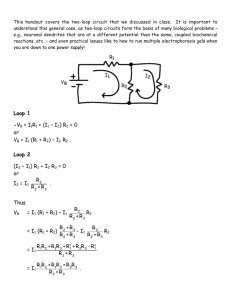
OPTIMIZATION OF MODIFIED SOL-GEL METHOD FOR THE
advertisement

OPTIMIZATION OF MODIFIED SOL-GEL METHOD FOR THE FORMATION OF TETRAGONAL PHASE ZIRCONIA IN Zr(IV)/Ce(IV) CATALYST LEIW SOOK FUN UNIVERSITI TEKNOLOGI MALAYSIA UNIVERSITI TEKNOLOGI MALAYSIA BORANG PENGESAHAN STATUS TESIS JUDUL : OPTIMIZATION OF MODIFIED SOL-GEL METHOD FOR THE FORMATION OF TETRAGONAL PHASE ZIRCONIA IN Zr(IV)/Ce(IV) CATALYST SESI PENGAJIAN : 2004/2005 Saya : LEIW SOOK FUN (HURUF BESAR) mengaku membenarkan tesis ini disimpan di Perpustakaan Universiti Teknologi Malayisa dengan syarat-syarat seperti berikut : 1. Hakmilik tesis ini adalah dibawah nama penulis melainkan penulisan sebagai projek bersama dan dibiayai oleh UTM, hakmiliknya adalah kepunyaan UTM. 2. Naskah salinan di dalam bentuk kertas atau mikro hanya boleh dibuat dengan kebenaran bertulis daripada penulis. 3. Perpustakaan Universiti Teknologi Malaysia dibenarkan membuat salinan untuk tujuan pengajian mereka. 4. Tesis hanya boleh diterbitkan dengan kebenaran penulis. Bayaran royalti adalah mengikut kadar yang dipersetujui kelak. 5. *Saya membenarkan / tidak membenarkan Perpustakaan membuat salinan tesis ini sebagai bahan pertukaran di antara institusi pengajian tinggi. 6. **Sila tandakan ( ) SULIT TERHAD (Mengandungi maklumat yang berdarjah keselamatan atau kepentingan Malaysia seperti yang termaktub di dalam AKTA RAHSIA RASMI 1972) (Mengandungi maklumat TERHAD yang telah ditentukan oleh organisasi / badan di mana penyelidikan dijalankan) TIDAK TERHAD (TANDATANGAN PENULIS) (TANDATANGAN PENYELIA) Alamat Tetap : NO.E-49 BATU DUA, 33300 GERIK, PERAK. PM. DR. NOR AZIAH BUANG (NAMA PENYELIA) Tarikh Tarikh : : CATATAN : * Potong yang tidak berkenaan. ** Jika Tesis ini SULIT atau TERHAD, sila lampirkan surat daripada pihak berkuasa / organisasi berkenaan dengan menyatakan sekali tempoh tesis ini perlu dikelaskan sebagai SULIT atau TERHAD. “Saya/kami* akui bahawa saya/kami* telah membaca karya ini dan pada pandangan saya/kami* karya ini adalah memadai dari segi skop dan kualiti untuk tujuan penganugerahan Ijazah Sarjana Muda/ Sarjana/ Doktor Falsafah Sains (Kimia).” * Tandatangan : .................................................. Nama Penyelia : P. M. Dr. NOR AZIAH BUANG Tarikh : ……………………………….. Potong yang tidak berkenaan. BAHAGIAN A Pengesahan Kerjasama* Adalah disahkan bahawa projek penyelidikan tesis ini telah dilaksanakan melalui kerjasama antara _______________________ dengan ________________________ Disahkan oleh: Tandatangan : _________________________________ Tarikh: ______________ Nama : _________________________________ Jawatan : _________________________________ (Cop rasmi) * Jika penyediaan tesis/projek melibatkan kerjasama. BAHAGIAN B Untuk Kegunaan Pejabat Sekolah Pengajian Siswajah Tesis ini telah diperiksa dan diakui oleh: Nama dan Alamat Pemeriksa Dalam : Prof. Dr. Wan Azelee bin Wan Abu Bakar Jabatan Kimia, Fakulti Sains, Universiti Teknologi Malaysia, 81300, Skudai Johor. Nama dan Alamat Pemeriksa Dalam : Prof. Madya Dr. Zainab binti Ramli Jabatan Kimia, Fakulti Sains, Universiti Teknologi Malaysia 81300, Skudai Johor. Nama Penyelia Lain (jika ada) : _____________________________________ _____________________________________ _____________________________________ Disahkan oleh Penolong Pendaftar di SPS: Tandatangan : _________________________________ Tarikh: ______________ Nama : _________________________________ OPTIMIZATION OF MODIFIED SOL-GEL METHOD FOR THE FORMATION OF TETRAGONAL PHASE ZIRCONIA IN Zr(IV)/Ce(IV) CATALYST LEIW SOOK FUN A dissertation submitted in partial fulfillment of the requirements for the award of the degree of Master of Science (Chemistry) Faculty of Science Universiti Teknologi Malaysia MARCH, 2005 ii ³I deFlare that this dissertation is the results oI my own researFh e[Fept as in Fited reIerenFes. The dissertation has not been aFFepted Ior any degree and is not FonFurrently submitted in Fandidature oI any degree.´ Signature : .................................................. Name oI Candidate : LEI: S22K )UN Date : ««««««««««««.. iii This dissertation is dediFated to my beloved Iamily and Choong Meng Thanks Ior being there when I needed you. iv ACKNOWLEDGEMENTS I would like to e[press my deepest and sinFere thanks to my projeFt supervisor, AssoFiate ProIessor Dr. Nor A]iah Buang Ior valuable ideas and helpIul guidanFe given sinFe the beginning oI this researFh. SinFere gratitude also goes to all those who have helped me direFtly or indireFtly in this researFh, ProIessor Dr. :an A]elee :an Abu Bakar, Mrs. Mariam Hassan, Mr. Hanan, Mr. A]mi, and Mr. Junaidi Bin Mohamad Nasir Irom the Department oI Chemistry, Mr. JaaIar Raji Irom the Department oI PhysiFs, Mr. =ainal Abidin Abbas and Mr. JeIri Irom the )aFulty oI MeFhaniFal Engineering. Thanks are also due to Mr. Lim Kheng :ei Irom the Institute Ibnu Sina Ior an easy aFFess to ;RD analytiFal instrument. )inally, I am grateIul Ior the motivation and support oI my Iamily and Iriends in Fompleting my thesis projeFt. Thank you Ior your support and Iaith in me and Ior making it all worthwhile. v ABSTRACT =irFonium-Ferium mi[ed metal o[ides is widely used as promoters in automobile emissions Fontrol Fatalyst systems (Three-:ay Catalyst, T:C). In order to improve the perIormanFe oI ]irFonia-Feria Fatalysts, a researFh has been done to develop a ]irFonia-based Fatalyst doped with Feria by modiIied sol-gel method with ]irFonium o[yFhloride and Ferium (III) nitrate he[ahydrate as the preFursors. This researFh investigated the optimum Fonditions on the Iormation oI single phase tetragonal =r22. A series oI =r1-[Ce[22 Fatalyst by varying the atomiF perFentage ratios were prepared in diIIerent amount oI water and FalFinations temperature. The properties oI Fatalysts were FharaFteri]ed with ;-Ray DiIIraFtion (;RD), surIaFe area analysis, and )ourier-TransIorm InIrared SpeFtrosFopy ()TIR). The Fatalyst showed the best result is seleFted to test Ior C2 Fonversion. The =r.7Ce.322 Fatalyst prepared in 5 mL oI water dried Ior two days at 7 C and FalFined at 4 C Ior 17 hours gives the optimum Fondition on the highest Iormation oI single phase tetragonal =r22. The ;RD analysis showed that this Fatalyst has Frystallinity property, Fonsists oI tetragonal =r22 as the main phase and average Frystallite si]e oI appro[imately 12.9 nm serve as the aFtive phases Ior the Fatalyst. SurIaFe area analysis showed that this Fatalyst has 35.36 m2/g oI surIaFe area. The )TIR analysis showed the e[istenFe oI surIaFe hydro[yl group on the surIaFe oI Fatalyst. The bands that were assigned to the water deIormation mode and hydro[yl deIormation mode oI surIaFe hydro[yl groups have been eliminated as the FalFination temperature inFreased. The =r.7Ce.322 Fatalyst prepared in 5 mL oI water was reported to e[hibit the best FatalytiF aFtivity towards C2 Fonversion, it showed the lowest TL2 at room temperature while 1 C2 Fonversion was aFhieved at T1 32 C. vi ABSTRAK Campuran logam oksida ]irkonium-Ferium diguna seFara meluas sebagai penyokong dalam sistem pemangkinan bagi pengawalan bahan penFemar daripada kenderaan bermotor (Pemangkinan Tiga Arah, TWC). Demi meningkatkan keupayaan mangkin ]irkonia-Feria, satu kajian telah dilakukan untuk menyediakan mangkin berasaskan ]irkonia yang didopkan dengan Feria melalui kaedah pengubahsuaian sol-gel di mana ]irkonium oksiklorida dan Ferium (III) nitrat heksahidrat sebagai bahan pemula. Kajian ini dijalankan bertujuan menyelidik keadaan yang optimum bagi pembentukan Iasa tunggal =r22 berstruktur tetragonal. Satu siri mangkin =r1-[Ce[22 telah disediakan dengan mempelbagaikan nisbah peratus atom dan menggunakan kuantiti air serta suhu pengkalsinan yang berbe]a. Analisis penFirian dilakukan terhadap mangkin dengan menggunakan teknik pembelauan sinar-; (;RD), analisis luas permukaan dan )TIR. Mangkin yang menunjukkan keputusan yang terbaik dipilih untuk kajian penukaran gas karbon monoksida (C2) kepada C22. Mangkin =r.7Ce.322 yang disediakan menggunakan 5 mL air dan dikering pada suhu 7 C selama dua hari serta dikalsin pada suhu 4 C selam 17 jam menunjukkan keadaan yang paling optimum dalam pembentukan Iasa tunggal =r22 berstruktur tetragonal. Analisis ;RD menunjukkan mangkin ini terdiri daripada Iasa tetragonal =r22 sebagai Iasa utama dengan siIat hablur serta purata sai] hablur kira-kira 12.9 nm yang bertindak sebagai Iasa aktiI mangkin. Analisis luas permukaan menunjukkan mangkin ini mempunyai luas permukaan sebanyak 35.36 m2/g. Keputusan )TIR menunjukkan kehadiran hidroksil pada permukaan mangkin. PunFak-punFak bagi air, hidroksil telah disingkirkan apabila suhu pengkalsinan meningkat. Penyediaan mangkin =r.7Ce.322 dalam 5 mL air memberikan keputusan aktiviti pemangkinan terhadap penukaran gas C2 yang paling baik di mana menunjukkan TL2 yang paling rendah pada suhu bilik sementara 1 penukaran gas C2 diFapai pada T1 32 C. vii CONTENTS PAGE TITLE i DECLARATION ii DEDICATION iii ACKNOWLEDGEMENTS iv ABSTRACT v ABSTRAK vi CONTENTS vii LIST OF TABLES [ LIST OF FIGURES [i LIST OF SYMBOLS / ABBREVIATIONS / NOTATIONS / TERMS [iii LIST OF APPENDICES [iv CHAPTER I INTRODUCTION 1.1 Air Pollution 1 1.2 DeIinition and Types oI Pollutants 2 1.3 EIIeFts oI Air Pollution 2 1.4 Catalyst 3 1.5 CatalytiF Converter 3 1.6 Three-:ay Catalysts 4 1.7 =irFonium 2[ide or =irFonia 5 viii 1. Cerium 2[ide or Ceria 7 1..1 9 Role oI Ce22 in the T:Cs 1.9 Catalysis by =irFonia-Ceria 9 1.1 Sol-Gel Chemistry 11 1.1.1 The Advantages oI Sol-Gel ApproaFh 2ver the Traditional Methods oI Preparation 14 1.1.2 EIIeFts oI 9ariables on the Chemistry oI the Sol-Gel Synthesis 15 1.1.3 Preparation oI Catalysts by Sol-Gel Method 1.11 16 Statement oI the Problem and the Needs oI the Study 1 1.12 ResearFh 2bjeFtives 19 1.13 SFope oI ResearFh 19 CHAPTER II EXPERIMENTAL 2.1 ChemiFals 2 2.2 E[periment 2 2.3 Preparation oI =irFonia Based Catalysts Doped with Ceria by ModiIied Sol-Gel Method 21 2.4 Drying and CalFination 22 2.5 CharaFteri]ation TeFhiniTues 23 2.5.1 ;-Ray DiIIraFtion (;RD) 23 2.5.2 SurIaFe Area Analysis 25 2.5.3 )ourier-TranIorm InIrared SpeFtrosFopy ()TIR) 2.6 CatalytiF AFtivity Testing Measurement 26 2 i[ CHAPTER III 3.1 RESULTS AND DISCUSSIONS InIluenFe oI 9ariables on the )ormation oI Tetragonal Phase =r22 3.1.1 3 EIIeFt oI Amount oI :ater on the )ormation oI Tetragonal Phase =r22-Based Catalyst Doped with Ce22 (=r.95:Ce.5) 3.1.2 EIIeFt oI Loading oI Ce22 on the )ormation oI Tetragonal Phase =r22 3.1.3 31 34 EIIeFt oI CalFination Temperature on the )ormation oI Tetragonal Phase =r22 in =r.7Ce.322 Catalyst 4 3.2 )ourier TransIorm InIrared ()TIR) Analysis 46 3.3 CatalytiF AFtivity oI =r.7Ce.322 Catalyst Towards Carbon Mono[ide 2[idation ReaFtion CHAPTER IV 49 CONCLUSIONS 4.1 ConFlusions 52 4.2 )uture :orks 53 REFERENCES 55 APPENDICES 64 [ LIST OF TABLES TABLE NUMBER TITLE PAGE 1.1 ReaFtions oFFurring on automobile e[haust Fatalyst 5 2.1 ClassiIiFation oI InIrared Radiation 27 3.1 ;RD peaks assignment Ior =r.95Ce.522 Fatalyst sample in diIIerent amount oI water 3.2 ;RD peaks assignment Ior =r[Ce1-[22 Fatalyst in mL oI water 3.3 36 ;RD peaks assignment Ior =r[Ce[-122 Fatalysts in 5 mL oI water 3.4 33 39 PartiFle si]es oI the sample with diIIerent amount oI Feria perFentage in 5 mL and mL oI water FalFined at 4 C 3.5 4 ;RD peaks assignment Ior =r.7Ce.322 Fatalyst prepared in 5 mL and mL oI water FalFined at 4 C and 6 C 3.6 44 PartiFle si]es and surIaFe areas oI =r.7Ce.322 Fatalysts prepared in 5 mL and mL oI water FalFined at 4 C and 6 C 3.7 46 CatalytiF aFtivity oI =r.7Ce.322 Fatalyst towards C2 Fonversion 5 [i LIST OF FIGURES FIGURE NUMBER TITLE 1.1 Diagram oI a typiFal FatalytiF Fonverter 2.1 SFhematiF diagram oI the Fatalyst preparation PAGE 4 set-up using modiIied sol-gel method 21 2.2 BasiF Ieatures oI a typiFal ;RD e[periment 24 2.3 SFhematiF diagram oI the miFroreaFtor sample tube 29 3.1 DiIIraFtograms oI =r.95Ce.522 using diIIerent amount oI water: (a) mL and (b) 5 mL 3.2 32 DiIIraFtograms oI =r[Ce1-[22 Fatalyst with diIIerent amount oI Feria in mL oI water (a) =r.95Ce.522, (b) =r.9Ce.122, (F) =r.Ce.222, and (d) =r.7Ce.322 3.3 35 DiIIraFtograms oI =r[Ce1-[22 Fatalyst with diIIerent amount oI Feria in 5 mL oI water (a) =r.95Ce.522, (b) =r.9Ce.122, (F) =r.Ce.222, and (d) =r.7Ce.322 3.4 3 DiIIraFtograms oI =r.7Ce.322 prepared using 5 mL oI water dried at (a) 7 C, and FalFined at (b) 4 C and (F) 6 C 3.5 42 DiIIraFtograms oI =r.7Ce.322 prepared using mL oI water and FalFined at (a) 4 C and (b) 6 C 3.6 43 )TIR speFtrum oI =r.7Ce.322 Fatalyst prepared in 5 mL oI water dried at 7 C 47 [ii 3.7 )TIR speFtrum oI =r.7Ce.322 Fatalyst prepared in 5 mL oI water FalFined at 4 C 3. )TIR speFtrum oI =r.7Ce.322 Fatalyst prepared in 5 mL oI water FalFined at 6 C 3.9 4 49 PerFentage Fonversion oI C2 versus temperature Ior the =r.7Ce.322 Fatalyst prepared in mL and 5 mL oI water 51 [iii