Interpreting the ACC-AHA Guidelines on Cardiovascular Disease Prevention and Cholesterol Management

Interpreting the ACC-AHA Guidelines on
Cardiovascular Disease Prevention and
Cholesterol Management
Nathan D. Wong, PhD, FACC, FAHA
Professor and Director
Heart Disease Prevention Program
Division of Cardiology
University of California, Irvine
Past President, American Society for Preventive
Cardiology
Disclosures
Research contracts to institution from Amgen,
Bristol Myers-Squibb, and Regeneron
Consultant, Amgen and Re-Engineering Healthcare,
Quinne Manuel
C-DAY
Cholesterol?
Controversy?
Confusion?
Conundrum?
Courage…...?
Nov. 12, 2013
I have some bad news for you. While your cholesterol has remained the same, the research findings have changed.
New CVD Prevention Guidelines
1) AHA/ACC Guideline on Cardiovascular Risk
Assessment (November)
2) AHA/ACC Guideline on Lifestyle Management
(November)
3) AHA/ACC Guideline on Obesity Management
(November)
4) AHA/ACC Guideline on Cholesterol Management
(November)
5) AHA Advisory on Blood Pressure Control
(November)
6) Guidelines on Hypertension by Members Appointed to JNC-8 (December)
7) ASH/ISH Guidelines on Hypertension (December)
NHLBI Charge to the Expert Panel
Evaluate higher quality randomized controlled trial (RCT) evidence for cholesterol-lowering drug therapy to reduce ASCVD risk
Use Critical Questions (CQs) to create the evidence search from which the guideline is developed
• Cholesterol Panel: 3 CQs
• Risk Assessment Work Group: 2 CQs
• Lifestyle Management Work Group: 3 CQs
RCTs and systematic reviews/meta-analyses of RCTs independently assessed as fair-to-good quality
Develop recommendations based on RCT evidence
• Less expert opinion than in prior guidelines
Preventive cardiology efforts begin with assessment of cardiovascular disease risk
Recommendation to begin with a global risk assessment using the Pooled Cohort Equations to estimate 10-year ASCVD Risk
(other risk assessment algorithms include the
European SCORE, PROCAM, or Framingham scores)
Why Use Risk Scores?
1) Risk functions provide an “economic and efficient method of identifying persons at high cardiovascular risk who need preventive treatment….” (Kannel 1976)
2) Intensity of treatment should match a person’s risk
(ACC Bethesda Conf, JACC 1996).
3) A physician’s estimate is only accurate 24% of the time (Pignone, 2003).
4) Can be used to communicate risk in patients and motivate adherence to lifestyle and other therapies.
5) Routine use of global risk scores leads to greater use of guideline-based therapy and improved outcomes
(Sheridan, 2008).
ATP III Update ( 2004 )
LDL-C Treatment Targets based on 10-year
Hard CHD Risk from Framingham Risk Score
Risk Category LDL-C Goal
Initiate
TLC
Consider
Drug Therapy
High risk:
CHD or CHD risk equivalents
(10-year risk >20%)
Moderately high risk:
2+ risk factors
(10-year risk 10% to
20%)
Moderate risk:
2+ risk factors (risk
<10%)
Lower risk:
0-1 risk factor
<100 mg/dL
(optional goal:
<70 mg/dL)
<130 mg/dL
(optimal <100 mg/dL)
<130 mg/dL
<160 mg/dL
Adapted from Grundy SM, et al. Circulation . 2004;110:227-239.
100 mg/dL
130 mg/dL
130 mg/dL
160 mg/dL
100 mg/dL
(<100 mg/dL: consider drug Rx)
130 mg/dL
(100-129 mg/dL: consider Rx )
160 mg/dL
190 mg/dL
ASCVD Risk Calculator: Development
NHLBI charge to the workgroup: To Develop or
Recommend an Approach to Quantitative Risk
Assessment That Could be Used to Guide Care
Risk Assessment Work Group judged new risk tool was needed that was Inclusive of African Americans and with expanded endpoint including stroke
Sought cohorts representative of the U.S. population as a whole
•
Community- or population-based
•
Whites and African Americans (at a minimum)
•
Recent follow-up data of at least 10 years
10-year follow-up from other ethnicities not available at time of development
ASCVD Risk Calculator: Development (cont.)
Pooled Cohort Equations
•
Atherosclerosis Risk in Communities (ARIC)
•
Cardiovascular Heath Study (CHS)
•
Coronary Artery Risk Development in Young
Adults (CARDIA)
•
Framingham Original and Offspring
Hard ASCVD
•
CHD death, nonfatal MI, fatal/nonfatal stroke
(but does not include angina, PCI/CABG or other
CVD such as HF or PVD)
Models tested using traditional RFs + newer markers when possible
Internal and external validation
Recommendations for Assessment of 10-
Year Risk of a First Hard ASCVD Event
I IIa IIb III The race- and sex-specific Pooled Cohort Equations* to predict 10-year risk of a first hard ASCVD event should be used in non-Hispanic African Americans and non-Hispanic whites, 40 to 79 years of age.
I IIa IIb III Use of the sex-specific Pooled Cohort Equations for non-Hispanic whites may be considered for estimation of risk in patients from populations other than African Americans and non-Hispanic whites.
*Derived from the ARIC study, CHS, CARDIA study, Framingham original and offspring cohorts
.
