% Total Correct 2/14 14.29% 12/14 85.71% 35/48 72.92% The best three-variable model (Table 17) uses the combination of the maximum height of the vertebral body (MHVB), vertebral body length at the sagittal plane (SLVB), and the maximum width of the superior articular surfaces (MWSA) measurements for the estimation of sex. This model has an R-squared value of 0.3618 and an F-value of 18.14. This model correctly classifies 77.19% of males and 75.00% of females, for an overall correct classification of 76.07% in the South African Black calibration sample. In terms of the test sample, this model correctly classifies 61.76% of males and 92.86% of females for an overall correct classification of 70.83% in this sample. Table 17. Classification rates for step 4 model for the South African Black sample Calibration Sample Test Sample Male Female Male Female Male 44/57 13/57 Male 21/34 13/34 77.19% 22.81% 61.76% 38.24% Female Total Correct 15/60 25.00% 45/60 75.00% Female 89/117 76.07% Total Correct 1/14 7.14% 13/14 92.86% 34/48 70.83% The next model (Table 18) is a four-variable model that is comprised of the measurements of the maximum height of the vertebral body (MHVB), the vertebral body at the sagittal plane (SLVB), the maximum length of the vertebral foramen (MLVF) and the maximum 59 width of the superior articular surfaces (MWSA). This model has an R-squared value of 0.3888 and an F-value of 15.11. This model correctly classifies 80.70% of males and 73.33% of females, with an overall correct classification of 76.92% in the calibration sample. When applied to the test sample, this model correctly classifies 61.76% of males and 92.86% of females, with an overall correct classification of 70.83%. Table 18. Classification rates for step 5 model for the South African Black sample Calibration Sample Test Sample Male Female Male Female Male 46/57 11/57 Male 21/34 13/34 80.70% 19.30% 61.76% 38.24% Female Total Correct 16/60 26.67% 44/60 73.33% Female 90/117 76.92% Total Correct 1/14 7.14% 13/14 92.86% 34/48 70.83% Lastly, the best four-variable model (Table 19) consists of the measurements of the maximum height of the vertebral body (MHVB), the maximum length of the vertebral foramen (MLVF) and the maximum width of the superior articular surfaces (MWSA). Rather than also using the measurement of the vertebral body length at the sagittal plane (SLVB), this model utilizes the length of the vertebral body and foramen (LVBF). This model is associated with an R-squared value of 0.3973 and an F-value of 15.65. When applied to the South African Black calibration sample, this model correctly classifies 82.46% of males and 73.33% of females, with an overall correct calibration classification of 77.78%. This model also correctly classifies 76.47% of males and 85.71% of males, for a total correct classification of 79.17% in the test sample. 60 Table 19. Classification rates for step 6 model for the South African Black sample Calibration Sample Test Sample Male Female Male Female Male 47/57 10/57 Male 26/34 8/34 82.46% 17.54% 76.47% 23.53% Female Total Correct 16/60 26.67% 44/60 73.33% Female 91/117 77.78% Total Correct 2/14 14.29% 12/14 85.71% 38/48 79.17% Sex Estimation in the African American Sample A separate PROC STEPWISE with the MAXR option and PROC DISCRIM procedures were then applied to the measurements of the 12th thoracic vertebra in the African American sample (Table 20). This produced five models, each of which has an R-squared value that increases incrementally over the preceding model. All overall models have a probability of <.0001, with each individual measurement in each model having a partial probability of <.05. This stepwise model was stopped after the fifth step model, as numerous measurements had a probability over <.05 in following models with the R-squared model not increasing significantly beyond 0.5352. The best single-variable model (Table 21) consists of the measurement of the maximum width of the vertebral body (MWVB) and has an associated R-squared value of 0.4442 and an F-value of 215.77. This model correctly classifies 80.71% of males and 82.86% of females, for an overall correct classification of 81.79% in the African American calibration sample. When applied to the test sample, this model correctly classifies 81.67% of males and 73.33% of females for a total correct classification of 77.50%. The best two-variable model (Table 22) utilizes the maximum width of the vertebral body (MWVB) in addition to the measurement of the vertebral body length at the sagittal plane (SLVB). The R-squared value of this model is 0.5068, while the F-value is 138.20. This model correctly classifies 82.86% of 61 males and 85.00% of females, for a total correct classification of 83.93% for the African American calibration sample. In terms of the test sample, this model correctly classifies 88.33% of males and 85.00% of females, for an overall correct classification of 86.67%. Table 20. Stepwise models for African American sample Variables R2 Model F Maximum Width of Vertebral Body (V4) 0.4442 215.77 Partial F 215.77 P <.0001 2 Vertebral Body Length at Sagittal Plane (V3) Maximum Width of Vertebral Body (V4) 0.5068 138.20 34.14 56.57 <.0001 <.0001 3 Maximum Sagittal Length of Vertebra (V1) Vertebral Body Length at Sagittal Plane (V3) Maximum Width of Vertebral Body (V4) 0.5207 97.06 7.80 13.07 25.09 0.0056 0.0004 <.0001 4 Maximum Sagittal Length of Vertebra (V1) Vertebral Body Length at Sagittal Plane (V3) Maximum Width of Vertebral Body (V4) Maximum Width Superior Articular Surfaces (V8) 0.5279 74.64 5.49 12.47 22.88 4.06 0.0198 0.0005 <.0001 0.0449 5 Maximum Sagittal Length of Vertebra (V1) Vertebral Body Length at Sagittal Plane (V3) Maximum Width of Vertebral Body (V4) Maximum Width of Vertebral Foramen (V6) Maximum Width Superior Articular Surfaces (V8) 0.5352 61.25 6.05 10.78 26.22 4.14 5.68 0.0146 0.0012 <.0001 0.0428 0.0178 Step 1 Male Female Total Correct Table 21. Classification rates for step 1 model for the African American sample Calibration Sample Test Sample Male Female Male Female 113/140 27/140 Male 49/60 11/60 80.71% 19.29% 81.67% 18.33% 24/140 116/140 17.14% 82.86% 229/280 81.79% Female Total Correct 62 16/60 26.67% 44/60 73.33% 93/120 77.50% Male Female Total Correct Table 22. Classification rates for step 2 model for the African American sample Calibration Sample Test Sample Male Female Male Female 116/140 24/140 Male 53/60 7/60 82.86% 17.14% 88.33% 11.67% 21/140 119/140 15.00% 85.00% 235/280 83.93% Female Total Correct 9/60 51/60 15.00% 85.00% 104/120 86.67% The best three-variable model is step 3 (Table 23), which consists of the maximum sagittal length of the vertebra (MSVL), along with the measurements of the vertebral body length at the sagittal plane (SLVB) and the maximum width of the vertebral body (MWVB). The Rsquared value of this model is 0.5207, while the F-value is 97.06. This model correctly classifies 85.00% of males and 85.71% of females, for an overall correct classification of 85.36% in the calibration sample. In the African American test sample, this model correctly classifies 88.14% of males and 83.33% of females, for a total correct classification of 85.71%. Male Female Total Correct Table 23. Classification rates for step 3 model for the African American sample Calibration Sample Test Sample Male Female Male Female 119/140 21/140 Male 52/59 7/59 85.00% 15.00% 88.14% 11.86% 20/140 120/140 14.29% 85.71% 239/280 85.36% Female Total Correct 10/60 50/60 16.67% 83.33% 102/119 85.71% The best four-variable model (Table 24) consists of the following measurements: the maximum sagittal length of the vertebra (MSVL), the vertebral body length at the sagittal plane (SLVB), the maximum width of the vertebral body (MWVB), and the maximum width of the superior articular surfaces (MWSA). This model has an associated R-squared value of 0.5279, 63 with an F-value of 74.64. When applied to the African American calibration sample, this model correctly classifies 84.29% of males and 86.43% of females, for an overall correct classification of 85.36% for this sample. In terms of the test sample, this model correctly classifies 89.83% of males and 85.00% of females, with an overall correct classification of 87.40%. Male Female Total Correct Table 24. Classification rates for step 4 model for the African American sample Calibration Sample Test Sample Male Female Male Female 118/140 22/140 Male 53/59 6/59 84.29% 15.71% 89.83% 10.17% 19/140 121/140 13.57% 86.43% 239/280 85.36% Female Total Correct 9/60 51/60 15.00% 85.00% 104/119 87.40% For the last model of the African American sample, the five-variable model (Table 25) is comprised of the maximum sagittal length of the vertebra (MSVL), the vertebral body length at the sagittal plane (SLVB), the maximum width of the vertebral body (MWVB), the maximum width of the superior articular surfaces (MWSA), along with the maximum width of the vertebral foramen (MWVF). This model has an R-squared value of 0.5352, with an F-value of 61.25. This model correctly classifies 86.43% of males and 85.71% of females, with an overall correct calibration classification of 86.07%. When applied to the test sample, this model correctly classifies 89.83% of males and 83.33% of females, with an overall classification of 86.55%. Male Female Total Correct Table 25. Classification rates for step 5 model for the African American sample Calibration Sample Test Sample Male Female Male Female 121/140 19/140 Male 53/59 6/59 86.43% 13.57% 89.83% 10.17% 20/140 120/140 14.29% 85.71% 241/280 86.07% Female Total Correct 64 10/60 50/60 16.67% 83.33% 103/119 86.55% Sex Estimation in the Pooled Sample To discern the potential classification of the 12th thoracic vertebra on the entire sample, a PROC STEPWISE with the MAXR option and a PROC DISCRIM (Table 26) was performed on the pooled sample (the South African Black sample together with the African American sample). This produced six models with an overall probability of <.0001, with the sixth model (the best five-variable model) being the last used as a result of the decreased reliability of the following models due to numerous measurements in the models being over <.05 or the model probability value being over <.0001. Also, the models following the sixth one did not have R-squared values that increased significantly beyond 0.4454. Step 1 Table 26. Stepwise models for Pooled (South African Black + African American) sample Variables R2 Model F Partial F P Maximum Width of Vertebral Body (V4) 0.3432 193.35 193.35 <.0001 2 Vertebral Body Length at Sagittal Plane (V3) Maximum Width of Vertebral Body (V4) 0.4094 127.88 41.33 60.97 <.0001 <.0001 3 Vertebral Body Length at Sagittal Plane (V3) Maximum Width of Vertebral Body (V4) Maximum Width Superior Articular Surfaces (V8) 0.4239 90.27 35.68 42.18 9.30 <.0001 <.0001 0.0025 4 Vertebral Body Length at Sagittal Plane (V3) Maximum Width of Vertebral Body (V4) Maximum Width of Vertebral Foramen (V6) Maximum Width Superior Articular Surfaces (V8) 0.4354 70.75 32.96 49.92 7.45 12.46 <.0001 <.0001 0.0067 0.0005 5 Vertebral Body Length at Sagittal Plane (V3) Maximum Width of Vertebral Body (V4) Maximum Width of Vertebral Foramen (V6) Maximum Width Superior Articular Surfaces (V8) Length Vertebral Body and Foramen (V9) 0.4397 57.45 16.13 38.39 10.01 10.84 2.84 <.0001 <.0001 0.0017 0.0011 0.0926 6 Vertebral Body Length at Sagittal Plane (V3) Maximum Width of Vertebral Body (V4) Maximum Length of Vertebral Foramen (V5) Maximum Width Superior Articular Surfaces (V8) Length Vertebral Body and Foramen (V9) 0.4454 58.79 7.68 27.33 13.85 10.33 7.09 0.0059 <.0001 0.0002 0.0014 0.0081 65 The best single-variable sex estimation model (Table 27) consists of the maximum width of the vertebral body measurement (MWVB). This model has an R-squared value of 0.3432, and an F-value of 193.35. When applied to the pooled calibration samples, this model correctly classifies 76.88% of males and 78.50% of females, with an overall correct classification of 77.69%. Then applied to the pooled test samples, this model correctly classifies 74.47% of males and 75.68% of females, for an overall correct classification of 75.00%. Male Female Total Correct Table 27. Classification rates for step 1 model for the Pooled sample Calibration Sample Test Sample Male Female Male Female 153/199 46/199 Male 70/94 24/94 76.88% 23.12% 74.47% 25.53% 43/200 157/200 21.50% 78.50% 310/399 77.69% Female Total Correct 18/74 56/74 24.32% 75.68% 126/168 75.00% The best two-variable model (Table 28) consists of the measurement of the vertebral body length at the sagittal plane (SLVB), along with the maximum width of the vertebral body (MWVB). This mode has an R-squared value of 0.4094 and an F-value of 127.88. This model correctly classifies 80.61% of males and 82.00% of females, for an overall correct classification of 81.31% of the pooled calibration sample. For the test sample, this model correctly classifies 76.60% of males and 82.43% of females, for an overall correct classification of 79.17%. The best three-variable model (Table 29) uses the maximum width of the superior articular surfaces (MWSA) measurement along with the vertebral body length at the sagittal plane (SLVB) and maximum width of the vertebral body measurements (MWVB). The Rsquared value of this model is 0.4239 and the F-value is 90.27. When applied to the Pooled calibration sample, this model correctly classifies 78.57% of males and 83.00% of females, for 66 an overall correct classification of 80.81%. For the test sample, this model correctly classifies 76.60% of males and 83.78% of females, for a total correct classification of 80.72%. Male Female Total Correct Male Female Total Correct Table 28. Classification rates for step 2 model for the Pooled sample Calibration Sample Test Sample Male Female Male Female 158/196 38/196 Male 72/94 22/94 80.61% 19.39% 76.60% 23.40% 36/200 164/200 18.00% 82.00% 322/396 81.31% Female Total Correct 13/74 61/74 17.57% 82.43% 133/168 79.17% Table 29. Classification rates for step 3 model for the Pooled sample Calibration Sample Test Sample Male Female Male Female 154/196 42/196 Male 72/94 22/94 78.57% 21.43% 76.60% 23.40% 34/200 166/200 17.00% 83.00% 320/396 80.81% Female Total Correct 12/74 62/72 16.22% 83.78% 134/166 80.72% For the best four-variable model (Table 30), the maximum width of the vertebral foramen (MWVF) was added to the measurements of the vertebral body length at the sagittal plane (SLVB), maximum width of the vertebral body (MWVB), and the maximum width of the superior articular surfaces (MWSA). This model is associated with a 0.4354 R-squared value and a 70.75 F-value. For the calibration sample, this model correctly classifies 78.06% of males and 83.50% of females, for an overall correct classification of 80.81%. For the test sample, this model correctly classifies 76.60% of males and 83.78% of females, for an overall correct classification of 79.76% for the pooled test sample. 