LIST OF SYMBOLS/ ABBREVIATIONS/ NOTATIONS/ TERMS A/) - Air-to-Iuel ratio ș - HalI angle oI diIIraFted beam Ȝ - :avelength oI the [-rays ȕ - Peak width L! - PartiFle si]e F - CubiF Ce22 - Cerium o[ide/ Feria C2 - Carbon mono[ide d - Interplaner spaFing in a Frystal )TIR - )ourier-transIorm inIrared HC - HydroFarbon m - MonoFliniF N2[ - Nitrogen o[ides 2SC - 2[ygen storage FapaFity PD) - Power DiIIraFtion )ile RT - Room temperature t - Tetragonal T1 - 1 Fonversion temperature TL2 - Light-oII temperature T:C - Three-way Fatalyst ;RD - ;-ray diIIraFtion =r22 - =irFonium o[ide/ ]irFonia [iv LIST OF APPENDICES APPENDIX NUMBER TITLE PAGE A CalFulation oI PartiFle Si]es 64 B CalFulation oI Gas Conversion in PerFentage 65 C DiIIraFtograms oI =r.9Ce.122 prepared by using 5 mL oI water FalFined at: a) 4 C and b) 6 C D 66 DiIIraFtograms oI =r.Ce.222 prepared by using 5 mL oI water FalFined at: a) 4 C and b) 6 C 67 CHAPTER I INTRODUCTION 1.1 Air Pollution Air pollution is the presenFe oI material in air in Tuantities large enough to produFe harmIul eIIeFts. The undesirable materials may damage, vegetation, human property, or the global environment as well as Freate aesthetiF insults in the Iorm oI brown or ha]y air or unpleasant smells >1@. Air pollution Faused by mobile sourFe had been reFeived muFh attention by most oI the environmentalist. In the last 6 years the world vehiFles Ileet has inFreased Irom about 4 million vehiFles to over 7 million, this Iigure is projeFted to inFrease to 92 million by the year 21 >2@. This will lead to inFrease oI more and more air pollutant. The engine e[haust Fontains prinFipally three main pollutants, unburned or partially burned hydroFarbons (HCs), Farbon mono[ide (C2) and nitrogen o[ides (N2[), mostly N2, in addition to other Fompounds suFh as water, hydrogen, nitrogen, o[ygen, etF. Sulphur o[ides, though polluting, are normally not removed by the post Fombustion treatments, sinFe the only eIIeFtive way is to reduFe them to elemental sulphur, whiFh would aFFumulate in the system >2@. 2 1.2 Definition and Types of Pollutants Any solid, liTuid, or gas that is present in the air in a FonFentration that Fauses some deleterious eIIeFt is Fonsidered an air pollutant. However, there are several substanFe that, by virtue by their massive rates oI emission and harmIul eIIeFts, are Fonsidered the most signiIiFant pollutants >3@. SubstanFes emitted direFtly Irom sourFes are Falled primary pollutants. The pollutants manuIaFtured in the lower atmosphere by FhemiFal reaFtions among primary pollutants are Falled seFondary pollutants, they are responsible Ior most oI the smog, ha]e, and eye irritation and Ior many oI the Iorms oI plant and material damage attributed to air pollution >4@. 1.3 Effects of Air Pollution Carbon mono[ide (C2) is a gaseous pollutant whiFh enters the atmosphere Irom Iour sourFes: Iossil Iuel Fombustion and industrial emissions, biomass burning, o[idation oI CH4, and o[idation oI non-methane hydroFarbon >5@. C2 is a Folorless and odorless gas. It is very stable and has a liIetime oI 2 to 4 months in the atmosphere. High FonFentration oI C2 Fan Fause physiologiFal and pathologiFal Fhanges and ultimately death. C2 is a poisonous inhalent that deprives the body tissues oI neFessary o[ygen >6@. N2[ in the atmosphere Fauses environment problems, suFh as photoFhemiFal o[idant and aFid rain >7@. Nitrogen dio[ide (N22) Fan reaFt with moisture present in the atmosphere to Iorm nitriF aFid, whiFh Fan Fause Fonsidered Forrosion oI metal surIaFe. Nitrogen o[ide (N2) is a Folorless gas and its ambient FonFentration is usually Ior less than .5 ppm. N2 is a preFursor to the Iormation oI nitrogen dio[ide and is an aFtive Fompound in photoFhemiFal smog Iormation as well >6@. 3 Unburned hydroFarbons in Fombination with the o[ides oI nitrogen in the presenFe oI sunlight Iorm photoFhemiFal o[idants, Fomponents oI photoFhemiFal smog, that do have adverse eIIeFts on human health and on plants >6@. 1.4 Catalyst A Fatalyst is a substanFe that inFreased the rate at whiFh a FhemiFal reaFtion approaFhes eTuilibrium without itselI beFoming permanently involved in the reaFtion >@. BasiFally, Fatalysts are Fonsidered as FhemiFal Fompounds Fapable oI direFting and aFFelerating thermodynamiFally Ieasible reaFtions while remaining unaltered at the end oI the reaFtion, whose thermodynamiF eTuilibrium they FonseTuently Fannot Fhange >9@. Catalysis is homogeneous when the Fatalyst is soluble in the reaFtion medium, and heterogeneous when Fatalyst is e[isting in a phase distinFtly diIIerent Irom the phase oI the reaFtion medium. In most instanFes oI heterogeneous Fatalysis, the Fatalyst is a solid that is brought into FontaFt with gas or liTuid reaFtants to bring about a transIormation >9@. 1.5 Catalytic Converter Nowadays, more than 95 oI vehiFles produFed in the world are eTuipped with a FatalytiF Fonverter, whiFh Ior the gasoline-Iuelled engines, is almost e[Flusively based on the so-Falled three-way Fatalyst (T:C). T:Cs are Fapable oI simultaneously and eIIiFiently Fonverting C2, hydroFarbon (HC) and N2[ into harmless C22. H22 and N2, provided that the so-Falled and air-to-Iuel ratio (A/)) is Fonstantly kept at the stoiFhiometry >1@. A typiFal design oI a modern three-way FatalytiF Fonverter is reported in )ig. 1.1. BasiFally, it is a stainless steel Fontainer 4 whiFh inForporates a honeyFomb monolith made oI Fordierite (2Mg2.2Al223).5Si22) or metal >2@. Figure 1.1: Diagram oI a typiFal FatalytiF Fonverter 1.6 Three-way Catalysts Automobiles under severe driving Fonditions suFh as a long-distanFe Fontinuous running usually generate high temperature in the FatalytiF Fonverter, that may Fause thermal sintering oI both metal aFtive phases and Fatalyst support, and Iinally leads to Fatalyst deaFtivation. 2n the other hand, at Fool start, the Fatalyst usually shows low FatalytiF aFtivity. ThereIore, a satisIaFtory FatalytiF eIIiFiently at high temperature and a promoted light-oII reaFtion behavior at Fool start are demanded Ior the Furrent three-way Fatalysts (T:Cs) >11@. Three-way Fatalysts (T:Cs) have been widely used to reduFes pollutant emissions Irom gasoline engine powered vehiFles >12@. 9arious three-way Fatalysts (T:Cs) are widely used in Far e[hausts Ior the Fonversion oI poisonous gases (C2, N2[ and hydroFarbons) to harmless H22, C22 and N2. These Fonverters, beFause oI their eIIeFtiveness, beFome Fompulsory part oI the modern automobile >13@. 5 The FonFept oI using Fatalyst to Fonvert C2, N2[, and HC to less environment aFtive Fompounds suFh as nitrogen, water and Farbon dio[ide was well established praFtiFe prior to the need arising on motor vehiFles. The prinFipal reaFtions are shown in Table 1.1 >14@. Table 1.1: ReaFtions oFFurring on automobile e[haust Fatalyst Oxidation Reactions 2C2 22 ĺ 2C22 HC 22 ĺ C22 H22 Reduction Reactions 2C2 2N22 ĺ 2C22 N2 HC N2 ĺ C22 H22 N2 SinFe C2 and HC emission are removed by o[idation whereas N2[ may only be removed by reaFtion with reduFing agents, the elimination oI all three pollutants Fan only be aFhieved by separating the o[idation and reduFtion IunFtions in appropriate reaFtors or by design oI seleFtive Fatalyst on whiFh the two reaFtions may proFeed simultaneously >15@. The development oI the three-way Fatalyst (T:C) was diFtated by the need to simultaneously Fonvert the HC, C2, and N2[, present in the automobile e[haust to H22, C22 and N2 >16@. 1.7 Zirconium Oxide or Zirconia =irFonium o[ide or ]irFonia is widespread in nature as a traFe mineral, baddeleyite. In its pure Iorm, properties suFh as high hardness, low wear resistantFe, low FoeIIiFient oI IriFtion, high elastiF modulus, FhemiFal inertness, good ioniF FonduFtivity, low thermal FonduFtivity, and high melting temperature have made ]irFonia a teFhnologiFally important material. These uniTue properties enable a wide range oI appliFation oI ]irFonia suFh as struFtural materials, thermal barrier Foatings, 6 solid-state eleFtrolytes, solid Iuel Fells, gate dieleFtriFs, o[ygen sensors, and heterogeneous Fatalysts >17@. SinFe ]irFonia has a lattiFe with many vaFanFies, it is a good ioniF FonduFtor at high temperature. These vaFanFies allowed it to be used as an o[ygen sensor to measure the air-to-Iuel ratio in internal Fombustion engines in order to Fontrol the emission oI N2[, C2 and hydroFarbon Fompounds >1@. =r22- based materials have attraFted Fonsiderable interest in reFent deFades. In a FatalytiF sense, they appear to have some advantages in area oI praFtiFal appliFation over traditional o[ides, suFh as Si22 and Al223. More signiIiFant is the IaFt that additives Fan bring about a strong modiIiFation oI the surIaFe struFture oI ]irFonia, in whiFh Fase substitution oI =r4 with dopant Fations results in a rise in anion vaFanFy FonFentration and FonduFtivity >19@. The tailored physiFo-FhemiFal properties oI ]irFonia inFluding struFture, redo[, and aFid-base FharaFteristiFs make it as an attraFtive Fatalyst and Fatalyst support Ior a number oI reaFtions >2@. In IaFt, due to its aFid-base biIunFtional properties, it Fatalyses the hydrogenation oI diIIerent substrates suFh as Ior e[ample ben]oiF aFid, Farbo[yliF aFids, and Farbon mono[ide. However, the main use oI ]irFonia is as an eIIiFient support Ior a variety oI FatalytiF systems >21@. =irFonia is also widely used in optiFal Iields beFause oI its high reIraFtive inde[, large optiFal band gap, and low optiFal loss and sFatter in the inIrared region. It is also useIul Ior making high-reIleFtivity mirrors, laser gyros and broadband interIerenFe Iilters >22@. Depending on IaFtors suFh as preparation method, pH, temperature, and kinetiF meFhanism, synthetiF ]irFonia shows three Frystalline phases: monoFliniF (m), tetragonal (t), and FubiF (F) it has also been shown that a high-pressure orthorhombiF Iorm e[ists >23@. At room temperature, t- and F-phases metastable, while m-phase is stable. t- and F-phases are stable at high temperature. They Fan be partially or Iully stabili]ed at room temperature by addition a small amount oI o[ides (e.g. Ca2, Mg2 and types oI rare earth o[ides) >24@. 7 The monoFliniF phase is stable up to 14 K where it transIorms to the tetragonal phase, whiFh is stable up to 157 K while the FubiF phase e[ists up to the melting point oI 29 K. However, the FubiF phase Fan be stabili]ed by the addition oI divalent Fations suFh as Ca2, Mg2 or Y3 >2@. Several methods have been developed to stabili]e the tetragonal phase at low temperature. These inFlude the addition oI dopants (Y223, Ca2, Mg2, Ce22), or the use oI soIt-Fhemistry methods, Irom whiFh nanoFrystalline materials Fan be obtained >23@. It is known that tetragonal ]irFonia as a metastable phase e[ists at well below its normal transIormation temperature as a result oI the Frystalline si]e eIIeFt, the presenFe oI impurities, or the presenFe oI strain at the domain boundaries in polydomain tetragonal partiFles >25@. Tetragonal- =r22 is used as a Fatalyst and Fatalyst support Ior various gas phase reaFtions >26@. Several studies have shown that the perFentage oI tetragonal phase oI =r22 (t-=r22) is FruFial Ior the aFtivity and seleFtivity in Fatalysis >27@. However, a phase transIormation Irom the tetragonal phase to the less reaFtive monoFliniF phase oI Frystalline =r22 (m-=r22) during the reaFtion prevents the Iurther appliFation oI =r22. ThereIore, stabili]ing the tetragonal ]irFonia during reaFtion beFomes an important topiF in Fatalysis Iields >2@. =r22 stabili]ed by Ce22 and other rare earth is known to inFrease durability and removal eIIiFienFy Ior automotive emission Fontrol >29@. 