2013 Prevention Guidelines
ASCVD Risk Estimator
ASCVD Risk Estimator
•
Provides 10-year
ASCVD risk for those
40-79 and lifetime risk for those 20-59
•
This is intended to drive discussions of greater adherence to heart-healthy lifestyle
•
Initiates risk discussion with patient
•
Not an automatic prescription for a statin
Recommendations for Use of Newer Risk Markers
After Quantitative Risk Assessment
I IIa IIb III If, after quantitative risk assessment, a riskbased treatment decision is uncertain, assessment of ≥1 of the following—family history, hs-CRP, CAC score, or ABI —may be considered to inform treatment decision making.†
I IIa IIb III
No Benefit
Routine measurement of carotid intima-media thickness is not recommended in clinical practice for risk assessment for a first ASCVD event.†
†Based on new evidence reviewed during ACC/AHA update of evidence.
If the risk decision is uncertain, the following factors can help inform the treatment decision and revise the risk estimate upward:
1) LDLC ≥ 160 mg/dl
2) Family hx premature ASCVD (prior CAD or stroke in male first degree relative age <55 or female first degree relative <65)
3) Increased lifetime risk
4) hsCRP≥ 2 mg/L
5) CAC score ≥ 300 or 75 th %tile
6) ABI < 0.9
Ankle- Brachial Index (ABI)
Measure of lower-extremity occlusive peripheral arterial disease
Ankle SBP *
Brachial Artery SBP
ABI
ABI < 0.90 is abnormal
Sensitivity = 90% specificity= 98% for stenosis >
50%.
Can detect asymptomatic disease .
* dorsalis pedis or posterior tibial artery by doppler probe
ABI and Total Mortalty
(ABI Collaboration, JAMA 2008)
Coronary Calcium and Atherosclerosis:
Pathology Evidence
Coronary calcium invariably indicates the presence of atherosclerosis, but atherosclerotic lesions do not always contain calcium (1-3).
Calcium deposition may occur early in life, as early as the second decade, and in lesions that are not advanced (4-5).
Correlates with plaque burden; highly sensitive for angiographic disease
1) Wexler et al., Circ 1996; 94: 1175-92, 2) Blankenhorn and Stern, Am J Roentgenol 1959;
81: 772-7, 3) Blankenhorn and Stern, Am J Med Sci 1961; 42: 1-49, 4) Stary, Eur Heart J
1990; 11(suppl E): 3-19, 5) Stary, Arteriosclerosis 1989; 9 (suppl I): 19-32.
Cumulative Incidence of Any Coronary
Event: MESA Study
(Detrano et al., NEJM 2008)
Annual CHD Event Rates (in %) by Calcium Score Events by
CAC Categories in Subjects with DM, MetS, or Neither Disease
(Malik and Wong et al., Diabetes Care 2011)
Coronary Heart Disease
4
Annual
CHD
Event
Rate
4
3.5
3
2.5
2
1.5
1
0.5
0
0
0.4
0.1
0.2
0.4
0.8
1.5
1.3
2.1
1.9
2.2
3.5
DM
MetS
Neither MetS/DM
1-99
100-399
400+
Coronary Artery Calcium Score
ACCF/AHA 2010 Guideline: CAC Scoring for CV risk assessment in asymptomatic adults aged 40 and over with diabetes (Class IIa-B)
Can Screening for Atherosclerosis Identify Those Most
Likely to Benefit from Lipid-Lowering Therapy?
2010 ACC/AHA Greenland et al. Risk Assessment
ACC/AHA 2013 Guideline: IIb-B
“..Assessing CAC is likely to be the most useful of the current approaches to improving risk assessment among individuals found to be at intermediate risk after formal risk assessment”
Intermediate Risk
MESA Subjects
(n=1330)
C-statistics:
FRS alone 0.623
FRS+CAC 0.784 (p<0.001)
FRS+CIMT 0.652 (p=0.01)
FRS+FMD 0.639 (p=0.06)
FRS+CRP 0.640 (p=0.03)
FRS+FamHx 0.675
(p=0.001)
FRS+ABI 0.650 (p=0.01)
Yeboah J et al, JAMA 2012
d d
Lifestyle management remains the cornerstone for reducing cardiovascular disease risk including achieving and maintaining optimal risk factor levels
Critical Question: Among adults, what is the effect of dietary patterns and/or macronutrient composition on CVD risk factors, when compared to no treatment or to other types of interventions?
Advise adults who would benefit from
LDL-C or BP lowering* to:
I IIa IIb III Consume a dietary pattern that emphasizes intake of vegetables, fruits, and whole grains; includes low-fat dairy products, poultry, fish, legumes, nontropical vegetable oils and nuts; and limits intake of sweets, sugar-sweetened beverages, and red meats.
•
Adapt this dietary pattern to appropriate calorie requirements, personal and cultural food preferences, and nutrition therapy for other medical conditions (including diabetes).
•
Achieve this pattern by following plans such as the
DASH dietary pattern, the U.S. Department of
Agriculture (USDA) Food Pattern, or the AHA Diet.
*Refer to 2013 Blood Cholesterol Guideline for guidance on who would benefit from
LDL-C lowering.
Physical Activity
I IIa IIb III Lipids:
In general, advise adults to engage in aerobic physical activity to reduce LDL-C and non –HDL-C: 3 to 4 sessions a week, lasting on average 40 minutes per session, and involving moderate- to vigorousintensity physical activity.
I IIa IIb III BP:
In general, advise adults to engage in aerobic physical activity to lower BP: 3 to 4 sessions a week, lasting on average 40 minutes per session, and involving moderate- to vigorous-intensity physical activity.
