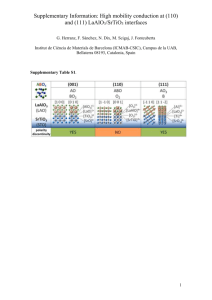
THE SOL-GEL SYNTHESIS AND CHARACTERIZATION OF STRONTIUM IONS
advertisement

THE SOL-GEL SYNTHESIS AND CHARACTERIZATION OF STRONTIUM TITANATE DOPED WITH Pr AND Al IONS MUTIA SUHAIBAH BINTI ABDULLAH A thesis submitted in fulfillment of the requirement for the award of the degree of Master of Science (Physics) Faculty of Science Universiti Teknologi Malaysia NOVEMBER 2010 iii iv ACKNOWLEDGEMENTS I would like to present this thesis expressing the deepest and sincere gratitude before Him for the inspiration and guidance. My utmost gratitude goes to my supervisor, Prof. Dr Rosli Hussin for allowing me to join his team, for his expertise, kindness, patience and most of all, for introducing me to phosphor. I believe that one of the main gains of this 2-years program was working with Dr. Rosli and gaining his trust and knowledge. My thanks and appreciation goes to my group members Musdalilah Ahmad Salim, Siti Aishah Fuzi, Suhailah Abdullah, Nur Shahira Alias, Dayang Nurfazliana, Muhamad Syawal and Safwan Ahmad Pauzi for their continuous support, insightful comments, friendship and pleasant discussions and also for all the fun we have had in the last two years. Sincere gratitude is expressed to all the lab assistants especially En Jaafar for technical and experimental assistance especially the maintenance of the equipment in our lab, En Zainal from Faculty of Mechanical Engineering UTM and Cik Siti Khadijah Mohd Bakhori from N.O.R Laboratory, School of Physic, Universiti Sains Malaysia and for their cheerful help in the running of XRD and RAMAN and PL. I am also extremely grateful to Universiti Teknologi Malaysia generally and Physic department, Faculty of Science especially for providing experiment facilities. I am greatly indebted to my family; My parents, Hj Abdullah bin Daud and Hjh Fatilah Hasni bt Hj Abd Latif for their never-ending love and support in all my efforts, and for giving me the foundation to be who I am, my siblings for their encouragement and prayers, which enabled me to complete this endeavor. Thanks go to the Ministry of Science, Technology and Innovation (MOSTI) for the financial support through National Science Fellowship (NSF) scholarship. I also would like to thank all whose direct and indirect support helped me in completing the thesis in time. v ABSTRACT Strontium Titanate (SrTiO3) doped with Praseodymium (Pr) as an oxide compound phosphor show a potential application for a field emission display (FED). Addition of Aluminum ions (Al3+) has been attracting interest as a sensitizer to improve the luminescent efficiency of phosphors. In this study, the influence of Al3+ as a dopant on the crystallization, surface morphology and luminescent properties of SrTiO3:Pr,Al nanophosphors were investigated. Nanophosphor with the nominal composition of SrTiO3 undoped and doped with Pr3+ and Al3+ were synthesized at relatively low temperature by the sol-gel method. The crystal structures and average grain sizes were examined using x-ray diffraction (XRD) and scanning electron microscopy (SEM). XRD patterns indicated that crystalline SrTiO3 which has been synthesized at calcining temperature of 800⁰C for 2 h present a cubic structure. Moreover, the improvement of single crystalline phase is confirmed by increasing the temperature. SEM micrographs indicate that the addition of Pr3+ and Al3+ influence the texture and morphology of the samples. Nanoparticles samples with various sizes of 25-55 nm were obtained as a function of Pr and Al concentration using Scherrer‟s equation. Fourier Transform Infrared (FTIR) spectra from SrTiO3 doped and undoped are also reported. The effect of doping on the infrared spectral of SrTiO3 structure is clearly shown in the low-frequency regions but overlapped with O-Ti-O bending mode. Raman spectroscopy was employed to investigate the evolution of the cubic phase in the nanocrystals during annealing and doping concentration. By addition of dopants, Raman spectra show formation of secondorder Raman Scattering of SrTiO3. The luminescent properties of SrTiO3:Pr,Al phosphor were investigated by Photoluminescence (PL) spectroscopy. Under 325 nm excitation, SrTiO3:Pr,Al phosphor exhibited a strong red emission, peaking at about 615 nm. The intensity of emission spectra was enhanced by the addition of Al3+ ions. vi ABSTRAK Strontium Titanate (SrTiO3) didop dengan Praseodymium (Pr) sebagai sebatian fosfor oksida mempunyai potensi tinggi dalam penggunaan paparan pancaran medan (FED). Penambahan ion Aluminium (Al) sebagai pemeka bertujuan untuk meningkatkan kecekapan pendarcahaya bahan fosfor. Dalam kajian ini, pengaruh Al3+ sebagai bahan dop ke atas penghabluran, morfologi permukaan dan sifat pendarcahaya nanofosfor SrTiO3: Pr telah dikaji. Nanofosfor dengan komposisi nominal SrTiO3 tidak berdop dan dop dengan Pr3+ and Al3+ telah disintesis pada suhu rendah menggunakan kaedah sol-gel. Struktur hablur dan purata saiz zarah diperiksa menggunakan pembelauan sinar-X (XRD) dan mikroskopi imbasan elektron (SEM). Corak XRD menunjukkan bahawa hablur SrTiO3 yang telah disintesis pada suhu pengalsinan 800⁰C selama 2 jam menghasilkan struktur kubus. Tambahan lagi, penambahbaikan fasa satu hablur disahkan dengan peningkatan suhu. Mikrograf SEM menunjukkan bahawa penambahan Pr3+ dan Al3+ mempengaruhi tekstur dan morfologi sampel. Sampel berzarah nano dengan pelbagai saiz antara 25-55 nm diperolehi dengan kebergantungan pada kepekatan Pr3+ dan Al3+ menggunakan persamaan Scherrer. Spektrum Transformasi Fourier Infra merah (FTIR) untuk SrTiO3 yang berdop dan tidak berdop juga turut dilaporkan. Kesan pendopan pada struktur SrTiO3 dalam spektrum inframerah jelas ditunjukkan dalam kawasan frekuensi rendah tetapi bertindan dengan mod pembengkokan O-Ti-O. Spektroskopi Raman digunakan untuk mengkaji evolusi fasa kiub dalam hablur nano semasa pengalsinan dengan kepekatan bahan dop berubah. Dengan penambahan bahan dop, spektrum Raman menunjukkan pembentukan Sebaran Raman Urutan Kedua. Sifat pendarcahaya fosfor SrTiO3:Pr,Al dikaji menggunakan Spektroskopi Fotopendarcahaya. Di bawah pengujaan 325 nm, fosfor SrTiO3:Pr,Al menunjukkan pancaran merah yang kuat pada puncak 615 nm. Keamatan spektrum meningkat dengan penambahan ion Al3+. vii TABLE OF CONTENTS CHAPTER 1 2 TITLE PAGE DECLARATION ii DEDICATION iii ACKNOWLEDGEMENTS iv ABSTRACT v ABSTRAK vi TABLE OF CONTENTS vii LIST OF TABLES xi LIST OF FIGURES xii LIST OF SYMBOLS AND ABBREVIATIONS xv INTRODUCTION 1.1 General Introduction 1 1.2 Statement of Problem 4 1.3 Objectives of Study 4 1.4 Significance of the Study 5 THEORETICAL BACKGROUND 2.1 Introduction 6 2.2 Luminescence 7 2.2.1 8 2.3 Process that create luminescence Phosphor Material 11 viii 3 2.4 Phosphor based 14 2.5 Titanium Dioxide (TiO2) 14 2.6 Alkaline earth titanate 19 2.7 Phosphor doped rare-earth 21 2.7.1 23 Praseodymium 2.8 Phosphor Synthesis 25 2.9 Sol-gel method 26 2.9.1 Preparation of precursor solutions 27 2.9.2 Hydrolysis 27 2.9.3 Gelation 28 2.9.4 Aging and drying 30 2.9.5 Annealing and porosity control. 