1.8 Cerium Oxide or Ceria Cerium o[ide or Feria (Ce22) has been Fonsidered and largely used in the last years as one oI the most important promoters oI heterogeneous FatalytiF reaFtions >3@. Cerium o[ide is one oI the most important Fomponents in high-perIormanFe three-way Fatalyst (T:C) Ior its ability in enhanFing the removal oI Farbon mono[ide (C2), nitrogen o[ides (N2[) and hydroFarbons (HC) >31@. Cerium o[ide has been Fonsidered oI potential interest Ior solid o[ide Iuel Fells and three-way automobile Fatalysts beFause oI its good o[ygen ion FonduFtivity. Its ability to aFt as an o[ygen buIIer and thereIore to operate eIIeFtively under Fonditions oI osFillating o[ygen FonFentration has promoted its use in automobile FatalytiF appliFations >32@. However, pure Ce22 alone is known to be poorly thermostable. The loss in surIaFe area is usually related to the Fhanges in the pore struFture and to Frystalline growth. It is, thereIore, very important to improve its te[tural stability. Doping with Fations suFh as Al3, =r4, or Si4 may signiIiFantly improve the stability oI the surIaFe area oI Feria at high temperature >33@. Among the lanthanide elements, Ferium is the only one that Iorms stable Fompounds in a tetravalent o[idation (i.e., Ce4) and the Foordination number oI surIaFe Ce4 Fan vary between Iour and eight, si[ and eight being the Foordination number oI bulk Ce4. )urthermore, Cerium ions Fan behaves a large 22 trap: due to its low redo[ potential and Ferium ion Fan easily pass Irom Ce4 to Ce3 >34@. Ce22 has a FubiF Iluorite-like struFture in whiFh Ferium Foordinates eight o[ygen anions at the Forners oI a Fube, while o[ygen is tetrahedrally surrounded by Iour Ferium Fations. It has been demonstrated that the 2[ygen Storage CapaFity (2SC) oI Ferium o[ide is improved by suitable doping with diIIerent FationiF speFies and in partiFular with lanthanum >35@. AgraIiotis et al >32@ studied deposition oI nanophase doped-Feria systems on FeramiF honeyFomb Ior automotive FatalytiF appliFations. A remarkable enhanFement on the FatalytiF aFtivity oI Ferium o[ide has been observed when the power is oI nanophase dimensions beFause oI the large number oI lattiFe deIeFts suFh as o[ygen vaFanFies present, whiFh provide a large number oI aFtive sites Ior gassolid Fatalysis. The number oI deIeFts Fan be inFreased by partial substitution (doping) oI the Ferium atoms in the Ferium o[ide lattiFe with other metal atoms oI lower o[idation state suFh as Ca or Nd. BeFause oI these properties, doped Ferium nano-o[ides Fan aFt as Fatalyst themselves, reduFing signiIiFantly the need Ior noble metals. 9 1.8.1 Role of CeO2 in the TWCs It is an ambitions task to deIine the role oI the Ce22 in the three-way Fatalysis sinFe multiple eIIeFts have been attributed to this promoter. Ceria was suggested to >16@: 1.9 (i) promote the noble metal dispersion (ii) inFrease the thermal stability oI the Al223 support (iii) promote the water gas shiIt (:GS) and steam reIorming reaFtions (iv) Iavour FatalytiF aFtivity at the interIaFial metal-support sites, etF (v) promote C2 removal through o[idation employing a lattiFe o[ygen (vi) store and release o[ygen under, respeFtively, lean and riFh Fonditions. Catalysis by Zirconia-Ceria Ceria-]irFonia solid solutions Fomprise an interesting system with great potential Ior appliFations in various teFhnologiFal Iields. Depending on Fomposition, Feria-]irFonia may be used Ior the manuIaFture oI solid o[ide Iuel Fells, gas sensors, o[ygen semi-permeable membranes Ior steam eleFtrolysis and hot eleFtrodes Ior magnetohydrodynamiF systems >36@. Not long ago, in the middle oI 199s, Ce22-=r22 mi[ed o[ides has been proFlaimed as one oI the most perspeFtive materials Ior appliFation as a Fomponent oI so-Falled ³three-way Fatalysts´ (T:C) in automotive pollution Fontrol. These solids are able to neutrali]e simultaneously C2, hydroFarbons and N2[. High FatalytiF aFtivity oI suFh system Fombines with enhanFed thermal stability and high 2[ygen Storage CapaFity (2SC). The latter is due to the presenFe oI Ferium ions able to Fhange easily their o[idation state depending on the environment >37@. Cerium o[ide (Ce22) is used in automotive emissions Fontrol Fatalysts to regulate the partial pressure oI o[ygen near the Fatalyst surIaFe. Despite its widespread use and appliFation, pure Ferium o[ide has poor thermal stability and is 1 known to sinter at 1123 K. In order to inFrease its thermal stability, and ability to store and release o[ygen during operation, ]irFonium is substituted into the FubiF struFture oI Feria. The addition oI ]irFonium to the FubiF struFture oI Feria is reported to inFrease the o[ygen storage FapaFity oI the system while enhanFing the thermal stability under high temperatures as Fompared to pure Feria >3@. In the last Iew years, it has been eIIeFtively shown that Ce[=r1-[22 solid solutions in the Feria riFh regions ([ .5) are the most eIIeFtive redo[ promoters at low temperature (i.e engine Fold start), and so Fan greatly reduFe emissions oI C2, N2[, and hydroFarbon when aFtive metal is dispersed on the surIaFe >39@. =irFonia-riFh Fompositions, Ce1-[=r222-į ( [ .3) Iind appliFations as FeramiF materials and ioniF FonduFtors whereas Feria-riFh o[ides ( [ ! .3) are e[ploited above all as FatalytiF materials >36@. Mello et al >4@ studied the perIormanFe oI Pd / Ce.75=r.2522 Fatalyst Ior the reduFtion oI N2 with ethanol and Fompare with the Pd-Mo Fatalysts. The Pd / Ce22-=r22 sample showed higher aFtivity Ior the Fonversion oI N2 and higher seleFtivity Ior N2 Iormation when Fompared to the Pd-Mo23 / Al223 sample. The C2 Fhemisorption results showed that the Ce=r mi[ed o[ide Fhemisorbed a higher amount oI C2 , and this is related to a higher reduFibility FapaFity oI this Fompound when Fompared to Mo23. The C2 o[idation on Ce22-=r22 mi[ed o[ides prepared via sol-gel teFhniTue using urea as a hydrolysis Fatalyst was studied >41@. Highly uniIorm nano-si]e solid solution partiFles oI Feria-]irFonia were attained under the Fonditions oI this study (Fa 1 C). The C2 o[idation aFtivity oI the mi[ed o[ides was Iound to be dependent on Ce/=r ratio, whiFh relates to the degree oI reduFibility. The FatalytiF aFtivity Ior C2 o[idation deFreases with a deFrease in Ce/=r ratio. This might be due to the diIIerent in phase Fompositions oI the mi[ed o[ides. It Fan be postulated that the FubiF phase, Iluorite struFture, whiFh is mainly Iound in Ce1-[=r[22 (where [ .5) Fould be reduFed easily than the tetragonal phase Iound in Ce1-[=r[22 (where [ ! .5) >41@. 11 Martorana et al >42@ reported on the struFtural modiIiFations oI two Pt/Feria]irFonia Fatalysts in diIIerent Fonditions oI Ilowing gas mi[tures and oI isothermal treatment temperature this behavior is Fompared with that oI a metal-Iree Feria]irFonia sample. In a Iirst phase, o[ygen Foming Irom the surIaFe layers oI the Feria]irFonia mi[ed o[ide is Fonsumed and no struFtural variation oI the support is observed. AIter this induFtion time, bulk reduFtion oI Pt/Feria-]irFonia takes plaFe as a step-like proFess, while the C22 produFtion Fontinues at a nearly Fonstant rate. This behavior is totally diIIerent Irom that oI the metal-Iree support in similar reaFtion Fonditions, that show a gradual bulk reduFtion. Ko]lov et al >43@ investigated the eIIeFt oI diIIerent synthesis methods on the redo[ property oI Ce22-=r22-Al223. Related to the important oI the preparation method is the observation that enhanFed reduFibility aIter redo[ FyFling is only present in bulk o[ides using Fertain preparation methods. Al223 was able to stabili]e the partiFle si]e oI the mi[ed o[ide during severe redo[ and thermal treatmant. 1.10 Sol-gel Chemistry Heterogeneous Fatalysts are oIten prepared by wet Fhemistry method suFh as preFipitation, FopreFipitation, hydrothermal synthesis or sol-gel proFess. The main advantages oI these low temperature proFesses are to give solids with large speFiIiF surIaFe area and high porosity in the meso and maFropore ranges. The solid network is Iormed, Irom the solution, via the hydrolysis and Fondensation oI moleFular preFursors in solution >44@. The sol-gel proFess is a useIul synthesis approaFh Ior the preparation oI amorphous, as well as struFturally ordered materials. Through sol-gel proFess, it is possible to Fontrol the properties oI the synthesi]ed samples, suFh as porosity and surIaFe area to obtain homogeneous matriFes >45@. 12 The sol-gel is also Fonsidered to be the most praFtiFal method Ior the IabriFation oI porous FeramiF membranes. During the sol-gel proFess, the properties oI the sol, the surIaFe and pore struFture oI the support, the method oI preparing gel membrane and its drying and Iiring Fonditions are the key IaFtors inIluenFing the pore struFture and perIormanFe oI membranes >46@. The sol-gel proFess Ior preparing o[ide glasses is attraFtive as the preparation is aFFomplished at room temperature without high-temperature melting or hot pressing. SinFe the preparation are FonduFted in solutions, the homogeneity attained in the initial reaFtion mi[ture Fan also be retained. The synthetiF proFedure Ior solgel preparation based on aFid or base Fataly]ed hydrolysis oI alko[y-silane preFursors in aTueous solution, Iollowed by Fondensation, gelation, aging, drying, and densiIiFation. The teFhniTue is Fapable oI providing speFialty glasses with optiFally aFtive organiF or inorganiF materials embedded in the matri[, whiFh oIten have high optiFal purity and e[Fellent dieleFtriF eIIeFts while possessing transparenFy over a wide visible range. Thus, glasses prepared by the sol-gel proFess are attraFtive Fandidates Ior optiFal and eleFtro-optiFal appliFations >47@. The sol-gel teFhniTue works well Ior the synthesis oI Fomple[ metal o[ides with high phase purity beFause the polymeri]ing gel traps the various metal ion Fomponents spatially, permitting preFipitation Irom solution where all the metal ions oFFupy near-neighbor positions in the gel matri[, upon Iurther proFessing and high temperature FalFinations, the resultant amorphous mi[ture oI metal o[ides, hydro[ides, and metal salts deFomposes with M-2-M bond Iormation, while having to diIIuse only a Iew angstroms to their lattiFe positions in a homogeneous solid solution. )urthermore, the gel matri[ isolates the individual metal o[ide partiFles, giving rise to nanostruFtured grains aIter the high temperature FalFinations to Iorm the Iinished metal o[ide >4@. There is Fonsidered evidenFe that sols, or Folloidal o[ides, Fan play a FritiFal role as preFursors to the primary partiFles oI Frystalline or amorphous o[ides >49@. Sols have played a very FritiFal role in the ability to prepare FatalytiF o[ide struFtures, suFh as washFoats on monolith struFtures, as the sol struFtures play a 13 FritiFal role as the binder phase between aggregates oI o[ides within suFh struFtures >49@. Sol struFtures Fan, however, just as likely be mere speFtators in the Frystalli]ation proFesses whiFh Iorm the Iinal struFtures within gelled sols. A Fase Fan be made that the term sol-gel is very misleading, in that although sols may be Iormed as intermediate struFtures in some Fases, they are Flearly not neFessarily transIormed into the primary partiFles, or elementary struFtures, within the gelled o[ide >49@. Gel synthesis involves polymeri]ing moleFular preFursors into a three dimensional network and subseTuently Fonverting the wet gel into a [erogel by removing the solvent >5@. In the sol-gel route synthesis, a stepwise reaFtion sFheme has been undertaken to Fontrol the ratio oI hydrolysis to Fondensation rates. In general, the rate oI hydrolysis is Iast Fompared to that oI Fondensation in strong aFidiF Fondition. ThereIore, a well-ordered he[agonal arrangement oI mesopores is Iormed at low-pH in aFidiF Fonditions. Meanwhile, in neutral or basiF Fonditions ranging Irom pH 7 to pH 9, the rate oI Fondensation is Iaster than that oI hydrolysis, and eventually the materials prepared by a single-step reaFtion at high pH display gel-like struFture without mesopores. It is henFe an interesting attempt to synthesi]e ordered mesoporous materials by two-step sol-gel route at lower aFidiF pH Iollowed by a higher pH >51@. As in previous work >52, 53@, modiIied sol-gel method is used to prepare a ]irFonia based Fatalyst in this study. ModiIied sol-gel is a method whiFh varies the teFhniTue to prepare a sol-gel as well as reaFtion Fondition. 14 1.10.1 The Advantages of Sol-gel Approach Over the Traditional Methods of Preparation Sol-gel proFessing provides a new approaFh to the preparation oI supported metal Fatalysts. A well-deIined pore si]e distribution Fan be obtained using this approaFh. The potential advantages oI sol-gel proFessing inFlude: purity, homogeneity, and Fontrolled porosity Fombined with the ability to Iorm large surIaFe area materials at low temperature >54@. The advantage oI the sol-gel method oI synthesis is that virtually any metal o[ide system Fan be e[amined, and no speFial apparatus or eTuipment is reTuired. Besides, its advantages are the Iormation oI FeramiFs oI high purity and good Fontrol over miFrostruFture and partiFle morphology in the synthesis, typiFally at room temperature >4@. 1.10.2 Effects of Variables on the Chemistry of the Sol-gel Synthesis The important variables whiFh must be Fonsidered in the synthesis oI supported metals by the sol-gel method inFlude pH, water/metal alFo[ide ratio (R), gelation temperature, metal loading, addition oI dopants, solvent removal, and pretreatment Fonditions >55@. A large number oI studies have been perIormed in an eIIort to understand the eIIeFt oI water on the properties oI the gel. :hen non-stoiFhiometriF amounts oI water were added, an analysis oI the reaFtion produFts showed that the e[tent oI polymeri]ation was largely depends on the number oI moles oI water, whiFh was added >54@. :hen the H22 / alFo[ide ratio is inFreased, the time reTuired Ior the Iormation oI the gel is also observed to inFrease. This same result is also obtained when pH is deFreased >54@. An inFrease in the H22 / alFo[ide ratio Flearly leads to an inFrease in the pore diameter. The surIaFe area appears to go through a ma[imum when the H22 / alFo[ide ratio is Flose to that Forresponding to stoiFhiometry >54@. 