Cardiac Rehabilitation:
Benefits Following a Myocardial Infarction (MI)
Effect of cardiac rehabilitation in randomized controlled trials following a MI
1.5
1.15
1 *
0.76
*
0.75
* *
0.5
0
All Cause Death CV Mortality Nonfatal Recurrence
Cardiac rehabilitation reduces CV events after a MI
*p<0.0125
CV=Cardiovascular
Oldridge NB et al. JAMA 1988;260:945-950
……even modest weight loss (3-5% of body weight) can result in clinically meaningful benefits for triglycerides, blood glucose, glycated hemoglobin, and development of diabetes (type 2)….
. Advise overweight and obese individuals who would benefit from weight loss to participate for ≥6 months in a comprehensive lifestyle program adhering to a lower-calorie diet and in increasing physical activity
Prescribe on-site, highintensity (i.e., ≥14 sessions in 6 months) comprehensive weight loss interventions provided in individual or group sessions by a trained interventionist (dietitian, exercise physiologist, etc.)
What’s New in the Cholesterol Guideline?
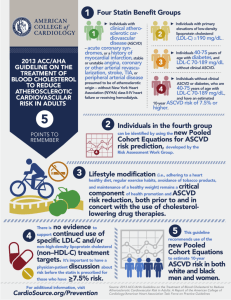
1) Focus on ASCVD reduction: 4 Statin Benefit Groups
2) New Perspective on LDL-C and/or Non-HDL-C Treatment Goals
3) Emphasis on the Clinician-Patient Risk Discussion
4) Global Risk Assessment for Primary Prevention
5) Safety Recommendations
Critical questions addressed the evidence for LDL-C and non-HDL-C goals primary and secondary prevention and the evidence for reduction in
ASCVD events in relation to cholesterol lowering drugs available.
What’s Similar to ATP 3?
Emphasis on lifestyle measures as crucial
Focus on treatment of LDL cholesterol
(LDL-C)
Greatest intensity of treatment for patients at highest risk
Preference for statins over other medications lowering LDL-C (although this is greater in the new ACC/AHA Guideline)
4 Statin Benefit Groups
Clinical ASCVD*
LDL –C >190 mg/dL, Age >21 years
Primary prevention – Diabetes: Age 40-75 years, LDL–C
70-189 mg/dL
Primary prevention - No Diabetes
† : ≥7.5%‡ 10-year
ASCVD risk, Age 40-75 years, LDL –C 70-189 mg/dL,
*Atherosclerotic cardiovascular disease
† Requires risk discussion between clinician and patient before statin initiation.
‡
Statin therapy may be considered if risk decision is uncertain after use of ASCVD risk calculator.
These identified statin benefit groups to not imply that other groups
(e.g., those outside the specified age ranges) will not benefit and should not be treated
Individuals Not in a Statin Benefit Group
In those for whom a risk decision is uncertain:
These factors may inform clinical decision making:
• Family history of premature ASCVD
• Elevated lifetime risk of ASCVD
• LDL–C ≥160 mg/dL
• hs-CRP ≥2.0 mg/L
• Coronary artery calcium (CAC) score
≥300 Agaston units
• Ankle brachial index (ABI)<0.9
Their use still requires discussion between clinician and patient
Clinician - Patient Risk Discussion
Before Statin Rx Especially Primary
✔
Estimate 10 yr
Prevention
✔
Review potential for benefit
ASCVD Risk (when no CVD) from heart-healthy lifestyle /
Review other risk factors consider referral to dietitian and
& risk factor control exercise physiologist
✔
Review potential for
- benefit from statins and potential for adverse effects & drug-drug interactions
✔ Patient Preferences
* Factors if risk decision uncertain :
LDLC ≥ 160, family hx premature ASCVD , increased lifetime risk, hsCRP≥ 2, CAC score ≥ 300 or 75 th % , ABI < 0.9
Summary of Statin Initiation Recommendations to
Reduce ASCVD Risk (Revised Figure)
Summary of Statin Initiation Recommendations to
Reduce ASCVD Risk (Revised Figure)
That’s what it says “one tablespoon full 30 times a day”
Intensity of Statin Therapy
High- Moderate- and Low-Intensity Statin Therapy (Used in the RCTs reviewed by the Expert Panel)*
High-Intensity Statin Therapy
Daily dose lowers LDL-C on average, by approximately ≥50%
Moderate-Intensity Statin
Therapy
Daily dose lowers
LDL-C on average, by approximately 30% to <50%
Low-Intensity Statin
Therapy
Daily dose lowers LDL-C on average, by <30%
Atorvastatin (40†)-80 mg
Rosuvasatin 20 (40) mg
Atorvastatin 10 (20) mg
Rosuvastatin (5) 10 mg
Simvastatin 2040 mg‡
Pravstatin 40 (80) mg
Lovastatin 40 mg
Fluvastatin XL 80 mg
Fluvastatin 40 mg bid
Pitavastatin 2-4 mg
Simvastatin 10 mg
Pravastatin 10-20 mg
Lovastatin 20 mg
Fluvastatin 20-40 mg
Pitavastatin 1 mg
*Individual responses to statin therapy varied in the RCTs and should be expected to vary in clinical practice.
There might be a biologic basis for a less-than-average response.
†Evidence from 1 RCT only: down-titration if unable to tolerate atorvastatin 80 mg in IDEAL (Pedersen et al).
