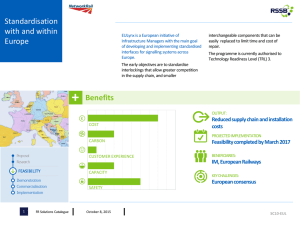
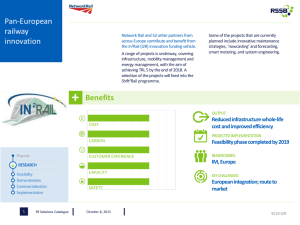
UST Faculty Research Proposals UST/HCC STEM Scholars & Articulation Program
advertisement

UST Faculty Research Proposals UST/HCC STEM Scholars & Articulation Program US Department of Education Grant Year 2015-2016 DR. ALBERT RIBES, Biology Department. XRCC4 and XLF telomeric localization It is estimated that each cell has to deal with ~10 double strand breaks (DSB) each day, mostly created by replication errors, reactive oxygen species and irradiation. Non-Homologous End Joining (NHEJ) is a DNA repair mechanism that plays crucial roles in preventing genome instability that ensues in the presence of DSB. Most NHEJ components have been identified and their roles can be classified into three categories: DSB detection and synapsis (Ku and DNA-PKcs), DNA end processing (Artemis, and polymerases) and ligation (XLF, XRCC4, LigIV). Surprisingly several NHEJ factors like Ku and DNAPKcs are also present at telomeres where they aid in protecting the natural ends of chromosomes form nucleolytic degradation. Our lab has some unpublished data suggesting that both XLF and XRCC4 can bind TRF1 (Figure1), an essential component of human telomeres and we are currently using Protein Fluorescence Complementation Assays (PFCA) to investigate what parts of XRCC4 and XLF are required for their telomeric localization, and whether or not, post-translation modification can influence this process. During past funding cycles we have identified RAP1 as a new interacting member for the both XRCC4 and XLF (Figure 1). Since RAP1 also play roles at telomeres, it is possible that its interaction with XLF and XRCC4 may be related to their telomeric localization. Using fluorescence microscopy we have determined the nuclear localization for both the XRCC4-RAP1 and XLF-RAP1 interactions. For these interactions, the distinctive punctate staining observed for their respective interactions with TRF1 is lacking, due to the presence of RAP1 beyond telomeric tracks. YFP YFP-DAPIContrast Merge Cell Contrast Figure1. XLF Interacts with RAP1. Mammalian cells were transfected with indicated constructs. Pictures were taken 48 hr. after transfection, showing staining consistent with interaction within nucleus between XLF and RAP1 We have collected enough evidence, both through microscopy and fluorescence measurements, to demonstrate that four interactions (XLF-RAP1, XRCC4-RAP1, XLF-TRF1 and XLRCC4-XLF) are occurring in live cells. We also have unpublished evidence that XRCC4-XLF form filaments in live cells and that filament formation is not required for their telomeric localization. The only pieces of evidence that prevent us from publishing our results are the lack of the western blot controls. This past year we have developed a working western blot protocol and successfully performed several western blots (Figure 2). For this year we propose to focus our efforts in generating all necessary western blots and start assembling our data in form of figures for publication. Specifically, we propose to create western blots for the following pairs of interactions: XRCC4-RAD21 XRCC4-TRF1 XRCC4-TRF2 XRCC4-RAP1 XLF-RAD21 XLF -TRF1 XLF -TRF2 XLF -RAP1 Figure 2. XRCC4 interacts with RAP1. A. Fluorescence measurements in same transfected cells indicate the intensity of interaction between XLF and RAP1. Each bar represents three different measurements for three different transfection for a total of 9 measurements for each different V1/V2 combination. B. Western blots corresponding with the transfection. We also propose to perform co-immunoprecipiation experiments to confirm that TRF1 interaction with XRCC4 or XLF are not only detected by co-IP but also with other techniques. We have created a FLAG tagged version of TRF1 and a MYC tag version of XRCC4 and XLF and we plan to use magnetized proteinA beads to pull perform co-IP experiments as depicted in Fig 3 Figure3. Co-immunoprecipitation experiments to detect the presence of XRCC4-TRF1 and XLFTRF1 interactions. Figure depicts expexted results in case or absence of interaction using magnetized proteinA beads and FLAG antibody in extracts from cells transfected with either XRCC4-Myc and TRF1FLAG or XLF-Myc and TRF1-FLAG. ****************************** DR. SHIVAS AMIN, Biology Department. Reintroduction and monitoring of coastal prairie reestablishment Houston’s metropolis is booming with construction and growth; however, this expansion comes at a price. Much of the native coastal prairie that once inhabited the Houston Area is now gone and many of our endemic species are suffering. Local organizations are restoring what they can, but much is unknown about prairie reestablishment. For example, established restoration procedures can unexpectedly fail in well-maintained locations. One possible explanation could be an imbalance in the soil microbial ecosystems. To account for this, many restoration projects are “seeded” with commercially obtained bacterial cultures; however, this