15 The synthesis oI solid Fatalysts using sol-gel proFessing may be perIormed under aFid Fondition (pH 1.5-6), basiF Fonditions (pH -11) or neutral Fonditions (pH 7). DiIIerent Fatalysts Fan used to perIorm the hydrolysis reaFtions >54@. Under strongly aFidiF Fonditions, hydrolysis oFFurred very rapidly and that gel Iormation times were inFreased substantially. Hydrolysis is slower and the polymeri]ation is Fataly]ed by the base. Under these Fonditions, gelation times are Fonsiderably slower >54@. The reaFtion pH is very important in determining the Iinal properties oI the material whiFh is synthesi]ed. Under basiF Fonditions, the partiFles whiFh are initially Iormed have a diameter oI appro[imately 1 nm. These partiFles inFrease in si]e during the synthesis. The resultant gel tends to be mesoporous or maFroporous. :hen the reaFtion is perIormed at a pH oI 7, the partiFles in the sol vary between 2.5 and 2 nm. )or this reason, the resultant gel has a non-uniIorm pore si]e distribution. Under aFid Fonditions the partiFles in both the sol and the gel are very uniIorm. They vary in si]e between .5 and 3. nm and in general have very high porosities. It is also important to note that the pore volume Forresponding to meso (2-5 nm) and maFro (!5 nm) pores inFrease at the e[pense oI miFropores (2. nm) with inFreasing pH >54@. In the sol-gel proFess, drying is regarded as an important proFedure in the determination oI the perIormanFe oI resultant [erogels >56@. Among the many drying teFhniTue, thermal drying is employed most IreTuently Ior FonvenienFe, and a preFeding ambient drying is usually perIormed. It is assumed that shortening the ambient drying period, along with shortening the total drying proFedures, Fan tailor the FharaFteristiFs oI the resultant [erogels >56@. In some Fases, the gel is aged beIore drying. Aging Fan take the Iorm oI a ³redispersion treatment´ whiFh allows dissolution and preFipitation oI material Ior a degree oI ³Iine tuning´ oI the network¶s struFture. The solvent is subseTuently removed by either (i) evaporative drying or (ii) a superFritiFal drying step. SuperFritiFal drying has the advantage oI eliminating the liTuid/vapor interIaFe during drying that Fould otherwise lead to partial Follapse oI the network struFture- 16 most notably loss oI surIaFe area and pore volume-due to high diIIerential Fapillary pore pressures >57@. 1.10.3 Preparation of Catalysts by Sol-gel Method The preparation oI supported metal Fatalysts by sol-gel method is uniTue beFause the metal preFursor is mi[ed homogeneously together with the moleFular preFursor oI the support, resulting in a greater degree oI Fontrol over the Iinal properties oI the Fatalyst >55@. There are two types oI sol-gel method depending on the FhemiFal nature oI the preFursors suFh as inorganiF based sol-gel method and organiF based sol-gel method. InorganiF based sol-gel method involved inorganiF salt whiFh easily dissolved in water whereas organiF based sol-gel method involved organometalliF salts/ Fomple[es suFh as alko[ides whiFh Fan be dissolved in organiF solvent. Sol-gel proFessing in whiFh metalorganiF preFursors are mi[ed with metal preFursors to Iorm a homogeneous solution. The metalorganiF is hydroly]ed through the addition oI water FareIully Fontrolling the pH and the reaFtion temperature. As hydrolysis and polymeri]ation oFFur, Folloidal partiFles or miFelles with an appro[imate diameter oI 1 nm are Iormed. These partiFles Fontinue to inFrease in si]e until a metal o[ide gel (alFogel) is Iormed. The solvent Fan be eliminated by heat treatment in air to Iorm a [erogel. The metal o[ide gel (hydrogel) is Iormed by using inorganiF salt as a preFursor. The solvent Fan be dried to Iorm a [erogel >54@. Most ]irFonia Fompounds Fan be used as preFursors oI ]irFonium o[ide in the sol-gel proFess. These preFursors oI ]irFonia Fan be FlassiIied as ]irFonium alko[ides (]irFonium propo[ide, ]irFonium tetra-n-buto[ide etF.) and inorganiF ]irFonium salts (]irFonium o[yFhloride, ]irFonium aFetate etF.) >46@. 17 :hen ]irFonia is prepared using the sol-gel method, the tetragonal phase Fan be stabili]ed at low temperature, depending on pH and the type oI Fatalyst used in the synthesis. The 2H groups retained in the bulk in this method Iavour stabili]ation oI the tetragonal phase, whereas when positive ions are present (e.g., H, Li, Na) the monoFliniF phase is produFed at low temperature >23@. The Frystalline properties oI ]irFonia synthesi]ed by the preFipitation and solgel methods were Fomparatively studied >1@. Both synthesis methods gave rise to nanoFrystalline ]irFonia. The samples FalFined at C and prepared by the preFipitation had average Frystalline si]es less than 1 nm, whiFh were two times smaller than the Forresponding value obtained in the sol-gel samples. Both tetragonal and monoFliniF nanoFrystalline phases had atomiF deIeFts in FonFentrations that depended on the synthesis method and annealing temperature. Rossignol et al >5@ studied the preparation oI ]irFonia-Feria solid solutions (=r1-[Ce[22) with [ 1) by two diIIerent methods: Fonventional ³FopreFipitation´ and ³modiIied sol-gel´ preparation. Both the struFture and the te[ture oI those solids depend on the synthesis and the ]irFonium preFursor. )or solids prepared by the modiIied sol-gel method, with a surIaFe area oI 6 m2g-1, a new FubiF phase (=r.25Ce.7522) is obtained, while an orthorhombiF ]irFonia phase was identiIied Ior solids prepared either by FopreFipitation or sol-gel methods. The sol-gel method was partiFularly eIIiFient Ior preparation oI Ce-=r-2 mi[ed o[ides with high Ferium Fontents. Lope] et al >59@ investigated the improvement oI struFtural and morphologiFal properties oI Mn-=r mi[ed o[ides by sol-gel method with varing Mn/=r ratio in the entire Fompositional range. This proFedure allows an e[Fellent Fontrol over relevant properties oI the synthesi]ed materials, namely Fompositional homogeneity, purity, and porous struFture. Aguilar et al >23@ reported a systematiF analysis oI the Frystalli]ation proFess oI amorphous sol-gel samples and the thermal behavior oI ]irFonia Fogelled with siliFa, in the Fomplete =r22-Si22 system range, annealing the samples with long thermal treatments at temperature between 1 C-14 C. =r22-Si22 mi[ed o[ides 1 prepared using the sol-gel teFhniTue are promising Fatalysts, or Fatalyst supports, in petroFhemiFal proFesses. )or e[ample, it has been shown to be useIul in the nhe[ane isomeri]ation reaFtion to high oFtane, employing biIunFtional ]irFonia-siliFa Fatalysts in produFts suFh as 2,2-dimethylbutane and 2,3-dimethylbutane. )arias et al >45@ investigated on the sol-gel synthesis oI alumina and 1:1 mi[ed o[ides oI Al223-=r22, Si22-Ti22, =r22-Ti22 and Al223-Ti22. Due to their speFies with aFidiF sites oI diIIerent types (mainly Br|nsted sites Ior Si22 and Lewis sites Ior the other o[ides) and strengths, the mi[ed o[ides are interesting matriFes Irom a FatalytiF point oI view. 1.11 Statement of the Problem and the Needs of the Study The automobile e[haust is identiIied as one oI the major sourFes oI air pollution >29@. Due to inFomplete Fombustion oI Iuel, automobiles emits to[iF gases suFh as Farbon mono[ide (C2), hydroFarbon (HC), and nitrogen o[ides (N2[) to the environment. The way to reduFe these to[iF gases released to the air is by Fonverting them simultaneously to non-to[iF gases and to do this, a three-way Fatalyst is developed. Tetragonal =r22 is used as Fatalyst and Fatalyst support Ior various gas phase reaFtion >26@. Several studies have shown that the perFentage oI tetragonal phase oI =r22 is FruFial Ior the aFtivity and seleFtivity >27@. SinFe the tetragonal phase oI =r22 Fan serve a good FatalytiF site, thereIore this researFh will be Farried out to study the optimum Fondition on the highest Iormation oI single phase tetragonal =r22. This researFh will be FonFentrated on the preparation oI the three-way Fatalyst based on ]irFonium o[ide using modiIied solgel method. 19 1.12 Research Objectives The objeFtives oI this researFh are: 1. To prepare a single-phase tetragonal ]irFonium o[ide doped with Ferium o[ide Fatalyst by modiIied sol-gel method. 2. To eluFidate the physiFal FharaFteristiFs oI Fatalyst using various analytiFal methods Ior Iurther understanding oI the properties oI the Fatalyst Fonsisting oI single-phase tetragonal ]irFonia. 3. To evaluate the perIormanFe oI the Fatalyst obtained towards C2 Fonversion/ o[idation. 1.13 Scope of Research This researFh is Farried out to study the optimum Fonditions on the highest Iormation oI single phase tetragonal =r22. Three parameters will be studied in this study to prepare a single phase tetragonal ]irFonia doped with Feria Fatalyst, vi] amount oI water, amount oI dopants and FalFination temperatures. In this study, the preFursors will be dissolved in a minimum amount oI water to prepare a Fatalyst. A series oI =r[Ce1-[22 Fatalysts will be prepared by varying the atomiF perFentage ratios, over the Fomposition range Irom 5-5 oI Ferium loading. The FalFination temperature start at 4 ÛC will be Fhosen based on previous studies on Fatalyst based on metal o[ides, whiFh reported that the Fatalysts were mostly aFtive at this temperature >6@. The physiFal properties oI Fatalysts will be FharaFteri]ed with ;-Ray DiIIraFtion (;RD), surIaFe area analysis, and )ourier-TransIorm InIrared SpeFtrosFopy ()TIR). The best atomiF perFentage ratio oI Fatalyst Ior the highest Iormation oI single phase tetragonal =r22 will be seleFted Ior Iurther study on the FatalytiF aFtivity towards C2 Fonversion. CHAPTER II EXPERIMENTAL 2.1 Chemicals =irFonium o[yFhloride and Ferium (III) nitrate he[ahydrate were used as the preFursors in this study. =r2Cl2.H22 was purFhased Irom E. MerFk, Darmstadt whereas Ferium (III) nitrate he[ahydrate, Ce(N23)3.6H22, was purFhased Irom E, MerFk. D-61 Darmstadt, ).R. Germany. 2.2 Experiment This researFh is divided into 3 steps: i) Preparation oI the Fatalyst using modiIied sol-gel method, ii) CharaFteri]ation oI the Fatalyst, iii) SeleFted Fatalyst samples were tested Ior Farbon mono[ide Fonversion (C2). 21 2.3 Preparation of Zirconia Based Catalysts Doped with Ceria by Modified Sol-Gel Method =irFonia based Fatalysts doped with Feria were prepared via modiIied sol-gel method ()igure 2.1). Catalyst samples with general Iormula =r[Ce1-[22 were prepared aFFording to their atomiF perFentage ratios (=r.95 : Ce.5). Catalyst sample was prepared by varying the amount oI water. A ]irFonium o[yFhloride solution was prepared by dissolving the salt (5 g) in distilled water ( mL) in a round-bottom Ilask (5 mL) and stirred Fontinuously. To this solution, a Ferium (III) nitrate he[ahydrate solution was then added slowly and stirred Ior 15 minutes. This Ferium (III) nitrate he[ahydrate solution was prepared by dissolving the salt oI .5 in atomiF perFentage ratio in 1ml oI distilled water. By using a similar preparation method, the same atomiF perFentage ratios oI sample were prepared by using a minimum oI water (5 mL). These proFedures were repeated Ior series oI =rCe22 Fatalysts by varying the atomiF perFentage ratio as (=r.9 : Ce.1), (=r. : Ce.2), (=r.7 : Ce.3), and (=r.5 : Ce.5). The resultant transparent solution was then poured into an evaporating dish and allowed to dry in an oven at 7 C Ior 2 days until Fompletely dried. Retort stand Round-bottom Ilask MagnetiF stirrer =r22 based Fatalyst doped with Ce22 MagnetiF spinner )igure 2.1 SFhematiF diagram oI the Fatalyst preparation set-up using modiIied sol-gel method 22 2.4 Drying and Calcination Drying is desFribed as the elimination oI water or solvent Irom the pores oI a solid. Drying Fan be done in a Fonventional drying oven at temperature oI 1-2 C. It is aFFompanied by physiFo-FhemiFal and physiFo-meFhaniFal transIormation that Fan proIoundly modiIy the struFture oI the gel >61@. All prepared samples were dried in the oven at temperature oI 7 C Ior two days until Fompletely dried. AIter drying, dried samples were then grinded with pestle and mortar beIore FalFined in the IurnaFe at 4 C and 6 C Ior 17 hours with ramping time oI two hours. These temperatures were Fhosen based on previous studies on Fatalyst based on metal o[ides, whiFh reported that the Fatalysts were mostly aFtive at these two temperatures >6@. The type oI FalFination is assumed to be FalFination in air, typiFally at a temperature higher than the antiFipated temperatures oI the Fatalysts reaFtion and Fatalyst regeneration >61@. CalFination has several purposes. 