‡Although simvastatin 80 mg was evaluated in RCTs, initiation of simvastatin 80 mg or titration to 80 mg is not recommended by the FDA due to the increased risk of myopathy, including rhabdomyolysis.
Targets?
LDL cholesterol and benefit in clinical trials
Is lower better ?
30
4S - Placebo
25
Rx - Statin therapy
PRA – pravastatin
ATV - atorvastatin
Secondary Prevention
4S - Rx
20
15
10
5
80
(2.1)
100
(2.6)
120
(3.1)
140
(3.6)
LDL-C achieved mg/dL (mmol/L)
LIPID - Placebo
LIPID - Rx
TNT
HPS - Rx
TNT – ATV80
CARE - Rx
TNT – ATV10
PROVE-IT - PRA
PROVE-IT – ATV
CARE - Placebo
HPS - Placebo
AFCAPS - Placebo
Primary Prevention
WOSCOPS – Placebo
AFCAPS - Rx
6
WOSCOPS - Rx
JUPITER
ASCOT - Placebo
ASCOT - Rx
0
40
(1.0)
60
(1.6)
160
(4.1)
180
(4.7)
200
(5.2)
Adapted from Rosensen RS. Exp Opin Emerg Drugs 2004;9(2):269-279
LaRosa JC et al. N Engl J Med 2005;352:e-version
New Perspective on LDL –C & Non-HDL–C Goals
• Lack of RCT evidence to support titration of drug therapy to specific LDL –C and/or non-HDL–C goals
• Strong evidence that appropriate intensity of statin therapy should be used to reduce ASCVD risk in those most likely to benefit
• Quantitative comparison of statin benefits with statin risk ( evaluation of net clinical benefit )
• Nonstatin therapies – did not provide
ASCVD risk reduction benefits or safety profiles comparable to statin therapy
TNT: Primary Efficacy Outcome Measure: Major
Cardiovascular Events*
0.15
Atorvastatin 10 mg
Atorvastatin 80 mg
0.10
Mean LDL-C level = 101 mg/dL
Relative risk reduction
22%
0.05
Mean LDL-C level = 77 mg/dL
HR=0.78 (95% CI 0.69, 0.89); P<.001
0
0 1
Time (Years)
* CHD death, nonfatal non
–procedure-related MI, resuscitated cardiac arrest, fatal or nonfatal stroke.
2 3 4 5 6
LaRosa et al. N Engl J Med.
2005;352:1425-1430.
CTT Meta-analysis 26 Trials
Proportional effects on MAJOR VASCULAR EVENTS per mmol/L LDL-C reduction, by baseline LDL-C
Relative risk (CI) per mmol/L LDL-C reduction
No. of events (% pa)
Statin/more Control/less
More vs less statin
<
2.0
2,<2.5
2.5,<3.0
3,<3.5
3.5
Total
Statin vs control
<
2.0
2,<2.5
2.5,<3.0
3,<3.5
3.5
Total
All trials
<
2.0
2,<2.5
2.5,<3.0
3,<3.5
3.5
Total
99% or
704 (17.9%)
1189 (18.4%)
1065 (20.1%)
517 (20.4%)
303 (23.9%)
3837 (19.4%)
206 (9.0%)
339 (7.7%)
801 (8.2%)
1490 (10.8%)
4205 (12.6%)
7136 (11.0%)
910 (14.7%)
1528 (14.0%)
1866 (12.4%)
2007 (12.3%)
4508 (13.0%)
10973 (13.0%)
95% CI
795 (20.2%)
1317 (20.8%)
1203 (22.2%)
633 (25.8%)
398 (31.2%)
4416 (22.3%)
217 (9.7%)
412 (9.1%)
1022 (10.5%)
1821 (13.3%)
5338 (15.9%)
8934 (13.8%)
1012 (16.4%)
1729 (15.9%)
2225 (14.7%)
2454 (15.2%)
5736 (16.5%)
13350 (15.8%)
0.5
0.75
Statin/more better
1
0.71 (0.52 - 0.98)
0.77 (0.64 - 0.94)
0.81 (0.67 - 0.97)
0.61 (0.46 - 0.81)
0.64 (0.47 - 0.86)
0.72 (0.66 - 0.78)
0.87 (0.60 - 1.28)
0.77 (0.62 - 0.97)
0.76 (0.67 - 0.86)
0.77 (0.71 - 0.84)
0.80 (0.77 - 0.84)
0.79 (0.77 - 0.81)
0.78 (0.61 - 0.99)
0.77 (0.67 - 0.89)
0.77 (0.70 - 0.85)
0.76 (0.70 - 0.82)
0.80 (0.76 - 0.83)
0.78 (0.76 - 0.80)
1.25
Control/less better
Primary prevention 5 to <10% 5-year major CVD risk
Per 1 mmol reduction LDL-C with a statin*
• Significantly greater 34% reduction in relative risk of major CVD than higher risk groups
• 17% reduction in total mortality
*1 mmol/L (39 mg/dl) LDL-C reduction was the average in the primary prevention RCTs excluding JUPITER
CTT Collaborators. Lancet 2012; 380: 581-590
Primary Prevention Statin Therapy
Thresholds for initiating statin therapy derived from 3 exclusively primary prevention RCTs
Placebo group 10 yr event rates:
JUPITER – 7.6%; MEGA 5.1%;
AFCAPS-TEXCAPS 6.9%
Guideline Panel’s recommendation:
As a matter of caution, to avoid over-treating, the
Panel identified those with risk ≥7.5% as a group in which statins provide benefit.
