AP PHYSICS 2 SUMMARY CHAPTER 12 – FIRST LAW OF THERMODYNAMICS
advertisement
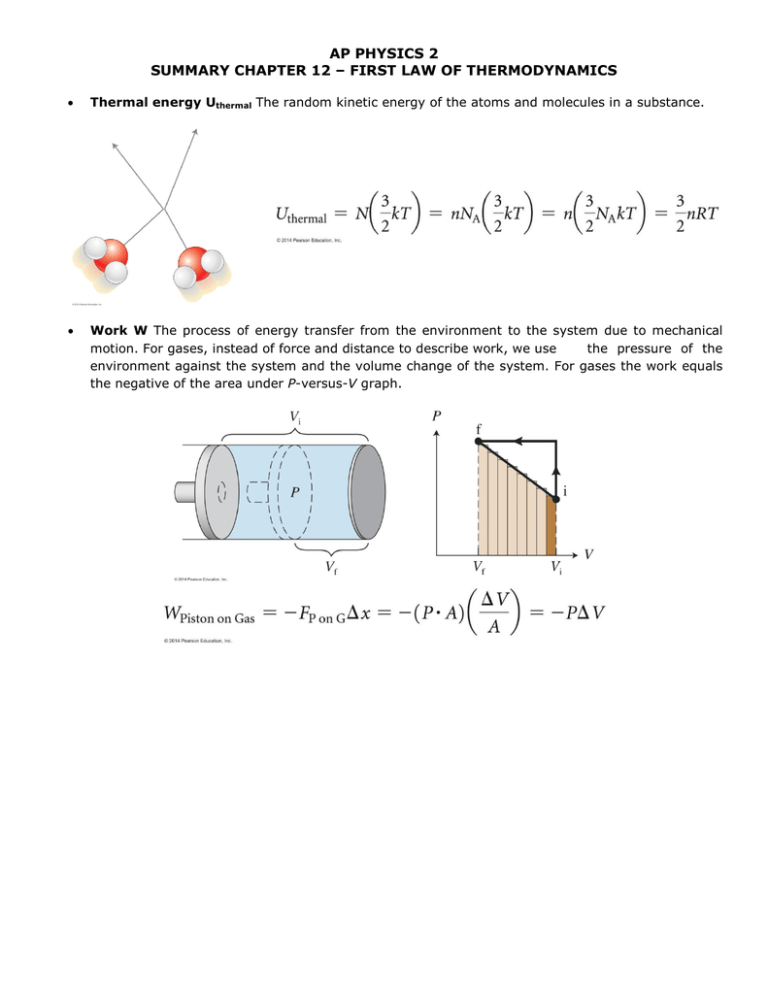
AP PHYSICS 2 SUMMARY CHAPTER 12 – FIRST LAW OF THERMODYNAMICS • Thermal energy Uthermal The random kinetic energy of the atoms and molecules in a substance. • Work W The process of energy transfer from the environment to the system due to mechanical motion. For gases, instead of force and distance to describe work, we use the pressure of the environment against the system and the volume change of the system. For gases the work equals the negative of the area under P-versus-V graph. • Heating Q Process of energy transfer from the environment to the system due to differences in their temperatures. Q > 0 if energy is transferred into the system; Q < 0 if energy is transferred out of the system. • First law of thermodynamics A system’s energy can change 𝚫Uint = (Uf - Ui) during a process due to the work W done on the system by the environment and/ or due to the heating Q of the system by the environment. • Consequences of heating (no work done on the system) o Temperature change The system’s temperature changes when there is no phase change occurring. o Phase changes System melts (𝚫U > 0) or freezes (𝚫U < 0) at constant temperature. System boils (𝚫U > 0) or condenses (𝚫U < 0) at constant temperature. • Thermodynamic energy transfer mechanisms o Conduction Hconduction Energy transfer from particle to particle via contact. o Convection Hconvection Energy transfer by particles moving from one place to another. o • Evaporation Hevaportation Energy transfer due to evaporation/condensation on surface. Radiation Hradiation Energy transfer via absorption or emission of radiation.