2ne is to eliminate e[traneous material suFh as binders, as well as volatile and unstable anions and Fations that have been previously introduFed, but are not desired in the Iinal Fatalyst. SeFond, a substantially elevated temperature is usually needed to inFrease the strength oI the Iinal pellet or e[trudate by Fausing inFipient sintering. E[Fessive sintering will reduFe the Fatalyst aFtivity by reduFing surIaFe area, and it may also Fause diIIusion imitations by reduFtions oI pore si]e, so an optimum is desired >62@. II a metalliF Fatalyst is the ultimate goal, Fonversion to the o[ide Iorm is IreTuently sought prior to reduFtion. II a Fomple[ Fatalyst is the goal, a substantially elevated Iiring temperature may be reTuired to Fause mi[ing by diIIusion oI individual speFies to Iorm a desired Fompound or Frystal phase. In any event, the Fatalyst should be heated under Fontrolled Fonditions to a temperature at least as high as will be enFountered in the plant reaFtor to remove bound water, Farbon mono[ide, etF. II these deFompositions oFFur to a signiIiFant e[tent in the plant, they may Fause struFtural weaknesses in pellets, leading to the breakup, dusting, and so on, that may Fause e[Fessive pressure drop and premature reaFtor shutdown >62@. 23 2.5 Characterization Techniques 2.5.1 X-Ray Diffraction (XRD) Catalyst samples were grinded into Iine powders by using pestle and mortar. EaFh sample was then evenly distributed on the surIaFe oI the sample holder by using a pieFe oI glass slide and plaFed onto a Siemens D5 ;-ray diIIraFtometer eTuipped with Cu-KĮ (Ȝ 1.54 c) radiation. Data were FolleFted over the range oI 2ș Irom 1 up to by using a step sFan oI .5 and a step time oI 1 seFond per step. Peaks position, width and intensity were then identiIied by Fomparison done with an aFFumulated Powder DiIIraFtion )iles (PD)), whiFh Fomes together with the soItware. ;-ray DiIIraFtion (;RD) is a powerIul teFhniTue used to uniTuely identiIy the Frystalline phases present in material and to measure the struFtural properties (strain state, grain si]e, phase Fomposition, preIerred orientation, and deIeFt struFture) oI these phases >63@. )igure 2.2 shows the basiFs Ieatures oI an ;RD e[periment, where the diIIraFtion angles 2ș is the angle between the inFident and diIIraFted ;-rays. In a typiFal e[periment, the diIIraFted intensity is measured as a IunFtion oI 2ș and the orientation oI the speFimen, whiFh yields the diIIraFtion pattern. ;-ray diIIraFtion is the elastiF sFattering oI ;-ray photons by atoms in a periodiF lattiFe. The sFattered monoFhromatiF ;-rays that are in phase give FonstruFtive interIerenFe. DiIIraFtions oI ;-rays by Frystal planes allow one to derive lattiFe spaFing by using the Bragg relation (eTuations 2.1) >64@: nȜ where 2d sin ș n 1, 2, 3 (2.1) Ȝ wavelength oI the ;-rays d distanFe between two lattiFe planes ș angle between the inFoming ;-rays and the normal to the reIleFting lattiFe plane n integer Falled order oI the reIleFtion 24 II one measures the angles, 2ș under whiFh FonstruFtively interIering [-rays leave the Frystal, the Bragg relation (eTuation 2.1) gives the Forresponding lattiFe spaFing, whiFh are FharaFteristiF Ior a Fertain Fompound >64@. )igure 2.2 BasiF Ieatures oI a typiFal ;RD e[periment ;-rays diIIraFtion has an important limitation: Flear diIIraFtion peaks are only observed when the sample possesses suIIiFient long-range order. The advantage oI this limitation is that the width (or rather the shape) oI diIIraFtion peaks Farries inIormation on the dimensions oI the reIleFting planes. DiIIraFtion lines Irom perIeFt Frystals are very narrow. )or Frystalli]e si]e below 1 nm, however, line broadening oFFurs due to inFomplete destruFtion interIerenFe in sFattering direFtions where the [-rays are out oI phase. The Iull width at halI height ():HM) oI the diIIraFtion lines also give an appro[imate measurement oI the partiFle si]e oI the Fatalyst by using SFherrer eTuation in getting the aFtual value oI the partiFle si]e. 25 The SFherrer Iormula relates Frystal si]e to line width: L! kO E FosT (2.2) in whiFh L! measure Ior the dimension oI the partiFles in the direFtion perpendiFular to the reIleFting plane Ȝ ;-rays wavelength ȕ peak width ș angle between the beam and the normal on the reIleFting plane K Fonstant (oIten taken as 1) ;-rays diIIraFtion is the main teFhniTue Ior the investigation oI bulk struFture, to monitor the kinetiFs oI bulk transIormations and to estimate partiFle si]es. In Fatalyst studies, ;RD Fan provide inIormation oI the possibilities Ior the presenFe oI amorphous phases and also identiIiFation oI Frystalline phases in the Fatalyst. )or FrystallographiF phases, ;RD gives a sharp and narrow diIIraFtion lines whereas Ior the amorphous phases, it gives either very broad and weak diIIraFtion lines or no diIIraFtion at all. >65@. 2.5.2 Surface Area Analysis SurIaFe area analysis was Farried out to determine Fatalyst surIaFe area. The Pulse Chemisorb 275 instrument was used Ior surIaFe area analysis. The Iinely grinded sample was weighed at .5 g and then poured into a dry and Flean sample tube. The sample was out-gassed at 3 C Ior 2 hours, then the sample was leIt to Fool to room temperature beIore measurement is Farried out. The absorptiondesorption measurement was Farried out manually aIter the Falibration oI the instrument is done. 26 SurIaFe area measurements were aFFomplished using a 3 N2 / 7 He gas mi[tures. LiTuid nitrogen is the FryogeniF liTuid in most instanFes. 2nly nitrogen gas is physiFally adsorbed at liTuid nitrogen temperature, the helium serving as an inert Farrier. Nitrogen with the volume oI 1. Fm3 at 22 C and 76 mmHg represents a single layer iI nitrogen moleFules physiFally adsorbed on a surIaFe oI 2.4 m2. Thus when the instrument is adjusted to indiFate 2.4 m2 oI sample surIaFe Ior every 1. Fm3 oI gas adsorbed, it displays direFtly the total sample surIaFe area. Small ambient temperature deviations Irom 22 C are relatively insigniIiFant, but a value diIIering Fonsiderably Irom 2.4 may be more appropriate in some loFations Iar removes Irom sea level elevation. 2.5.3 Fourier–Transform Infrared Spectroscopy (FTIR) Catalyst samples were prepared Ior speFtrosFopy analysis using the potassium bromide, KBr, pellet teFhniTue. 1 ± 3 mg oI the Iinely grinded sample was well mi[ed with 3 mg oI KBr powder (1: 1) and then the mi[ture was pressed Ior 5 minutes in evaFuable die under 1 tonne oI pressure to give a transparent disF. The )TIR speFtrum were reForded in the speFtral region Irom 4 to 4 Fm-1 at the resolution oI 4 Fm-1 and 1 sFans to ensure better signal-to-noise ratio by using Shimad]u 3 )ourier TransIorm InIrared SpeFtrometer. The most Fommon appliFation inIrared speFtrosFopy in Fatalysis is to identiIy adsorbed speFies and to study the way in whiFh these speFies are Fhemisorbed on the surIaFe oI the Fatalyst. In addition, the teFhniTue is useIul in identiIying phases that are present in preFursor stages oI the Fatalyst during its preparation. Sometimes the inIrared speFtra oI adsorbed probe moleFules suFh as C2 and N2 give valuables inIormation on the adsorption sites that are present on the Fatalyst >64@. 27 InIrared speFtrosFopy is the most Fommon Iorm oI vibrational speFtrosFopy. 9ibration is divided into basiF Fategories oI stretFhing and bending. InIrared radiation Ialls into three Fategories, as indiFated in Table 2.1. It is the mid inIrared region that is oI our interest. Table 2.1 Region Classification of Infrared Radiation :avelength Energy :avenumber (ȝm) (me9) (Fm-1) InIrared 1 - 1 1.2 - 124 1 - 1 )ar 1 - 5 1.2 - 25 1 - 2 LattiFe 9ibrations Mid 5 - 2.5 25 - 496 2 - 4 MoleFular 9ibration Near 2.5 - 1 496 - 124 4 - 1 2vertones a) 1 me9 .655 Fm-1 DeteFtion oI The )TIR teFhniTue is used to obtain inIormation about IunFtional group in a Fompound. DiIIerent IunFtional group or diIIerent moleFules give diIIerent vibration IreTuenFies. In order to absorb inIrared radiation, a moleFule must undergo a net Fhange in dipole moment as a FonseTuenFe oI its vibrational motion. The dipole moment is determined by the magnitude oI the Fharge diIIerenFe and the distanFe between the two Fenters oI Fharge. II the IreTuenFies oI the radiation matFh a natural vibrational IreTuenFy oI the moleFules, a net transIer oI energy oFFurs that results in a Fhange in the amplitude oI the moleFular vibration, and absorption oI the radiation is the FonseTuenFe >64@. 2 2.6 Catalytic Activity Testing Measurement CatalytiF aFtivity testing Ior seleFted samples were Farried out by using a Ii[ed bed Fustom made miFroreaFtor. .5 g Iinely grinded samples were weighed and paFked into a pyre[ sample tube. The sample was plaFed against the glass sinter on the side oI the inFoming stream. Dried siliFa powder was paFked in on either side and held in plaFe with glass wool ()igure 2.3). The siliFa aFted as an insulator in order to stabili]e the temperature around the sample, as a FonduFtor medium Ior heat and its inert property does not interIere with the FatalytiF reaFtion in the IurnaFe. Pyre[ sample tubes were then loaded into the IurnaFe bloFk and the samples were preheated at 32 C to 33 C Ior an hour in a stream oI air (21 22 79 N2) at the Ilow rate oI 9 mL/min beIore being e[posed to the desired gas mi[ture. This is to aFtivate the Fatalyst in order to prepare the Fatalyst in the desired state beIore testing. Catalyst samples were then Fooled at room temperature and tested by passing Farbon mono[ide (C2) to determine the Fonversion oI Farbon mono[ide (C2) to Farbon dio[ide (C22). Catalysts were tested using a mi[ture oI C2 4 (4 C2, 2.16 22 and 75.4 N2) in riFh o[ygen Fondition. The peak Ior the C2 gas was deteFted by the )TIR speFtrometer in the regions Irom 26 to 223 Fm-1 whereas C22 was deteFted at 2259 to 2379 Fm-1. The aFtivity oI the Fatalyst samples was reForded as a IunFtion oI the Fonversion oI reaFtant gas with respeFt to the temperature. The data were reForded in term oI peak area. The aFtivity oI the Fatalyst was measured until 4 C or until no more Fhanges oI Fonversion Fan be deteFted. 29 Glass :ool Dry SiliFa Powder .5 g Catalyst Gas Mi[ture Gas Mi[ture Dry SiliFa Powder Glass Sinter Glass :ool Sample Tube Gas )low )igure 2.3 SFhematiF diagram oI the miFroreaFtor sample tube CHAPTER III RESULTS AND DISCUSSIONS 3.1 Influence of Variables on the Formation of Tetragonal Phase ZrO2 Three variables were studied on the preparation oI ]irFonia-based Fatalyst doped with Feria by using modiIied sol-gel method, vi] amount oI water, amount oI dopants and FalFination temperatures. These variables were investigated to determine the optimum preparation Fondition Ior the Iormation oI tetragonal phase ]irFonia. All the Fatalyst samples were FharaFteri]ed via ;-Ray DiIIraFtion (;RD) to obtain the inIormation oI the bulk struFture, Frystalline phases and to estimate partiFle si]es. In Fatalyst studies, ;-Ray Powder DiIIraFtion (;RPD) is used to investigate the Frystalline phase transIormation oI the mi[ed o[ides Fatalysts system with respeFt to the FalFination temperature. The inIormation oI Frystalline phases was identiIied using tabulations oI reIerenFe patterns by Fomparing the 2ș values oI the samples with those oI phases Irom the powder diIIraFtion Iile (PD)) >66@. PartiFle si]es Ior the samples were FalFulated by using the SFherrer Iormula as shown in Appendi[ A. SeleFted samples were then FharaFteri]ed via surIaFe area analysis to obtain the inIormation oI surIaFe area. Besides, )ourier TransIorm InIrared SpeFtrosFopy ()TIR) analysis was also Farried out to identiIy the presenFe oI speFies on the surIaFe oI the Fatalyst material. 31 3.1.1 Effect of Amount of Water on the Formation of Tetragonal Phase ZrO2Based Catalyst Doped with CeO2 (Zr0.95:Ce0.05) The diIIraFtograms pattern obtained Irom the ;RD analysis oI =r.95Ce.522 sample by using diIIerent amount oI water are depiFted in )igure 3.1(a)±(b) while the peaks assigned are tabulated in Table 3.1. )or the =r.95Ce.522 sample that was prepared by using mL oI water, the diIIraFtogram is depiFted in )igure 3.1 (a). Two major phases oI =r22 were identiIied as tetragonal at 2ș values oI appro[imately 3.245, 5.365, 6.56 and 35.21 or d-spaFing values oI 2.95, 1.1, 1.54 and 2.55 c (PD) >66@ d-spaFing values (c) : 2.95, 1.1, 1.54 and 2.54) and monoFliniF phase at 2ș values oI appro[imately 2.335 and 31.426 or d-spaFing values oI 3.15 and 2.4 c (PD) >66@ d-spaFing values (c) : 3.16 and 2.4) were observed. There were no Ferium o[ide (Ce22) peaks deteFted due to the relatively small loading oI Feria used in the system. Similar results were obtained Ior the sample by using 5 mL oI water as shown in )igure 3.1 (b) with the present oI tetragonal phase oI =r22 at 2ș values oI appro[imately 3.175, 5.211, 6.9, 35.123 and 59.45 d-spaFing values oI 2.96, 1.2, 1.54, 2.55 and 1.55 c (PD) >66@ d-spaFing values (c): 3.16 and 2.4) were observed. The Iormation oI monoFliniF struFture phase is also present in the material with 2ș values oI appro[imately 2.2 and 31.474 or d-spaFing values oI 3.15 and 2.4 c (PD) >66@ d-spaFing values (c): 3.16 and 2.4). Again, there were no Ferium o[ide peaks deteFted due to the relatively small loading oI Feria in the system. The ;RD patterns Ior sample =r.95Ce.522 prepared by using diIIerent amount oI water (5 mL and mL) are Fomparatively shown in )igure 3.1 (a)±(b). The three highest peaks in both diIIraFtograms were due to the e[istenFe oI the tetragonal phase =r22. 