Statins and Diabetes Risk
1) Meta-analysis of 170,255 patients from 76 randomized clinical trials show overall a 9% increased risk for development of incident diabetes among statin users (Sattar, Lancet 2010)
2) Increased risk of diabetes only seen in those with multiple risk factors; obesity, elevated triglycerides, glucose, and hypertension are more strongly related than statins to development of diabetes
3) Many persons on statins have pre-diabetes to begin with; statins may accelerate the progression to diabetes, but on average diabetes occurs 5 weeks later in those on placebo.
4) For every case of diabetes associated with statin use, approximately 5-9 cardiovascular events are prevented
5) Most experts contend the benefits outweigh the risks regarding increases in glucose / A1c.
A moderate intensity statin shows net clinical benefit down to a
10-year ASCVD risk of <5% where NNT is 60-<100 and
NNT=100
A 7.5% 10-year ASCVD threshold provides net clinical benefit from NNT of 30 vs. NNH of 33.
Monitoring Statin Therapy: Lipid
Measurements Still Recommended
I IIa IIb III
Adherence to medication and lifestyle, therapeutic response to statin therapy, and safety should be regularly assessed. This should also include a fasting lipid panel performed within 4 to
12 weeks after initiation or dose adjustment, and every 3 to 12 months thereafter. Other safety measurements should be measured as clinically indicated.
Insufficient Response to Statin Therapy
I IIa IIb III
In individuals who have a less-thananticipated therapeutic response (>=50% on a high intensity statin or 30-<50% on a moderate intensity statin) or are intolerant of the recommended intensity of statin therapy, the following should be performed:
Reinforce medication adherence.
Reinforce adherence to intensive lifestyle changes.
Exclude secondary causes of hyperlipidemia .
Insufficient Response to Statin
Therapy May Warrant Non-Statin Drug
In individuals at higher ASCVD risk receiving the maximum tolerated intensity of statin therapy who continue to have a less-than-anticipated therapeutic response, addition of a nonstatin cholesterol-lowering drug(s) (ideally with proven efficacy from clinical trials) may be considered if the ASCVD risk-reduction benefits outweigh the potential for adverse effects.
Non-Statin Therapies
1) Ezetimibe –IMPROVE-IT showed additional risk reduction
2) Bile Acid Resins
3) Niacin (no benefit from AIM-HIGH and HPS2 TRIVE)
4) Fibrates (Fenofibrate – no overall benefit from ACCORD
Lipid Trial)
5) Therapies for HoFH (Lomitapide, Mipomersin)
Emerging Therapies in Development
1) CETP Inhibitors (Anacetrapib and Evacetrapib)
2) PCSK9 Inhibitors
Improve-IT Study Design
Patients stabilized post ACS ≤ 10 days:
LDL-C 50 –125*mg/dL (or 50–100**mg/dL if prior lipid-lowering Rx)
*3.2mM
**2.6mM
N=18,144 Standard Medical & Interventional Therapy
Simvastatin
40 mg
Uptitrated to
Simva 80 mg if LDL-C > 79
(adapted per
FDA label 2011)
Ezetimibe / Simvastatin
10 / 40 mg
Follow-up Visit Day 30, every 4 months
90% power to detect
~9% difference
Duration: Minimum 2 ½-year follow-up (at least 5250 events)
Primary Endpoint: CV death, MI, hospital admission for UA, coronary revascularization (≥ 30 days after randomization), or stroke
Cannon CP AHJ 2008;156:826-32; Califf RM NEJM 2009;361:712-7; Blazing MA AHJ 2014;168:205-12
LDL-C and Lipid Changes
1 Yr Mean
Simva
EZ/Simva
Δ in mg/dL
LDL-C
69.9
53.2
-16.7
TC
145.1
125.8
-19.3
TG
137.1
120.4
-16.7
HDL
48.1
48.7
+0.6
hsCRP
3.8
3.3
-0.5
Median Time avg
69.5 vs. 53.7 mg/dL
Primary Endpoint — ITT
Cardiovascular death, MI, documented unstable angina requiring rehospitalization, coronary revascularization (≥30 days), or stroke
HR 0.936 CI (0.887, 0.988) p=0.016
Simva — 34.7%
2742 events
NNT= 50
EZ/Simva — 32.7%
2572 events
6% relative risk reduction, but 2% absolute risk reduction
7-year event rates
IMPROVE-IT: How Much Really is the IMPROVEment?
Expert Analysis Dec 18, 2014
Nathan D. Wong, PHD, F.A.C.C.
; Michael Blaha, MD, MPH
http://www.acc.org/latest-in-cardiology/articles
1) Provides the first evidence from a large-scale trial that additional lipid treatment beyond a statin provides further
CVD event reduction in high-risk ACS patients
2) Involved exclusively high-risk persons with a recent
ACS so may not apply to lower risk persons….implications of this trial beyond the study population should be generalized with caution.