treatment is not always met with success. Therefore, it is necessary to determine the impact of the microbial community on prairie restoration. To aid in this process, we will determine if any correlation exists between the composition of the bacterial microbiome and native plant diversity. I propose the continuation of a collaborative project where students work with local institutions to study the reestablishment of native habitats within the city limits of Houston by focusing on key species necessary for prairie establishment. For the past two years, we have identified and monitored prairie-sampling sites within the Houston Area in various stages of succession. Currently, it is not known what types of organisms will recolonize prairie soil. Therefore, I propose that HSI-STEM students conduct soil samplings and plant diversity surveys in these prairies. As a control, students will also monitor adjacent grasslands that do not contain native plants. Throughout this project, students will 1) Learn ecological survey techniques and use databases to record all of their observations and 2) Determine chemical properties and microbial diversity of soil using established protocols. This project is in the third year of existence. Over the previous year we have accomplished the following: 1. Established collaborations with local plant experts. These experts have provided invaluable advice on what equipment to use, how they perform their own surveys, and knowledge on identifying local plant species. 2. Established local sampling sites and standardized survey methods. We have developed a transecting method for analyzing plant diversity within our specific prairie sites. We have recently narrowed our focus to a few critical prairie sites. 3. Utilizing technology. We used programs like HanDbase, Google Sheets and Dropbox to consolidate and quickly share data with our collaborators. 4. Gathering data. We have gathered baseline data on the presence of prairie grasses and flowering plants. A method is currently being developed to efficiently extract total DNA from soil. Our future goals for the upcoming year: 1. Gather more data. We must continue to monitor prairie sites throughout the year as plant composition changes. We must also begin collecting soil samples. 2. Extracting total DNA from soil. We have tried a variety of methods to extract prokaryotic DNA from prairie soil. We have yet to find the best technique. 3. Perform High-throughput sequencing. We will use Roche 454 sequencing of 16S ribosomal units to determine microbial diversity and relative abundance. 4. Diversify microbial DNA extraction. We are currently extracting prokaryotic DNA, but we would like to branch out to extracting the DNA of eukaryotic microbes like fungi. 5. Bioinformatics pipeline. We must develop a series of short python based scripts that will execute BLAST and perform phylogenic analysis of the obtained sequence data. **************************************** DR. HENRY FOUST, Mathematics Department. Delineating the entropy changes due to detonation and deflagration within the rotational detonation engine. Problem Statement Theoretically, combustion chambers run as a constant pressure or constant volume process. Assuming an ideal, perfect gas, it is straight forward to get the appropriate entropy and energy balances for the following cases: a) Constant Volume Combustion Chamber b) Constant Pressure Combustion Chamber c) General Combustion Chamber The rotational detonation engine, or “RDE”, (See Figure 1), in terms of entropy changes through the combustion chamber, runs somewhere between a constant volume and constant pressure combustion process. The “where” changes from run-to-run. Figure 1 – Rotational Detonation Engine at Air Force Research Laboratory, Wright Patterson Air Force Base To understand a detonation associated with the RDE refer to Figure 2, the Hugoniot Shock Curve represents the detonation shock that comes before the reaction zone where the reactants are consumed. The detonation actually occurs theoretically from the von Neumann point along the Raleigh line to the CJ condition, which is the end of the reaction zone and a sonic condition. You’ll notice in Figure 2 the actual RDE runs at pressures below the von Neumann Pressure and so the Rayleigh line for the RDE is a partial line. Figure 3 provides another interpretation of the above features. One possible reason for this (see Figure 4) is that the “detonation” reacts about 80% of the reactants and the other 20% (more or less) is deflagration, which releases less energy per given mass. It’s likely that if the combustion were 100% detonation, then the RDE would act more as a constant volume combustor, but it does not. Entropy (S) versus Temperature (T) graphs for each of the three processes where the RDE is the general combustor process have been developed. These S versus T graphs can be directly related to thermal efficiency. The proposed research is to further an effort the PI began while a USAF Summer