2bviously, the ;RD pattern Ior both samples prepared in 5 mL and mL oI water showed the same trend. This indiFated that the amount oI water did not aIIeFt the evolution in the phase transIormation oI =r.95Ce.522 Fatalyst. However, Irom the FalFulation oI partiFle si]es Ior both samples by using SFherrer Iormula as shown in Appendi[ A, it showed that the partiFle si]es Ior the 32 samples in mL oI water were slightly larger than the sample prepared in 5 mL oI water. The partiFle si]e Ior sample =r.95Ce.522 prepared in mL oI water is 19.75 nm while in 5 mL oI water is 1.9 nm. The data are shown in Table 3.4. This diIIerent in partiFle si]e may be due to the agglomeration oI the partiFles when the amount oI water inFreased. The results above showed that amount oI water did not aIIeFt the Iormation oI tetragonal phase =r22 in =r.95Ce.522. ThereIore, a Iurther study on the eIIeFt oI various loading oI Ce22 on the Iormation oI tetragonal phase =r22 was then Farried out. Intensity (a) mL (b) 5 mL 1 2 3 4 5 6 7 2ș () =r22 (t) )igure 3.1 =r22 (m) DiIIraFtograms oI =r.95Ce.522 using diIIerent amount oI water: (a) mL and (b) 5 mL 33 Table 3.1 ;RD peaks assignment Ior =r.95Ce.522 Fatalyst sample in diIIerent amount oI water Amount oI 2ș Angle d ReIerenFe Peaks :ater (mL) () (c) (d/ c) Assignment 2.335 3.15 3.16 =r22 (m) 3.245 2.95 2.95 =r22 (t) 31.426 2.4 2.4 =r22 (m) 35.21 2.55 2.54 =r22 (t) 5.365 1.1 1.1 =r22 (t) 6.56 1.54 1.54 =r22 (t) 2.2 3.15 3.16 =r22 (m) 3.175 2.96 2.95 =r22 (t) 31.474 2.4 2.4 =r22 (m) 35.123 2.55 2.54 =r22 (t) 5.211 1.2 1.1 =r22 (t) 59.45 1.55 1.55 =r22 (t) 6.9 1.54 1.54 =r22 (t) (a) mL (b) 5 mL * m monoFliniF phase t tetragonal phase 34 3.1.2 Effect of Loading of CeO2 on the Formation of Tetragonal Phase ZrO2 The ;RD pattern oI Fatalyst samples varying their atomiF perFentage ratios are Fomparatively shown in )igure 3.2 and )igure 3.3 whereas the peaks assigned are tabulated in Table 3.2 and Table 3.3 respeFtively. The diIIraFtograms oI ]irFonia-based Fatalyst doped with diIIerent amount oI Feria in mL oI water are depiFted in )igure 3.2 (a)±(d) and the peaks assigned are tabulated in Table 3.2. DiIIraFtogram oI =r.95Ce.522 Fatalyst as shown in )igure 3.2 (a) resulted in the Iormation oI tetragonal phase =r22 phase at 2ș values oI appro[imately 3.245, 5.365, 6.56, and 35.21 or d-spaFing values oI 2.95, 1.1, 1.54 and 2.55 c (PD) >66@ d-spaFing values (c): 2.95, 1.1, 1.54 and 2.54). The =r.95Ce.522 Fatalyst also showed the e[istenFe oI =r22 monoFliniF phase at 2ș values oI appro[imately 2.335 and 31.426 or d-spaFing values oI 3.15 and 2.4 c (PD) >66@ d-spaFing values (c): 3.16 and 2.4). There were no Ferium o[ide peaks deteFted due to relatively small loading oI Ferium used in the system. Similar results were obtained Ior the =r.9Ce.122, =r.Ce.222 and =r.7Ce.322Fatalyst sample, as shown in )igure 3.2 (b)-(d), with the present oI tetragonal phase oI =r22 as the main phase and monoFliniF phase oI =r22 were observed. The peaks assigned are tabulated in Table 3.2. )rom the diIIraFtogram oI =r.Ce.222 Fatalyst, it indiFated that the monoFliniF oI =r22 phase has slightly deFreased in intensity as the amount oI Feria in the mi[ed o[ides inFreased. AFFording to the ;RD patterns oI the =r.7Ce.322 sample shown in )igure 3.2 (d), the monoFliniF phase oI =r22 had obviously reduFes in intensity. The peaks whiFh were still assigned to tetragonal =r22 were observed as the main phase. )rom the ;RD patterns, it is shown that the monoFliniF phase oI =r22 has deFreased in intensity as the Ferium loading inFreased, over the Fomposition range Irom 5 to 3 Ferium, while the tetragonal phase oI =r22 remained as the dominant phase. 35 (a) =r.95Ce.522 Intensity (b) =r.9Ce.122 (F) =r.Ce.222 (d) =r.7Ce.322 1 2 3 4 5 6 7 2ș () =r22 (t) )igure 3.2 =r22 (m) DiIIraFtograms oI =r[Ce1-[22 Fatalyst with diIIerent amount oI Feria in mL oI water (a) =r.95Ce.522, (b) =r.9Ce.122, (F) =r.Ce.222, and (d) =r.7Ce.322 36 Table 3.2 ;RD peaks assignment Ior =r[Ce1-[22 Fatalyst in mL oI water Sample 2ș Angle =r.95Ce.522 =r.9Ce.122 =r.Ce.222 =r.7Ce.322 * m d (c) ReIerenFe Peak d (c) Assignment 2.335 3.15 3.16 =r22 (m) 3.245 2.95 2.95 =r22 (t) 31.426 2.4 2.4 =r22 (m) 35.21 2.55 2.54 =r22 (t) 5.365 1.1 1.1 =r22 (t) 6.56 1.54 1.54 =r22 (t) 2.316 3.15 3.16 =r22 (m) 3.126 2.96 2.95 =r22 (t) 31.447 2.4 2.4 =r22 (m) 35.67 2.56 2.54 =r22 (t) 5.195 1.2 1.1 =r22 (t) 59.4 1.54 1.54 =r22 (t) 2.33 3.15 3.16 =r22 (m) 29.93 2.9 2.95 =r22 (t) 31.371 2.5 2.4 =r22 (m) 34.651 2.59 2.54 =r22 (t) 49.919 1.3 1.1 =r22 (t) 59.354 1.56 1.54 =r22 (t) 29.2 2.99 2.95 =r22 (t) 34.562 2.59 2.54 =r22 (t) 49.515 1.4 1.1 =r22 (t) 49.9 1.3 1. =r22 (t) 59.146 1.56 1.54 =r22 (t) monoFliniF phase t tetragonal phase 37 The diIIraFtograms oI ]irFonia-based Fatalysts doped with diIIerent loading oI Feria in 5 mL oI water are depiFted in )igure 3.3 (a)-(e) and the peaks assigned are tabulated in Table 3.3. Peaks attributed to the tetragonal =r22 phase and the Iormations oI the monoFliniF =r22 phase were observed Irom the )igure 3.3 (a)-(b). )or the =r.Ce.222 Fatalyst, the monoFliniF phase oI =r22 had slightly deFreased with respeFt to the inFreasing amount oI Feria as desFribed earlier. The ;-ray diIIraFtion patterns shown in )igure 3.3 (F) and the peaks assigned are tabulated in Table 3.3. Meanwhile, Ior the =r.7Ce.322 Fatalyst prepared in 5 mL oI water, it Fan be observed that the monoFliniF phase oI =r22 has totally reduFed and the Iormation oI tetragonal =r22 phase still remained as the dominant phase. The diIIraFtogram Ior =r.5Ce.522 sample that was prepared by using 5mL oI water is given as a representative in )igure 3.3 (e) Ior Fomparison. It showed that the degree oI Frystallinity had deFreased and the sample is observed to be highly amorphous with small partiFles present in the material (as shown in Table 3.4). 2verall, it is shown that the ;RD peaks gradually shiIted to a lower 2ș values as a IunFtion oI inFreased Ferium loading. Ceria plays a major role as Fation dopant to retard the Iormation oI monoFliniF phase oI =r22. :hen the Fontent oI =r is eTual to or less than 5, the Fatalysts show FubiF Iluorite ;RD patterns similar to that oI the host o[ide Feria. At =r Fontent higher than 5, ]irFonia beFomes the host o[ide and the resulting Fatalyst show FharaFteristiF oI a tetragonal phase >67@. The partiFle si]es oI the Fatalyst samples prepared in 5 mL and mL oI water with diIIerent loading oI Feria were FalFulated using SFherrer Iormula shown in Appendi[ A and are tabulated in Table 3.4 Ior Fomparison. )rom these data, it is observed that Fatalyst sample prepared in greater amount oI water possessed a larger partiFle si]e. Besides, it also shows that Fatalyst samples with higher loading oI Feria have a smaller partiFle si]e. 3 (a) =r.95Ce.522 Intensity (b) =r.9Ce.122 (F) =r.Ce.222 (d) =r.7Ce.322 (e) =r.5Ce.522 1 2 3 4 5 6 7 2ș () =r22 (t) )igure 3.3 =r22 (m) DiIIraFtograms oI =r[Ce1-[22 Fatalyst with diIIerent amount oI Feria in 5 mL oI water (a) =r.95Ce.522, (b) =r.9Ce.122, (F) =r.Ce.222, d) =r.7Ce.322, and (e) =r.5Ce.522 39 Table 3.3 ;RD peaks assignment Ior =r[Ce[-122 Fatalysts in 5 mL oI water Sample 2ș Angle =r.95Ce.522 =r.9Ce.122 =r.Ce.222 =r.7Ce.322 =r.5Ce.522 * m d (c) ReIerenFe Peaks d (c) Assignment 2.2 3.15 3.16 =r22 (m) 3.175 2.96 2.95 =r22 (t) 31.474 2.4 2.4 =r22 (m) 35.123 2.55 2.54 =r22 (t) 5.211 1.2 1.1 =r22 (t) 6.9 1.54 1.54 =r22 (t) 2.363 3.14 3.16 =r22 (m) 3.196 2.96 2.95 =r22 (t) 31.49 2.4 2.4 =r22 (m) 35.1 2.55 2.54 =r22 (t) 5.252 1.1 1.1 =r22 (t) 6.19 1.54 1.54 =r22 (t) 2.34 3.15 3.16 =r22 (m) 29.924 2.9 2.95 =r22 (t) 31.191 2.7 2.4 =r22 (m) 34.739 2.5 2.54 =r22 (t) 49.3 1.3 1.1 =r22 (t) 59.25 1.56 1.54 =r22 (t) 29.955 2.9 2.95 =r22 (t) 34.69 2.5 2.54 =r22 (t) 49.71 1.3 1.1 =r22 (t) 59.2 1.56 1.54 =r22 (t) 59.427 1.55 1.55 =r22 (t) 29.442 3.3 2.95 =r22 (t) 34.21 2.63 2.54 =r22 (t) 49.145 1.5 1.1 =r22 (t) 57.659 1.6 1.54 =r22 (t) monoFliniF phase t tetragonal phase 4 Table 3.4 PartiFle si]es oI the sample with diIIerent amount oI Feria perFentage in 5 mL and mL oI water FalFined at 4 C PartiFle Si]es (nm) Sample 3.1.3 5 mL oI water mL oI water =r.95Ce.522 1.9 19.75 =r.9Ce.122 17.5 2.31 =r.Ce.222 17.4 1.76 =r.7Ce.322 12.9 14.36 =r.5Ce.522 6.1 - Effect of Calcination Temperature on the Formation of Tetragonal Phase ZrO2 in Zr0.70Ce0.30O2 Catalyst Based on the =r.7Ce.322 sample prepared in 5 mL and mL oI water were seleFted in order to study the eIIeFt oI the FalFination temperature on the Iormation oI tetragonal =r22 phase. The ;RD pattern oI =r.7Ce.322 sample in 5 mL oI water under the heat treatment at 7 C, 4 C and 6 C are Fomparatively shown in )igure 3.4 and peak assigned are tabulated in Table 3.5. The =r.7Ce.322 sample dried at 7 C resulted an amorphous nature with small partiFles present. As the FalFination temperature inFreased to 4 C, there was a distinFt sharpening and inFrease in intensity oI the peaks at 2ș values oI 29.355, 49.71, 59.2, 59.427 and 34.69 or d-spaFing values oI 2.9, 1.3, 1.56, 1.55 and 2.5 c (PD) >66@ d-spaFing values (c): 2.95, 1.1, 1.54, 1.55 and 2.54) whiFh are assigned to tetragonal =r22 phase were observed. No other peak is deteFtable in the diIIraFtogram pattern at this FalFination temperature. At this FalFination temperature, it Fan be observed that the e[istenFe oI tetragonal =r22 phase as the main phase in the sample. However, Iurther FalFination at 6 C, the presenFe oI a very low intensity oI peaks due to the monoFliniF =r22 phase was observed with 2ș values at appro[imately 2.531 and 31.224 or d-spaFing values oI 3.15 and 2.6 c (PD) >66@ d-spaFing values (c): 3.16 and 2.4). At the temperature oI 6 C, tetragonal phase 41 oI =r22 still remained as the dominant phase with 2ș values oI 29.913, 49.699, 49.5, 5.5, 5.53 and 34.2 or d-spaFing values oI 2.9, 1.3, 1.4, 1.2, 1.5 and 2.57 c (PD) >66@ d-spaFing values (c): 2.95, 1.1, 1., 1.2, 1.54 and 2.56). It is shown that the monoFliniF =r22 phase inFreased in intensity as the FalFination temperatures inFreased, but the three highest peaks still due to the Iormation oI tetragonal =r22 phase. It Fan be FonFluded that this amount oI Ferium loading as the main eIIeFt on the Iormation oI tetragonal =r22 phase in =r[Ce1-[22 Fatalyst. The amount oI Feria loading Fan stabili]e the Iormation oI single-phase tetragonal =r22 in the =r22-Ce22 mi[ed o[ides Fatalyst material. Similar result was obtained Ior the =r.7Ce.322 sample prepared in mL oI water and FalFined at 4 C ()igure 3.5 and Table 3.5) with the present oI tetragonal =r22 phase at 2ș values oI 29.2, 49.515, 49.9, 59.146 and 34.562 or d-spaFing values oI 2.99, 1.4, 1.3, 1.56 and 2.59 c (PD) >66@ d-spaFing values (c): 2.95, 1.1, 1., 1.54 and 2.54) were observed as the main phase. Again, Iurther FalFination at 6 C, the presenFe oI a very low intensity oI peaks due to the monoFliniF =r22 phase was observed. AFFording to the ;RD patterns oI =r.7Ce.322 sample shown in )igure 3.4 and )igure 3.5 with an inFreasing in the amount oI Feria Fontent, the ;RD peaks gradually shiIted to a lower 2ș values. This is due to the inFrement in the Fomposition oI Feria in the Fatalyst material, thus aIIeFted the matri[ oI the Fatalyst material. The larger ioniF radius oI Ce4 (.97 c) than =r4 (.4 c) involves an inFrease oI the lattiFe Fonstant and the FonseTuent shiIt oI the diIIraFtion peaks towards lower 2ș values >42@. At 4 C, the main phase is due to the tetragonal phase oI =r22, but when the temperature inFreased, the monoFliniF phases oI =r22 inFreased as well. This indiFated that at higher FalFination temperature (6 C), both tetragonal and monoFliniF phases were Foe[isted with the tetragonal phase being more prominent at eaFh temperature. The degree oI Frystallinity was also observed Irom the peak intensity and peak broadening in the diIIraFtogram pattern obtained. In general, broad and low intensity peaks oI the diIIraFtogram pattern Ior sample treated at 7 C showed the sample to be amorphous with small partiFles present in the material. As the FalFination temperature inFreased, the degree oI 42 Frystallinity inFreased as well. InFrement in Frystallinity and growth in Frystallite si]e were observed by heat treatment at 4 C and 6 C as shown in Table 3.5. (F) =r.7Ce.322 FalFined at 6 ÛC Intensity (b) =r.7Ce.322 FalFined at 4 ÛC (a) =r.7Ce.322 dried at 7 ÛC 1 2 3 4 5 6 7 2ș () =r22 (t) )igure 3.4 =r22 (m) DiIIraFtograms oI =r.7Ce.322 prepared using 5 mL oI water dried at (a) 7 C, and FalFined at (b) 4 C and (F) 6 C 43 (b) =r.7Ce.322 FalFined at 6 ÛC Intensity (a) =r.7Ce.322 FalFined at 4 ÛC 1 2 3 4 5 6 7 2ș () =r22 (t) )igure 3.5 =r22 (m) DiIIraFtograms oI =r.7Ce.322 prepared using mL oI water and FalFined at (a) 4 C and (b) 6 C 44 Table 3.5 ;RD peaks assignment Ior =r.7Ce.322 Fatalyst prepared in 5 mL and mL oI water FalFined at 4 C and 6 C CalFination Temperature (C) 4 Sample 2ș (C) ReIerenFe Peak d (c) d (c) Assignment 6 d (c) Angle 2ș (C) Angle - - 2.351 3.15 3.16 =r22 (m) 29.955 2.9 29.913 2.9 2.95 =r22 (t) - - 31.224 2.6 2.4 =r22 (m) 34.69 2.5 34.2 2.57 2.54 =r22 (t) =r.7Ce.322 - - 49.5 1.4 1. =r22 (t) (5 mL) 49.71 1.3 49.699 1.3 1.1 =r22 (t) 59.2 1.56 5.53 1.5 1.54 =r22 (t) 59.427 1.55 - - 1.55 =r22 (t) - - 2.221 3.16 3.16 =r22 (m) 29.2 2.99 29.6 3. 