31 2.10 X-ray Diffraction (XRD) 32 2.11 Scanning Electron Microscope (SEM) 35 2.12 Raman Studies 36 2.13 Fourier Transform Infrared (FTIR) Spectroscopy 39 2.14 Photoluminescence Studies 42 EXPERIMENTAL AND CHARACTERIZATIONS 3.1 Introduction 44 3.2 Sample Preparation 45 3.3 Experimental Characterization 47 3.3.1 X-ray Diffraction (XRD) studies 48 3.3.2 Scanning Electron Microscope (SEM) 49 3.3.3 Raman Spectroscopy 50 3.3.4 Fourier Transform Infrared (FTIR) 51 Spectroscopy 3.3.5 Photoluminescence Spectroscopy 52 ix 4 RESULTS AND DISCUSSIONS 4.1 Introduction 53 4.2 Structural Studies 54 4.2.1 54 Phase formation 4.2.1.1 The influence of calcined temperature on host structure 54 4.2.1.2 The influence of dopant addition to the host structure 56 4.2.1.3 The effect of dopant concentration 4.3 Grain size and morphology 4.4 Compositional analyses 62 4.5 Infrared (IR) Spectra 63 4.5.1 analysis 58 The influence of calcined temperature on host structure 4.5.2 4.5.3 4.6 4.6.2 63 The influence of dopant addition to the host structure 67 The influence of dopant concentration 69 4.5.3.1 Pr addition 69 4.5.3.2 Al addition 69 Raman Spectra 4.6.1 60 71 The influence of calcined temperature on host structure 71 The influence of dopant addition to the host 75 structure 4.6.3 4.7 The effect of dopant concentration 76 4.6.3.1 Pr addition 76 4.6.3.2 Al addition 77 Photoluminescence Studies 80 4.7.1 The influence of dopant addition 80 4.7.2 The influence of Pr concentration 82 4.7.3 The influence of Al concentration 82 x 5 CONCLUSIONS AND RECOMMENDATIONS 5.1 5.2 REFERENCES Conclusions 87 5.1.1 Structural studies 87 5.1.2 Luminescence studies 88 Recommendations 89 91 xi LIST OF TABLES NO TITLE PAGE 2.1 Luminescence phenomena and the methods of excitation 9 2.2 Phosphor Devices 15 2.3 Typical physical and mechanical properties of titania 16 2.4 Optical properties of titania. 17 2.5 Electronic Configurations of Trivalent Rare-Earth Ions in the 23 Ground State 2.6 List of some commonly used Metal Alkoxides and 29 recommended solvents for solids 3.1 Concentration of praseodymium and aluminum in the prepared 47 samples of strontium titanate phosphor 4.1 Position (cm-1) and Assignment of IR Bands 65 4.2 Raman modes comparison with the literature values 73 xii LIST OF FIGURES NO 2.1 TITLE Marine creatures like jellyfish produce their own light through PAGE 7 phosphorescence 2.2 Possible physical process following absorption of a photon by a 11 molecule 2.3 Properties and applications of Titanium Dioxide 18 2.4 Crystal structure of TiO2 group (a) Rutile (b) Anatase (c) 18 Brookite 2.5 SrTiO3 (perovskite) structure 20 2.6 Structure of SrTiO3 in the cubic phase 20 2.7 Structural units of the cubic SrTiO3crystal sectioned by three 21 different planes shown as shaded: a) the (100) surface contains O2−and Sr2+ ions, b) the (110) surface contains Ti4+, O2−and Sr2+ atoms, c) the (001) surface contains Ti4+ and O2−ions. 2.8 Emission spectrum of Y2O2S:Pr3+ (0.3%) at room temperature 24 2.9 Energy level of Pr3+ ions 25 2.10 Common configurations for an XRD unit 33 2.11 Principle of X-ray Diffraction 33 2.12 A schematic diffractogram showing the presence of two phases 34 (with peaks at different angular positions) originating from a material with small and large crystallites. The broad hump is due to an amorphous phase. 2.13 Principle of Scanning Electron Microscope 36 2.14 Example of SEM images of (a) Materials (b) Insects (c) Cabbage 36 leaf xiii 2.15 Light scattered from a molecules 38 2.16 Schematic representation of a Raman spectrometer 38 2.17 Schematic sketch of the essential features of a Fourier transform 40 infrared (FTIR) spectrometer. 2.18 Major types of vibrational modes a) Stretching vibrations b) 41 Bending vibrations 2.19 An example of an FTIR spectrum 41 2.20 Process of luminescence 43 3.1 Schematic steps involved in the sol-gel process used for the 46 preparation of SrTiO3: Pr, Al 3.2 X-ray Diffractometer (Siemens Diffractometer D5000) at Faculty 48 of Mechanical Engineering, Universiti Teknologi Malaysia, Skudai. 3.3 Scanning Electron Microscopy (JEOL JSM-6701F) at Institute of 49 Ibnu Sina, Universiti Teknologi Malaysia, Skudai. 3.4 Equipment used for Raman spectroscopy at N.O.R laboratory, 50 School of Physics, Universiti Sains Malaysia. 3.5 FTIR spectroscopy at Chemistry Department, Universiti 51 Teknologi Malaysia, Skudai. 4.1 XRD pattern of SrTiO3 at various calcination temperatures 55 4.2 XRD pattern of SrTiO3 at 800⁰C 56 4.3 XRD pattern for (a) SrTiO3 (b) SrTiO3:1mol%Pr (c) 57 SrTiO3:1mol%Pr,1mol%Al 4.4 XRD pattern for various concentration of (a) Pr3+ (b) Al3+ when 59 3+ Pr fixed at 1 mol % 4.5 Illustration of substitution process in SrTiO3 sample as addition 60 of Pr and Al 4.6 Particle size obtained using the Scherrer‟s equation as a function 61 of (a) Pr concentration (b) Al concentration when Pr3+ fixed at 1 mol % 4.7 Scanning electron micrographs of (a) SrTiO3 (b)SrTiO3:1mol%Pr (c) SrTiO3:1mol%Pr,1mol%Al calcined at 800⁰C 62 xiv 4.8 EDAX spectra of (a) SrTiO3 (b) SrTiO3:1mol%Pr (c) 64 SrTiO3:1mol%Pr, 1mol%Al phosphor 4.9 FTIR spectra for undoped SrTiO3 with different calcinations 66 temperatures 4.10 FTIR spectra of (a) undoped SrTiO3 (b) SrTiO3: Pr (c) SrTiO3: 68 Pr, Al phosphor which calcined at 800⁰C 4.11 FTIR spectra of the SrTiO3: xPr with 0 ≤ x ≤ 1 mol % which 70 calcined at 800⁰C 4.12 FTIR spectra of the SrTiO3: 1mol%Pr, yAl with 0 ≤ y ≤ 1 mol% 72 which calcined at 800⁰C 4.13 Raman spectrum of the SrTiO3 samples calcined at 600-800⁰C 74 for 2 h. 4.14 Raman spectra of SrTiO3 sample by addition of dopant which 76 calcined at 800⁰C 4.15 Raman spectra of SrTiO3:xPr with various Pr concentrations 78 (0≤ x ≤1 mol %) which calcined at 800⁰ C 4.16 Raman spectra of SrTiO3:1 mol% Pr, yAl with 0≤ y≤1 mol % 79 which calcined at 800⁰ C 4.17 Photoluminescence (PL) spectra of SrTiO3, SrTiO3:Pr3+ and 81 SrTiO3:Pr, Al 4.18 Energy level of Pr3+ 81 4.19 Photoluminescence spectra of the SrTiO3:xPr (0≤x≤1 mol %) 83 phosphors under 325 nm excitation 4.20 PL spectra of SrTiO3:Pr, yAl (Pr3+:1 mol%) with various molar 84 ratios of Al (0≤y≤1 mol %) under the 325nm laser excitation at room temperature 4.21 Proposed scheme of energy transfer from the defects created around Al3+ levels to the (3P0, 1, 2 , 1I6, 1D2) energy levels of Pr3+. 86 xv LIST OF SYMBOLS AND ABREVIATIONS ZnS Zinc Sulphide TiO2 Titanium Dioxide UV Ultraviolet SrTiO3 Strontium Titanate Pr3+ Praseodymium (III) ions Al 3+ Aluminum (III) ions SEM Scanning Electron Microscopy XRD X-ray Diffraction HCl Hydrochloric Acid TMOS Tetramethoxysilane TEOS Tetraethoxysilane EDAX Energy Dispersive X-ray Analysis TTIP Titanium tetraisopropoxide Pr(NO3)3.6H2O Praseodymium Nitrate Hexahydrate Al(NO3)3.9H2O Aluminium Nitrate Nonahydrate Sr(NO3)2 Strontium Nitrate Wavelength d spacing between the planes D averaged dimension of crystallites FTIR Fourier Transform Infrared ICDD International Centre for Diffraction Data SiO2 Silicon Dioxide TeO2 Tellurite Dioxide P2O5 Phosphorous Dioxide CHAPTER 1 INTRODUCTION 1.1 General Introduction Luminescence is a science related to spectroscopy. First observed in an extract of Ligrium nephiticiem by Monardes in 1565, it took until 1852 to be fully described by Stokes who reported the theoretical basis for the mechanism of absorption (excitation) and emission. Today luminescence, in its varied forms, is one of the fastest growing and most useful analytical techniques in science. Applications can be found in areas as diverse as materials science, environmental science, microelectronics, physics, chemistry, biology, biochemistry, medicine, toxicology, pharmaceuticals, and clinical chemistry. This rapid growth occurred only in the past couple of decades and is principally driven by the unique needs of the life sciences. Luminescence is defined as the generation of light without heat which usually occurs at low temperatures, and is thus a form of cold body radiation. It can be caused by the movement of electrons within a substance from more energetic states to less energetic states. Examples of luminescence in nature that had been observed from ancient times are fireflies, insects, fishes, mushrooms and luminescent bacteria. A luminescent material is often called phosphor, which means „light bearer‟ (Joseph, 2 2001). Phosphor is a substance that exhibits the phenomenon of phosphorescence (sustained glowing after exposure to energized particles such as electrons). Of course we are familiar with phosphor; we meet them everyday. If this should come as a surprise, switch on the fluorescent lamp, relax in front of the television set or take a look at the screen of our computer. All of that are the examples of phosphor application in our daily life. Research on phosphors and their applications requires