Faculty Fellow at AFRL/WPAFB. Utilizing entropy and conservation principles developed for the three types of combustion processes determine a means to relate the % detonation to the S versus T graphs. Referring to Figure 4, the lower right purple portion is unreacted gases where right above it, separated by the darker purple line, is combusted products, which is much warmer. Deflagration occurs across this boundary. CFD results could provide the temperature gradients across this boundary, and along with other considerations readily available, the reactions due to deflagration can be characterized. This deflagration process will be related to the S versus T graphs for the two theoretical processes and the general (RDE) process. This research effort would provide a diagnosis tool to tell the staff engineers at AFRL what changes to the RDE are showing improvement in terms of thermal efficiency. Figure 2 – Hugoniot Curve(s) and Rayleigh Line(s) Figure 3 – Detonation Shock and attached reaction zone Figure 4 – CFD Results for the RDE Likely Results • • • • • Establish a relationship between detonation/deflagration processes occurring in RDE (chemical kinetics) and S versus T graphs for RDE and theoretical combustion processes We’ll all get a solid foundation in the theory of detonation Student presentation/poster at 2016 UST Research Week Student poster at 2016 HCC Research Week Possible national conference presentation, conference proceedings and journal paper submissions References Lee (2008). The Detonation Phenomenon; Cambridge University. Zucker and Biblarz (2002). Fundamentals of Gas Dynamics, 2nd edition; John Wiley and Sons; New York, NY. Fickett and Davis (1979). Detonation Theory and Experiment; Dover Publications, Mineola, NY. F. Zang, editor (2012). Shock Wave Science and Technology Reference Library, volume 6, Detonation Dynamics; Springer, Berlin, Germany. White (2000). Fluid Mechanics; Wiley and Sons, New York, NY. Murthy and Curran, ed. (1996). Developments in High-Speed-Vehicle Propulsion Systems, Chapter 9, American Institute of Aeronautics and Astronautics, Reston, VA. Davison (2008). Fundamentals of Shock Wave Propagation in Solids; Springer, Berlin, Germany. Zeldovich, Barenblatt, Librovich, and Makhviladze (1985). The Mathematical Theory of Combustion and Explosions; Consultants Bureau, New York, NY. http://www.nrl.navy.mil/content_images/11_FA2.pdf http://digitalcommons.uconn.edu/cgi/viewcontent.cgi?article=6482&context=dissertations ****************************************** DR. BIRGIT MELLIS, Chemistry & Physics Department. Synthesis, analysis and application of gold and other metal nanoparticles The project I am planning for the coming year consists of synthesizing gold nanoparticles with different cylcodextrins as ligand shells. The synthesized nanoparticles will be analyzed regarding quality, monodispersity and size with UV-Vis, Dynamic Light Scattering (DLS) and other suitable methods. Promising nanoparticle systems will then be used in photothermal studies (with a 532 nm laser) with the goal of future implementation in biomedical applications such as imaging and therapy. The HCC contribution to this project will mainly consist of the synthesis and preliminary analysis of the gold nanoparticle systems (with UV-Vis measurements). This project would be best suited for an inorganic or organic chemist with an interest in synthesis methods. As a condensed matter physicist I want to concentrate on the analysis part in this project. But I will provide the instructions for the synthesis and we can compare our results to goldnanoparticle systems with other ligand shells previously synthesized at UST. All further experiments, such as DLS and photothermal experiments can be conducted at UST. I enjoyed working with a team of HCC colleagues on a project in the field of Modern Physics a year ago and I am looking forward to this new collaboration. *************************************************** DR. JAVORIS HOLLINGSWORTH, Chemistry Department. Design and synthesis of new nanotechnology Annually, the World Health Organization reports approximately 3-5 million cases of cholera, 100,000150,000 of which result in death. This diarrheal disease is typically transmitted by consumption of contaminated water and food, which is closely associated with inadequate environmental management. Hence, cholera cases are very common in developing parts of the world, such as the Indian subcontinent and sub-Saharan Africa. The primary research objective involves the development of bacteria-grabbing nanotechnology that may reduce the transmission of deadly diseases, such as cholera. In general, the goal is to push the limits of polypeptoid production by establishing new cost-effective synthetic strategies. The prepared materials will be suitable for environmental and biomedical applications. By attaching bacteriagrabbing polypeptoids onto the surface of magnetic core-shell particles, the engineered system will permit the selective removal of