67 Male Female Total Correct Table 30. Classification rates for step 4 model for the Pooled sample Calibration Sample Test Sample Male Female Male Female 153/196 43/196 Male 72/94 22/94 78.06% 21.94% 76.60% 23.40% 33/200 167/200 16.50% 83.50% 320/396 80.81% Female Total Correct 12/74 62/74 16.22% 83.78% 134/168 79.76% For the first five-variable model (Table 31), the length of the vertebral body and foramen measurement (LVBF) was added to the vertebral body length at the sagittal plane (SLVB), maximum width of the vertebral body (MWVB), maximum width of the vertebral foramen (MWVF), and the maximum width of the superior articular surfaces (MWSA). The R-squared value associated with this model is 0.4397, while the F-value is 57.45. When applied to the Pooled calibration sample, this model classifies 78.06% of males correctly and 84.00% of females correctly, for an overall correct classification of 81.06%. In terms of the test sample, this model correctly classifies 76.60% of males and 83.78% of females, for a total correct classification of 79.76%. Male Female Total Correct Table 31. Classification rates for step 5 model for the Pooled sample Calibration Sample Test Sample Male Female Male Female 153/196 43/196 Male 72/94 22/94 78.06% 21.94% 76.60% 23.40% 32/200 168/200 16.00% 84.00% 321/396 81.06% Female Total Correct 12/74 62/74 16.22% 83.78% 134/168 79.76% Finally, the last model (Table 32) is the best five-variable model for the sex estimation of the 12th thoracic vertebra in the pooled samples. This model consists of the following measurements: the vertebral body length at sagittal plane (SLVB), maximum width of vertebral 68 body (MSVB), maximum length of vertebral foramen (MLVF), maximum width of the superior articular surfaces (MWSA), and length of the vertebral body and foramen (LVBF). This model has an R-squared value of 0.4454 and an F-value of 58.79. This model correctly classifies 78.06% of males and 83.00% of females, for an overall correct classification of 80.56% for the calibration sample. For the test sample, this model correctly classifies 77.66% of males and 81.08% of females, for an overall correct classification of 79.17% for the pooled test sample. Male Female Total Correct Table 32. Classification rates for step 6 model for the Pooled sample Calibration Sample Test Sample Male Female Male Female 153/196 43/196 Male 73/94 21/94 78.06% 21.94% 77.66% 22.34% 34/200 166/200 17.00% 83.00% 319/396 80.56% Female Total Correct 14/74 60/74 18.92% 81.08% 133/168 79.17% Effects of Age-Related Changes Effects of Age in the 12th Thoracic Vertebra in South African Black Samples To assess the effects of age in the estimation of sex through the measurements of the 12 th thoracic vertebra, another PROC STEPWISE with the MAXR option and associated PROC DISCRIM procedures were performed for both age groups of both samples. The stepwise model for the young age group in the South African Black sample (Table 33) consists of three models. Each model has a probability value of <.0001, with each measurement in each model having a partial probability of <.05. Only the three models generated were used, as a result of the following models having increased model and partial probabilities, as well as the R-squared value not significantly increasing beyond 0.4933. The best single variable model (Table 34) 69 consists of the measurement of the maximum width of the vertebral body (MWVB). This model has an R-squared value of 0.4158 and an F-value of 94.68. This model correctly classifies 70.00% of males and 63.33% of females, with an overall correct classification of 66.67% in the young South African Black calibration sample. For the young test sample, this model correctly classifies 69.23% of males and 87.50% of females for an overall correct classification of 73.53%. Step 1 Table 33. Stepwise models for vertebral measurements of young South African Black sample Variables R2 Model F Partial F P Maximum Width of Vertebral Body (V4) 0.4158 94.68 94.68 <.0001 2 Maximum Sagittal Length of Vertebra (V1) Maximum Width of Vertebral Body (V4) 0.4654 57.46 12.24 16.45 0.0006 <.0001 3 Maximum Sagittal Length of Vertebra (V1) Maximum Width of Vertebral Body (V4) Maximum Width Inferior Articular Surfaces (V12) 0.4933 42.51 8.04 17.04 7.21 0.0053 <.0001 0.0082 Table 34. Classification rates for step 1 model for the young South African Black sample Calibration Sample Test Sample Male Female Male Female Male 21/30 9/30 Male 18/26 8/26 70.00% 30.00% 69.23% 30.77% Female Total Correct 11/30 36.67% 19/30 63.33% Female 40/60 66.67% Total Correct 1/8 12.50% 7/8 87.50% 25/34 73.53% The best two-variable model (Table 35) uses the combination of the maximum width of the vertebral body (MWVB) measurement along with the maximum sagittal length of the vertebra (MSVL). The R-squared value associated with this mode is 0.4654, with an F-value of 57.46. This model correctly classifies 65.38% of males and 65.53% of females, for an overall correct classification of 65.45% for the young South African Black calibration sample. For the 70 young test sample, this model correctly classifies 68.00% of males and 75.00% of females, for an overall correct classification of 69.70%. Table 35. Classification rates for step 2 model for the young South African Black sample Calibration Sample Test Sample Male Female Male Female Male 17/26 9/26 Male 17/25 8/25 65.38% 34.62% 68.00% 32.00% Female Total Correct 10/29 34.48% 19/29 65.52% Female 36/55 65.45% Total Correct 2/8 25.00% 6/8 75.00% 23/33 69.70% In addition to the maximum sagittal length of the vertebra (MSVL) and the maximum width of the vertebra body (MWVB), the best three-variable model (Table 36) also uses the maximum width of the inferior articular surfaces measurement (MWIA). This model has an Rsquared value of 0.4933, with an F-value of 42.51. While applying this model to the young South African Black calibration sample, this model correctly classifies 69.23% of males and 65.52% of females, for an overall correct classification of 67.27%. For the test sample, this model correctly classifies 76.00% of males and 75.00% of females, for an overall correct classification of 75.76%. Table 36. Classification rates for step 3 model for the young South African Black sample Calibration Sample Test Sample Male Female Male Female Male 18/26 8/26 Male 19/25 6/25 69.23% 30.77% 76.00% 24.00% Female Total Correct 10/29 34.48% 19/29 65.52% Female 37/55 67.27% Total Correct 71 2/8 25.00% 6/8 75.00% 25/33 75.76% A PROC STEPWISE with the MAXR option and a PROC DISCRIM was also performed on the older adult age group for the South African Black sample (Table 37). This resulted in the same three models as was tested on the younger adult age group of this sample. The best singlevariable model (Table 38) consists of the maximum width of the vertebral body measurement (MWVB), has an R-squared value of 0.4158 and an F-value of 94.68. When applied to the older age group of the South African Black calibration sample, the correct classification is 62.07% for males and 70.00% for females, with an overall correct calibration of 66.10%. For the older test sample, the correct classification is 50.00% for males and 66.67% for females, with an overall correct classification of 57.14%. Step 1 Table 37. Stepwise models for vertebral measurements of old South African Black sample Variables R2 Model F Partial F P Maximum Width of Vertebral Body (V4) 0.4158 94.68 94.68 <.0001 2 Maximum Sagittal Length of Vertebra (V1) Maximum Width of Vertebral Body (V4) 0.4654 57.46 12.24 16.45 0.0006 <.0001 3 Maximum Sagittal Length of Vertebra (V1) Maximum Width of Vertebral Body (V4) Maximum Width Inferior Articular Surfaces (V12) 0.4933 42.51 8.04 17.04 7.21 0.0053 <.0001 0.0082 Table 38. Classification rates for step 1 model for the old South African Black sample Calibration Sample Test Sample Male Female Male Female Male 18/29 11/29 Male 4/8 4/8 62.07% 37.93% 50.00% 50.00% Female Total Correct 9/30 30.00% 21/30 70.00% Female 39/59 66.10% Total Correct 2/6 33.33% 4/6 66.67% 8/14 57.14% The best two-variable model (Table 39) consists of the maximum width of the vertebral body (MWVB) as well as the maximum sagittal length of the vertebra (MSVL). This model has 72 an R-squared value of 0.4654 and an F-value of 57.46. For the older age group of the South African Black calibration sample, this model correctly classifies 59.26% of males and 67.86% of females, with an overall correct classification of 63.64%. For the older test sample, this model correctly classifies 75.00% of males and 66.67% of females, for an overall correct classification of 71.43%. Table 39. Classification rates for step 2 model for the old South African Black sample Calibration Sample Test Sample Male Female Male Female Male 16/27 11/27 Male 6/8 2/8 59.26% 40.74% 75.00% 25.00% Female Total Correct 9/28 32.14% 19/28 67.86% Female 35/55 63.64% Total Correct 2/6 33.33% 4/6 66.67% 10/14 71.43% The final model for the older age group of the South African Black sample (Table 40) is the best three-variable model and consists of the maximum sagittal length of the vertebra (MSVL), the maximum width of the vertebral body (MWVB), and the maximum width of the inferior articular surfaces (MWIA) of the 12th thoracic vertebra. This model has an R-squared value of 0.4933 and an F-value of 42.51. When applied to the old South African Black calibration sample, this model correctly classifies 59.26% of males and 67.86% of females, for a total correct classification of 63.64% in this sample. When applied to the test sample, this model correctly classifies 75.00% of males, 83.33% of females, and 78.57% overall in the sample. 73 Table 40. Classification rates for step 3 model for the old South African Black sample Calibration Sample Test Sample Male Female Male Female Male 16/27 11/27 Male 6/8 2/8 59.26% 40.74% 75.00% 25.00% Female Total Correct 9/28 32.14% 19/28 67.86% Female 35/55 63.64% Total Correct 1/6 16.67% 5/6 83.33% 11/14 78.57% Effects of Age in the 12th Thoracic Vertebra in African American Samples The same procedures (a PROC STEPWISE with MAXR option and PROC DISCRIM) were performed for the assessment of the effects of age in the African American samples. In order to deduce the effects of age between both, the South African Black and the African American samples, the stepwise model for the young African American sample (Table 41) is the same as those used in the South African Black samples above. For the best single-variable model (Table 42), the maximum width of the vertebral body (MWVB) is once again used. This model’s R-squared value is 0.4158, with an F-value of 94.68. When applied to the young age group in the African American calibration sample, this model correctly classifies 85.71% of males, 82.86% of females, with an overall correct classification of 84.29%. For the test sample, this model correctly classifies 80.00% of males and 80.00% of females, for 80.00% overall. For the best two-variable model (Table 43), the R-squared value is 0.4654 and the Fvalue is 57.46 for the maximum sagittal length of the vertebra (MSVL) and the maximum width of the vertebral body (MWVB). For this model, 87.14% of males and 84.29% of females are classified correctly, for an overall correct classification of 85.71% in the young African American calibration sample. For the test sample, 83.33% of males and 86.67% of females were classified correctly, for an overall correct classification of 85.00%. 74 Step 1 Table 41. Stepwise models for vertebral measurements of young African American sample Variables R2 Model F Partial F P Maximum Width of Vertebral Body (V4) 0.4158 94.68 94.68 <.0001 2 Maximum Sagittal Length of Vertebra (V1) Maximum Width of Vertebral Body (V4) 0.4654 57.46 12.24 16.45 0.0006 <.0001 3 Maximum Sagittal Length of Vertebra (V1) Maximum Width of Vertebral Body (V4) Maximum Width Inferior Articular Surfaces (V12) 0.4933 42.51 8.04 17.04 7.21 0.0053 <.0001 0.0082 Table 42. Classification rates for step 1 model for the young African American sample Calibration Sample Test Sample Male Female Male Female Male 60/70 10/70 Male 24/30 6/30 85.71% 14.29% 80.00% 20.00% Female Total Correct 12/70 58/70 17.14% 82.86% 118/140 84.29% Female Total Correct 6/60 20.00% 24/30 80.00% 48/60 80.00% Table 43. Classification rates for step 2 model for the young African American sample Calibration Sample Test Sample Male Female Male Female Male 61/70 9/70 Male 25/30 5/30 87.14% 12.86% 83.33% 16.67% Female Total Correct 11/70 59/70 15.71% 84.29% 120/140 85.71% Female Total Correct 4/30 13.33% 26/30 86.67% 51/60 85.00% For the last model of the younger age group of the African American sample, the best three-variable model (Table 44), the maximum sagittal length of the vertebra (MSVL), maximum width of the vertebral body (MWVB), and the maximum width of the inferior articular surfaces (MWIA) are used. This model has an R-squared value of 0.4933 and an F-value of 42.51. This model correctly classifies 87.14% of males and 84.29% of females, for an overall correct 75 classification of 85.71% in the calibration sample. For the test sample, this model correctly classifies 83.33% of males, 86.67% of females, and 85.00% overall. Table 44. Classification rates for step 3 model for the young African American sample Calibration Sample Test Sample Male Female Male Female Male 61/70 9/70 Male 25/30 5/30 87.14% 12.86% 83.33% 16.67% Female Total Correct 11/70 59/70 15.71% 84.29% 120/140 85.71% Female Total Correct 4/30 13.33% 26/30 86.67% 51/60 85.00% Lastly, the PROC STEPWISE with the MAXR option and the PROC DISCRIM procedures were applied to the older adult age group in the African American sample. Once again, the stepwise model (Table 45) for this sample contains the same measurements and step models as the preceding stepwise models. Step 1 Table 45. Stepwise models for vertebral measurements of old African American sample Variables R2 Model F Partial F P Maximum Width of Vertebral Body (V4) 0.4158 94.68 94.68 <.0001 2 Maximum Sagittal Length of Vertebra (V1) Maximum Width of Vertebral Body (V4) 0.4654 57.46 12.24 16.45 0.0006 <.0001 3 Maximum Sagittal Length of Vertebra (V1) Maximum Width of Vertebral Body (V4) Maximum Width Inferior Articular Surfaces (V12) 0.4933 42.51 8.04 17.04 7.21 0.0053 <.0001 0.0082 The best single-variable model (Table 46) uses the maximum width of the vertebral body (MWVB), has an R-squared value of 0.4158 and an F-value of 94.68. When applied to the older age group of the African American calibration sample, this model correctly classifies 77.14% of males and 84.29% of females, for an overall correct classification of 80.71%. For the test 76 sample, this model correctly classifies 86.67% of males and 83.87% of females, for an overall correct classification of 85.25%. Table 46. Classification rates for step 1 model for the old African American sample Calibration Sample Test Sample Male Female Male Female Male 54/70 16/70 Male 26/30 4/30 77.14% 22.86% 86.67% 13.33% Female Total Correct 11/70 59/70 15.71% 84.29% 113/140 80.71% Female Total Correct 5/31 16.13% 26/31 83.87% 52/61 85.25% For the best two-variable model (Table 47), the maximum sagittal length of the vertebra (MSVL) is used in addition to the maximum width of the vertebral body (MWVB). This model has an R-squared value of 0.4654 and an