the use of a number of fields in science and technology. For advanced application, phosphors are widely used in plasma display panels (PDPs), field emission displays (FEDs), luminous paint and safety indicator. The long afterglow phosphors are a special kind of luminescent materials with long persistent phosphorescence lasting for several hours at room temperature. As novel functional materials, these long afterglow phosphors are drawing more and more attention in recent years because of a constantly growing market for their applications. In the early time, Co and Cu doped zinc sulphide (ZnS: Cu, Co) was considered a main kind of phosphorescent materials. However, the material itself is not stable enough during its application. It can only maintain phosphorescence no more than a few hours. Sulfide absorb the moisture from the surrounding environment to form sulfate that causes the destruction of sulfide lattice, and thus the material no longer shows long afterglow (Chang and Mao, 2004). Therefore, it is substituted by the strontium aluminates. In the past decade, new kinds of long persistent phosphors, which overcome the shortcoming of the above mentioned sulfides, were invented. In the mean time, titanate-based phosphors also were increasingly investigated due to the potential interest of these materials. Phosphors based on oxide matrices are attractive host materials for the development of advanced phosphors due to their ease of synthesis and stability. Titanium dioxide (TiO2) could be a possible candidate among oxide compounds which have been known to exhibit an absorption band in the soft ultra violet (UV) range, because TiO2 has served as a photonic catalyst in this energy range. Recently, perovskite titanate MTiO3 (M=Mg, Ca, Sr, Ba) have attracted great interest both in scientific and technological field. The oxide perovskite strontium titanate (SrTiO3) is 3 expected to be chemically stable and good candidates for optical host materials. SrTiO3 is a well-known material because of its good properties, such as high dielectric constant, high charge storage capacity, good insulating property, excellent chemical and physical stability and excellent optical transparency in the visible range. In addition, SrTiO3 is suitable for host matrix as phosphors (Guo et al., 2006; Yamamoto et al., 2002). Up to now, rare earth ion doped luminescent materials, due to their characteristic emission bands ranging from ultraviolet to visible, to infrared wavelength region, have attracted much attention and become an interesting topic in the field of luminescent material. The luminescence properties of titanate perovskite doped with trivalent praseodymium were increasingly investigated since the mid1990s due to the potential interest of these red emitting phosphors for display applications. Several studies have already reported on the luminescence of Pr3+ doped SrTiO3 powder samples. The addition of Al3+ or Ga3+ into SrTiO3: Pr reported to greatly enhance the emission intensity. Traditionally, phosphor are synthesized in powder form by procedures involving crushing, grinding, ball milling and high-temperature solid state reactions. However, it is difficult to obtain reliable emission intensities with such methods, probably because of inhomogeneous distributions of metal ions, phase separations or the accumulation of impurities. Moreover as luminescent materials, the phosphorescent properties are greatly affected by the grain size, when the grain size reaches nano scale, many new properties can be obtained. For this reason, recent investigations have addressed the development of alternative synthetic procedures and more homogenous materials with improved emission efficiency achieved by using sol-gel process. The sol-gel method, possessing advantages of well controlling the stoichiometry, particle size and morphology, is a potential method for preparing inorganic materials. The sol-gel method of phosphor preparation is regarded as a wet 4 method. A kind of metal organic compounds known as alkoxides of metals is used as precursors. These metal-organic alkoxides could either be in liquid form or are soluble in certain organic solvents. Through the use of the appropriate reagents, the processes of hydrolysis and gelation can be induced to produce homogeneous gels from the mixture of alkoxides. To obtain powder or ceramic samples, gels can be baked, sintered and powderized as in other traditional methods. The sol-gel method is advantageous in as much as thin films or coatings of the phosphor can be formed on substrates directly and/or the sol-gel can be molded into designated forms. In this study, the sol-gel method was utilized for preparing SrTiO3 phosphors. 1.2 Statement of problem Many works on luminescence properties of other materials like aluminates, silicates and phosphate via sol gel method were done. Even though there are several reports on luminescence properties of SrTiO3:Pr, Al, there is no clear-cut understanding on the relation between luminescence properties and structure. Thus, in this study we present the luminescence spectra of this system which is prepared via sol-gel method and indicate the close relationship between such emission and the molecular arrangements in the respective structures. 1.3 Objectives of study The objectives of this study are as follows: To synthesize nano-particle phosphor strontium titanate doped with rare-earth or transition metal. To determine the crystalline phase and structure of host material. 5 To determine the influence of the dopant concentration on luminescent characteristic of phosphor system. 1.4 To determine the mechanism of luminescence of SrTiO3; Pr, Al. Significance of the study In the fast growing field of luminescent material, there are lots of these material applications in many fields such as painting, safety sign and so on. In this research, we want to develop phosphor based titanates because of its promising luminescence performance. By the end of this research, we expected to have a system that has excellent properties as phosphor materials which has low calcination temperature, nanoscale size and high intensity. This study also looks forward to find out the knowledge base of phosphor properties and applications in scientific and technological field. CHAPTER 2 THEORETICAL BACKGROUND 2.1 Introduction As stated in Chapter 1, luminescent materials have wide applications in areas like laser technology, display device biomedical engineering, pollution monitoring etc. The tremendous interdisciplinary appeal for luminescence has resulted in a growing number of researchers desiring to quickly employ new and emerging luminescence techniques without the time consuming effort of becoming an expert in physical spectroscopy. Chapter 2 gave an overview of literature relevant to this study. This chapter commence with general explanations about luminescence, its history and process involved in luminescence. This is followed by discussions about phosphor materials associated with its history and applications of these materials. A review of the works already done, the various properties and applications of phosphor were discussed below. The use of alkaline earth and rare-earth in phosphor system were also discussed along with their properties. The most important factor in this phosphor material system is the host and dopant which will be discussed in section 2.5 onwards. A discussion of the experimental characterization will end up this chapter. 