bacteria (e.g. Vibrio cholerae) from contaminated water via an external field. This preventative strategy may have a significant impact on public health worldwide. **************************************************** DR. SARAH GHAOUI, Chemistry Department (Computational Chemistry). Investigating reaction mechanisms and molecular structure of organometallic compounds using computational methods My theoretical research focuses on investigating reaction mechanisms and molecular structure of organometallic compounds using computational methods. Molecular orbital calculations of small molecules are carried out at the density functional level. Gold nanoparticles (GNP) have been intensely studied for the last twenty-five years and are still the focus of research especially in the field of medicine. Due of their nontoxicity, and the ability to synthesize the GNP in different shapes and sizes, via different pathways and under different conditions, GNP can also act as drug delivery instruments. The mechanism for the synthesis of gold nanoparticles has been successful using different stabilizing agents to control the size of the nanoparticles. Yet, little is known about the mechanism of its synthesis, or its binding to protein molecules. A detailed understanding of the mechanism and kinetics of these reactions is required in order to gain control of the shape and size of the synthesized gold particles, and the delivery methods. Computational methods employing the Gaussian program will be utilized to determine geometries and energies of species involved as well as inspecting the orbital overlap for clarifications. ****************************************************** DR. CRYSTAL YOUNG, Chemistry & Physics Department (Organic). Fossil fuels A future shortage of fossil fuels and our dependence on exports from politically unstable regions may lead to an energy crisis. Not only is this problem an economic one, but also an environmental issue. The increased use of hydrocarbons while not sustainable, may also be contributing to global warming and dangerous changes to the biosphere. My approach to this problem is two-fold. One option is using solar energy as a supplement, which may be harvested using organic photovoltaic cells. The other is by reducing some of the demand by designing more energy efficient systems like organic light-emitting diodes. Polymer-based photovoltaic systems are attractive materials for use in solar cells because of their facile processing and their capacity to afford low-cost, flexible, and lightweight devices with nominal negative environmental impact.1–3 With the advent of the bulk heterojunction in which the electron donor and acceptor are both present in a continuous phase, poly(phenylene vinylene) (PPV) derivatives have been shown to be efficient electron-donating components (Figure 1).1 In particular, several studies have sought to determine the ideal morphological parameters to maximize efficiency of the easily synthesized and commonly used dialkoxy-PPVs (DAO-PPVs) in organic photovoltaic (OPV) systems.1–3 Due, however, to their extended conjugation length and enhanced electron delocalization, DAO-PPVs are highly susceptible to photooxidation by singlet oxygen during device operation, which severely deteriorates device performance and ultimately reduces device lifetime.4–5 Specifically, the electron-donating alkoxy groups lower the oxidation potential of the PPV backbone, thereby enhancing the oxidative degradation of the conjugated polymer. PPV derivatives also have potential as the emissive layer in blue organic light-emitting diode (OLED) devices due to their large semiconductor band gap and high photoluminescent quantum yields.6–8 Blue OLEDs with long device lifetimes are necessary for displays as well as for use in creating white OLEDs for lighting applications. Similar problems plague DAO-PPVs in OLED devices as in OPV systems. Not only does the strong electron-donation from the alkoxy side chains enhance the oxidative cleavage of the polymer chain, but also the reduction in the HOMO-LUMO gap causes a red shift in the emission spectra. To address this problem, I have explored the synthesis and use of an alternative class of PPV -- dialkyl PPVs (DA-PPVs) -- where the strongly donating alkoxy groups are replaced with more weakly donating alkyl groups (see Figure 1). When compared to DAO-PPVs, DA-PPVs exhibit higher oxidation potentials; moreover, the alkyl groups will simultaneously provide two radical scavenging sites (i.e., the benzylic positions) for every repeat unit in the chain. With these modifications, I anticipate that these new materials would offer enhanced operational performance and longer device lifetimes. Furthermore, the band gap of these materials can be readily tuned by judiciously incorporating additional substituents on the phenyl rings and/or the vinylic moieties along the polymer backbone. My previous work in the Lee group on organic photovoltaic devices showed that DA-PPV was markedly less soluble