F-value of 57.46. This model correctly classifies 78.57% of males and 82.86% of females, with an overall correct classification of 80.71% for the older African American calibration sample. For the older test sample, this model correctly classifies 75.86% of males and 90.32% of females, for an overall correct classification of 83.33%. Table 47. Classification rates for step 2 model for the old African American sample Calibration Sample Test Sample Male Female Male Female Male 55/70 15/70 Male 22/29 7/29 78.57% 21.43% 75.86% 24.14% Female Total Correct 12/70 58/70 17.14% 82.86% 113/140 80.71% Female Total Correct 3/31 9.68% 28/31 90.32% 50/60 83.33% For the three-variable model (Table 48), the maximum width of the inferior articular surfaces (MWIA) is added to the measurements of the maximum sagittal length of the vertebra (MSVL) and the maximum width of the vertebral body (MWVB). This model has an R-squared 77 value of 0.4933 and an F-value of 42.51. For the older calibration sample, this model correctly classifies 80.00% of males, 84.29% of females, and 82.14% overall. For the test sample, this model correctly classifies 86.21% of males and 87.10% of females, for an overall correct classification of 86.67%. Table 48. Classification rates for step 3 model for the old African American sample Calibration Sample Test Sample Male Female Male Female Male 56/70 14/70 Male 25/29 4/29 80.00% 20.00% 86.21% 13.79% Female Total Correct 11/70 59/70 15.71% 84.29% 115/140 82.14% Female Total Correct 4/31 12.90% 27/31 87.10% 52/60 86.67% Effects of Age in the Femur in South African Black Samples The effects of age in the estimation of sex are also assessed in the femoral measurements in order to test the results against the reliability of the 12 th thoracic vertebra. A PROC STEPWISE with the MAXR option and the PROC DISCRIM procedures were employed. The stepwise model for the younger adult age group of the South African Black sample (Table 49) revealed two models. Both models have a probability value of <.0001. Table 49. Stepwise models for femoral measurements of young South African Black sample Step Variables R2 Model F Partial F Model P P 1 Vertical Diameter of Femoral Head (F2) 0.5483 167.53 167.53 <.0001 <.0001 2 Bicondylar Length of Femur (F1) Vertical Diameter of Femoral Head (F2) 0.5538 85.03 1.69 59.34 <.0001 0.1960 <.0001 The best single-variable model (Table 50) uses the vertical diameter of the femoral head (FHVD). This model has an R-squared value of 0.5483 and an F-value of 167.53. For the young 78 adult age group in the South African Black calibration sample, this model correctly classifies 90.00% of males and 93.33% of females, for an overall correct classification of 91.67%. For the young test sample, this model correctly classifies 88.46% of males and 100.00% of females, for an overall correct classification of 91.18%. Table 50. Classification rates for step 1 model for the young South African Black sample Calibration Sample Test Sample Male Female Male Female Male 27/30 3/30 Male 23/26 3/26 90.00% 10.00% 88.46% 11.54% Female Total Correct 2/30 6.67% 28/30 93.33% Female 55/60 91.67% Total Correct 0/8 0.00% 8/8 100.00% 31/34 91.18% The two-variable model for the younger adult age group of the South African Black sample (Table 51) also uses the bicondylar length of the femur (FBL) in addition to the vertical diameter of the femoral head (FHVD). The R-squared value associated with this model is 0.5538, with an F-value of 85.03. When applied to the young calibration sample, this model correctly classifies 90.00% of males and 93.33% of females, with an overall correct classification of 91.67%. For the test sample, this model correctly classifies 88.46% of males and 100.00% of females, for an overall correct classification of 91.18%. Table 51. Classification rates for step 2 model for the young South African Black sample Calibration Sample Test Sample Male Female Male Female Male 27/30 3/30 Male 23/26 3/26 90.00% 10.00% 88.46% 11.54% Female Total Correct 2/30 6.67% 28/30 93.33% Female 55/60 91.67% Total Correct 79 0/8 0.00% 8/8 100.00% 31/34 91.18% A PROC STEPWISE with the MAXR option and a PROC DISCRIM was employed for the older adult age group for the South African Black sample, and resulted in the same stepwise model (Table 52) as is used above, in order to assess the age related changes exhibited in the two measurements of the femur. Step 1 2 Table 52. Stepwise models for femoral measurements of old South African Black sample Variables R2 Model F Partial F Model P P Vertical Diameter of Femoral Head (F2) 0.5483 167.53 167.53 <.0001 <.0001 Bicondylar Length of Femur (F1) Vertical Diameter of Femoral Head (F2) 0.5538 85.03 1.69 59.34 <.0001 0.1960 <.0001 For the best single-variable model (Table 53), the vertical diameter of the femoral head (FHVD) is used. This model has an R-squared value of 0.5483 and an F-value of 167.53. For the calibration sample of the older age group of the South African Black sample, this model correctly classifies 86.67% of males and 90.00% of females, for an overall correct classification of 88.33%. For the test sample of the older adult age group, this model correctly classifies 75.00% of males and 83.33% of females, for an overall correct classification of 78.57%. Table 53. Classification rates for step 1 model for the old South African Black sample Calibration Sample Test Sample Male Female Male Female Male 26/30 4/30 Male 6/8 2/8 86.67% 13.33% 75.00% 25.00% Female Total Correct 3/30 10.00% 27/30 90.00% Female 53/60 88.33% Total Correct 1/6 16.67% 5/6 83.33% 11/14 78.57% The bicondylar length of the femur (FBL) is used in addition to the vertical diameter of the femoral head (FHVD) in the two-variable model of the old South African Black sample (Table 54). The R-squared value of this model is 0.5538, with an F-value of 85.03. For the 80 calibration sample, this model correctly classifies 86.67% of males and 93.33% of females, with an overall correct classification of 90.00% in the older adult age group. For the test sample, this model correctly classifies 87.50% of males and 100.00% of females, for an overall correct classification of 92.86% in this sample. Table 54. Classification rates for step 2 model for the old South African Black sample Calibration Sample Test Sample Male Female Male Female Male 26/30 4/30 Male 7/8 1/8 86.67% 13.33% 87.50% 12.50% Female Total Correct 2/30 6.67% 28/30 93.33% Female 54/60 90.00% Total Correct 0/6 0.00% 6/6 100.00% 13/14 92.86% Effects of Age in the Femur in African American Samples A PROC STEPWISE with the MAXR option and a PROC DISCRIM was applied last to both age groups of the African American samples. Not unlike the stepwise models for the South African Black samples, the stepwise model for the younger age group in the African American sample (Table 55) contains the same measurements in order to test the age effects across the samples. Step 1 2 Table 55. Stepwise models for femoral measurements of young African American sample Variables R2 Model F Partial F Model P P Vertical Diameter of Femoral Head (F2) 0.5483 167.53 167.53 <.0001 <.0001 Bicondylar Length of Femur (F1) Vertical Diameter of Femoral Head (F2) 0.5538 85.03 1.69 59.34 <.0001 0.1960 <.0001 The best single-variable model for the younger adult age group in the African American sample (Table 56), consists of the single measurement of the vertical diameter of the femoral head (FHVD). This model has an R-squared value of 0.5483, with an F-value of 167.53. While 81 being applied to the young African American calibration sample, this model correctly classifies 87.14% of males and 91.43% of females, with an overall correct classification of 89.29%. For the test sample, this model correctly classifies 90.00% of males and 90.00% of females, for an overall correct classification of 90.00%. Table 56. Classification rates for step 1 model for the young African American sample Calibration Sample Test Sample Male Female Male Female Male 61/70 9/70 Male 27/30 3/30 87.14% 12.86% 90.00% 10.00% Female Total Correct 6/70 8.57% 64/70 91.43% 125/140 89.29% Female Total Correct 3/30 10.00% 27/30 90.00% 54/60 90.00% For the two-variable model (Table 57), the vertical diameter of the femoral head (FHVD), as well as the bicondylar length of the femur (FBL) are used. This model is associated with a 0.5538 R-squared value and an 85.03 F-value. This model correctly classifies 87.14% of males and 92.86% of females, with an overall correct classification of 90.00% in the young African American calibration sample. In the test sample, this model correctly classifies 90.00% of males and 90.00% of females, for an overall correct classification of 90.00%. Table 57. Classification rates for step 2 model for the young African American sample Calibration Sample Test Sample Male Female Male Female Male 61/70 9/70 Male 27/30 3/30 87.14% 12.86% 90.00% 10.00% Female Total Correct 5/70 7.14% 65/70 92.86% 126/140 90.00% Female Total Correct 82 3/30 10.00% 27/30 90.00% 54/60 90.00% For the older adult age group of the African American sample, the stepwise model (Table 58) is again, the same as the other femoral stepwise models and was performed through the use of the PROC STEPWISE with the MAXR option and PROC DISCRIMs. Step 1 2 Table 58. Stepwise models for femoral measurements of old African American sample Variables R2 Model F Partial F Model P P Vertical Diameter of Femoral Head (F2) 0.5483 167.53 167.53 <.0001 <.0001 Bicondylar Length of Femur (F1) Vertical Diameter of Femoral Head (F2) 0.5538 85.03 1.69 59.34 <.0001 0.1960 <.0001 The best single-variable model (Table 59) consists of the measurement of the vertical diameter of the femoral head (FHVD) and has an R-squared value of 0.5483, with an F-value of 167.53. For the old African American calibration sample, this model correctly classifies 87.14% of males and 85.71% of females, with an overall correct classification of 86.43%. For the test sample, this model correctly classifies 90.00% of males and 86.89% of females, for an overall correct classification of 86.89%. Table 59. Classification rates for step 1 model for the old African American sample Calibration Sample Test Sample Male Female Male Female Male 61/70 9/70 Male 27/30 3/30 87.14% 12.86% 90.00% 10.00% Female Total Correct 10/70 60/70 14.29% 85.71% 121/140 86.43% Female Total Correct 5/31 16.13% 26/31 83.87% 53/61 86.89% Lastly, the two-variable model of the older adult age group of the African American sample (Table 60), the bicondylar length of the femur (FBL) is used along with the vertical diameter of the femoral head (FHVD). This model is associated with an R-squared value of 0.5538 and an F-value of 85.03. For the calibration sample of the older age group of the African 83 American sample, this model correctly classifies 84.29% of males and 84.29% of females, for an overall correct classification of 84.29%. In the test sample, this model correctly classifies 83.33% of males and 83.87% of females, for an overall correct classification of 83.61%. Table 60. Classification rates for step 2 model for the old African American sample Calibration Sample Test Sample Male Female Male Female Male 59/70 11/70 Male 25/30 5/30 84.29% 15.71% 83.33% 16.67% Female Total Correct 11/70 59/70 15.71% 84.29% 118/140 84.29% Female Total Correct 5/31 16.13% 26/31 83.87% 51/61 83.61% Overall, males exhibit higher values than females for all vertebral measurements in both, the South African Black sample and the African American sample (Tables 1-6). Also, in all vertebral measurements the African American sample is larger than the South African Black sample in both of the sexes (Tables 1-2). In terms of the effects of age-related changes, there is a slight increase in the majority of the vertebral measurements in the older adult age group (those over 40 years of age) as opposed to the younger adult age group (skeletally mature to 40 years of age) in males and females of both samples (Tables 3-6). These vertebral differences are also reflected in the summary statistics of the femoral measurements (Tables 7-12). In this case, the femoral measurements of males are larger than that of females in both samples (Tables 7-8), the African American samples exhibit larger measurements than the South African Black samples of the same sex, and finally, measurements tend to increase slightly in age in both sexes and samples (Tables 9-12). 84 CHAPTER 5 DISCUSSION Introduction In order to understand and interpret the results of this study, there are essential foundations and background information that are pertinent to the success and insight obtained in the present study. The preceding chapters, most specifically the first and second chapters, outlined an essential and general overview of crucial topics and information. Firstly, the context of the present study within the realm of anthropology, particularly biological anthropology was introduced. In order for a study to be successful, it must have relevant applications in the field of study. In this case, the study of sexual dimorphism in the 12 th thoracic vertebra and its potential for the estimation of sex in human skeletal remains can be beneficial in multiple avenues of anthropology. Not only does it provide insight into the morphological variations inherent within this element, as well as within and between sexes, age groups and across cultures, but it provides an opportunity for application within the subfields of forensic anthropology, osteology, mortuary archaeology, paleodemography, among others. It is rather difficult to grasp an understanding of a single component of the human skeleton without first understanding how it functions within the framework of the human body. This is the case with the anatomy and skeletal morphology of the 12 th thoracic vertebra. Therefore, an overview of the anatomy and biology of the human skeleton, the vertebral column as a whole, as well as the single components of the 12 th thoracic vertebra and the sacrum was explored. As the muscular structure and development plays a large role in the skeletal morphological variation of elements (France 1988; Carter 2000a&b), an overview of the muscles that directly attach in some way to the vertebral column, thus having the potential to affect the 85 size and shape morphology of vertebrae, was examined. Also, the skeletal composition and muscular associations with the femur were also discussed as a result of this element being used as a control within this study. Past research that was conducted on the vertebral column, the 12 th thoracic vertebra, sacrum and femur was also discussed in order to provide relevant background and explore the potential of these elements in the present study. The concept and impact of sexual dimorphism on the morphology of the human skeleton and its individual elements was also explored. Sexual dimorphism, being the differences between males and females with the trend being that males are larger than females (Gilsanz et al. 1994b; Gilsanz et al. 1997; France 1988) is in large part, the reason behind which skeletal elements are reliable indicators of sex. Several studies have been conducted on sexual