7 2.2 Luminescence Luminescence phenomena have attracted the attention of human since time immemorial. Puzzling questions were related to the observation of emission of visible light by a number of living organisms including fireflies, insects, fishes, mushrooms and luminescent bacteria. How can an animal or a plant produce light? Figure 2.1 shows an example of a living organism which is luminous. Figure 2.1 Marine creatures like jellyfish produce their own light through phosphorescence This interesting and mysterious phenomenon attracted the attention of many scientists during the last four centuries. However, it was not subjected to systematic study until the middle of the 19th century. The phenomenon of certain kinds of substances emitting light on absorbing various energies without heat generation is called luminescence. Luminescence is a science closely related to spectroscopy, which is the study of the general laws of absorption and emission of radiation by matter (Vij, 1998). In other words, it is "cold light", light from other sources of energy, which can take place at normal and lower temperatures. In luminescence, some energy source kicks an electron of an atom out of its ground state which is the lowest-energy state into an excited state which is also called the higher-energy state; then the electron gives back the energy in the form of light so it can fall back to its ground state. 8 A systematic scientific study of the subject of luminescence is of recent origin, from the middle of nineteenth century. In 1852 English Physicist Stokes identified this phenomenon and formulated his law of luminescence now known as Stoke‟s Law, which state that the wavelength of the emitted light is greater than that of the excited radiation. The phenomenon of certain kinds of substance emitting light on absorbing various energies without heat generation is called luminescence. Luminescence is obtained under variety of excitation sources (Fouassier, 1984). The wavelength of emitted light is characteristic of the luminescent substance and not of the incident radiation. The various luminescence phenomena are given names based on the type of radiation used to excite the emission (Table 2.1). 2.2.1 Process that create luminescence The changes that take place in atoms during luminescence are the same as those that occur during incandescence. Electrons in atoms absorb energy from some source and jump to a higher energy level. After a fraction of a second, the electrons fall back to their original level, giving off the energy they had previously absorbed. Various procedures can be used to get this process started, that is, to get electrons to jump higher energy levels. In incandescence, the process is heating. In luminescence, it can be any one of a number of other processes. Luminescence emission occurs as a result of photo effect inside the complicated discrete luminescence centers. The process generally involves two steps: (a) Excitation It is a process where a primary particle or a photon of energy E gives up some or all of its energy to raise electrons from occupied low energy levels to unoccupied higher energy levels. On the basis of band theory of solids with respect to electronic levels, the forbidden gap can be imagined to contain 9 Table 2.1: Luminescence phenomena and the methods of excitation Luminescence phenomena Methods of excitation Photoluminescence Light or photons Bioluminescence Bio-chemical energy (results from a biologically catalyzed reaction) Sono-luminescence Sound waves Electro-luminescence Electric field (light created from a gas in the path of an electrical discharge) Chemi-luminescence Chemical energy (results from a chemical reaction) Tribo-luminescence Mechanical energy (results from the crushing of crystals) Cathodo-luminescence Electrons or cathode rays Radio luminescence Nuclear radiation or Ionizing radiation Fluorescence Ionizing radiation, UV and visible light Phosphorescence Ionizing radiation, UV and visible light Thermoluminescence Ionizing radiation, UV and visible light some acceptor/donor levels. Interaction of ionizing radiation with the solids can transfer sufficient energy to electrons in the valence band for transferring them to the conduction band. A good number of these liberated electrons return immediately to the ground state accompanied or unaccompanied by light emission (causing fluorescence or phosphorescence or internal heating). A fraction of these can be captured at the donor levels call traps with the corresponding holes at the acceptor levels or traps. The donor/acceptor levels are metastable states associated with crystal defects including impurities. 10 (b) Emission Emission occurs when internal energy from one system is transformed into energy that is carried away from that system by electromagnetic radiation. An emission spectrum for any given system shows the range of electromagnetic radiation it emits. When an atom has energy transferred to it, either by collisions or as a result of exposure to radiation, it is said to be experiencing excitation, or to be "excited." Fluorescence Refer to Figure 2.2, absorption of UV radiation by a molecule excites it from a vibrational level in the electronic ground state to one of the many vibrational levels in the electronic excited state. This excited state is usually the first excited singlet state. A molecule in a high vibrational level of the excited state will quickly fall to the lowest vibrational level of this state by losing energy to other molecules through collision. The molecule will also partition the excess energy to other possible modes of vibration and rotation. Fluorescence occurs when the molecule returns to the electronic ground state, from the excited singlet state, by emission of a photon. If a molecule which absorbs UV radiation does not fluoresce it means that it must have lost its energy some other way. Phosphorescence A molecule in the excited triplet state may not always use intersystem crossing to return to the ground state. It could lose energy by emission of a photon. A triplet/singlet transition is much less probable than a singlet/singlet transition. The lifetime of the excited triplet state can be up to 10 seconds, in comparison with 10-5 s to 10-8 s average lifetime of an excited singlet state. Emission from triplet/singlet transitions can continue after initial irradiation. Internal conversion and other radiationless transfers of energy compete so successfully with phosphorescence that it is usually seen only at low temperatures or in highly viscous media (Figure 2.2). 11 Figure 2.2 Possible physical process following absorption of a photon by a molecule 2.3 Phosphor Material Phosphors are transition metal compounds or rare earth compounds of various types. It is mainly inorganic materials, which are prepared by proper heat treatment. Almost all good inorganic phosphors consists of crystalline “host material” in which small amounts of certain impurities, the “activators” are dissolved (Ronda et al., 1998; William et al., 2006). The activators are primarily responsible for the luminescence. Other impurities, the “co-activators”, are necessary in some (not in all) cases to dissolves the activator impurities into the host crystal. Coactivators do not, or only to a very minor degree, participate in the luminescence process. Both activators and (if necessary) co-activators are diffused into the host crystal at elevated temperatures, the “firing”. The firing temperature often is little below the melting temperature of the host material. 