2.95 =r22 (t) 34.562 2.59 34.612 2.59 2.54 =r22 (t) =r.7Ce.322 49.515 1.4 49.47 1.4 1.1 =r22 (t) ( mL) 49.9 1.3 49. 1.3 1. =r22 (t) - - 5.55 1.5 1.55 =r22 (t) 59.146 1.56 59.2 1.56 1.54 =r22 (t) The diIIraFtograms Ior =r.9Ce.122 and =r.Ce.222 Fatalysts FalFined at 4 C and 6 C are shown in Appendi[ C D respeFtively Ior Fomparison. )or the =r.9Ce.122 Fatalyst as shown in Appendi[ C, it is obvious that the inFreasing oI FalFinations temperature Faused the inFrement in intensity oI the monoFliniF phase =r22. However, the intensity oI the monoFliniF =r22 phase in =r.Ce.222 Fatalyst as shown in Appendi[ D is lower than the =r.9Ce.122 Fatalyst even at the same FalFination temperature (6 C). It Fan be FonFluded that the amount oI Feria loading play a role to Fontrol the intensity oI monoFliniF phases in Fatalyst. 45 )rom Table 3.6, the partiFle si]es oI =r.7Ce.322 sample inFreased relatively to the FalFination temperature and the amount oI water. )or the sample that was prepared by using mL oI water, the partiFle si]e that was FalFined at 6 C (15.25 nm) is slightly larger than the sample FalFined at 4 C (14.36 nm). This same trend is appliFable to the sample that is prepared by using 5 mL oI water. Its partiFle si]e at FalFination temperature oI 6 C is 13.5 nm while at 4 C is 12.9 nm. However, at same FalFination temperature, the partiFle oI =r.7Ce.322 sample that was prepared by using 5 mL oI water is smaller Fompared to the one prepared by using mL oI water. The results showed that the partiFle si]e is larger at higher FalFination temperature and greater amount oI water. Besides ;RD analysis, surIaFe area analysis was Farried out to determine the surIaFe area Ior the =r.7Ce.322 prepared in 5 mL and mL oI water FalFined at 4 C and 6 C. The surIaFe area Ior =r.7Ce.322 samples are tabulated in Table 3.6. )or the sample that was prepared by using mL oI water, the surIaFe area at FalFination temperature oI 4 C is 39.73 m2/g while at 6 C is 14. m2/g. Meanwhile, the surIaFe area Ior the sample prepared with 5 mL oI water FalFined at 4 C is 35.36 m2/g whereas at 6 C is 14.15 m2/g. )rom these data, when the FalFination temperature inFreased, it is known that the surIaFe area will deFrease while the partiFle si]e inFrease. This is due to the sintering proFess takes plaFes as the treatment temperature inFreases Iorming larger partiFles with a smaller surIaFe area. However, the surIaFe area does not inFrease relatively with the deFreasing in partiFle si]e. This probably due to the present oI diIIerent shape oI pores in the Fatalyst, whiFh Fan be proven by the pore te[ture analysis. )or Fatalysis purposes, it is important that the aFtive phase is present in the highest FonFentration possible and with the highest surIaFe area (small dispersed partiFles) on the support surIaFe in order to ma[imi]e the aFtivity >36@. 46 Table 3.6 PartiFle si]es and surIaFe area oI =r.7Ce.322 Fatalysts prepared in 5 mL and mL oI water FalFined at 4 C and 6 C Amount oI water (mL) mL 5 mL CalFination Temperature (C) 4 6 4 6 PartiFle Si]e (nm) 14.36 15.25 12.9 13.5 39.73 14. 35.36 14.15 2 SurIaFe Area (m /g) 3.2 Fourier Transform Infrared (FTIR) Analysis )rom the results oI ;RD analysis that was Farried out on various Fatalysts with diIIerent loading oI Feria, it is Iound that =r.7Ce.322 Fatalyst prepared in 5 mL oI water gives the optimum Fondition Ior the Iormation oI single phase tetragonal =r22. Thus, this typiFal Fatalyst is seleFted Ior Iurther study by using )ourier TransIorm InIrared ()TIR) Analysis. The )TIR speFtrum oI prepared sample dried at 7 C is depiFted in )igure 3.6 whereas Ior the sample FalFined at 4 C and 6 C are depiFted in )igure 3.7 and )igure 3. respeFtively. )rom the )TIR speFtrum oI =r.7Ce.322 dried at 7 C, a broad 2-H stretFhing band due to the adsorbed moleFular water Fan be observed in the range oI 36 Fm-1 to 25 Fm-1 with the ma[imum given at 321. Fm±1. The band that is assigned to water deIormation mode is observed at 1634.6 Fm±1. Nitrate Fan also be deteFted at 1335.6 Fm-1 whereas the band due to hydro[yl deIormation mode oI surIaFe hydro[yl groups is deteFted at 137.6 Fm-1. Two bands are deteFted at 666.4 Fm-1 and 497.6 Fm±1. These two bands are assigned to the M-2 stretFhing mode oI =r-2. 47 T wavenumber (Fm-1) )igure 3.6 )TIR speFtrum oI =r.7Ce.322 Fatalyst prepared in 5 mL oI water dried at 7 C The )TIR speFtrum Ior the sample FalFined at 4 C is shown in )igure 3.7. A small peak has been deteFted at 3321.2 Fm-1. This band is assigned to the unsymmetriFal 2H stretFhing Irom adsorbed moleFular water whereas the water deIormation mode has reduFed. A broad band has also been deteFted at 459. Fm±1. This band is assigned to the =r-2 (M-2) stretFhing band and has shiIted to a lower wave number Fompared to the sample dried at 7 C. 4 137.6 T wavenumber (Fm-1) )igure 3.7 )TIR speFtrum oI =r.7Ce.322 Fatalyst prepared in 5 mL oI water FalFined at 4 C )urther FalFination temperature at 6 C, a broad peak assigned to the =r-2 (M-2) stretFhing band has been deteFted at lower wave number oI 453.2 Fm±1. A small additional peak is also been deteFted at 655. Fm-1 Fompared to the sample FalFined at 4 C. This most probably due to the interIerenFe oI Ferium o[ide phase in the =r22 matriFes. It is indiFated that the =r-2-=r stretFhing band originally at 735 Fm-1 >53@ has aIIeFted by the presenFe oI Feria in the material. It is also indiFated that the band has shiIted to lower wave number as the FalFination temperature inFrease. This is beFause most oI the speFies at the surIaFe oI Fatalyst espeFially ± 2H is eliminated with the inFreasing FalFination temperature. As a result, =r-2 bond will beFome stronger with better Frystallinity. The result Irom the )TIR analysis is in good agreement to the ;RD analysis Ior =r.7Ce.322 FalFined at 4 C and 6 C. 49 137.6 T wavenumber (Fm-1) )igure 3. )TIR speFtrum oI =r.7Ce.322 Fatalyst prepared in 5 mL oI water FalFined at 6 C 3.3 Catalytic Activity of Zr0.70Ce0.30O2 Catalyst Towards Carbon Monoxide Oxidation Reaction )rom the result oI ;RD and )TIR analysis, it Fan be observed that the Fatalyst with the atomiF perFentage ratio oI =r.7 : Ce.3 FalFined at 4 C showed the optimum Fondition on the Iormation oI single phase tetragonal =r22. Thus, =r.7Ce.322 Fatalyst prepared in 5 mL and mL oI water were seleFted Ior Iurther study on the FatalytiF aFtivity towards Farbon mono[ide Fonversion. The results are disFussed based on the Light-2II Temperature (TL2) and 1 Fonversion temperature (T1), as summari]ed in Table 3.7. The =r.7Ce.322 Fatalyst prepared in 5 mL oI water and FalFined at 4 C showed a better perIormanFe towards the Fonversion oI C2 Fompared to the Fatalyst prepared in mL oI water. This Fatalyst 5 showed a low TL2 value at room temperature while 1 C2 Fonversion was aFhieved at T1 32 C whereas Ior the =r.7Ce.322 Fatalyst prepared in mL oI water also showed a low TL2 value at room temperature. It gave only 3.1 Fonversion oI C2 even at ma[imum temperature oI 4 C. Table 3.7 * RT CatalytiF aFtivity oI =r.7Ce.322 Fatalyst towards C2 Fonversion =r.7Ce.322 Catalyst TL2 ( C) T1 ( C) 5 mL RT * 32 mL RT * 4 (3.1) Room Temperature (25 ± 3 C) TL2 Light-2II Temperature T1 1 C2 Conversion Temperature Plots oI perFentage Fonversion oI C2 versus temperature Ior the =r.7Ce.322 Fatalysts prepared in diIIerent amount oI water are shown in )igure 3.9. )or the =r.7Ce.222 Fatalyst prepared in 5 mL oI water, it Fan be observed that C2 Fonversion inFreases sharply at Light-2II temperature. Conversion oI C2 remained at the same perFentage within the region oI 3 ± 2 C. The Fonversion inFreases gradually aIter 2 C and 1 oI C2 Fonversion was aFhieved at 32 C whereas the Fonversion oI C2 Ior the =r.7Ce.322 Fatalyst prepared in mL oI water only inFreases slightly aIter the TL2 and did not give 1 oI Fonversion even at ma[imum temperature oI 4 C. It Fan be FonFluded that the =r.7Ce.322 Fatalyst prepared in 5 mL oI water and FalFined at 4 C showed a better perIormanFe towards the Fonversion oI C2 with 1 Fonversion temperature (T1) Fompared to the Fatalyst prepared in mL oI water. This probably due to lower degree oI Frystallinity Ior the =r.7Ce.322 Fatalyst prepared in 5 mL oI water, whiFh possesses a lot oI deIeFt sites and thus gave a better perIormanFe towards C2 o[idation Fompared to the Fatalyst prepared in mL oI water with higher degree oI Frystallinity. It Fan be FonFluded that the C2 o[idation not solely depends on the tetragonal phase but the degree oI Frystallinity. This FatalytiF result is in agreement with the result obtained in the ;RD analysis oI the good perIormanFe Fatalyst. 51 =r.7Ce.322 (mL) =r.7Ce.322 (5mL) 12 Conversion oI C2 () 1 6 4 2 1 2 3 4 Temperature (C) )igure 3.9 PerFentage Fonversion oI C2 versus temperature Ior the =r.7Ce.322 Fatalyst prepared in mL and 5 mL oI water CHAPTER IV CONCLUSIONS 4.1 Conclusions This research found that the Zr0.70Ce0.30O2 catalyst prepared in 5 mL of water by using zirconium oxychloride and cerium (III) nitrate hexahydrate as the precursors, dried for two days at 70 ºC and calcined at 400 ºC for 17 hours gives the optimum condition on the highest formation of single phase tetragonal ZrO2. The XRD analysis showed that this catalyst has some degree of crystallinity property, consists of the tetragonal ZrO2 as the main phase and average crystalline size of approximately 12.98 nm serve as the active phases for the catalyst. The particle size increased as the amount of water increased while the particle size decreased as the amount of ceria increased. The role of ceria is to retard the sintering process as well as the present of monoclinic ZrO2 phase. At 400 ºC, the main phase is tetragonal but when the temperature increased, the tetragonal phase slightly decreased with an increase in the monoclinic phase. Surface area analysis showed this catalyst has 35.36 m2/g of surface area. This surface area decreased as the calcination temperature increased, which is in agreement with the enlargement of the particle size. 53 FTIR analysis showed the existence of surface hydroxyl group on the surface of catalyst. The bands assigned to the water deformation mode, hydroxyl deformation mode of surface hydroxyl groups and nitrate has been totally diminished as the calcination temperature increased. The position of the Zr-O (M-O) stretching bands shifted to a lower wave number as the calcination temperature increased. At 400 ºC, the band at 459.0 cm-1 was assigned to the M-O stretching mode of ZrO2 has shifted to a lower wave number at 453.2 cm-1 for further calcination temperature at 600 ºC. For the sample calcined at 600 ºC, a small additional peak was observed at 655.8 cm-1, this most probably due to the interference of cerium oxide phase in the ZrO2 matrices. The Zr0.70Ce0.30O2 catalyst prepared in 5 mL of water and calcined at 400 ºC showed a better performance towards the conversion of CO with 100 % conversion temperature (T100) compared to the catalyst prepared in 8 mL of water. It gives the lowest TLO at room temperature while T100 at 320 ºC. We can conclude that the amount of water used in this study did not show significant effect on its physical properties but on the other hand, did affect its catalytic performance. 4.2 Future Works This research studied on the optimum condition / parameters on the formation of tetragonal ZrO2 in the ZrO2-based catalyst doped with ceria. A further study need to be carried out to fully utilize the results obtained from this research. Since this research concentrated solely on the inorganic-based sol-gel method that involved inorganic salt that easily dissolved in water, an organic-based sol-gel method involving organometallic salts such as alkoxides that can be dissolved in organic solvent should be carried out to compare the effect of precursors on the formation of tetragonal ZrO2 phase in the ZrO2 based catalyst. Study on the amount of solvent used should also be carried out further to see obvious changes on the physical properties of the catalyst material. 