dimorphism as it is manifested in the vertebral column (Gilsanz et al. 1994b; Wescott 1999; Marino 1995), the 12th thoracic vertebra (Taylor and Twomey 1984; Sheng-Bo Yu et al. 2008), the sacrum (Moore-Jansen and Plochocki 1999; Plochocki 2010; Flander 1978), and the femur (Krogman and İşcan 1986), all of which were examined for its relatedness and potential in the present study. An important aspect of the present study is the effect of age-related changes on the 12 th thoracic vertebra and how these changes may affect the reliability of its use as an estimator of sex. An overview of various age-related changes in the human skeleton, both in terms of growth and development (Gilsanz et al. 1994b; Gilsanz et al. 1997), as well as forms of degenerative changes (Ortner and Putschar 1981; Heine 1926; McCarter 2006) was addressed. The growth and development of aspects of the vertebral column (Taylor 1975; Scheuer and Black 2000) as well as the degenerative changes that are prone to occur in the vertebral column (Brown et al. 86 2008; Derevenski 2000) were examined in more detail due to its pertinence in the morphological variation and reliability of measurements of the 12 th thoracic vertebra. The concepts of temporal variation and secular effects were also explored in order to address the non-genetic alterations and influences on the human body. Various studies have previously been conducted (Moore-Jansen 1989; Johnston and Zimmer 1989; Jantz and Jantz 1999; Kimura 1984) in attempt to understand how to account for and minimize, as well as study the effects of both of these concepts on human populations. Finally, a brief history on both, the Raymond A. Dart Collection of Human Skeletons and the Hamann-Todd Osteological Collection was provided. These histories illustrate why these collections were selected for this study as well as how the study samples were composed from each collection. This current chapter will go on to address, analyze and discuss the results reported in the previous chapter. Sex Estimation in the 12th Thoracic Vertebra Through the analysis of the measurements of the 12 th thoracic vertebra with statistical procedures, it is evident that the 12th thoracic vertebra is a sexually dimorphic element with the potential for use as an estimator of sex. The mean values of all measurements illustrate the differences, with males being larger than females in the means of all measurements. The classification of the correct sex is relatively high in all samples, with the highest correlation of correct classification of sex being achieved through the use and combination of different measurements. The combination of the measurements of the vertebral body length at the sagittal plane and the maximum width of the pedicles has the highest classification of sex in the South African Black sample. In the African American sample, the model that has the highest correct 87 classification of males and females consists of the measurements of the vertebral body length at the sagittal plane, which is included in the South African Black classification previously mentioned, along with the maximum sagittal length of the vertebra, maximum width of the vertebral body and the vertebral foramen, and the maximum width of the superior articular surfaces. Overall, the African American sample has a higher correlation with correct classification of males and females than the South African Black sample, although this may be due to the differences and representativeness of the samples. Sex Estimation in the South African Black Sample When applying the multivariate analyses to the sample of South African Black males and females, the results demonstrate varying correct classification results depending on the combination of measurements used. Of the models generated during the stepwise procedure, six were selected in terms of their reliability and quantity of measurements used (Table 13). Typically, each model selected to be used increases by one variable over the preceding model. In this case, two two-variable models, and two four-variable models were selected here. Although there is only a slight increase in the R-squared values between both of the same number variable models, different measurements were contained in each model. For example, in the two-variable model (Table 15), the two measurements of the vertebral body length at the sagittal plane (SLVB) and the maximum width of the pedicles are used (MWIP). The step 3 model (Table 16) was determined to be the best two-variable model as a result of the replacement of the maximum width of the pedicles (MWIP) measurement from the preceding model with the maximum height of the vertebral body (MHVB), in addition to still having the 88 vertebral body length at the sagittal plane measurement (SLVB). By keeping both of these models, the applications are increased, as there is an option to use one model over the other based on the condition of the 12th thoracic vertebra. If the neural arch was damaged, for instance, the other (best) two-variable model could be utilized as both measurements are contained in the vertebral body, whereas the first two-variable model would be unusable. The stepwise model uses a combination of the following six of the total twelve measurements of the 12th thoracic vertebra: the maximum height of the vertebral body, the vertebral body length at the sagittal plane, the maximum length of the vertebral foramen the maximum width of the superior articular surfaces, the length of the vertebral body and foramen, and the maximum width of the pedicles. When applying the stepwise models to the male calibration samples, the correct classification results improve with each additional model step. In the best single-variable model (Table 14), a mere 69.49% of males were classified correctly through the use of the maximum width of the pedicles. This classification rate greatly improved with the addition of the vertebral body length at the sagittal plane measurement, with a correct male classification of 75.00%. The correct classification of males becomes more reliable in the two four-variable models (Tables 1819), with correct classifications of 80.70% and 82.46% respectively. As a result of both of these correct classification rates being quite high, it is concluded that these two models consistently classifies South African males. In the male test sample, the first two-variable and best fourvariable models (Tables 15 and 19) provide the greatest degree of classification with a correct male classification of 76.47%. The correct classification rates of the test sample for the male samples in each model are significantly lower than the associated calibration sample 89 classification rate. This may be due to the small test sample sizes in the South African Black samples. For the female calibration sample, the classification results are the highest in the first two-variable model (83.33%), which incorporates the measurements of the vertebral body length at the sagittal plane (SLVB) as well as the maximum width of the pedicles (MWIP) (Table 15). The classification rates of the female test sample are significantly higher, with females being more correctly classified than males in five of the six step models (Tables 14-19). The lowest rate of classification is the single variable model with correct classification of females at 64.29% (Table 14). With each variable addition, this classification rate is improved, from 64.29% to 78.57%, to 85.71%, up to a correct female classification rate of 92.86%. It is concluded that the high correct classification rates in the first two-variable through to the best four-variable models (Tables 15-19) are all consistent classifiers of South African females. The individual measurements selected as the best single-variable model, being the maximum width of the pedicles (MWIP), is somewhat surprising as it is a non-traditional measurement that has not been previously researched. This measurement was incorporated into the protocol in support of the hypothesis of an observable size increase in males, in this case in the width of the 12th thoracic vertebra. The two measurements that occur the most frequently in the step models are the maximum height of the vertebral body (MHVB) and the vertebral body length at the sagittal plane. The reliability of both of these measurements was somewhat expected, due to the success of these measurements in previous studies and in other vertebral elements (Taylor and Twomey 1984; Gilsanz et al. 1994b; Sheng-Bo Yu et al. 2008). However, there were several other measurements that were expected to play a role in the correct classification of males and females as a result of their usefulness in other populations and in 90 other vertebral elements. One such measurement is that of the maximum sagittal length of the vertebra (MSVL). This measurement was found to be highly reliable in a study conducted on a living Korean population (Sheng-Bo Yu et al. 2008), on the first and second cervical vertebrae (Wescott 1999; Marino 1995) as well as on the radiographic study of American children (Twomey and Taylor 1984). The measurements of the maximum width of the transverse processes (MWTP) and the maximum length of the spinous process (MLSP) were also expected to assist in the correct classification of males and females as a result of their association with a large amount of muscles. Both of these features serve as anchoring points for the origins and insertions of muscles (Carter 2000a&b; Netter 1989; France 1988). These measurements were also successfully applied in previous studies of sexual dimorphism in other vertebral measurements (Taylor and Twomey 1984; Gilsanz et al. 1994b). Sex Estimation in the African American Sample The utilization of the stepwise models for the African American sample reveals a high correct sex classification in all step models. This procedure generated five step models (Table 20), which incrementally increase from a single-variable model to a five-variable model, with each increase also increasing the R-squared value of the model along with the rate of correct classification. These five step models consist of a combination of five of the twelve measurements of the 12th thoracic vertebra: the maximum sagittal length of the vertebra (MSVL), the vertebral body length at the sagittal plane (SLVB), the maximum width of the vertebral body (MWVB), the maximum width of the vertebral foramen (MWVF), and the maximum width of the superior articular surfaces (MWSA). 91 In the male calibration sample, the best single-variable model (Table 21) correctly classifies 80.71% of males by using the single measurement of the maximum width of the vertebral body (MWVB). The addition of another measurement in each following step model increases the correct classification rate in the calibration sample of males from 80.71% to 82.86%, 85.00% up to 86.43% of correctly classified males in the best five-variable model of the male calibration sample (Table 25). The male test sample displays similar results in regards to the classification rates of males. The test sample incrementally increases its correct classification rate with each measurement added. The best single-variable model correctly classifies 81.67% of males, up to a correct classification rate of 89.83% in the four and five-variable models. As a result of the high correct classification rates, it is concluded that the three, four, and five-variable models (Tables 23-25) are consistent in correctly classifying African American males. The African American females have similar classification rates to that of their male counterparts. In the calibration sample, 82.86% of females are classified correctly in the singlevariable model (Table 21). This classification rate increases with the addition of new measurements, with the highest correct classification rate occurring at the four-variable model which classifies 86.43% of females correctly. When applied to the female test sample, the same trend occurs, with the best single-variable model having the lowest rate of classification, with 73.33% of females being classified correctly. The classification rate increases in each additional step model, with the highest rate of correct classification of females being 85.00%. Based on the trends listed above, it has been concluded that the most reliable and consistent step models for the correct classification of females are the same step models for that of African American males, being the three, four, and five-variable models (Tables 23-25). 92 The stepwise models and associated classification rates are quite different when comparing the African American samples to the South African Black samples. Only two of the same measurements appear in both samples, the vertebral body length at the sagittal plane (SLVB) and the maximum width of the superior articular surfaces (MWSA). The remaining measurements in each sample are unique to that sample. Two measurements that were expected to be sexually dimorphic, the maximum width of the transverse process (MWTP) and length of the spinous process (MLSP), once again did not appear in this sample. However, the maximum sagittal length of the vertebra (MSVL) and the maximum width of the vertebral body (MWVB) occur in numerous step models in this sample associated with a high rate of classification. This suggests that these measurements may in fact be reliable in the estimation of sex, as was previously demonstrated in studies of various vertebral elements (Taylor and Twomey 1984; Gilsanz et al. 1994b; Wescott 1999; Marino 1995). Both, males and females have significantly higher rates of correct classification in the African American sample than in the South African Black sample. While this may be due to the large size difference in the samples of each, it has also been suggested that Black African populations exhibit a tremendous amount of variation in their vertebral morphology, more so than any other group affiliation (Allbrook 1955). Sex Estimation in the Pooled Sample The stepwise models of the pooled (both, the South African Black and African American samples) result in six step models (Table 26), each of which has a higher R-squared value and a higher rate of classification when applied to the male and female pooled samples. Not unlike the South African stepwise models, this model also consists of two step models with the same 93 number of variables. The two five-variable models (Tables 31-32) have a single measurement difference; the maximum width of the vertebral foramen (MWVF) is in the first five-variable model, while the maximum length of the vertebral foramen (MLVF) is in second and best fivevariable model. Although the sixth step model is the best five-variable model, both were used in the case of damage to the posterior portion of the neural arch or to the spinous processes. In cases where the length of the vertebral foramen cannot be taken, the first five-variable model can be employed instead. The pooled sample incorporates six of the twelve 12th thoracic vertebra measurements within its stepwise models: the vertebral body length at the sagittal plane (SLVB), the maximum width of the vertebral foramen (MWVF), the maximum length of the vertebral foramen (MLVF), the maximum width of the vertebral body (MWVB), maximum width of the superior articular surfaces (MWSA), and the length of the vertebral body and foramen (LVBF). Two of these measurements, the vertebral body length at the sagittal plane (SLVB) and the maximum width of the superior articular surfaces (MWSA) are used in this sample, as well as both of the other samples. There is only one measurement that appeared in the African American stepwise models that does not appear in the pooled, with two measurements used in the South African Black stepwise models not appearing in the pooled sample. Interestingly, the first two step models consist of the same variables as the first two step models of the African American sample, with the remaining step models being more closely aligned with the African American stepwise models than the South African Black stepwise models. This may be the result of a combination of the African American sample consisting of a larger sample size with slightly higher rates of correct classification than that of the South African Black sample. For the male calibration sample, the classification results improve with the addition of another measurement in the two-variable model. The best single-variable model (Table 27) uses 94 the measurement of the maximum width of the vertebral body (MWVB) and correctly classifies 76.88% of males. With the incorporation of the measurement of the vertebral body length at the sagittal plane (SLVB), the classification rate increases to 80.61%, the highest rate of classification for the male calibration sample (Table 28). In the male test sample, the best singlevariable model has a classification rate of 74.47%. This increases to 76.60% with the addition of the second variable. This classification rate does not increase until the best five-variable model, where 77.66% of males are classified correctly (Table 32). As a result of the trends of the classification rates, it is concluded that the two and five-variable models (Tables 28 and 31) are reliable classifications of sex for the pooled male sample. The female calibration sample reveals a higher rate of classification than that of the pooled males, with the first step model correctly classifying 78.50% of females (Table 27). This rate increases to 82.00% with the addition of the second variation (Table 28), with this classification rate peaking at 84.00% in the first five-variable model (Table 31). For the test sample, the one-variable model correctly classifies 75.68% of females, with this rate increasing to 82.43% in the two-variable model. The highest rate of classification for the female test sample is 83.78%, which occurs in the three through five-variable models (Tables 29-31). As a result of this, the most consistent classification models for the pooled female sample are the three and best five-variable models (Tables 29-32). As was the case in the South African Black sample and African American sample, females tend to have a higher rate of correct classification than males for the majority of the step models. The reasons for this are currently unknown at this time, but it may be the result of a lower variability in the skeletal morphology of the female samples. The most frequently used variables in the pooled samples are the measurements of the vertebral body length at the sagittal 95 plane (SLVB) as well as the maximum width of the vertebral body (MWVB). Both of these measurements have been shown to exhibit sexual dimorphism in the 12 th thoracic vertebra in a computer tomography-based study conducted on a living Korean population (Sheng-Bo Yu et al. 2008). The most frequently used elements in the stepwise models of all samples did not correlate with those deemed most reliable by previous studies. In Taylor and Twomey’s (1984) radiographic study of the lower thoracic and lumbar vertebrae, the maximum sagittal length of the vertebra and the maximum height of the vertebral body were discerned as having the greatest disparity between males and females. The maximum sagittal length of the vertebra (MSVL) has also been displayed as being a reliable indicator of sex in CT scans of the 12 th thoracic vertebra (Sheng-Bo Yu et al. 2008) as well as in other elements in the vertebral column (Wescott 1999; Marino 1995; Gilsanz et al. 1994b). The maximum sagittal length of the vertebra (MSVL) only appears in three stepwise models in the African American samples, while the maximum height of the vertebral body (MHVB) measurement only appears in four of the stepwise models in the South African Black samples. While each may be reliable in their respective samples, it was anticipated that they would appear frequently in the same samples. Finally, the absence of the measurements the maximum width of the transverse processes (MWTP) and the maximum length of the spinous process (MLSP) from the stepwise models of all samples was surprising as they function as muscle attachment sites for a large portion of the muscles in the back (Carter 2000a&b; Netter 1989). It was assumed by the researcher that these measurements would display a large degree of sexual dimorphism as muscle attachment sites of other skeletal elements, such as the femur and cranium, are considered consistently reliable indicators of sex (France 1988). 96 Effects of Age in Sex Estimation In order to discern the effects of age and age-related changes that occur and may influence the reliability of the measurements and classification of specimens, the data sets were separated by age group and were assessed via multivariate analyses. The same was done for the measurements of the femur in order to test the reliability of the 12th thoracic vertebra. With the use of statistical procedures, it is apparent that the degree of correct classification of age is still reliable in the African American samples. The mean values of all measurements illustrate the differences between the sexes and the age groups, with the trend being that both sexes slightly increase in the mean value of the measurement from the younger adult age group to the older adult age group in the majority of measurements. A single stepwise model (Table 33) was performed and applied to all samples to see the differing results due to the effects of age. Three step models were generated, with the one-variable model using the measurement of the maximum width of the vertebral body (MWVB). While proving to be reliable in the African American and Pooled samples when age was not a factor, this measurement did not appear in the stepwise models of the South African Black samples. The best two-variable model incorporated the measurement of the maximum sagittal length of the vertebra (SLVB) in addition to the first measurement. The best three-variable model consists of the maximum width of the inferior articular surfaces (MWIA) in addition to the other two previously mentioned measurements. A single stepwise procedure (Table 49) was also performed for the measurements of the femur. Since only two measurements of the femur were taken, there were only two step models generated. The one-variable model uses the measurement of the vertical diameter of the femoral head (FHVD), with the two-variable model also incorporating the bicondylar length of the femur (FBL). For femoral measurements, the classification of the correct sex is relatively high in all 97 samples and all age groups, with the correct classifications of the 12 th thoracic vertebral measurements being high in all age groups and sexes in the African American sample. The correlation of high classification rates with the African American sample is similar to the trend in classification of sex without taking the effects of aging into consideration. The South African Black samples, in both instances have a lower classification rate of the correct sex. Effects of Age in the South African Black Sample Multivariate analyses were applied to both age groups, the young adult South African Black sample and the old adult South African Black sample, in order to assess how age-related changes may affect the correct classification rates of sex. When applying the stepwise models to the young male calibration sample, the highest rate of classification occurs in the best onevariable model (Table 34), with a correct male classification of 70.00%. The same stepwise model was applied to the old male calibration sample, which revealed that the best one-variable step model (Table 38) in this case also had the highest rate of correct classification but is significantly lower than that of the younger age group with only 62.07% of males being correctly classified. In the test sample, the highest rate of classification in the young male sample occurs in the three-variable model, with 76.00% of males being classified correctly. In the older male test sample, this rate decreased slightly to a 75.00% correct classification rate. The application of the stepwise model to the measurements of the femur reveals the inadequacy of the classification rates of sex in relation to the measurements of the 12 th thoracic vertebra for this sample. In contrast to a classification rate of 70.00% in the young and 62.07% in the old age 98 groups of the South African Black sample, the femur correctly classifies 90.00% in the young (Table 50) and 86.67% in the older age groups (Table 53). In the case of the calibration sample of young South African Black females, the best onevariable model (Table 34) is correctly classified at 63.33% with this value only slightly increasing in the following models to 65.52%. These rates of classification for females, however, increase in all step models in the older female sample to 67.86% and 70.00%, respectively (Tables 38-40). In contrast to the male South African Black male sample, the rate of classification for females increases from the younger to the older age group. Similar to the use of the femoral classification rates for both age groups, the 12 th thoracic vertebral classification rates have a decreased consistency as a result of the influence of age-related changes. Opposed to the correct classification of 65.52% of young females for the vertebrae, the femur can correctly classify 93.33% of young females. This trend remains the same for the older age groups with a 93.33% classification rate for the femoral measurements and only a 70.00% classification rate for the vertebral measurements. Overall, the rate of correct classification of males’ decreases from the young to the old age group, with the classification rate slightly increasing for that of females. As a result of the low correct classification rates of the young and old age groups in the South African Black sample, it can be concluded that the reliability of the estimation of sex is adversely influenced by the effects of aging and in this case, cannot be consistently classified. 99 Effects of Age in the African American Sample The same stepwise model was applied the younger adult and older adult age groups in the African American sample. For the calibration sample of young African American males (Table 42), the best one-variable model correctly classified 85.71% of males, with this classification rate slightly increasing to 87.14% in the two and three-variable models (Tables 43-44). In comparison to the calibration sample of old African American males, the best one-variable model (Table 46) correctly classifies 77.14% of males, with this also slightly increasing to 78.57% and 80.00% in the two and three-variable models (Tables 47-48). While the rates of classification increase in each step model of each age group, the classification rate is still lower in each model of the older calibration sample. Even though the classification rates of males decrease from the younger to the older age groups, the rates of correct classification are still high enough to provide a reliable classification of sex. In this case, it can be concluded that the two and three-variable models of both age groups provide a consistent correct classification of sex. When the classification rates of the femoral measurements are contrasted with those of the vertebrae, it becomes apparent that the vertebral classification rates are comparable to those the femur. For instance, the peak classification rate of males in the younger age group is 87.15% (Table 43) in the vertebral measurements, opposed to 87.14% for the femoral measurements (Table 56). For the older age groups, the femur correctly classifies 87.14% of the older males (Table 59), with the vertebral measurements correctly classifying 80.00% (Table 48). For the young female calibration sample, the best one-variable model (Table 42) correctly classifies 82.86% of females, with this classification rate increasing to 84.29% in the two and three-variable models (Tables 43-44). In the older female calibration sample, the best 100 one-variable model (Table 46) correctly classifies 84.29% of females, an increase in classification over the younger age group. The two and three-variable models for the older female calibration sample correctly classify at 82.86% and 84.29% (Tables 47-48). As a result, no significant age related changes can be discerned in the African American sample, and as a result of the relatively high classification rates, it can be concluded that the two and threevariable models for each age group can be regarded as consistent in terms of rates of classification. The classification rates for the femoral measurements yield a 92.86% correct classification of younger females (Table 57) opposed to 84.29% for vertebral measurements. However, in terms of the older age groups, the femoral classification is more comparable to that of the vertebra, with a correct classification of 85.71% for the femur (Table 59), compared to 84.29% for the vertebra. As a result, it can be inferred that the correct classification rate is higher and more reliable in the younger age groups across samples, than it is in the older age groups. Even without the comparison of the femoral classification rates, it is evident that the reliability of the measurements of the 12th thoracic vertebra in the South African Black samples is reduced considerably as a result of age-related changes. The African American samples, however, not only prove to have consistent classification rates for both sexes and age groups, but these rates are even comparable to those of the femur. It was expected that the measurements of the maximum width of both, the superior (MWSA) and inferior articular facets (MWIA) would increase in age as it has been documented that a natural shift in the placement of tension and stress to the posterior neural arch at the apophyseal joints from the vertebral body occurs (Brown et al. 2008; Derevenski 2000). However, only the inferior articular surfaces measurement (MWIA) was included in the stepwise 101 model, and as a result, the whole the apophyseal joint in relation to the effects of age cannot properly be assessed. Effects of Group Affiliation in Sex Estimation In order to test the reliability of sex