3) A relative risk reduction of 6% (hazard ratio of 0.94) is not impressive, but the high baseline risk resulted in a reasonable NNT of 50; such added therapy is a reasonable option in high-risk ACS subjects
4) Not clear if patients starting at lower LDL-C levels
(eg from a high intensity statin) would derive similar benefit
Management of Muscle Symptoms on Statin Therapy
• It is reasonable to evaluate and treat muscle symptoms including pain, cramping, weakness, or fatigue in statin-treated patients according to the management algorithm
• To avoid unnecessary discontinuation of statins, obtain a history of prior or current muscle symptoms to establish a baseline before initiating statin therapy
Management of Muscle Symptoms on Statin Therapy
(con’t)
If unexplained severe muscle symptoms or fatigue develop during statin therapy:
• Promptly discontinue the statin
• Address possibility of rhabdomyolysis with:
CK
Creatinine
urine analysis for myoglobinuria
Statin Assignment using the 2013 ACC/AHA Cholesterol
Guideline Identifies More Persons with Significant CAD versus the 2001 NCEP ATP III Guideline (Johnson and
Dowe, JACC 2014)
1) 3,076 subjects studied who had CCTA plaque measured
2) From the NCEP ATP III Guideline based on
LDL-C thresholds for initiating statin use , 59% of subjects with >=50% left main stenosis and 40% with
>=50% stenosis of other branches would not have been treated .
3) Comparable numbers from the 2013 ACC/AHA
Cholesterol Guideline were 19% and 10%
4) The use of targets in the NCEP ATP III caused many with significant stenosis not to be identified for treatment.
Question 1
A 48 year old white woman with diabetes and microalbuminuria has a lipid panel
TC 197; TG 300; HDL-C 42; LDL-C 95
Her BMI is 34; weight up 4 lbs
Systolic BP 134. A1c 8.4%. Non-smoker
In addition to adherence to an optimal lifestyle what is the next step to reduce her risk for heart attack and stroke?
Question 1
A. Moderate intensity statin; no followup lipids needed
B. No need to use the risk estimator; she has diabetes
C. Moderate intensity statin, regular lipid follow-ups to determine adherence and response to therapy.
.
D. US guidelines don’t allow a non-statin to be added
E. Time spent on adherence not useful; missing 25% of doses per month not likely to affect potential for benefit
Question 1
Answer C.
She’s in a statin “benefit” group.
Why not an LDL-C or non HDL-C? The evidence points to proper intensity of therapy
Why not high intensity statin therapy?
She has only a 3.1% 10 year risk. Had she been a smoker, her risk would be 9.3%. Then appropriate measures to aid tobacco cessation as well as a more intensive dose of statin if tolerated would have been appropriate
.
Her A1c is elevated. Can a bile acid sequestrant be used? Yes
Non-statins can be used in any of the high risk groups if it is felt that optimal adherence to statin therapy gives a less than anticipated response.
Question 2
79 yo man had acute MI & received a stent to his LAD.
Home on atorvastatin 40 mg daily &usual 2 o prevention Rx
TC 150; TG 150; HDL-C 45; LDL-C 75; Non HDL-C 105
Which of these is a true statement?
A. Getting under 70 will provide an outcomes difference for him
B. Statin dose should be atorva 80 mg/day or rosuva 40 mg/d
C. Guidelines recommend lipids monitored periodically to assess adherence to statin/lifestyle; also for safety issues
D. No need to have lipids checked. It's a set it and go on strategy
Question 2
Answer is C. Since he is over 75, use of 40 mg atorvastatin would be reasonable, even though there is data to support high dose statin after age 75.
The issue of competing comorbidities, multiple medications, and greater potential for side-effects with a high-intensity statin affect the decision here. What is crucial is to monitor lipids periodically and discuss safety issues.
After checking lipids 4-12 weeks after the statin is started, they should be checked 3-12 months regularly with the interval dependent on how likely the patient is to have problems with adherence or safety issues
Question 3
60 yo overweight white man with diabetes and hypertension for past 10 years on metformin. He is a non-smoker. He comes in
25 minutes into an acute ST elevation anterior MI and has a PCI with a drug eluting stent placed in his LAD. His lipid panel on admission shows: TC 168 TG 220 HDL 30 LDL 94 His non
HDL-C was 138. He was on simvastatin 10 mg daily.
Post MI, he is given aspirin, clopidogrel, lisinopril, and a long acting beta blocker. He is discharged on atorvastatin 20 mg daily. Four weeks later, his LDL-C has fallen to 70 mg/dl.
He has met LDL-C targets of <100 and 70 or less for primary and secondary prevention. Is his care optimal?
Question 3
An example of how fixed “targets” can give less than optimal Rx
Although he attained the LDL-C target of <100 mg/dl, he needed a higher intensity statin. When he had an LDL-C of 94, this 60 yo man with diabetes and hypertension should have been on a moderate intensity statin.
After an acute coronary event, he now merited as a first consideration, a high intensity statin. The evidence based choice is atorvastatin 80 mg daily. In the PROVE-IT trial, those with acute MI randomized to a high intensity statin (atorvastatin 80 mg/d)) had improvement in outcomes as compared to those on a moderate intensity statin (pravastatin 40 mg/d)
Cannon C et al N Engl J Med 2004;350:1495-504.
FH is more common than many well known genetic diseases
• 1:300 – 1:500 worldwide
• Autosomal dominant
• 620,000 FH patients in US
• Average LDL is 220 mg/dl
• 20 fold increased risk of coronary heart disease
• Causes 20% of MIs before age 45 and 5% before age
60
Hopkins et al. J. Clinical Lipidology. 2011
Goldberg et al. J. Clinical Lipidology. 2011
FH exposes people to very high cholesterol from birth, thus reaching a threshold for CHD earlier in life
Cumulative exposure (cholesterol yrs) by age:
FH vs unaffected (healthy) individuals
10
Homozygous FH Heterozygous
FH
Healthy individuals
5
Threshold for
CHD: reached by age 40 for those with HeFH and age 20 for HoFH,
> 70 yrs in healthy individuals
0
1 20 40
Age (years)
60 80
Adapted from Horton et al. J Lipid Res.