than DAO-PPV. Despite the long alkyl chains on the polymers that were synthesized, solubility was too low for optimal comparisons to be made. To this end, I have investigated the alternating co-polymer derivatives of poly(meta-phenylene vinylene) (PmPV). Notably, PmPV derivatives have increased band gaps due to disruption of the conjugation, as well as improved solubility.9,10 Figure 1. Structures of dialkoxy-PPV (DAO-PPV) and its derivatives (top), and dialkylPPV (DA-PPV) and its derivatives (bottom). While DA-PPV oligomers have been reported,11,12 surprisingly few (if any) studies have described DA-PPVs having molecular weights greater than approximately 20,000. To address this, I have developed Wittig-type synthetic routes to defect-free poly[(m-phenylenevinylene)-co(2,5-dioctyl-p-phenylenevinylene)] as well as the corresponding defect-free alkoxy poly[(3octyloxy-m-phenylenevinylene)-co-(2,5-dioctyl-p-phenylenevinylene)]. In my investigation, DAO-PPV was used as a well-known benchmark polymer to evaluate the performance of the new dialkyl derivatives in working OLED and photovoltaic devices. Figure 2. Generalized device structure for an OLED and an OPV. In an OLED device, the electroluminescence of the organic component (e.g., PPV) is utilized for the emissive layer. Figure 2 shows a typical OLED configuration like those studied. The basic design consists of a glass substrate patterned with an indium tin oxide anode (ITO), followed by a conducting layer of poly(3,4-ethylenedioxythiophene) poly(styrenesulfonate) (PEDOT:PSS), and then the emissive layer (active layer) topped with a low-work-function metal as the cathode.13 When an electrical current is applied to the device, the recombination of a hole and electron in the emissive layer releases energy in the form of visible light at a wavelength dependent upon the band-gap of the material. Similarly, the structure of an OPV device includes all the same components with the active layer being composed of a mixture of the polymer and a fullerene derivative [6,6]-phenyl-C61-butyric acid methyl ester (PCBM). However, in this type of device instead of introducing electric current to create light, light is absorbed by the conjugated polymer (donor) and the excitons formed are separated into holes that travel toward the negative anode (ITO) and electrons that travel toward the cathode.14 Once the charges have been separated, they can then be collected for energy applications. In addition to these structures, I have been pursuing an investigation of the performance of the ether derivatives shown in Figure 3. These compounds would be ideal targets for undergraduate students to pursue. Using reactions and theories developed in lecture and lab, students would be able to take the next important step in the scientific process in designing experimental procedures of their own. The highly lucrative field of polymer chemistry would be opened to them, which would expand their skill set for several industries. A student could easily synthesize a compound and utilizing my contacts at the University of Houston, complete characterization of the final products in a short-term research project. Integration of practical application to the subject brings new depth to a student’s learning. Figure 3. PPV ether derivatives currently being synthesized. References 1. Hoppe, H.; Sariciftci, N. S. Morphology of Polymer/Fullerene Bulk Heterojunction Solar Cell. J. Mater. Chem. 2006, 16, 45–61. 2. Thompson, B. C.; Fréchet, J. M. Polymer–Fullerene Composite Solar Cells. J. Angew. Chem. 2008, 47, 58–77. 3. Yang, X.; Loos, J. Toward High-Performance Polymer Solar Cells: The Importance of Morphology Control. Macromolecules 2007, 40, 1353–1362 4. McElvain, J.; Antoniadis, H.; Hueschen, M.; Miller, J.; Roitman, D.; Sheats, J.; Moon, R. Formation and Growth of Black Spots in Organic Light-Emitting Diodes. J. Appl. Phys. 1996, 80, 6002–6007. 5. Friend, R. H.; Gymer, R. W.; Holmes, A. B.; Burroughes, J. H.; Marks, R. N.; Taliani, C.; Bradley, D. D. C.; Dos Santos, D. A.; Bredas, J. L.; Logdlund, M.; Salaneck, W. R. Electroluminescence in Conjugated Polymers. Nature 1999, 397, 121–128. 6. Burroughes, J. H.; Gradley, D. D. C.; Brown, A. R.; Marks, R. N.; Mackay, K.; Friend, R. H.; Burns, P. L.; Holmes, A. B. Light-Emitting Diodes Based on Conjugated Polymers. Nature 1990, 347, 539–541. 7. Friend, H. R.; Bradley, C. D. D.; Townsend, D. P. Photo-Excitation in Conjugated Polymers. J. Phys. D 1987, 20, 1367–1384. 8. Samuel, I. D. W.; Crystall, B.; Rumbles, G.; Burn, P. L.; Holmes, B. A.; Friend, H. R. The Efficiency and Time-Dependence of Luminescence from Poly (p-phenylene vinylene) and Derivatives. Chem. Phys. Lett. 1993, 213, 472–478. 9. Lipson, S.M.; O'Brien, D.F.; Byrne, H.J.; Davey, A.P.; Blau, W.J. Investigation of Efficiency and Photostability in Polymer Films. Synthetic Met. 2000, 553, 111–112. 10. Liao, L.; Pang, Y. Ding, L.; Karasz, F.E. Blue-Emitting Soluble Poly(mphenylenevinylene) Derivatives. Macromolecules 2001, 34, 7300–7305. 