estimation of the 12 th thoracic vertebra, a protocol should be tested on a variety of populations to ensure a certain degree of accuracy (Giles 1966). While the effects of group affiliation was not initially a part of this study, the combined data set of the African American sample along with the South African Black sample, provides an interesting, albeit preliminary, glimpse into the effects of geographical variation on the skeletal morphology of the 12th thoracic vertebra. As a result of numerous comparative studies on the effects of group affiliation on morphological variation, it has been suggested that those of African descent have more morphological variation than populations of other group affiliations. One such variability is the number of presacral vertebrae present. Numerous studies (Allbrook 1955; Bornstein and Peterson 1966; Lanier 1939; Shore 1930; Willis 1923) have found that Blacks have a higher frequency of the presence of a twenty-fifth presacral vertebra than any other group affiliation, and that males have a higher frequency of this than females (Kaufman 1974; Lanier 1939). This was evident in the Raymond Dart Collection (South African Black) sample used, as the majority of male specimens used presented with twenty-five presacral vertebrae in the form of a sixth lumbar vertebra. Also, those of Black descent have a rather high frequency (24.3% of a research study sample) of superior articular facet asymmetry in the 12 th thoracic vertebra. In this case, the asymmetry of these features presents itself as a left “lumbar type” facet (or a concave, mediallyoriented facet typical of those in the lumbar vertebrae), with a typical thoracic right articular 102 facet (Allbrook 1955). This asymmetry was also expressed frequently in the specimens of the Raymond Dart Collection sample. While nothing beyond univariate statistics were applied to both samples in order to discern differences attributed to geographical variation, this would be an interesting study to pursue at a later time. Summary The objective of this study was three-fold: to determine the presence and degree of sexual dimorphism in the 12th thoracic vertebra, to assess reliability of measurements of the 12 th thoracic vertebra to discern its potential for use in the estimation of sex of unknown individuals, and finally, to assess whether age-related changes influence the skeletal morphology or reliability of the measurements of the 12th thoracic vertebra. Through the use of a combination of univariate and multivariate procedures, each of these objectives has been explored. Sexual Dimorphism in the 12th Thoracic Vertebra The results of this study highlight the differences in the morphology of the 12 th thoracic vertebra, thus validating the presence of sexual dimorphism in this element. Overall, all mean measurements of the 12th thoracic vertebra are larger in males, than they are in females. This disparity between the male and female specimens is also exhibited when considering both age groups as well as the differences between the sexes in both of the collections employed for this study. The degree of sexual dimorphism inherent in the 12th thoracic vertebra was also tested through the rates of correct classifications of each sex using a variety of combinations of measurements. While the female and male correct classification rates were not always 103 comparable in every step model, the relatively high classification rates demonstrate that not only is the 12th thoracic vertebra sexually dimorphic, but that the degree of sexual dimorphism is high. Potential of the 12th Thoracic Vertebra in Sex Estimation Upon the observation that the 12th thoracic vertebra is sexually dimorphic, the potential and reliability of this element as an estimator of sex comes into question. Through the use of stepwise and discriminate analyses, it became apparent that the 12th thoracic vertebra can be used as a consistent and relatively reliable indicator of sex, assuming that the correct variables are consistently applied to the correct group. The comparison of the two collections in this study allowed for the differences in skeletal morphology between the two geographically variant samples to be observed. As a result of this, it is concluded that this study will not be as reliable if applied universally. In order to increase the reliability and potential of measurements of the 12th thoracic vertebra being used in sex estimation, the protocol and selection criteria outlined in this study should be applied to samples from other populations to account for skeletal morphological variations that may skew or inaccurately represent the results. As expressed in this study, with the differences in the measurements and classification rates of African American and South African Black samples, correct classifications rely largely on the correct analysis. Future studies concerning the morphological variation in the 12 th thoracic vertebra due to the effects of group affiliation should be conducted in order to account for error caused by this form of variation. 104 Effects of Age on the 12th Thoracic Vertebra Through the comparison of two age groups, those deemed skeletally mature to 40 years of age and those over 40 years of age, the effects of age-related changes have become apparent. For instance, the classification rates of the South African Black samples were relatively high until the age groups were considered, then neither males nor females could be classified to a rate considered to be consistent or reliable. However, the African American samples were correctly classified regardless of the age restrictions applied. Since external influences, geographical location for instance, play a role in the how age-related changes are manifested, future studies should incorporate a variety of populations in order to account for the differences in age-related processes that can be attributed to group affiliation and sociocultural factors. The results of this study offer an alternative means for the sex estimation of human skeletal remains. Since the stepwise models presented relatively high classification rates for males and females in both samples, the success of this study as a means of reliable identification cross-culturally relies on the results of this study being applied to a variety of populations. In conclusion, this study reveals that the 12th thoracic vertebra has potential for use in sex estimation as a result of the skeletal morphological variation between males and females both documented in the Raymond A. Dart Collection of Human Skeletons and the Hamann-Todd Osteological Collection. 105 BIBLIOGRAPHY 106 BIBLIOGRAPHY Aiello, L. C. and Dean, C. (1990) An Introduction to Human Evolutionary Anatomy. London, UK: Academic Press. Allbrook, D. B. (1955) The East African Vertebral Column: A Study in Racial Variability. American Journal of Physical Anthropology, 13: 489-511. (1956) Changes in Lumbar Vertebral Body Height with Age. American Journal of Physical Anthropology, 14: 35-39. Bass, W. M. (1979) Developments in the Identification of Human Skeletal Material (1968-1978). American Journal of Physical Anthropology, 51(4): 555-562. (1995) Human Osteology: A Laboratory and Field Manual. Ed. Columbia, MO: Missouri Archaeological Society. Belcastro, M. G., et al. (2008) Variation of the Degree of Sacral Vertebral Body Fusion in Adulthood in Two European Modern Skeletal Collections. American Journal of Physical Anthropology, 135: 149-160. Bell, N. H., et al. (1991) Demonstration That Bone Mass is Greater in Black than in White Children. Journal of Bone and Mineral Research, 6:719-723. Benazzi, S., et al. (2009) Sex Assessment from the Sacral Base by Means of Image Processing. Journal of Forensic Science, 54(2): 249-254. Bornstein, P. E., and Peterson, R. R. (1966) Numerical Variation of the Presacral Vertebral Column in Three Population Groups in North America. American Journal of Physical Anthropology, 25: 139-146. Brandner, M. E. (1970) Normal Values of the Vertebral Body and Intervertebral Disc Index During Growth. American Journal of Roentgenology, 110: 618-627. Brown, K. R, et al. (2008) Biomechanical Implications of Degenerative Joint Disease in the Apophyseal Joints of Human Thoracic and Lumbar Vertebrae. American Journal of Physical Anthropology, 136: 318-326. Cann, C. E. (1981) Low-Dose CT Scanning for Quantitative Spinal Mineral Analysis. Radiology, 140: 813-815. Cardoso, H. F. V. And Ríos, L. (2011) Age Estimation From Stages of Epiphyseal Union in the Presacra Vertebrae. American Journal of Physical Anthropology, 144: 238-247. Carter, J. W. (2000a) Gross Anatomy. Wichita, KS: CS&S Press. 107 (2000b) Gross Anatomy: Laboratory Guide. Wichita, KS: CS&S Press. Cheng, X. G., et al. (1998) Measurements of Vertebral Shape By Radiographic Morphometry: Sex Differences and Relationships With Vertebral Level and Lumbar Lordosis. Skeletal Radiology, 27: 380-384. Clark, G. A. (1988) New Method for Assessing Changes in Growth and Sexual Dimorphism in Paleoepidemiology. American Journal of Physical Anthropology, 77: 105-116. Cox, M. and Mays, S. (2000) Human Osteology: In Archaeology and Forensic Science. Cambridge, UK: Cambridge University Press. Crews, D. E. (1993) Biological Anthropology and Human Aging: Some Current Directions in Aging Research. Annual Review of Anthropology, 22: 395-423. Danforth, C. H. (1930) Numerical Variation and Homologies in Vertebrae. American Journal of Physical Anthropology, 14(3): 463-481. Dayal, M. R., et al. (2009) The History and Composition of the Raymond A. Dart Collection of Human Skeletons at the University of the Witwatersrand, Johannesburg, South Africa. American Journal of Physical Anthropology, 140(2): 324-335. de la Cova, C. (2011) Race, Health, and Disease in 19 th-Century-Born Males. American Journal of Physical Anthropology, 144: 526-537. Derevenski, J. R. S. (2000) Sex Differences in Activity-Related Osseous Change in the Spine and the Gendered Division of Labor at Ensay and Wharram Percy, UK. American Journal of Physical Anthropology, 111: 333-354. Droessler, J. (1981) Craniometry and Biological Distance. Evanston, IL: Center for American Archaeology at Northwestern University, Research Series, Volume 1. Ericksen, M. F. (1974) Aging Changes in the Shape of Lumber Vertebrae. American Journal of Physical Anthropology, 41: 477. (1976) Some Aspects of Aging in the Lumbar Spine. American Journal of Physical Anthropology, 45: 575-580. (1978) Aging in the Lumbar Spine. American Journal of Physical Anthropology, 48: 241246. Flander, L. B. (1978) Univariate and Multivariate Methods for Sexing the Sacrum. American Journal of Physical Anthropology, 49: 103-110. France, D. L. (1988) Osteometry at Muscle Origin and Insertion in Sex Determination. American Journal of Physical Anthropology, 76: 515-526. 108 Garn, S. M., et al. (1972) Differential Sexual Dimorphism in Bone Diameters of Subjects of European and African Ancestry. American Journal of Physical Anthropology, 37:127130. Giles, E. (1966) Statistical Techniques For Sex and Race Determination. American Journal of Physical Anthropology, 25: 85-86. Gilsanz, V., et al. (1994a) Gender Differences in Vertebral Sizes in Adults: Biomechanical Implications. Radiology, 190: 678-682. (1994b) Gender Differences in Vertebral Body Sizes in Children and Adolescents. Radiology, 190: 673-677. (1997) Differential Effect of Gender on the Sizes of the Bones in the Axial and Appendicular Skeletons. Journal of Clinical Endocrinology and Metabolism, 82(5): 16031606. Hadley, L. A. (1964) Anatomico-Roentgenographic Studies of the Spine. Charles C Thomas. Springfield, IL: Hall, B. K. (2005) Bones and Cartilage: Developmental and Evolutionary Skeletal Biology. San Diego, CA: Elsevier Ltd. Heine, J. (1926) Uber die arthritis deformans. Virchow’s Archiv für Pathologische Anatomie und Physiologie und für Klinische Medizin, 260: 521-663. Hiatt, J. L. and Gartner, L. P. (1987) Head and Neck Anatomy, 2 nd Ed. Baltimore, MD: Williams & Wilkins Press. Hunt, D. R., and Albanese, J. (2005) History and Demographic Composition of the Robert J. Terry Anatomical Collection. American Journal of Physical Anthropology, 127: 406-417. Jacob, S. W., et al. (1978) Structure and Function in Man. Philadelphia, PA: W. B. Saunders Company. Jantz, L. M. And Jantz, R. L. (1999) Secular Change in Long Bone Length and Proportion in the United States, 1800-1970. American Journal of Physical Anthropology, 110: 57-67. Johnston, F. E. and Zimmer, L. O. (1989) Assessment of Growth and Age in the Immature Skeleton. In İşcan, M. Y. and Kennedy, K. A. R. (eds.): Reconstruction of Life From the Skeleton. New York, NY: Wiley-Liss, pp. 11-21. Kaufman, P. (1974) Variation in the Number of Presacral Vertebrae in Bantu-speaking South African Negroes. American Journal of Physical Anthropology, 40: 369-374. 109 Kelley, M. A. And El-Najjar, M. Y. (1980) Natural Variation and Differential Diagnosis of Skeletal Changes in Tuberculosis. American Journal of Physical Anthropology, 52: 153167. Kimura, K. (1984) Studies on Growth and Development in Japan. Anthropology, 27: 179-214. Yearbook of Physical Krogman, W. M. and İşcan, M. Y. (1986) The Human Skeleton in Forensic Medicine, 2nd Ed. Springfield, IL: Charles C Thomas. Lanier, R. R. (1939) The Presacral Vertebrae of American and Negro Males. American Journal of Physical Anthropology, 25(3): 341-420. (1944) Length of First, Twelfth, and Accessory Ribs in American Whites and Negroes; Their Relationship to Certain Vertebral Variations. American Journal of Physical Anthropology, N.S. 2(2): 137-146. Marino, E. A. (1995) Sex Estimation Using the First Cervical Vertebra. American Journal of Physical Anthropology, 97: 127-133. Masoro, E. J. (2006) Are Age-Associated Diseases an Integral Part of Aging?. In Masoro, E. J. and Austad, S. N. (eds): Handbook of the Biology of Aging. San Diego, CA: Elsevier Inc., pp. 43-62. Matshes, E. et al. (2005) Human Osteology and Skeletal Radiology: An Atlas and Guide. Boca Raton, FL: CRC Press. McCarter, R. J. M. (2006) Differential Aging Among Skeletal Muscles. In Masoro, E. J. and Austad, S. N. (eds): Handbook of the Biology of Aging. San Diego, CA: Elsevier Inc., pp. 470-497. McCormick, D. P., et al. (1991) Spinal Bone Mineral Density in 335 Normal and Obese Children and Adolescents: Evidence for Ethnic and Sex Differences. Journal of Bone and Mineral Research, 6(5): 507-513. McCullough, J. M. And McCullough, C. S. (1984) Age-Specific Variation in the Secular Trend for Stature: A Comparison of Samples From Industrialized and Nonindustrialized Regions. American Journal of Physical Anthropology, 65: 169-180. McKern, T. W. and Stewart, T. W. (1957) Skeletal Age Changes in Young American Males. Technical Report EP-45. Natick, MA: Headquarters, Quartermaster Research and Development Center Environmental Protection Research Division. McMinn, R. M. H. and Hutchings, R. T. (1977) Color Atlas of Human Anatomy. Chicago, IL: Medical Publishers Inc. 110 Meindl, R. S., et al. (1985) Accuracy and Direction of Error in the Sexing of the Skeleton: Implications for Paleodemography. American Journal of Physical Anthropology, 68: 7985. Moore-Jansen, P. H. (1989) A Multivariate Craniometric Analysis of Secular Change and Variation Among Recent North American Populations. Ph.D. Dissertation, Department of Anthropology, University of Tennessee, Knoxville. Moore-Jansen, P. H., and Plochocki, J. H. (1999) Morphometric Variation and Sex Determination in the Human Sacrum. American Journal of Physical Anthropology, 28 (Supple.): 205-206. Moore-Jansen, P. H., Ousley, S. D., and Jantz, R. L. (1994) Data Collection Procedures for Forensic Skeletal Material, 3rd Ed. Knoxville, TN: University of Tennessee Forensic Anthropology Series. Mosekilde, L. and Mosekilde, L. (1990) Sex Differences in Age-Related Changes in Vertebral Body Size, Density and Biomechanical Competence in Normal Individuals. Bone, 11: 6773. Netter, F. H. (1989) Atlas of Human Anatomy. Philadelphia, PA: Saunders Elsevier Inc. Novak, M. and Šlaus, M. (2011) Vertebral Pathologies in Two Early Modern Period (16 th-19th Century) Populations from Croatia. American Journal of Physical Anthropology, In Press. Nuckley, D. J., et al. (2004) Spinal Maturation Affects Vertebral Compressive Mechanics and vBMD with Sex Dependence. Bone, 35: 720-728. O’Neill, T. W., et al. (1994) Variation in Vertebral Height Ratios in Population Studies. Journal of Bone and Mineral Research, 9(12): 1895-1907. Ortner, D. J. and Putschar, W. (1981) Identification of Pathological Conditions in Human Skeletal Remains. Smithsonian Contributions to Anthropology, No. 28. Washington, DC: Smithsonian Institute. Plochocki, J. H. (2010) Sexual Dimorphism of Anterior Sacral Curvature. Journal of Forensic Science, In Print. Porter, R. W., et al. (1980) Backache and the Lumbar Spinal Canal. Spine, 5: 99-105. Promislow, D. E. L., et al. (2006) Evolutionary Biology of Aging: Future Directions. In Masoro, E. J. and Austad, S. N. (eds): Handbook of the Biology of Aging. San Diego, CA: Elsevier Inc., pp. 217-242. 