12 Phosphor materials characteristics: High luminescence efficiency Excellent mechanical strength High flow ability High packing density of phosphor The scientific research on phosphors has a long history going back more than 100 years. A prototype of the ZnS-type phosphors, an important class of phosphors for television tubes, was first prepared by Théodore Sidot, a young French chemist, in 1866 rather accidentally (Bol et al. 2002). It seems that this marked the beginning of scientific research and synthesis of phosphors. From the late 19th century to the early 20th century, Philip E.A. Lenard and co-workers in Germany performed active and extensive research on phosphors, and achieved impressive results (William and Marvin, 2004). They prepared various kinds of phosphors based on alkaline earth chalcogenides (sulfides and selenides) and zinc sulfide, and investigated the luminescence properties. They established the principle that phosphors of these compounds are synthesized by introducing metallic impurities into the materials by firing. The metallic impurities, called luminescence activators, form luminescence centers in the host. Lenard and coworkers tested not only heavy metal ions but various rare-earth ions as potential activators. Pohl (1920) and co-workers in Germany investigated Tl+-activated alkali halide phosphors in detail in the late 1920s and 1930s (Bose, 1992). They grew single-crystal phosphors and performed extensive spectroscopic studies. They introduced the configurational coordinate model of luminescence centers in cooperation with F. Seitz in the United State and established the basis of present-day luminescence physics. 13 Humbolt Leverenz and co-workers at Radio Corporation of America (U.S.) also investigated many practical phosphors with the purpose of obtaining materials with desirable characteristics to be used in television tubes. Detailed studies were performed on ZnS type phosphors. Since the end of World War II, research on phosphors and solid-state luminescence has evolved dramatically. Chang-Tai Xia et al reported on BaLiF3(Eu2+): A promising X-ray storage phosphor (Xia and Shi, 1997), Yong Gao et al. (1996) works on luminescence properties of SrB4O7: Eu, Tb phosphors while Qin Fei et al. (2005) studied on luminescent properties of Sr2MgSi2O7 and Ca2MgSi2O7 long lasting phosphors activated by Eu2+, Dy3+. Turning to the applications of phosphors, one notes the more recent appearance of various new kinds of electronic displays using phosphors, such as electroluminescent displays, vacuum fluorescent displays, plasma displays, and field emission displays; this is, of course, in addition to the classical applications such as fluorescent lamps, television tubes, X-ray screens, etc. The major and important applications of phosphors are in light sources, display devices, and detector systems. Table 2.2 lists various kinds of phosphor devices according to the method used to excite the phosphor. It gives a summary of phosphor devices by the manner in which the phosphors are applied. No further explanation of the table is necessary. Research and developments of these applications belong to the fields of illuminating engineering, electronics, and image engineering. Therefore, research and technology in phosphors require a unique combination of interdisciplinary methods and techniques, and form a fusion of the above mentioned fields. 14 2.4 Phosphor based Host materials play an important role in order to make the best phosphor. The host materials are typically oxides, sulfides, nitrate, halides or carbonates. Among these host materials, oxide phosphors have been found to be a candidate in field emission display (FED) and plasma display panel (PDP) devices as they are sufficiently conductive to release electric charges stored on the phosphor particle surfaces. The luminescent efficiency and thermal stability can be maintained under prolonged Coulomb loading because of oxides are resistant to high-density electron irradiation. Various long lasting oxide phosphors have been investigated such as SiO2, TiO2, TeO2 and P2O5. Among them, TiO2 could be a good host to excite rare earth ions more efficiently to result in with intense luminescence phenomenon from them (Wang et al., 2007). Luminescence of the titanate-type compounds has the potential for their use in optoelectronic applications. TiO2 possesses a good mechanical resistance and stability even in some corrosive environments and therefore it is widely used in the development of stable host matrices such as BaTiO3, SrTiO3 and CaTiO3. TiO2 has been investigated widely by many researchers. Byong Kee Moon et al. (2006) studied on spectroscopy of nanocrystalline TiO2:Eu3+. Lucas Alonso Rocha et al (2005) also studied on europium incorporated into titanium oxide by the sol-gel method. Zhimin Liu et al (2005) investigated on solvothermal synthesis of mesoporous Eu2O3–TiO2 composites. 2.5 Titanium Dioxide (TiO2) Titanium dioxide, also known as titania is the naturally occurring oxide of titanium, chemical formula TiO2. Pure titanium dioxide does not occur in nature but 15 is derived from ilmenite or leuxocene ores. It is also readily mined in one of the purest forms, rutile beach sand. Table 2.2: Phosphor Devices 16 These ores are the principal raw materials used in the manufacture of titanium dioxide pigment. The first step is to purify the ore, and is basically a refinement step. Either the sulphate process, which uses sulphuric acid as an extraction agent or the chloride process, which uses chlorine, may achieve this. After purification the powders may be treated (coated) to enhance their performance as pigments. Physical and mechanical properties of sintered titania are summarized in Table 2.3, while the optical properties of titania are provided in Table 2.4. Table 2.3: Typical physical and mechanical properties of titania. Property Density 4 gcm-3 Porosity 0% Modulus of Rupture 140MPa Compressive Strength 680MPa Poisson‟s Ratio 0.27 Fracture Toughness 3.2 Mpa.m-1/2 Shear Modulus 90GPa Modulus of Elasticity 230GPa Microhardness (HV0.5) 880 Resistivity (25°C) 1012 ohm.cm Resistivity (700°C) 2.5x104 ohm.cm Dielectric Constant (1MHz) 85 Dissipation factor (1MHz) 5x10-4 Dielectric strength 4 kVmm-1 Thermal expansion (RT- 9 x 10-6 1000°C) Thermal Conductivity (25°C) 11.7 WmK-1 17 Table 2.4: Optical properties of titania. Phase Refractive Density Crystal Index (g.cm-3) Structure Anatase 2.49 3.84 Tetragonal Rutile 2.903 4.26 Tetragonal Applications for sintered titania are limited by its relatively poor mechanical properties. It does however find a number of electrical uses in sensors and electrocatalysis. By far its most widely used application is as a pigment, where it is used in powder form, exploiting its optical properties. The most important function of titanium dioxide however is in powder form as a pigment for providing whiteness and opacity to such products such as paints and coatings (including glazes and enamels), plastics, paper, inks, fibres and food and cosmetics. Titanium dioxide is by far the most widely used white pigment. Titanium dioxide is very white and has a very high refractive index – surpassed only by diamond. The refractive index determines the opacity that the material confers to the matrix in which the pigment is housed. Hence, with its high refractive index, relatively low levels of titania pigment are required to achieve a white opaque coating. The high refractive index and bright white colour of titanium dioxide make it an effective opacifier for pigments. The material is used as an opacifier in glass and porcelain enamels, cosmetics, sunscreens, paper, and paints. One of the major advantages of the material for exposed applications is its resistance to discoloration under UV light. Figure 2.3 below shows major properties and applications served by titanium dioxide. 