11. Thorn-Csanyi, E.; Kraxner, P. All-trans Oligomers of 2,5-dialkyl-1,4-phenylenevinylenesMetathesis Preparation and Characterization. Macromol. Chem. Phys. 1997, 198, 3827– 3843. 12. Bijnens, W.; Van Der Borght, M.; Manca, J.; De Ceuninck, W.; De Schepper, L.; Vanderzane, D.; Gelan, J.; Stals, L. A New Precursor to Electroconducting Conjugated Polymers: Synthesis and Opto-Electrical Properties of Luminescent Devices Based on These PPV Derivatives. Opt. Mater. 1998, 9, 150–153. 13. Liu, S.-W.; Lee, J.-H.; Lee, C.-C.; Chen, C.-T.; Wang, J.-K. Charge Carrier Mobility of Mixed-Layer Organic Light-Emitting Diodes. Appl. Phys. Lett. 2007, 91, 142106. 14. Thompson, B. C.; Fréchet, J. M. Polymer–Fullerene Composite Solar Cells. J. Angew. Chem. 2008, 47, 58–77. ******************************************************* DR. RICHA CHANDRA, Chemistry & Physics Department. Bioanalytical chemistry of serum lipoproteins This research program involves several areas of chemistry including analytical chemistry and aspects of biochemistry and physical chemistry. There are opportunities for students with an inclination to chemistry and biochemistry as well as students interested in studies involving physics, biology, nutritional and biomedical sciences to develop experiments within the larger project. This is a highly multidisciplinary project with opportunities for a variety of undergraduate research students to find niche projects within the overall research goals. The focus of the research is developing and utilizing an array of bioanalytical tools and methods to isolate and characterize lipoprotein fractions from human serum samples for further studies in relation to cardiovascular disease progression. Researchers in our group center efforts on method development, which involves a thorough examination of primary literature and instrumentation, the analytical process, and the statistical evaluation of data. Currently, the main preparative techniques we use are a high-speed centrifugal flotation and immunoseparation. We plan to use preparative high-pressure liquid chromatographic (HPLC) to further isolate lipoproteins of interest. We use dynamic light scattering (DLS), a mainstay in the physics world, to determine size distributions of lipoproteins that are responsible for the metabolism of dietary fats. Biochemical techniques that are in our consort of analytical tools include SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) for protein analysis and enzymatic triglyceride analyses. We will also be utilizing isoelectric focusing gel electrophoresis (IEF) to analyze the different isoforms of proteins involved. As this project advances, there is room for potential collaborative studies that will involve medical and life science applications as well. Students in this research group will experience firsthand the potential for clinical applications and the impact of bioanalytical research involving cardiovascular chemistry. Students in this research group will • develop a mastery of laboratory research skills involving analytical and biochemical methodology, • learn to read and review the primary literature and apply knowledge to research project goals, • become adept at keeping an organized laboratory notebook with a detailed record of experiments, • become independent scientific thinkers capable of designing a hypothesis and experimental plans as well as analyzing and interpreting experimental results, • and learn to present their ideas and experimental results both in scientific formal writing and in poster presentations including local and national ACS Conferences and at the Annual HSI-STEM Grant Research Symposium. Background and Significance Cardiovascular disease (CVD) today remains one of the leading causes of mortality in the United States. In recent years, health providers have encouraged people to change their lifestyles and to take advantage of the many new pharmacological approaches to lower their plasma cholesterol.2 The incidence of CVD is on the decline, but according to 2007 mortality data from the American Heart Association, CVD still accounts for more than 2200 deaths per day. The American Heart Association recently established new Impact Goals for 2020 to emphasize cardiovascular health in the next decade. Obesity is described as an epidemic in the American population in children and adults, and the development of CVD in the form of atherosclerotic plaque may be largely due to diet and lifestyle in this population. Most scientific efforts have focused on LDL (low density lipoproteins, d = 1.019-1.063 g/mL) and their susceptibility to oxidative stress in the formation of atherosclerotic plaque.4 Triglyceride-rich lipoproteins (TRL) are the population of lipoproteins in human serum that are involved in the metabolism of dietary fats and contain most plasma triglycerides.5 An elevation of triglyceride-rich lipoproteins (TRL), which are the presumed metabolic precursors to LDL, is positively correlated to the development of heart