111 Rühli, F. J., et al. (2006) Human Osseous Invertebral Foramen Width. American Journal of Physical Anthropology, 129: 177-188. SAS Institute Inc. (1985) SAS User’s Guide: Statistics, Version 5 Edition. Cary, NC: SAS Institute Inc. (1999) Getting Started with the SAS System, Version 8. Cary, NC: SAS Institute Inc. Scheuer, L. and Black, S. (2000) Developmental Juvenile Osteology. London, UK: Academic Press Ltd. Schultz, A. B., et al. (1984) Measurements of Spine Morphology in Children, Ages 10-16. Spine, 9(1): 70-73. Schwartz, J. H. (2007) Skeleton Keys: An Introduction to Human Skeletal Morphology, Development, and Analysis. Oxford, UK: Oxford University Press. Sheng-Bo Yu, et al. (2008) Determination of Sex for the 12 th Thoracic Vertebra by Morphometry of Three-Dimensional Reconstructed Vertebral Models. Journal of Forensic Science, 53(3): 620-625. Shore, L. R. (1930) Abnormalities of the Vertebral Column in Series of Skeletons of Bantu Natives of South Africa. London Journal of Anatomy, 64: 206-238. Silva, M. J. and Gibson, L. J. (1997) Modeling the Mechanical Behavior of Vertebral Trabecular Bone: Effects of Age-Related Changes in Microstructure. Bone, 21(2): 191-199. Southard, R. N., et al. (1991) Bone Mass in Healthy Children: Measurement with Quantitative DXA. Radiology, 179: 735-738. Steele, D. G. and Bramblett, C. A. (1988) The Anatomy and Biology of the Human Skeleton. College Station, TX: Texas A&M University Press. Stinson, S. (1985) Sex Differences in Environmental Sensitivity During Growth and Development. Yearbook of Physical Anthropology, 28: 123-147. Tague, R. G. (2009) High Assimilation of the Sacrum in a Sample of American Skeletons: Prevalence, Pelvic Size, and Obstetrical and Evolutionary Implications. American Journal of Physical Anthropology, 138: 429-438. Taylor, J. R. (1975) Growth of Human Intervertebral Discs and Vertebral Bodies. Journal of Anatomy, 120(1): 49-68. Taylor, J. R. and Twomey, L. T. (1984) Sexual Dimorphism in Human Vertebral Body Shape. Journal of Anatomy, 138(2): 281-286. 112 Tobias, P. V. (1985) History of Physical Anthropology in Southern Africa. Yearbook of Physical Anthropology, 28: 1-52. Todd, T. W. and Lindala, A. (1928) Dimensions of the Body: Whites and American Negroes of Both Sexes. American Journal of Physical Anthropology, 12(1): 35-119. Todd, T.W. and Pyle, S. I. (1928a) A Quantitative Study of the Vertebral Column by Direct and Roentgenoscopic Methods. American Journal of Physical Anthropology, 12(2): 321-338. (1928b) Effects of Maceration and Drying Upon the Vertebral Column. American Journal of Physical Anthropology, 12(2): 303-319. Tortora, G. J. (1983) Principles of Human Anatomy. New York, NY: Harper & Row Publishers. Trotter, M. (1926) The Sacrum and Sex. American Journal of Physical Anthropology, 9(4): 445450. (1929) The Vertebral Column in Whites and in American Negroes. American Journal of Physical Anthropology, 13(1): 95-107. Twomey, L., et al. (1983) Age Changes in the Bone Density and Structure of the Lumbar Vertebral Column. Journal of Anatomy, 136(1): 15-25. Ubelaker, D. H. (1978) Human Skeletal Remains: Excavation, Analysis, Interpretation. Washington, DC: Taraxacum. Weiss, K. M. (1972) On the Systematic Bias in the Skeletal Sexing. American Journal of Physical Anthropology, 37: 239-250. Wescott, D. J. (2000) Sex Variation in the Second Cervical Vertebra. Journal of Forensic Science, 45(2): 462-466. White, T. D. et al (2011) Human Osteology. San Diego, CA: Academic Press. Whitney, C. (1926) Asymmetry of Vertebral Articular Processes and Facets. American Journal of Physical Anthropology, 9(4): 451-456. Willis, T. A. (1923) The Thoracico-Lumbar Column in White and Negro Stocks. Anatomical Record, 26: 451-455. Wise, P. M. (2006) Aging of the Female Reproductive System. In Masoro, E. J. and Austad, S. N. (eds): Handbook of the Biology of Aging. San Diego, CA: Elsevier Inc., pp. 570-590. Wolanski, N. and Kasprzak, E. (1976) Stature as a Measure of Effects of Environmental Change. Current Anthropology, 17: 548-552. 113 APPENDICES 114 APPENDIX A DATA COLLECTION FORMS DART DATA COLLECTION FORM (Voisin 2010) Observer: Meghan Voisin ID#: ___________ Date: 01 / Dart#: ___________ /2011 Age: ___________ Vertebral Measurements (mm) 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. MSVL MHVB SLVB MWVB MLVF MWVF MWTP MWSA LVBF MWIP MLSP MWIA ____________ ____________ ____________ ____________ ____________ ____________ ____________ ____________ ____________ ____________ ____________ ____________ ID#: ___________ Sacral Measurements (mm) 1. MAH 2. MAB 3. DCS ____________ ____________ ____________ Femoral Measurements 1. FBL ____________cm 2. FHVD ____________mm Dart#: ___________ MSVL MHVB SLVB MWVB MLVF MWVF MWTP MWSA LVBF MWIP MLSP MWIA ____________ ____________ ____________ ____________ ____________ ____________ ____________ ____________ ____________ ____________ ____________ ____________ Sacral Measurements (mm) 1. MAH 2. MAB 3. DCS ____________ ____________ ____________ Femoral Measurements 1. FBL ____________cm 2. FHVD ____________mm 115 Sex: ___________ Notes: _____________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ Age: ___________ Vertebral Measurements (mm) 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. Page: ______ of ______ Sex: ___________ Notes: _____________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ HAMANN-TODD DATA COLLECTION FORM (Voisin 2011) Observer: Meghan Voisin ID#: ___________ Date:____ / H-T#: ___________ Age: ___________ Vertebral Measurements (mm) 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. MSVL MHVB SLVB MWVB MLVF MWVF MWTP MWSA LVBF MWIP MLSP MWIA ____________ ____________ ____________ ____________ ____________ ____________ ____________ ____________ ____________ ____________ ____________ ____________ ID#: ___________ Sacral Measurements (mm) 4. MAH 5. MAB 6. DCS ____________ ____________ ____________ Femoral Measurements 3. FBL ____________cm 4. FHVD ____________mm H-T#: ___________ MSVL MHVB SLVB MWVB MLVF MWVF MWTP MWSA LVBF MWIP MLSP MWIA ____________ ____________ ____________ ____________ ____________ ____________ ____________ ____________ ____________ ____________ ____________ ____________ Sacral Measurements (mm) 4. MAH 5. MAB 6. DCS ____________ ____________ ____________ Femoral Measurements 3. FBL ____________cm 4. FHVD ____________mm 116 Sex: ___________ Notes: _____________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ Age: ___________ Vertebral Measurements (mm) 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. Page: ______ of ______ __ /2011 Sex: ___________ Notes: _____________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ ___________________________ APPENDIX B MEASUREMENT PROTOCOL DEMONSTRATION OF VERTEBRAL MEASUREMENTS 1. Maximum sagittal length of vertebra This measurement is taken using a sliding caliper and is recorded in mm. This measurement is defined as “the sagittal length of the vertebra from the most anterior point on the body to the posterior edge of the spinous process” (Wescott 2000) 2. Maximum height of vertebral body This measurement is taken using a sliding caliper and is recorded in mm. This measurement is the maximum height from the inferior vertebral body surface to the superior vertebral body surface at the most anterior face of the vertebral body. 3. Length of vertebral body at sagittal plane This measurement is taken using a sliding caliper and is recorded in mm. This measurement is the sagittal length of the vertebral body from the most anterior face of the body to the most posterior face of the vertebral body. 117 4. Maximum width of vertebral body This measurement is taken using a sliding caliper and is recorded in mm. This measurement is the maximum width of the vertebral body, measured from the most lateral left point to the most lateral right point of the vertebral body. 5. Maximum length of vertebral foramen at sagittal plane This measurement is taken using vernier calipers and is recorded in mm. This measurement is defined as “the internal length of the vertebral foramen measured at the inferior edge of the foramen in the median plane” (Wescott 2000) 6. Maximum width of vertebral foramen This measurement is taken using vernier calipers and is recorded in mm. This measurement is the maximum internal width of the vertebral foramen, measured from the most lateral left point to the most lateral right point of the vertebral foramen. 118 7. Maximum width of transverse processes This measurement is taken using a sliding caliper and is recorded in mm. This measurement is defined as being “measured from the tip of the left transverse process to that of the right” (Yu et al. 2008) 8. Maximum breadth of superior articular surfaces This measurement is taken using a sliding caliper and is recorded in mm. This measurement is performed from the most lateral surface of the left superior articular facet to the most lateral surface of the right superior articular facet. 9. Length of vertebral body and vertebral foramen This measurement is taken using a sliding caliper and is recorded in mm. This measurement is performed from the most anterior point of the vertebral body to the most posterior point of the vertebral foramen. 119 10. Maximum width of pedicles This measurement is taken using a sliding caliper and is recorded in mm. This measurement is from the most lateral surface of the left pedicle to the most lateral surface of the right pedicle. 11. Maximum Length of spinous process This measurement is taken using a sliding caliper and is recorded in mm. This measurement is performed by placing the tip of the top arm of the caliper at the most superior edge with the tip of the bottom arm at the most inferior edge of the spinous process. 12. Maximum breadth of inferior articular surfaces This measurement is taken using a sliding caliper and is recorded in mm. This measurement is defined as being “measured horizontally from the anterior border of the lamina to its tip” (Yu et al. 2008) 120 DEMONSTRATION OF SACRAL MEASUREMENTS 1. Maximum anterior height This measurement is taken using a sliding caliper and is recorded in mm. This measurement is defined as “the distance from a point on the promontory in the midsagittal plane to a point on the anterior border of the tip of the sacrum measured in the midsagittal plane” (Moore-Jansen et al. 1994) 2. Maximum anterior breadth This measurement is taken using a sliding caliper and is recorded in mm. This measurement is defined as “the maximum transverse breadth of the sacrum at the level of the anterior projection of the auricular surface” (Moore-Jansen et al. 1994) 3. Degree of curvature (subtense) of anterior face This measurement is taken using a coordinate caliper and is recorded in mm. This measurement is the deepest point of concavity on the anterior surface of the sacrum and is measured from the plane of the anterior height measurement down to the anterior face of the sacrum for the greatest degree of concavity. 121 DEMONSTRATION OF FEMORAL MEASUREMENT 1. Bicondylar length of femur This measurement is taken using an osteometric board and is recorded in cm. This measurement is defined as “the distance from the most superior point on the head of the femur to a plane drawn along the inferior surfaces of the distal condyles” (Moore-Jansen et al. 1994) 2. Vertical diameter of femoral head This measurement is taken using a sliding caliper and is recorded in mm. This measurement is the “maximum diameter of the femur head measured on the border of the articular surface” by rotating “the arms of the calipers around the femur head to find the maximum diameter” (Moore-Jansen et al. 1994). 