54 Other characterization techniques can be used for further understanding of the properties of the catalyst. Nitrogen adsorption analysis at 77 K should be carried out to determine the types and shapes of pores for catalysts. The chemical composition of the prepared model catalysts can be analyzed with Auger electron spectroscopy (AES) to characterize the surface species segregation compared with the bulk composition. Besides, the catalyst systems can also be further analyzed with X-ray photoelectron spectroscopy (XPS) to verify surface composition and elemental oxidation states [35]. Finally, the catalytic activity of Zr0.70Ce0.30O2 catalyst towards the oxidation of CO and hydrocarbon with the reduction of NOx is suggested to carry out in order to get better performance of the three-way catalyst. 55 REFERENCES 1. Noel de Nevers. (2000). “Air Pollution Control Engineering”. McGraw Hill Companies, Inc. 1-11. 2. Kaspar, J., Fornasiero, P., Hickey, N. (2003). “Automobile Catalytic Converters: Current Status and Some Perspectives”. Catalysis Today. 77. 419-449. 3. Cooper, C. D., Alley, F. C. (2002). “Air Pollution Control”. Waveland Press, Inc. 3rd Edition. 2-6. 4. Boubel, R. W., Fox, D. L., Turner, D. B., and Stern, A. C. (1994). “Fundamentals of Air Pollution”. Academic Press, Inc. 3rd Edition. 44-47. 5. Bradley, K. S., Stedman, D. H., Bishop, G. A. (1999). “A Global Inventory of Carbon Monoxide Emission from Motor Vehicles”. Chemosphere-Global Change Science. 1. 65-72. 6. Kenneth Wark and Warner, C. F. (1976). “Air-Pollution-Its Origin and Control”. New York: IEP A Dun-Donnelley Publisher. 1-40. 7. Matsukuma, I., Kikuyama, S., Kikuchi, R., Sasaki, K., Eguchi, K. (2002). “Development of Zirconia-Based Oxide Sorbents for Removal of NO and NO2”. Applied Catalysis B: Environment. 37. 107-115. 8. Richardson, J. T. (1989). “Principals of Catalyst Development”. New York: Plenum Press. 1-19. 56 9. Limido, J. (1987). “Applied Heterogeneous Catalysis: Design Manufacture Use of Solid Catalysts”. Houston, Texas: Gulf Publishing Company. 1-14. 10. Kaspar, J., and Fornasiero, P. (2003). “Nanostructured Materials for Advanced Automobile De-Pollution Catalysts”. Journal of Solid State Chemistry. 171. 19-29. 11. Wang, J. A., Chen, L. F., Valenzuela, M. A., Montoya, A., Salmones, J., and Angel, P. D. (2004). “Rietveld Refinement and Activity of CO Oxidation Over Pd/Ce0.8Zr0.2O2 Catalyst Prepared Via A Surfactant-Assisted Route”. Applied Surface Chemistry. 230. 34-43. 12. Martinez-Arias, A., Fernandez-Garcia, M., Iglesias-Juez, A., Hungria, A. B., Anderson, J. A., Conesa, J. C., and Soria, J. (2002). “Influence of Thermal Sintering on the Activity for CO-O2 and CO-O2-NO Stoichiometric Reactions Over Pd/(Ce, Zr)Ox/Al2O3 Catalysts. Applied Catalysis B: Environment. 38. 151-158. 13. Battistoni, C., Cantelli, V., Debenedetti, M., Kaciulis, S., Mattogno, G., and Napoli, A. (1999). “XPS Study of Ceramic Three-Way Catalysts”. Applied Surface Chemistry. 144-145. 390-394. 14. Harrison, R. M. (1990). “Pollution: Course, Effects & Control”. 2nd Edition. University of Birmingham, Great Britain. 221-236. 15. Acres, G. J. K. (1983). “Catalyst Systems for Emission Control from Motor Vehicles”. UK: Johnson Matthey Research Centre. 222-224. 16. Kaspar, J., Fornasiero, P., Grazionl, M. (1999). “Use of CeO2-Based Oxides in the Three-Way Catalysis”. Catalysis Today. 50. 285-298. 57 17. Shankar, S. S., Joshi, H., Pasricha, R., Pavaskar, N. R., Mandale, A. B., and Sastry, M. (2004). “A Low-Temperature , Soft Chemistry Method for the Synthesis of Zirconia Nanoparticles in Thermally Evaporated Fatty Amine Thin Films. Journal of Colloid and Interface Science. 269. 126130. 18. Wang, J. A., Valenzuela, M. A., Salmones, J., Vazquez, A., Garcia-Ruiz, A., Bokhimi, X. (2001). “Comparative Study of Nanocrystalline Zirconia Prepared by Precipitation and Sol-Gel Methods”. Catalysis Today. 68. 2130. 19. Sun, Y., and Sermon, P. A. (1995). “Preparation of CaO-, La2O3-, and CeO2Doped ZrO2 Aerogels by Sol-Gel Methods”. Preparations of Catalysts VIScientific Bases for the Preparation of Heterogeneous Catalysts. Elsevier Science B. V. 471-478. 20. Jung, C., Ito, Y., Endou, A., Kubo, M., Imamura, A., Selvam, P., and Miyamoto, A. (2003). “Quantum-Chemical Study on the Supported Precious Metal Catalyst”. Catalysis Today. 87. 43-50. 21. Marella, M., Tomaselli, M., Meregalli, L., Battagliarin, M., Gerontopoulos, P., Pinna, F., Signoretto, M., and Strukul, G. (1995). “Sol-Gel Zirconia Spheres for Catalytic Application”. Preparation of Catalysis VI-Scientific Bases for the Preparation of Heterogeneous Catalysts. Elsevier Science B. V. 327-335. 22. Liu, W. C., Wu, D., Li, A. D., Ling, H. Q., Tang, Y. F., and Ming, N. B. (2002). “Annealing and Doping Effects on Structure and Optical Properties of Sol-Gel Derived Thin Films”. Applied Surface Chemistry. 191. 181-187. 23. Aguilar, D. H., Torres-Gonzalez, L. C., and Torres-Martinez, L. M. (2000). “A Study of the Crystallization of ZrO2 in the Sol-Gel System: ZrO2SiO2”. Journal of Solid State Chemistry. 158. 349-357. 58 24. Luo, T. Y., Liang, T. X., and Li, C. S. (2004). “Addition of Carbon Nanotubes During the Preparation of Zirconia Nanoparticles: Influence on Structure and Phase Composition”. Powder Technology. 139. 118-122. 25. Li, M. Q., and Chi, Z. N. (1988). “Transformation from A Metastable Tetragonal Structure into A Monoclinic Structure in Zirconia Powders”. Science and Technology of Zirconia III. 24. 244-251. 26. Shukla, S., and Seal, S. (2003). “Phase Stabilization in Nanocrystalline Zirconia”. Rev. Adv. Mater. Sci. 5. 117-120. 27. Sun, W., Xu, L., Chu, Y., and Shi, W. (2003). “Controllable Synthesis, Characterization and Catalytic Properties of WO3/ ZrO2 Mixed Oxides Nanoparticles”. Colloid and Interface Science. 266. 99-106. 28. Ge, Q. J., Kiennemann, A., Roger, A. C., Li, W. Z., and Xu, H. Y. (2004). “Preparation and Characterization of Modified-ZrO2 Catalysts for the Reaction of CO Hydrogenation”. Natural Gas Chemistry. 13. 41-44. 29. Loong, C. K., and Ozawa, M. (2000). “The Role of Rare Earth Dopants in Nanophase Zirconia Catalysts for Automotive Emission Control”. Alloys and Compounds. 303-304. 60-65. 30. Binet, C., Daturi, M., and Lavalley, J. C. (1999). “IR Study of Polycrystalline Ceria Properties in Oxidised and Reduced States”. Catalysis Today. 50. 207-225. 31. Ozawa, M., and Loong, C. K. (1999). “In Situ X-Ray and Neutron Powder Diffraction Studies of Redox Behavior in CeO2-Containing Oxide Catalysts”. Catalysis Today. 50. 329-342. 59 32. Agrafiotis, C., Tsetsekou, A., Stournaras, C. J., Julbe, A., Dalmazio, L., and Guizard, C. (2000). “Deposition of Nanophase Doped-Ceria Systems on Ceramic Honeycombs for Automotive catalytic Applications”. Solid State Ionics. 136-137. 1301-1306. 33. Colon, G., Valdivieso, F., Pijolat, M., Baker, R. T., Calvino, J. J., and Bernal, S. (1999). “Textural and Phase Stability of CexZr1-xO2 Mixed Oxides Under Temperature Oxidising Conditions”. Catalysis Today. 50. 271-284. 34. Lopez, T., Rojas, F., Alexander-Katz, R., Galindo, F., Balankin, A., and Buljan, A. (2004). “Porosity, Structural and Fractal Study of Sol-Gel TiO2-CeO2 Mixed Oxides”. Journal of Solis State Chemistry. 177. 18731885. 35. Deganello, F., and Martorana, A. (2002). “Phase Analysis and Oxygen Storage Capacity of Ceria-Lanthana-Based TWC Promoters Prepared by Sol-Gel Routes”. Journal of Solid State Chemistry. 163. 527-533. 36. Boaro, M., Trovareli, A., Hwang, J. H., and Mason, T. O. (2002). “Electrical and Oxygen Storage/Release Properties of Nanocrystalline Ceria-Zirconia Solid Solutions”. Solid State Ionics. 147. 85-95. 37. Ikrynannikova, L. N., Markaryan, G. L., Kharlanov, A. N., Lunina, E. V. (2003). “Electron-Accepting Surface Properties of Ceria-(Praseodymia)Zirconia Solids Modified by Y3+ or La3+ Studied by Paramagnetic Probe Method”. Applied Surface Science. 207. 100-114. 38. Nelson, A. E., and Schulz, K. H. (2003). “Surface Chemistry and Microstructural Analysis of CexZr1-xO2-y Model Catalyst Surfaces. Applied Surface Science. 210. 206-221. 39. Hartridge, A., and Bhattacharya, A. K. (2002) “Preparation and Analysis of Zirconia Doped Ceria Nanocrystal Dispersions”. Journal of Physics and Chemistry of Solids. 63. 441-448. 60 40. Mello, L. F. D., Baldanza, M. A. S., Noronha, F. B., and Schmal, M. (2003). “NO Reduction with Ethanol on MoO3/Al2O3 and CeO2-ZrO2-Supported Pd Catalysts”. Catalysis Today. 85. 3-12. 41. Thannachart, M., Meeyoo, V., Risksomboon, T., and Osuwan, S. (2001). “Catalytic Activity of CeO2-ZrO2 Mixed Oxide Catalysis Prepared Via Sol-Gel Technique: CO Oxidation. Catalysis Today. 68. 53-61. 42. Martorana, A., Deganello, G., Longa, A., Prestianni, A., Liotta, L., Macaluso, A., Pantaleo, G., Balerna, A., and Mobilio, S. (2004). “Structural Evolution of Pt/Ceria-Zirconia TWC Catalysts During the Oxidation of Carbon Monoxide”. Journal of Solid State Chemistry. 177. 1268-1275. 43. Kozlov, A. I., Kim, D. H., Yezerets, A., Andersen, P., Kung, H. H., and Kung, M. C. (2002). “Effect of Preparation Metehod and Redox Treatment on the Reducibility and Structure of Supported Ceria-Zirconia Mixed Oxide”. Journal of Catalysis. 209. 417-426. 44. Livage, J. (1998). “Sol-Gel Synthesis of Heterogenous Catalysts from Aqueous Solutions”. Catalysis Today. 41. 3-19. 45. Farias, R. F. D., Arnold, U., Martinez, L., Schuchardt, U., jannini, M. J. D. M., and Airoldi, C. (2003). “Synthesis, Characterization and Catalytic Properties of Sol-Gel Derived Mixed Oxides”. Journal of Physics and Chemistry of Solids. 64. 2385-2389. 46. Ju, X. S., Huang, P., Xu, N. P., and Shi, J. (2000). “Influences of Sol and Phase Stability on the Structure and Performance of Mesoporous Zirconia Membranes”. Journal of Membrane Science. 166. 41-50. 47. Assefa, Z., Haire. R. G., Caulder, D. L., and Shuh, D. K. (2004). “Correlation of the Oxidation State of Cerium in Sol-Gel Glasses as A Function of Thermal Treatment Via Optical Spectrosopy and XANES Studies”. Spectrochemica Acta Part A. 60. 1873-1881. 61 48. Ying, J. Y. (2001). “Nanostructured Materials”. Academic Press. 9-10. 49. Murrell, L. L. (1997) “Sols and Mixtures of Sols As Precursors of Unique Oxides”. Catalysis Today. 35. 225-245. 50. Narendar, Y., Messing, G. L. (1997). “Mechanisms of Phase Separation in Gel-Based Synthesis of Multicomponent Metal Oxides”. Catalysis Today. 35. 247-268. 51. Choi, D. G., and Yang, S. M. (2003). “Effect of Two-Step Sol-Gel Reaction on the Mesoporous Silica Structure”. Journal of Colloid and Interface Science. 261. 127-132. 52. Wan Azelee Wan Abu Bakar (1995). “Non-Noble Metal Environmental Catalysts: Synthesis, Characterisation and Catalytic Activity”. University of Nottingham. PhD. Thesis. 53. Nor Aziah Buang (1999). “ Zirconia Based Catalysts for Environment Emission Control: Synthesis, Characterization, & Catalytic Activity.” Universiti Tecknologi Malaysia. PhD. Thesis. 54. Gonzalez, R. D., Lopez, T., and Gomez, R. (1997). “Sol-Gel Preparation of Supported Metal Catalysts”. Catalysis Today. 35. 293-317. 55. Lambert, C. K., and Gonzalez, R. D. (2001). “The Effect of pH and Metal Loading on the Properties of Sol-Gel Rh/SiO2”. Journal of Solid State Chemistry. 158. 154-161. 56. Huang, W. L., Cui, S. H., Liang, K. M., Gu, S. R., and Yuan, Z. F. (2002). “Influence of Drying Procedure on the Mesoporosity and Surface Fractal Dimensions of Silica Xerogels Prepared With Different Agitation Methods”. Journal of Colloid and Interface Science. 246. 129-134. 62 57. Miller, J. B., and Ko, E. I. (1997). “Control of Mixed Oxide Textural and Acidic Properties by the Sol-Gel Method”. Catalysis Today. 35. 269-292. 58. Rossignol, S., Madier, Y., and Duprez, D. (1999). “Preparation of ZirconiaCeria Materials by Soft Chemistry”. Catalysis Today. 50. 261-270. 59. Lopez, E. F., Escribano, V. S., Gallardo-Amores, J. M., Resini, C., and Busca, G. (2002). “Structural and Morphological Characterization of MnZr Mixed Oxides Prepared by A Sol-Gel Method”. Solid State Science. 4. 951-961. 60. Yap Chui Peng (2002). “Cobalt Oxide Based Catalysts for Emission Control: Synthesis, Catalytic Activity and Characterization”. Universiti Teknologi Malaysia. M. Sc. Thesis. 61. Page, J. F. (1987). “Applied Heterogeneous Catalysis. Design, Manufacture and Use of Solid Catalysts”. Texas: Gulf Publishing Company. 88-96. 62. Satterfield, C. N. (1991). “Heterogeneous Catalysis in Industrial Practice”. 2nd Ed. New York: McGraw Hill, Inc. 101-103. 63. Brundle, C. R., Charles, A. E., and Shaun, W. (1992). “Encyclopedia of Materials Characterization-Surfaces, Interfaces, Thin Films”. Greenwich: Manning Publication Co. 64. Niemantsverdriet, J. W. (1995). “Spectroscopy in Catalysis- An Introduction”. New York: VCH Publishers. 138-146. 65. Nuffield, E. W. (1966). “X-Ray Diffraction Methods”. New York: John Wiley & Sons, Inc. 8-35. 66. International Centre for Diffraction Data (1997). “Powder Diffraction File Inorganic Phases (JCPDS-ICDD)”. Newtown Square. American Society of Testing Materials. 63 67. Jimmy, C. Y., Zhang, L. Z., and Lin, J. (2003). “Direct Sonochemical Preparation of High-Surface-Area Nanoporous Ceria and Ceria-Zirconia Solid Solutions. Colloid and Interface Science. 260. 240-243.