disease. The size of TRL particles may be a potential indicator of cardiovascular disease risk. Smaller and denser variants of TRL particles also known as remnant lipoproteins have been strongly correlated with cardiovascular disease and were listed as an emerging atherogenic risk factor by the American Heart Association in 2001.6 The mechanism by which TRL cause atherosclerosis and cardiovascular disease is not well understood.7 Natural product antioxidants in the form of polyphenolic flavanoids and hydroxytyrosols (Figure 1) are proven to prevent chemical oxidative stress by a free radical scavenging mechanism. Flavones and their derivatives specifically prevent oxidative damage in LDL particles. Certain lipophilic hydroxytyrosyl esters in the form of fatty acid conjugates show a high antioxidant activity when compared to their counterparts and other known antioxidants such as vitamin E.8,9 Figure 1. Examples of natural product antioxidants: A) (+)-catechin found in red wine and green tea and B) hydroxytyrosol found in olive oil and fruits In cases of elevated plasma triglycerides or hypertriglyceridemia, TRL particles may undergo chemical oxidation similar to LDL leading them to become pro-atherogenic particles. It is worthwhile to examine the susceptibility of TRL particles to chemical oxidation as a function of their size and triglyceride content to better understand the mechanisms of CVD in hypertriglyceridemia. It is also worthwhile to examine the protective effects of natural product antioxidants for their ability to halt or slow the rate of the chemical oxidation of TRL particles. This may result in a lower incidence of CVD in these cases. Aims of the Study In the current study, as the next logical offshoot to the work I previously performed on analyzing the triglyceride rich lipoprotein (TRL) class,10 this research aims to elucidate the features of cardiovascular disease progression as it relates to the structural differences in TRL particles including size, density, protein and triglyceride content. The development of preparative and analytical methods to isolate and characterize TRL particles by the above features has a direct clinical application in establishing baseline measurements of a patient’s TRL status. This methodology would be highly advantageous in a clinical setting. The baseline data on the TRL particles and the preparative methodology also allows for further studies on the susceptibility and changes induced in TRL particles as a result of chemical oxidation. Upon chemical oxidation, changes to particle size, density, protein and triglyceride content will help shed light on the potential mechanisms of cardiovascular disease progression in cases of hypertriglyceridemia. Chemical oxidation may be the underlying mechanism that leads to the formation of proatherogenic denser and smaller TRL particles that are structurally akin to remnant lipoproteins formed in vivo. Examination of a variety of natural product antioxidants to determine their efficacy at slowing and preventing these structural changes is a natural offshoot of this research as a biochemical/bioorganic application. TRL particles by definition carry a higher concentration of triglycerides than other lipoproteins and are therefore more lipophilic. It is highly possible that lipophilic antioxidants, when incubated with plasma samples and TRL samples, may offer more antioxidant protection to lipoprotein particles both prior to and following chemical oxidation. The specific aims of this study are to 1) develop preparative methods to isolate lipoprotein fractions from human blood plasma, 2) analyze size distributions of the lipoprotein fractions isolated from the preparative methodologies, 3) analyze the protein and triglyceride content of the lipoprotein fractions, 4) establish standard methodology to exert chemical oxidative stress to plasma and lipoproteins fractions, 5) measure and analyze the effects of chemical oxidative stress on lipoprotein subclasses via size, protein and triglyceride analyses, 6) and study the antiooxidant protective effects of hydrophilic and lipophilic natural products on plasma and lipoprotein fractions, Approaches The first and second aims of this project are under current development with the use of a high-speed centrifugation method to float highly buoyant TRL particles (d < 1.006 g/mL) and subsequent analysis utilizing dynamic light scattering to characterize the lipoprotein fractions by size (diameter, nm). This technique is an adaptation of a triple spin flotation approach described elsewhere.11 By utilizing a peristaltic pump to carefully layer water above plasma samples prior to centrifugal flotation, we are able to effectively prepare samples for high-speed centrifugal flotation. Following each high-speed centrifugal spin at 14,000 rpm, we utilize the peristaltic pump to carefully aspirate fractions from the top to the bottom of the tube following the second spin. Remnant lipoproteins (RLP) which belong to the TRL class