2009;50:S172-S177
Da Vinci’s Mona Lisa: FH ?
Xanthelasma ??? ???
Leonardo da Vinci, in addition to his art is famed for his remarkable efforts in scientific work including investigations of atherosclerosis.
Indeed, careful clinical examination of the Mona Lisa reveals a yellow irregular leather-like spot at the inner end of the left upper eyelid and a well-defined swelling on the right hand beneath the index finger, probably signifying the first case of this disease.
www.powerofthegene.com
Died in 1516 @ age 37 suddenly , cause unknown
FH is a “Winnable battle”
Nordestgaard B G et al. Eur Heart J 2013;eurheartj.eht273
The FHF national patient registry
“What’s measured improves”
Peter F. Druker
Long term goal is to have
90% of FH patients
DIAGNOSED and TREATED
LDL Apheresis
FDA approved
Dextran sulfate adsorption (DSA)
Heparin-mediated extracorporeal precipitation
(HELP)
60-80% LDL reduction
Largest observational study - 72% event reduction
Small RCTs- regression
Potential vascular benefits
Endothelial function
Reduction in inflammatory markers (CRP, VCAM, MCP-1)
Mehta P, Baer J, Nell C, Sperling LS. Current Treatment Options in CV Medicine 2009,11:279-288
LDL apheresis
Heparin Pump
Re-Priming
Solution
Regeneration
Solution
Regeneration
Pump
Blood Pump
Plasma
Separator
Plasma Pump
LIPOSORBER
®
Column s
Blood Return Plasma Line
Waste Line
Time averaged LDL
LDL-C
Diet Therapy
Diet & Drug Therapy
LIPOSORBER
®
Treatment
Pre
Time Average
Post
Time
New Therapies to Lower LDL-C
Beyond Underlying Statin Therapy (courtesy P. Wilson)
Drug
Delivery
Action
Lomitapide
Oral
(pill)
MTP inhibitor
Mipomersen
Injection
(subq every week)
Anti-sense ApoB oligonucleotide
Proprotein convertase subtilisin/kexin type9
PCSK9 mAb
Injection
(subq every 4 weeks)
Antibodies to PCSK9
LDL-C
Effect in statin Rx
HoFH
40-50%
Availability REMS*
25%
50-70%
REMS* In research trials
Monitor
Concerns
Liver Ftn Tests Liver Ftn Tests
Hepatotoxicity
Drug interactions
Hepatotoxicity
Site injection rxn
Flu symptoms
Antibody development
Cost $300,000/pt/yr $300,000/pt/yr Not available
* REMS= Risk Evaluation and Mitigation Strategies
The Role of PCSK9 in the Regulation of LDL Receptor Expression
For illustration purposes only
Impact of PCSK9 mAb on LDL Receptor Expression
For illustration purposes only
LAPLACE-2: LDL-C Response at Mean of Weeks
10 and 12
Evolocumab Q2W & QM: 63 to 75% reductions in LDL-C versus placebo
Ezetimibe: 17 to 24% reductions in LDL-C versus placebo
Atorvastatin
80 mg
Rosuvastatin
40 mg
Atorvastatin
10 mg
Rosuvastatin
5 mg
Placebo Q2W
Placebo QM
Ezetimibe QD + Placebo Q2W
Ezetimibe QD + Placebo QM
All treatment differences versus placebo and ezetimibe were statistically significant (P<0.001).
No notable differences were observed between the mean of weeks 10 and 12 and week 12 alone.
LDL-C, low-density lipoprotein cholesterol; Q2W, biweekly; QM, monthly. Vertical lines represent 95% CIs.
Evolocumab Q2W
Evolocumab QM
Simvastatin
40 mg
82
GAUSS-2 in Statin Intolerant Pts:
Evolocumab Mean LDL-C % Change
Treatment Difference vs Ezetimibe
Average at weeks 10 and 12
At week 12
-37%
-38%
P<0.001
0
-18%
-20
-40
-60
-80
-56%
BL Day 1 Week 2 Week 4 Week 6 Week 8 Week 10 Week 12
Study drug administration
Biweekly SC
1: Ezetimibe (N = 51)
Study Week
2: Evolocumab 140 mg Q2W (N = 103)
BL, baseline. Vertical lines represent the standard error around the mean. Plot is based on observed data with no imputation for missing values. P values are multiplicity adjusted.
83
1) High risk subjects for cardiovascular disease whose LDL-C was suboptimal despite maximally tolerated statin and/or other lipid-lowering therapy.
2) Follow-up: 78 weeks. Subjects (N=2,341) with LDL-C >70 mg/dL, 18% of whom had heterozygous familial hypercholesterolemia (HeFH)
3) Randomized to alirocumab 150 mg Q2W SC or matching placebo.
4) Mean baseline LDL-C was 123 mg/dL and 122 mg/dL, respectively. The primary endpoint was percent LDL-C reduction from baseline at 24 weeks.