18 Properties Applications High dielectric constant Photocatalyst Good insulating properties PVC (rigid and flexible) High refractive index Solar cells Figure 2.3 Properties and applications of Titanium Dioxide Titanium dioxide occurs in nature as the well-known naturally occurring minerals rutile, anatase and brookite. The crystal structures of them are shown in Figure 2.4. The most common form is rutile, which is also the most stable form. Anatase and brookite both convert to rutile upon heating. (a) (b) (c) Figure 2.4 Crystal structure of TiO2 group (a) Rutile (b) Anatase (c) Brookite 2.6 Alkaline earth titanate 19 The Alkali Earth Metals comprise the second column from the left of periodic table. They are reactive metals that tend to oxidize in air. This makes it irritating to try to keep samples of them. Recently several alkali earth oxides such as MgO, SrO, CaO, and BaO were mixed up with TiO2 in an attempt to enhance the luminescence efficiency. Alkaline earth metal titanate having the formula MTiO3, wherein M is an alkaline earth metal (Ca, Sr, Ba), Alkaline-earth metal titanate belong to the important functional ceramics materials such as piezoelectricity, electro optic devices, semiconductor etc. MTiO3 has attracted interests as one kind of the promising oxide-based phosphors. Among of this alkaline earth titanate, recently strontium titanate (SrTiO3) becomes a choice for researchers to study. SrTiO3 is a well-known material because of its good properties, such as its high dielectric constant, high charge storage capacity, good insulating property, its chemical and physical stability and its excellent optical transparency in the visible range. In addition, it‟s vibrational frequency is quite low, which makes it suitable as host matrix for upconversion phosphors (Guo et al., 2006). SrTiO3 single crystal provides a good lattice match to most materials with perovskite structure which is show in Figure 2.5. At room temperature it exists in the cubic form, but transforms into the tetragonal structure at temperatures less than 105 K. The perovskite-type oxides, ABO3, are materials of which the physical properties, such as electric, magnetic, dielectric properties, and so on, have been widely investigated in relation to the crystal structure because the materials have relatively simple but various crystal structures that can be changed by substitutions of both A and B site ions with large fraction. The perovskite-type titanates show high levels of ionic and electronic conductivities and significant thermal, chemical and mechanical stability in a wide range of oxygen activities. Due to these properties, the 20 titanates have a lot of potential applications as electrodes for high temperature fuel cells and other electrochemical devices, catalysts, oxygen permeable membranes for oxygen separation and hydrogen production. Figure 2.5 SrTiO3 (perovskite) structure The SrTiO3 has cubic structure. The SrTiO3 primitive unit cell contains five atoms which is also the case for other ABO3 perovskites. Its unit cell possesses a simple cubic symmetry Pm3m and a lattice constant a= 3.89 Å. The structure of SrTiO3 in the cubic phase is a simple one (Figure 2.6), with Sr2+ ions at the cube corners, Ti4+ ions at the body centers, and O2− ions at the face centers. The structure can be considered as a rigid grouping of oxygen octahedra linked at their corners by shared oxygen ions. The Ti4+ ions thus lie at the center of each octahedron, while the Sr2+ ions lie outside the octahedral. Figure 2.6 Structure of SrTiO3 in the cubic phase The unit cells of cubic SrTiO3 lattice sectioned by three different planes, (100), (110) and (111), are shown in Figs. 2.7a-c. 21 Figure 2.7 Structural units of the cubic SrTiO3crystal sectioned by three different planes shown as shaded: a) the (100) surface contains O2−and Sr2+ ions, b) the (110) surface contains Ti4+, O2−and Sr2+ atoms, c) the (001) surface contains Ti4+ and O2−ions. 2.7 Phosphor doped rare-earth Luminescence of rare-earth ions is an active field of research. Much of this interest stems mainly from the unique physical properties of lanthanide oxides, which makes them promising candidates for a wide variety of applications, such as laser materials, optical amplifiers, phosphors and photocatalyst. Rare-earth elements are dopants donating interesting and often useful properties to host crystal. Rare-earth-doped materials have potential applications for phosphors, display monitor, X-ray imaging, scintillators, lasers and amplifiers for fiber-optic communications (Blasse and Grabmaier, 1994). Rare earth-based phosphors are one of the most important trichromatic luminescent materials, with which full-color display is produced by emissions in the blue, green and red regions, respectively, at 450,550 and 610 nm. The phosphor materials contain one or more 22 impurity ions or activators (A), typically present in 0.01-100 mol % concentrations. The actual emission is generated on these activator ions. Typical activators are rare earth- or transition-metal ions. The rare earths are used as activator due to their sensitivity to changes in the surroundings. The activators are primarily responsible for the luminescence. Doped ions and elements lead a band gap narrowing. The rare-earth elements usually comprise 17 elements consisting of the 15 lanthanides from Lanthanum (La: atomic number 57) to Lutetium (Lu: atomic number 71), of Scandium (Sc:atomic number 21), and of Yttrium (Y: atomic number 39). The electronic configurations of trivalent rare-earth ions in the ground states are shown in Table 2.5. As shown in the table, Sc3+ is equivalent to Argon, Y3+ to Krypton, and La3+ to Xenon in electronic configuration. The lanthanides from Ce3+ (Cerium ions) to Lu3+ have one to fourteen 4f electrons added to their inner shell configuration, which is equivalent to Xe. Ions with no 4f electrons, i.e., Sc3+, Y3+, La3+, and Lu3+, have no electronic energy levels that can induce excitation and luminescence processes in or near the visible region. In contrast, the ions from Ce3+ to Yb3+ (Ytterbium ions), which have partially filled 4f orbitals, have energy levels characteristic of each ion and show a variety of luminescence properties around the visible region. Many of these ions can be used as luminescent ions in phosphors, mostly by replacing Y3+, Gd3+ (Gadolinium ion), La3+, and Lu3+ in various compound crystals. Up to now, praseodymium has been developed for luminescence applications. 23 Table 2.5: Electronic Configurations of Trivalent Rare-Earth Ions in the Ground State 2.7.1 Praseodymium Trivalent praseodymium is well-known to emit efficiently between the blue and the red regions, depending on the host material, the concentration and the pumping conditions (Diallo et al., 1997). Pr3+-doped phosphor materials with full color luminescence have received substantial interest because the Pr3+ ions show a number of different emissions depending on the host lattice in which they are incorporated, for example red (from the 1D2 level), green (from the 3P0 level), blue (from the 1S0 level) and ultraviolet (from the 4f–5d state). Luminescence of Pr3+ consists of many multiplets, as follows: ~515 nm (3P0 → 3H4), ~670 nm (3P0 → 3F2), ~770 nm (3P0 → 3F4), ~630 nm (1D2 → 3H6), ~410 nm (1S0 → 1I6), and ultraviolet (5d → 4f) transitions. The relative intensities of the 24 peaks depend on the host crystals. As an example, the emission spectrum of Y2O2S:Pr3+ is shown in Figure 2.8. The radiative decay time of the 3P0 → 3HJ or 3FJ emission is ~10–5 s, which is the shortest lifetime observed in 4f → 4f transitions. For example, in Y2O2S host, decay times until 1/10 initial intensity are 6.7 μs for Pr3+, 2.7 ms for Tb3+, and 0.86 ms for Eu3+. The short decay time of Pr3+ is ascribed to the spin-allowed character of the transition. Since the short decay time is fit for fast information processing, Gd2O2S(F):Pr3+, Ce3+ ceramic has been developed for an Xray detector in X-ray computed tomography. The emission of the Pr3+ ion can be interpreted according to the energy level diagram of Pr3+ shown in Figure 2.9 Figure 2.8 Emission spectrum of Y2O2S:Pr3+ (0.3%) at room temperature. 25 Figure 2.9 Energy level of Pr3+ ions 2.8 Phosphor Synthesis Numerous methods have been reported for the synthesis of phosphor depending on the material of interest, size regime and application requirement. Whatever be the method, the attraction of the method depends on its capability for the reproduction of luminescence emission and the grain size. The various methods used for the preparation of phosphors are listed below. 