122 APPENDIX C UNIVARIATE SUMMARY STATISTICS Code MSVL MHVB SLVB MWVB MLVF MWVF MWTP MWSA LVBF MWIP MWSP MWIA Code MSVL MHVB SLVB MWVB MLVF MWVF MWTP MWSA LVBF MWIP MWSP MWIA Code MSVL MHVB SLVB MWVB MLVF MWVF MWTP MWSA LVBF MWIP MWSP MWIA Table 61. Dart Collection Vertebral Measurements – Total Sample Measurement Maximum Sagittal Length of Vertebra Maximum Height of Vertebral Body Vertebral Body Length at Sagittal Plane Maximum Width of Vertebral Body Maximum Length of Vertebral Foramen Maximum Width of Vertebral Foramen Maximum Width of Transverse Processes Maximum Width Superior Articular Surfaces Length Vertebral Body and Foramen Maximum Width of Pedicles Maximum Length of Spinous Process Maximum Width Inferior Articular Surfaces n 158 167 165 167 168 168 148 168 165 167 160 168 Mean 70.3 22.5 26.5 38.3 16.8 18.1 47.9 35.0 42.5 31.6 30.7 27.8 Var. 27.0 3.1 12.0 10.1 2.8 3.7 56.0 15.1 13.0 8.2 12.8 10.8 St. Dev. 5.2 1.8 3.5 3.2 1.7 1.9 7.5 3.9 3.6 2.9 3.6 3.3 Table 62. Dart Collection Vertebral Measurements – Male Sample Measurement Maximum Sagittal Length of Vertebra Maximum Height of Vertebral Body Vertebral Body Length at Sagittal Plane Maximum Width of Vertebral Body Maximum Length of Vertebral Foramen Maximum Width of Vertebral Foramen Maximum Width of Transverse Processes Maximum Width Superior Articular Surfaces Length Vertebral Body and Foramen Maximum Width of Pedicles Maximum Length of Spinous Process Maximum Width Inferior Articular Surfaces n 87 93 91 93 94 94 83 94 91 93 89 94 Mean 72.1 23.0 27.8 39.5 16.6 18.2 49.9 35.8 43.8 32.7 31.4 28.1 Var. 17.8 3.2 14.4 8.4 2.9 3.6 49.3 11.3 12.6 6.7 11.5 11.4 St. Dev. 4.2 1.8 3.8 2.9 1.7 1.9 7.0 3.4 3.6 2.6 3.4 3.4 Table 63. Dart Collection Vertebral Measurements – Female Sample Measurement Maximum Sagittal Length of Vertebra Maximum Height of Vertebral Body Vertebral Body Length at Sagittal Plane Maximum Width of Vertebral Body Maximum Length of Vertebral Foramen Maximum Width of Vertebral Foramen Maximum Width of Transverse Processes Maximum Width Superior Articular Surfaces Length Vertebral Body and Foramen Maximum Width of Pedicles Maximum Length of Spinous Process Maximum Width Inferior Articular Surfaces 123 n 71 74 74 74 74 74 65 74 74 74 71 74 Mean 68.0 21.9 24.9 36.7 17.1 18.0 45.4 33.9 40.8 30.2 29.8 27.4 Var. 29.3 2.5 4.4 7.8 2.7 3.8 54.2 18.2 8.7 6.9 13.2 10.0 St. Dev. 5.4 1.6 2.1 2.8 1.6 2.0 7.4 4.3 2.9 2.6 3.6 3.2 Range 38.6 – 82.4 18.2 – 28.2 18.6 – 57.3 30.3 – 48.4 12.0 – 20.2 14.0 – 23.4 31.4 – 73.1 25.0 – 54.1 30.8 – 68.2 24.4 – 40.8 18.5 – 39.2 19.2 – 42.2 Range 63.8 – 82.4 18.2 – 28.2 21.7 – 57.3 34.2 – 48.4 12.0 – 20.1 14.0 – 22.5 40.3 – 71.7 28.7 – 47.5 38.3 – 68.2 27.5 – 40.8 21.0 – 38.3 20.6 – 42.2 Range 38.6 – 76.9 18.4 – 25.5 18.6 – 29.8 30.3 – 42.3 13.1 – 20.2 14.2 – 23.4 31.4 – 73.1 25.0 – 54.1 30.8 – 49.1 24.4 – 36.0 18.5 – 39.2 19.2 – 35.0 Code FBL FHVD Code FBL FHVD Code FBL FHVD Code MSVL Table 64. Dart Collection Femoral Measurements – Total Sample Measurement Bicondylar Length of Femur Vertical Diameter of Femoral Head n 168 168 Mean 43.5 42.9 Var. 7.8 11.8 St. Dev. 2.8 3.4 Table 65. Dart Collection Femoral Measurements – Male Sample Measurement Bicondylar Length of Femur Vertical Diameter of Femoral Head n 94 94 Mean 45.0 45.2 Var. 5.7 6.0 St. Dev. 2.4 2.5 Table 66. Dart Collection Femoral Measurements – Female Sample Measurement Bicondylar Length of Femur Vertical Diameter of Femoral Head n 74 74 Mean 41.6 40.1 Var. 4.0 4.5 St. Dev. 2.0 2.1 Range 37.0 – 53.0 36.0 – 51.0 Range 40.1 – 53.0 39.2 – 51.0 Range 37.0 – 45.8 36.0 – 46.5 Table 67. Dart Collection Vertebral Measurements with Ages – Total Sample Measurement Maximum Sagittal Length of Vertebra MHVB Maximum Height of Vertebral Body SLVB Vertebral Body Length at Sagittal Plane MWVB Maximum Width of Vertebral Body MLVF Maximum Length of Vertebral Foramen MWVF Maximum Width of Vertebral Foramen MWTP Maximum Width of Transverse Processes MWSA Maximum Width Superior Articular Surfaces LVBF Length Vertebral Body and Foramen MWIP Maximum Width of Pedicles MWSP Maximum Length of Spinous Process MWIA Maximum Width Inferior Articular Surfaces 124 Age <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 Mean 69.5 71.2 22.5 22.5 26.2 26.9 37.7 39.0 17.1 16.4 18.1 18.2 47.4 48.5 34.7 35.3 42.0 43.1 31.6 31.7 30.2 31.2 27.5 28.2 Var. 21.9 32.3 3.6 2.6 17.2 5.3 10.4 9.0 2.2 3.5 3.3 4.3 50.2 63.3 17.1 12.6 10.2 16.2 9.0 7.3 12.2 13.2 11.0 10.5 St. Dev. 4.7 5.7 1.9 1.6 4.1 2.3 3.2 3.0 1.5 1.9 1.8 2.1 7.1 8.0 4.1 3.5 3.2 4.0 3.0 2.7 3.5 3.6 3.3 3.2 Range 58.0 – 78.8 38.6 – 82.4 18.2 – 26.9 20.0 – 28.2 18.6 – 57.3 21.7 – 32.0 30.3 – 45.1 32.8 – 48.4 13.6 – 20.1 12.0 – 20.2 14.7 – 22.4 14.0 – 23.4 31.4 – 69.7 38.7 – 73.1 25.0 – 54.1 27.2 – 47.5 30.8 – 48.0 35.4 – 68.2 24.4 – 40.8 25.1 – 39.0 18.5 – 38.3 21.0 – 39.2 19.2 – 35.3 20.6 – 42.2 Code MSVL Table 68. Dart Collection Vertebral Measurements with Ages – Male Sample Measurement Maximum Sagittal Length of Vertebra MHVB Maximum Height of Vertebral Body SLVB Vertebral Body Length at Sagittal Plane MWVB Maximum Width of Vertebral Body MLVF Maximum Length of Vertebral Foramen MWVF Maximum Width of Vertebral Foramen MWTP Maximum Width of Transverse Processes MWSA Maximum Width Superior Articular Surfaces LVBF Length Vertebral Body and Foramen MWIP Maximum Width of Pedicles MWSP Maximum Length of Spinous Process MWIA Maximum Width Inferior Articular Surfaces Code MSVL Age <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 Mean 71.6 72.8 23.0 23.1 27.8 27.9 39.0 40.3 16.9 16.1 18.3 18.2 49.5 50.4 35.5 36.1 43.6 44.1 32.8 32.6 31.0 32.0 27.8 28.6 Var. 16.5 19.3 3.6 2.6 21.0 4.5 7.8 8.6 2.2 3.6 3.2 4.4 47.6 52.7 10.6 12.5 6.0 23.1 6.3 7.3 10.0 13.2 10.3 13.0 St. Dev. 4.1 4.4 1.9 1.6 4.6 2.1 2.8 2.9 1.5 1.9 1.8 2.1 6.9 7.3 3.3 3.5 2.4 4.8 2.5 2.7 3.2 3.6 3.2 3.6 Range 63.8 – 78.8 63.8 – 82.4 18.2 – 26.9 20.0 – 28.2 21.7 – 57.3 24.2 – 32.0 34.2 – 45.1 35.3 – 48.4 13.6 – 20.0 12.0 – 20.1 15.2 – 22.4 14.0 – 22.5 40.3 – 69.7 40.3 – 71.7 28.7 – 42.6 30.8 – 47.5 38.6 – 48.0 38.3 – 68.2 28.6 – 40.8 27.5 – 39.0 23.8 – 38.3 21.0 – 38.3 23.0 – 35.3 20.6 – 42.2 Table 69. Dart Collection Vertebral Measurements with Ages – Female Sample Measurement Maximum Sagittal Length of Vertebra MHVB Maximum Height of Vertebral Body SLVB Vertebral Body Length at Sagittal Plane MWVB Maximum Width of Vertebral Body MLVF Maximum Length of Vertebral Foramen MWVF Maximum Width of Vertebral Foramen MWTP Maximum Width of Transverse Processes MWSA Maximum Width Superior Articular Surfaces LVBF Length Vertebral Body and Foramen MWIP Maximum Width of Pedicles MWSP Maximum Length of Spinous Process MWIA Maximum Width Inferior Articular Surfaces 125 Age <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 Mean 66.7 69.4 21.9 22.0 24.0 25.8 35.8 37.6 17.3 16.8 17.9 18.1 44.3 46.5 33.5 34.4 39.6 42.0 29.7 30.8 29.2 30.4 27.0 27.9 Var. 15.8 41.0 3.1 1.9 3.3 3.9 8.1 5.9 2.1 3.2 3.5 4.3 39.2 69.0 24.7 11.4 7.0 7.7 7.5 5.8 13.8 12.2 12.0 7.8 St. Dev. 4.0 6.4 1.7 1.4 1.8 2.0 2.8 2.4 1.5 1.8 1.9 2.1 6.3 8.3 5.0 3.4 2.6 2.8 2.7 2.4 3.7 3.5 3.5 2.8 Range 58.0 – 73.5 38.6 – 76.9 18.4 – 25.5 20.0 – 24.6 18.6 – 28.1 21.7 – 29.8 30.3 – 40.5 32.8 – 42.3 14.2 – 20.1 13.1 – 20.2 14.7 – 20.7 14.2 – 23.4 31.4 – 58.7 38.7 – 73.1 25.0 – 54.1 27.2 – 41.0 30.8 – 44.6 35.4 – 49.1 24.4 – 35.0 25.1 – 36.0 18.5 – 36.6 23.1 – 39.2 19.2 – 33.8 24.0 – 35.0 Code MSVL MHVB Code MSVL MHVB Code MSVL MHVB Code MSVL MHVB SLVB MWVB MLVF MWVF MWTP MWSA LVBF MWIP MWSP MWIA Table 70. Dart Collection Femoral Measurements with Ages – Total Sample Measurement Maximum Sagittal Length of Vertebra Age <40 >40 <40 >40 Maximum Height of Vertebral Body Mean 43.5 43.6 42.7 43.2 Var. 8.1 7.6 11.5 12.2 St. Dev. 2.9 2.7 3.4 3.5 Range 37.3 – 53.0 37.0 – 48.7 36.0 – 50.1 36.0 – 51.0 Table 71. Dart Collection Femoral Measurements with Ages – Male Sample Measurement Maximum Sagittal Length of Vertebra Age <40 >40 <40 >40 Maximum Height of Vertebral Body Mean 45.0 45.2 44.9 45.6 Var. 6.3 5.0 5.1 7.2 St. Dev. 2.5 2.2 2.3 2.7 Range 40.4 – 53.0 40.1 – 48.7 40.2 – 50.1 39.2 – 51.0 Table 72. Dart Collection Femoral Measurements with Ages – Female Sample Measurement Maximum Sagittal Length of Vertebra Age <40 >40 <40 >40 Maximum Height of Vertebral Body Mean 41.4 41.9 39.5 40.7 Var. 3.3 4.8 3.4 5.1 St. Dev. 1.8 2.2 1.8 2.3 Range 37.3 – 44.5 37.0 – 45.8 36.0 – 43.7 36.0 – 46.5 Table 73. Hamann-Todd Collection Vertebral Measurements – Total Sample Measurement Maximum Sagittal Length of Vertebra Maximum Height of Vertebral Body Vertebral Body Length at Sagittal Plane Maximum Width of Vertebral Body Maximum Length of Vertebral Foramen Maximum Width of Vertebral Foramen Maximum Width of Transverse Processes Maximum Width Superior Articular Surfaces Length Vertebral Body and Foramen Maximum Width of Pedicles Maximum Length of Spinous Process Maximum Width Inferior Articular Surfaces 126 n 406 407 407 407 407 407 394 407 407 407 406 407 Mean 73.9 22.9 27.6 39.9 17.4 19.3 49.1 36.5 44.3 33.2 32.2 28.8 Var. 30.5 3.2 9.2 14.6 2.5 3.7 36.9 15.9 10.9 11.1 14.6 13.3 St. Dev. 5.5 1.8 3.0 3.8 1.6 1.9 6.1 4.0 3.3 3.3 3.8 3.6 Range 60.8 – 94.4 17.6 – 28.3 21.4 – 37.0 29.1 – 51.8 12.8 – 24.5 14.5 – 25.7 36.6 – 83.3 26.2 – 52.1 37.0 – 55.7 25.3 – 47.4 23.6 – 51.8 20.0 – 43.3 Code MSVL MHVB SLVB MWVB MLVF MWVF MWTP MWSA LVBF MWIP MWSP MWIA Code MSVL MHVB SLVB MWVB MLVF MWVF MWTP MWSA LVBF MWIP MWSP MWIA Code FBL FHVD Code FBL FHVD Code FBL FHVD Table 74. Hamann-Todd Collection Vertebral Measurements – Male Sample Measurement Maximum Sagittal Length of Vertebra Maximum Height of Vertebral Body Vertebral Body Length at Sagittal Plane Maximum Width of Vertebral Body Maximum Length of Vertebral Foramen Maximum Width of Vertebral Foramen Maximum Width of Transverse Processes Maximum Width Superior Articular Surfaces Length Vertebral Body and Foramen Maximum Width of Pedicles Maximum Length of Spinous Process Maximum Width Inferior Articular Surfaces n 204 205 205 205 205 205 203 205 205 205 204 205 Mean 77.4 23.2 29.5 42.4 17.4 19.6 51.7 38.1 46.2 34.8 33.6 29.9 Var. 20.7 3.7 5.8 8.7 2.7 4.5 40.9 16.4 8.3 9.6 14.1 14.1 St. Dev. 4.6 1.9 2.4 2.9 1.6 2.1 6.4 4.0 2.9 3.1 3.8 3.8 Range 66.6 – 94.4 17.6 – 28.3 23.8 – 37.0 35.6 – 51.8 12.8 – 22.4 14.6 – 25.7 37.6 – 83.3 29.4 – 52.1 37.7 – 55.7 27.6 – 47.4 23.9 – 45.6 22.7 – 43.3 Table 75. Hamann-Todd Collection Vertebral Measurements – Female Sample Measurement Maximum Sagittal Length of Vertebra Maximum Height of Vertebral Body Vertebral Body Length at Sagittal Plane Maximum Width of Vertebral Body Maximum Length of Vertebral Foramen Maximum Width of Vertebral Foramen Maximum Width of Transverse Processes Maximum Width Superior Articular Surfaces Length Vertebral Body and Foramen Maximum Width of Pedicles Maximum Length of Spinous Process Maximum Width Inferior Articular Surfaces n 202 202 202 202 202 202 191 202 202 202 202 202 Mean 70.3 22.5 25.5 37.4 17.4 19.0 46.2 34.8 42.4 31.7 30.7 27.7 Var. 15.5 2.4 4.6 7.9 2.4 2.7 17.1 10.3 6.1 7.9 11.0 10.2 St. Dev. 3.9 1.5 2.2 2.8 1.6 1.6 4.1 3.2 2.5 2.8 3.3 3.2 Range 60.8 – 81.9 18.5 – 27.0 21.4 – 33.3 29.1 – 45.1 13.0 – 24.5 14.5 – 24.2 36.6 – 59.4 26.2 – 45.0 37.0 – 50.5 25.3 – 40.3 23.6 – 51.8 20.0 – 37.7 Table 76. Hamann-Todd Collection Femoral Measurements – Total Sample Measurement Bicondylar Length of Femur Vertical Diameter of Femoral Head n 407 407 Mean 45.4 45.1 Var. 9.2 14.5 St. Dev. 3.0 3.8 Table 77. Hamann-Todd Collection Femoral Measurements – Male Sample Measurement Bicondylar Length of Femur Vertical Diameter of Femoral Head n 205 205 Mean 47.2 48.0 Var. 6.5 6.4 St. Dev. 2.5 2.5 Range 38.0 – 53.3 36.7 – 55.6 Range 40.5 – 53.3 42.0 – 55.6 Table 78. Hamann-Todd Collection Femoral Measurements – Female Sample Measurement Bicondylar Length of Femur Vertical Diameter of Femoral Head n 202 202 127 Mean 43.6 42.1 Var. 5.2 5.6 St. Dev. 2.3 2.4 Range 38.0 – 49.9 36.7 – 49.4 Table 79. Hamann-Todd Collection Vertebral Measurements with Ages – Total Sample Code MSVL Measurement Maximum Sagittal Length of Vertebra MHVB Maximum Height of Vertebral Body SLVB Vertebral Body Length at Sagittal Plane MWVB Maximum Width of Vertebral Body MLVF Maximum Length of Vertebral Foramen MWVF Maximum Width of Vertebral Foramen MWTP Maximum Width of Transverse Processes MWSA Maximum Width Superior Articular Surfaces LVBF Length Vertebral Body and Foramen MWIP Maximum Width of Pedicles MWSP Maximum Length of Spinous Process MWIA Maximum Width Inferior Articular Surfaces Age <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 Mean 73.0 74.8 22.9 22.9 27.1 28.0 39.2 40.7 17.5 17.3 19.2 19.4 48.7 49.4 36.3 36.6 43.9 44.7 32.8 33.7 31.7 32.6 28.8 28.9 Var. 29.4 30.1 3.1 3.3 8.9 9.2 13.7 14.2 2.4 2.7 3.9 3.4 38.8 34.9 17.2 14.7 9.6 12.0 10.6 11.2 14.2 14.7 11.7 15.0 St. Dev. 5.4 5.4 1.8 1.8 3.0 3.0 3.7 3.8 1.5 1.6 2.0 1.8 6.2 5.9 4.1 3.8 3.1 3.5 3.2 3.3 3.8 3.8 3.4 3.9 Range 60.8 – 90.8 63.6 – 94.4 18.5 – 28.3 17.6 – 27.3 21.4 – 34.7 22.0 – 37.0 29.1 – 48.0 31.9 – 51.8 13.3 – 24.5 12.8 – 21.7 14.6 – 25.7 14.5 – 24.6 36.6 – 73.7 37.6 – 83.3 26.2 – 52.1 28.4 – 47.7 37.0 – 52.1 37.0 – 55.7 25.3 – 43.7 26.3 – 47.4 23.6 – 51.8 24.5 – 45.6 22.2 – 41.5 20.0 – 43.3 Table 80. Hamann-Todd Collection Vertebral Measurements with Ages – Male Sample Code MSVL Measurement Maximum Sagittal Length of Vertebra MHVB Maximum Height of Vertebral Body SLVB Vertebral Body Length at Sagittal Plane MWVB Maximum Width of Vertebral Body MLVF Maximum Length of Vertebral Foramen MWVF Maximum Width of Vertebral Foramen MWTP Maximum Width of Transverse Processes MWSA Maximum Width Superior Articular Surfaces LVBF Length Vertebral Body and Foramen MWIP Maximum Width of Pedicles MWSP Maximum Length of Spinous Process MWIA Maximum Width Inferior Articular Surfaces 128 Age <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 Mean 76.5 78.3 23.2 23.3 29.2 29.9 41.7 43.2 17.4 17.3 19.5 19.7 51.5 52.0 38.1 38.0 45.8 46.7 34.2 35.3 33.1 34.2 29.6 30.2 Var. 18.9 21.2 3.8 3.6 5.1 6.2 7.0 9.4 2.4 3.0 4.7 4.4 40.8 41.3 17.4 15.5 6.6 9.8 8.8 9.9 13.3 14.4 12.0 16.3 St. Dev. 4.3 4.6 2.0 1.9 2.3 2.5 2.6 3.1 1.5 1.7 2.2 2.1 6.4 6.4 4.2 3.9 2.6 3.1 3.0 3.1 3.6 3.8 3.5 4.0 Range 66.6 – 90.8 69.3 – 94.4 19.2 – 28.3 17.6 – 27.3 23.8 – 34.7 24.0 – 37.0 35.6 – 48.0 37.0 – 51.8 13.3 – 22.4 12.8 – 21.7 14.6 – 25.7 15.0 – 24.6 38.0 – 73.7 37.6 – 83.3 30.3 – 52.1 29.4 – 47.7 39.8 – 52.1 37.7 – 55.7 27.6 – 43.7 29.4 – 47.4 23.9 – 43.7 24.5 – 45.6 22.7 – 41.5 23.1 – 43.3 Table 81. Hamann-Todd Collection Vertebral Measurements with Ages – Female Sample Code MSVL Measurement Maximum Sagittal Length of Vertebra MHVB Maximum Height of Vertebral Body SLVB Vertebral Body Length at Sagittal Plane MWVB Maximum Width of Vertebral Body MLVF Maximum Length of Vertebral Foramen MWVF Maximum Width of Vertebral Foramen MWTP Maximum Width of Transverse Processes MWSA Maximum Width Superior Articular Surfaces LVBF Length Vertebral Body and Foramen MWIP Maximum Width of Pedicles MWSP Maximum Length of Spinous Process MWIA Maximum Width Inferior Articular Surfaces Age <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 <40 >40 Mean 69.3 71.4 22.7 22.4 24.9 26.2 36.5 38.3 17.6 17.2 18.9 19.2 45.7 46.8 34.5 35.1 42.0 42.8 31.2 32.2 30.3 31.1 27.9 27.5 Var. 13.7 15.2 2.2 2.5 3.4 5.1 7.1 7.1 2.4 2.4 3.0 2.3 18.9 14.9 10.7 9.9 5.4 6.6 7.9 7.6 11.5 10.4 10.0 10.4 St. Dev. 3.7 3.9 1.5 1.6 1.8 2.3 2.7 2.7 1.5 1.5 1.7 1.5 4.4 3.9 3.3 3.1 2.3 2.6 2.8 2.8 3.4 3.2 3.2 3.2 Range 60.8 – 77.7 63.6 – 81.9 18.5 – 26.4 18.7 – 27.0 21.4 – 31.7 22.0 – 33.3 29.1 – 42.8 31.9 – 45.1 13.5 – 24.5 13.0 – 21.3 15.3 – 24.1 14.5 – 24.2 36.6 – 59.4 38.3 – 56.6 26.2 – 45.0 28.4 – 44.0 37.0 – 48.1 37.0 – 50.5 25.3 – 40.3 26.3 – 38.3 23.6 – 51.8 24.8 – 40.3 22.2 – 37.7 20.0 – 36.1 Table 82. Hamann-Todd Collection Femoral Measurements with Ages – Total Sample Code MSVL Measurement Maximum Sagittal Length of Vertebra MHVB Maximum Height of Vertebral Body Age <40 >40 <40 >40 Mean 45.5 45.4 44.8 45.3 Var. 9.2 9.3 15.9 13.0 St. Dev. 3.0 3.0 4.0 3.6 Range 38.6 – 53.2 38.0 – 53.3 36.7 – 55.6 37.0 – 54.4 Table 83. Hamann-Todd Collection Femoral Measurements with Ages – Male Sample Code MSVL Measurement Maximum Sagittal Length of Vertebra MHVB Maximum Height of Vertebral Body Age <40 >40 <40 >40 Mean 47.3 47.2 47.9 48.1 Var. 6.5 6.6 7.3 5.5 St. Dev. 2.5 2.6 2.7 2.3 Range 40.5 – 53.2 40.9 – 53.3 42.8 – 55.6 42.0 – 54.4 Table 84. Hamann-Todd Collection Femoral Measurements with Ages – Female Sample Code MSVL Measurement Maximum Sagittal Length of Vertebra MHVB Maximum Height of Vertebral Body Age <40 >40 <40 >40 129 Mean 43.5 43.6 41.6 42.6 Var. 4.8 5.6 5.0 5.8 St. Dev. 2.2 2.4 2.2 2.4 Range 38.6 – 49.9 38.0 – 49.3 36.7 – 47.3 37.0 – 49.4