28-36% of Guideline-Recommended Patients Not on Statins: ACC NCDR
PINNACLE Registry (Maddox et al., JACC 2014)
Kaiser Permanente (KP) is utilizing the 2013 ACC/AHA
Cardiovascular Risk and Cholesterol Guidelines in an effort to prevent more heart attacks and strokes
KP is pivoting from a focus on LDL <100 to an emphasis on statin use rates in members of the four statin benefit groups outlined in the new guidelines
August 2014 - KP Southern California (KPSC) region made available to providers at the point of care the ACC/AHA 10-year ASCVD risk estimator
(A-Risk), with variables filled in automatically – A summary page of their electronic health record shows for example: "ASCVD risk 17%, start atorvastatin 40 mg daily…” also on the summary page is the last lipid panel result and date, and a medication list with dates of last refill
Pay for Performance? What should we measure?
On treatment LDL ? Should be be penalized if our patient does not reach LDL-C<100?
% reduction of LDL from baseline?
Appropriate intensity of statin used?
Meaningful discussion between clinician & patient
(informed clinical decision making)………
NCQA will retire the measure focusing on LDL-C treatment targets in persons with ASCVD and align with the latest guidelines focusing on statin therapy in patients with established ASCVD and not on
LDL-C control or screening.
Synopsis of Recommendations
1. Encourage adherence to a healthy lifestyle (provide support for medical nutrition therapy and physical activity counseling)
2. Statin therapy recommended for adult groups demonstrated to benefit
3. Statins have an acceptable margin of safety when used in properly selected individuals and appropriately monitored
4. Engage in a clinician-patient discussion before initiating statin therapy – especially for primary prevention in patients with lower ASCVD risk – includes discussion of global risk reduction, benefits and risks of treatment, and patient preferences
Stone NJ, et al. Ann Int Med. 2014 [epub ahead of print]
Synopsis of Recommendations
5. Use the newly developed pooled cohort equations for estimation 10-year ASCVD risk
6. Initiate proper intensity of statin therapy
7. Evidence is inadequate to support treatment to specific LDL-C or non-HDL-C goals
8. Regularly monitor patients for adherence to lifestyle and statin therapy
Stone NJ, et al. Ann Int Med. 2014 [epub ahead of print]
“Evidence-Based” Not “Evidence-
Bound ”
Three Key Dimensions
Scientific evidence
Patient preference
Clinical
Judgment
“Clinical practice guidelines such as these should not take the place of sound clinical judgment. These guidelines should enable a discussion between a patient and their health care provider about the best way to prevent a heart attack or stroke, based on the patient’s personal health profile and their preferences”
John Gordon Harold, MD, MACC
ACC Past President
Thank You!
UCI Heart Disease
Prevention Program www.heart.uci.edu
American Society for Preventive
Cardiology: www.aspconline.org
Microsomal Triglyceride Transfer Protein
(MTP)
•
•
MTP is an intracellular lipid-transfer protein found in the lumen of the endoplasmic reticulum (ER) responsible for binding and shuttling individual lipid molecules between membranes 1
Normal concentrations and function of MTP are necessary for the proper assembly and secretion of apo B-containing lipoproteins in the liver and intestines 2
Liver Cell
Cytoplasm
Lumen
ER
MTP
Intestinal
Epithelial Cell
Cytoplasm
Lumen
ER
MTP
1. Hussain M, et al. Journal of Lipid Research . 2003:44;22-32.
2. Liao W, et al. Journal of Lipid Research . 2003:44;978-985.
©2013 Aegerion Pharmaceuticals, Inc.
MTP Inhibitors – Mechanism of Action
MTP inhibitors 1,2
Prevent the assembly of apo B-containing lipoproteins in hepatocytes and enterocytes. This inhibits the synthesis of
VLDL and chylomicrons.
The inhibition of the synthesis of VLDL and intestinal chylomicron secretion lowers plasma lipids.
1. Wetterau JR, et al. Science . 1998:282;751-754.
2. Hussain MM, et al. Nutrition Metabolism . 2012:9;14.
©2013 Aegerion Pharmaceuticals, Inc.
Phase 2 Study Design
•
•
•
•
Single arm, open label study
16-week treatment duration - lomitapide as monotherapy (no background lipid-lowering therapies)
Dose escalated from a low starting dose (mean doses at each of the four titration steps were: 2.0, 6.7, 20.1, and 67.0 mg/day)
Low-fat diet (prescribed diet of <10% energy from fat)
6 Patients
Lomitapide
0.03 mg/kg
Lomitapide
0.1 mg/kg
Lomitapide
0.3 mg/kg
Lomitapide
1.0 mg/kg
Washout
4 weeks 4 weeks 4 weeks 4 weeks
Key Inclusion Criteria:
- Patients aged 18-40 yrs.
- Clinical Diagnosis of HoFH and one of the following
- documented functional mutation in both LDL receptor alleles
OR
- skin fibroblast LDL receptor activity <20% normal
OR
- TC >500 mg/dl + TGs <300 mg/dl + both parents with TC >250mg/dl
Cuchel, M. et al. NEJM 2007; 356:148-56.
4 weeks
©2013 Aegerion Pharmaceuticals, Inc.
Phase 2 HoFH Study: Efficacy
51% Reduction in LDL-C
Mean
Dose (mg): 2.0
6.7
20.1
51%
Reduction p<0.001
67.0
Cuchel, M. et al. NEJM 2007; 356:148-56.
©2013 Aegerion Pharmaceuticals, Inc.
Antisense Oligonucleotides and
Apo B Synthesis Inhibition
Brautbar A and Ballantyne CM. Nat Rev
Cardio 2011;8:253.
Mipomersen and LDL Lowering in
Homozygous FH
n=17 n=34
Baseline LDL-C: 405 mg/dl
200mg SC/Q week
Raal F. Lancet
2010;375:998-1006.