1. Co-precipitation methods 4. Solid-state reaction method 2. Combustion methods 5. Sol-gel method 3. Spray pyrolysis The solid-state reaction process is used intensively for phosphor synthesis, but this process often results in poor homogeneity and requires high calcinating temperature. Moreover, the grain size of phosphor powders prepared through solid- 26 state reaction method is in several tens of micrometers. Phosphors of small particles are obtained by grinding the larger phosphor particles. These processes easily introduce additional defects and greatly reduce luminescence efficiency. With the development of scientific technologies on materials, several chemical synthesis techniques, such as coprecipitation, sol–gel, microwave, Pechini and combustion synthesis methods have been applied to prepare phosphors (Zhao and Chen, 2007). Sol–gel process is an efficient technique for the preparation of phosphors due to the good mixing of starting materials and relatively low reaction temperature resulting in more homogeneous products than those obtained by direct solid state reactions (Jiang et al., 2004). 2.9 Sol-gel method The sol-gel method has been widely used to prepare a number of phosphors for displays and other materials that are of technical importance. The sol-gel method is a chemical technique that uses metal alkoxides for the synthesis and production of glasses or ceramics through a series of chemical processes, including hydrolysis, gelation, drying and thermal treatment. The sol-gel technique was developed as early as 1864; T. Graham prepared gels of silica from aqueous salts, while M. Ebelmen obtained silica gels from metal alkoxides. The potential of the sol-gel process was not appreciated until 1980, when it was “rediscovered” and found to be very useful in synthesizing various materials of practical importance, such as optical glasses and solid-state laser materials. Since then this method has received considerable attention and was investigated extensively. In general, a sol is defined as a colloid of solid particles suspended in a liquid; the particles consist of dense oxide or polymetric clusters formed by the 27 precursors and reagents. A gel, on the other hand, is a composite substance consisting of a continuous solid skeletal structure which results from the gelation of the sol; the gel forms cells which encapsulate colloidal liquids. 2.9.1 Preparation of precursor solutions The initial raw materials for sol–gel preparations consist of metal alkoxides either in the solid or in the liquid form (Table 2.6). An alkoxide is a metalorganic compound in which a hydrogen atom belonging to the hydroxyl (OH) group of an alcohol is replaced by a metal atom. As the sol–gel method is a wet chemical method, a proper solvent is needed to convert solid alkoxides, if used, into liquid form. Some alkoxide solutions are commercially available (see Table 2.6). Doping or activator ions are introduced either through another alkoxide solution or by using an aqueous solution of the doping ions. This liquid mixture of the metal alkoxides is stirred for an extended period. To stimulate hydrolysis, a mixture of water, alcohol, hydrochloric acid is added. 2.9.2 Hydrolysis A mixture of water, alcohol, and HCl is prepared, with HCl acting as a catalyst in this process. This acidic solution is added slowly (dropwise) into the precursor alkoxide mixture. The reaction of alkoxides with water is called hydrolysis. In hydrolysis, a hydroxyl (OH) group attaches itself to the metal atom by replacing the alkoxide (OR) group. Since the chemical and physical processes involved are similar for all metal alkoxides, silicon alkoxides are use as an illustrative example for the preparation of sol–gel materials. For these matrices, TMOS (tetramethoxysilane, Si(OCH3)4, liquid) or TEOS (tetraethoxysilane, Si(OC2H5)4, liquid) is commonly used. They react 28 readily but are not soluble in water. A solvent such as methanol (MeOH) or ethanol (EtOH) is normally used to produce the precursor solution. A typical hydrolysis reaction for these materials goes as OR3 Si OR H 2O OR3 Si OH ROH where R is the alkyl (alkylic) radical, CnH2n+1. R = CH3 for TMOS and R = C2H5 for TEOS. Hydrolysis can occur with any one of the OR groups of the molecule. If the sol–gels are to be doped, an aqueous solution containing the doping ion/ions is also blended in during the hydrolysis step. 2.9.3 Gelation With proper thermodynamic conditions, gelation occurs. Gelation is a continuous process in which two partially hydrolyzed molecules begin to connect and intertwine with each other with the release (condensation) of water when in an aqueous solution (OR) 3 Si OH HO Si (OR) 3 (OR) 3 Si O Si OR3 H 2O Alcohol, ROH, is released when an alcohol solution is employed: (OR) 3 Si OR HO Si OR3 OR3 Si O Si (OR) 3 ROH where ROH = C2H5OH for TEOS and CH3OH for TMOS. 29 Table 2.6: List of some commonly used Metal Alkoxides and recommended solvets for solid 30 With continuing gelation, larger structures are produced by polymerization, wherein chains of polymers can cross-link to form three-dimensional clusters. Small clusters suspended in the liquid constitute the sol, and through the gelation process these clusters begin to grow by combining with monomers or other clusters while releasing or condensing water or alcohol. Different metal alkoxides can also coalesce to form “compound” clusters. Several factors affect the rate of sol and gel formation including the temperature, the relative concentration of the alkoxide precursors, water, and solvent, and the pH of the total admixture. In most cases, sol–gel synthesis is carried out at room temperature, though both the sol and gel formation rates are known to increase with increasing temperature. Because water and alkoxysilanes are immiscible, a common solvent such as alcohol is also normally used as a homogenizing agent. 2.9.4 Aging and drying Aging leads to changes in the structure and other properties of the gel through further condensation, dissolution, and reprecipitation of monomers or oligomers. Syneresis or spontaneous shrinkage of the network of the gel takes place as bond or attraction between clusters induces a contraction of the network and expulsion of liquid from the pores. Drying by evaporation under normal conditions gives rise to pressure within the pores, which causes shrinkage of the gel network. Pressure gradients develop through the volume of the gels, so that the networks are compressed more at the surfaces than in the bulk. This may cause, if the gradients are too large, cracking of the sample. 31 After shrinkage stops, further evaporation drives the meniscus of the liquids into the bulk and the rate of evaporation decreases. The resulting dried gel is called a xerogel. Xerogels are useful in the preparation of dense ceramics and are also interesting because of their high porosity and large surface area; these materials are useful as catalytic substrates, filters, and vapor sensors. 