are isolated via an established immunoseparation assay and are also characterized by DLS for the first time. The third specific aim involves measurement of the triglyceride and protein content of lipoprotein particles via SDS-PAGE and enzymatic assays. Enzymatic assays utilizing UV-Vis spectroscopy allow us to quantify the triglyceride content of the TRL particles. My previous investigations indicate that there may be a reduction in triglyceride content upon chemical oxidation (unpublished results). The fourth and fifth aims of the project will be realized once we establish a method to chemically oxidize lipoprotein fractions. Similar to oxidation studies on LDL4, we may use copper ions to oxidize plasma samples. This closely mimics the hypothesized conditions of oxidation in arterial plaque formation. These conditions may reveal a potential mechanism by which atherogenic TRL particles decrease in size resulting in the formation of remnant lipoproteins. Measurements and analysis of TRL particle size and content are necessary to explore this hypothesis. As we complete the establishment of these methods to study TRL particles, the sixth and final specific aim of this project will involve the measurement of the resistance and susceptibility of plasma and lipoprotein fractions to chemical oxidation in the presence of various natural product antioxidants. FRAP (Ferric Reducing Ability of Plasma) assays12 of plasma and lipoprotein fractions will involve the oxidation of plasma and lipoproteins concurrently with the reduction of ferric ions to ferrous ions. This assay utilizes a ferrous ion-TPTZ (tripyridyltriazine) complex that quantitatively absorbs at 593 nm as a measure of “antioxidant” activity. Investigations will involve baseline plasma and lipoprotein FRAP measurements and measurements in the presence of catechin, resveratrol, and vitamin c as lipophilic and hydrophilic antioxidant candidates. Results from these measurements in conjunction with analyses of lipoprotein fractions in regard to size, triglyceride and protein content will be useful in determining the cardioprotective nature of natural product antioxidants in the maintenance lipoprotein structure in the presence of chemical oxidative stress. Impact of Results The research and preliminary results described here are novel and reveal information on the metabolism of the various lipoprotein classes in human blood. The research will further clarify our understanding of the development of cardiovascular disease and via bioanalytical methods provide potential clinical applications in the diagnosis, treatment and prevention of CVD incidence. This research plan is congruous with the newly established goals3 of the American Heart Association to promote cardiovascular health. This research project offers several opportunities for scientific collaboration as well as the development of courses related to the obesity epidemic examining the cardiovascular chemistry involved in the larger purview of a national social crisis. The methodology in this research spans areas of analytical, biological and bioorganic chemistry and has several potential applications in a clinical setting. References 1. Pohl, J.; Chandra, R.; Corpuz G.; McNeal C.; Macfarlane R.D. J. Pediatr. Gastroenterol. Nutr. 2008, 47(5), 507-513. 2. Ross, R., N Engl J Med. 1999, 340, 115-126. 3. Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Adams, R.J.; Berry, J.D.; Brown, T.M.; Carnethon, M.R.; Dai, S.; de Simone, G.; Ford, E.S.; Fox, C.S.; Fullerton, H.J.; Gillespie, C.; Greenlund, K.J.; Hailpern, S.M.; Heit, J.A.; Ho, M.; Howard, V.J.; Kissela, B.M.; Kittner, S.J.; Lackland, D.T.; Lichtman, J.H.; Lisabeth, L.D.; Makuc, D.M.; Marcus, G.M.; Marelli, A.; Matchar, D.B.; McDermott, M.M.; Meigs, J.B.; Moy, C.S.; Mozaffarian, D.; Mussolino, M.E.; Nichol, G.; Paynter, N.P.; Rosamond, W.D.; Sorlie, P.D.; Stafford, R.S.; Turan, T.N.; Turner, M.B.; Wong, N.D.; Wylie-Rosett, J., Circulation. 2011, 123, e18-e209. 4. Matsukawa, N.; Nariyama, Y.; Hashimoto, R.; Kojo, S., Bioorg. Med. Chem. 2003, 11, 40094013. 5. Havel, R.J., Triglyceride-Rich Lipoprotein Remnants. In Handbook of Lipoprotein Testing, Rifai, N.; Warnick, G.R.; Dominiczak, M.H., Eds. The American Association for Clinical Chmistry, Inc.: Washington D.C., 1997; 451-464. 6. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III), Journal of the American Medical Association. 2001, 285, 2486. 7. Shepherd, J., Plasma Triglycerides and the Risk for Vascular Disease. In Medscape Cardiology, Del Negro, A., Good, D., Gruber, L., Wang, K., Eds. Medscape: New York, NY, 2005; Vol. 9, http://www.medscape.com/viewarticle/512941. Richa Chandra, HSI-STEM Proposal, Page 7 of 7 8. Baltaze, J.; Poisson, J., J. Nat. Prod. 1995, 58, 1840-1847. 9. Procopio, A.; Celia, C.; Nardi, M.; Oliverio, M.; Paolino, D.; Sindona, G., J. Nat. Prod. 2011, 74, 2377-2381. 10. Chandra, R.; Macfarlane, R., Anal. Chem. 2006, 78 (3), 680–685. 11. Park, Y.; Grellner, W.J.; Harris, W.S.; Miles, J.M., Am. J. Physiol. E