2.9.5 Annealing and porosity control. Additional heat treatment of the sol–gel is required to produce pore-free ceramic materials; sintering at high temperatures results in densification driven by interfacial energy considerations. By heating, the gel constituents move by viscous flow or diffusion so as to reduce the solid–vapor interfacial areas, and hence reduce porosity. Removal of organics takes place by endothermic carbonization near 200°C, followed by exothermic oxidation at temperatures between 300 and 400°C. For the silicate system of our example, the exothermic process is suppressed if the gels are heated under inert conditions, where oxidation is prevented. The temperature interval between 400 and 525°C represents a region where considerable skeletal densification occurs with little associated weight loss. Structural relaxation, a process by which free excess volume is removed by diffusive motion of the network, is the predominant shrinkage mechanism in this temperature interval. The condensation (water or alcohol) and pyrolysis reactions that occur during heating liberate a large volume of gas that can generate high pressures, and because of low permeability of the small pores in the network, this may cause cracking when the samples are heated between room temperature and 400°C. At 800°C, there is partial densification of the sol–gel; by 900°C, the gel is completely densified leaving only a trace of silanols (SiOH). The sol–gel technique has the following advantages: a) High homogeneity of the chemical composition of the materials produced. b) High uniformity of doping ion distribution c) Processing temperature can be very low. 32 d) The microstructure (porosity and size of pores) of the materials can be controlled. e) Thin films and multilayer coatings of sol–gel materials can be readily prepared by spinning or dipping methods during the gelation period. f) The sol–gel procedures produce little unintentional contamination. No milling and grinding are needed, as these processes are known to contaminate samples. Fluxes such as B2O3, H3BO3 and NH4Cl which are commonly used in ceramic technology and contaminate the end products are no longer needed. When phosphor powders are prepared by the sol–gel method, powdering may be used and trace of foreign particles can be blended in. This “contamination” does not enter into the lattice of powders and will not affect the optical properties of the phosphor. 2.10 X-ray Diffraction (XRD) X-Ray Diffraction (XRD) is a high-tech, non-destructive technique for analyzing a wide range of materials, including fluids, metals, minerals, polymers, catalysts, plastics, pharmaceuticals, thin-film coatings, ceramics, solar cells and semiconductors. The XRD pattern is often spoken as the “FINGERPRINT” of a mineral or a crystalline substance, because it differs from pattern of every other mineral or crystalline substance. Common configuration of XRD units is shown in Figure 2.10 33 Figure 2.10 Common configurations for an XRD unit When a monochromatic x-ray beam with wavelength is incident on the lattice planes in a crystal planes in a crystal at an angle , diffraction occurs only when the distance traveled by the rays reflected from successive planes differs by a complete number n of wavelengths. This is described by Bragg‟s equation: n 2d sin where d is the spacing between the planes (see Figure 2.11). Figure 2.11 Principle of X-ray Diffraction (2.1) 34 By varying the angle , Bragg‟s Law conditions are satisfied by different dspacing in polycrystalline materials. A diffractometer collects intensities over an angular range by measuring 2θ, the angle between the incident and the diffracted beam. The resulting plot of the intensity as a function of the 2θ value is known as a diffractogram (see Figure 2.12) containing peaks (reflections) that are characteristic of the particular atomic arrangement(s) of the crystallites in the sample – the socalled „phases‟. When a mixture of different phases is present, the diffractogram exhibits the sum of the individual patterns. From such a diffractogram, information can be obtained about the identity of the phases (qualitative information), their atomic arrangement (structural information), their relative concentrations (quantitative information), as well as the possible presence of a non-crystalline amorphous phase. Figure 2.12 A schematic diffractogram showing the presence of two phases (with peaks at different angular positions) originating from a material with small and large crystallites. The broad hump is due to an amorphous phase. One possible method of calculating crystallite size from XRD line broadening is to use the method developed by Scherrer in 1918, which uses the Scherrer equation ( Cullity, 1978). D 0.9 / cos (2.2) 35 where D is the averaged dimension of crystallites; λ is the wavelength of X-ray; θ is Bragg angle; and β is the full width at half maximum of the peak. 2.11 Scanning Electron Microscope (SEM) Scanning Electron Microscopy (SEM) is a high resolution imaging technique, with a great depth of field. It shows topographical, structural and elemental information at magnifications of 10X to 200,000X. SEM utilizes electrons in place of light beams to achieve both higher magnification and greater clarity and depth of field over traditional microscopes. Preparing a sample for examination by an SEM is a complicated process. First, the sample must be dehydrated before placing in a vacuum chamber. If it is a non-metal, it must then be covered with a thin conductive covering to prevent "charging" by the electrons, usually gold foil. This process is called "sputter coating." Working principle of SEM is shown in Figure 2.13. A beam of electrons is produced from the "electron gun" located at the top of the SEM. These electrons are usually produced by the heating of a metallic filament like tungsten. The electron beam from the "gun" follows a path down through the microscope past electromagnetic lenses which focus the beam towards the sample. When the beam hits the sample, other electrons are dispersed or ejected from the sample. These electrons are called back scattered or secondary electrons. Specialized detectors placed in the SEM collect the ejected electrons and convert them to a signal sent to a viewing screen. The screen assembles the signal 36 into an image. SEM images can be controlled to a magnitude of x250 000 with a resolution around 10nm. Figure 2.14 show the example of SEM images. Figure 2.13 Principle of Scanning Electron Microscope (a) (b) (c) Figure 2.14 Example of SEM images of (a) Materials (b) Insects (c) Cabbage leaf 2.12 Raman Studies Raman is an invaluable technique for characterisation of materials. In the field of semiconductor characterisation, the use of Raman microscopes is now 37 widespread because it offers a non-destructive and quantitative microanalysis of structures and electronic properties. Because of its higher resolution with respect to FTIR spectroscopy and its versatility and simplicity in terms of sample handling and the possibility of acquisition of the whole spectra (4000 to 10 cm-1) with the same instrument. Raman has actually an increasing use versus other similar spectroscopic techniques. In particular, in addition to the well known application of identification of compounds, a large number of reported works rule for example on the characterization of concentration and mobility of free carriers, characterization of strain and crystal quality, determination of local crystal orientation, etc. It is worth to comment that while IR and Raman spectra analyze basically the same vibrational behavior of the molecule they are not exact duplicates since the selection rules and relative band intensities differ in many cases. When monochromatic radiation is incident upon a sample the light interact with the sample in the some fashion. It may be reflected, absorbed or scattered in some manner. It is the scattering of the radiation that occurs which can tell the Raman spectroscopist something of the samples molecular structure. If the frequency (wavelength) of the scattered radiation is analyzed, not only is the incident radiation wavelength seen (Rayleigh scattering) but also, a small amount of radiation that is scattered at some different wavelength (Stokes and AntiStokes Raman scattering). (approx. only 1 x 10-7 of the scattered light is Raman). It is the change in wavelength of the scattered photon which provides the chemical and structural information. Light scattered from a molecule has several components - the Rayleigh scatter and the Stokes and Anti-Stokes Raman scatter (Figure 2.15). In molecular systems, these frequencies are principally in the ranges associated with rotational, 38 vibrational and electronic level transitions. The scattered radiation occurs over all directions and may also have observable changes in its polarization along with its wavelength. The scattering process without a change of frequency is called Rayleigh scattering, and is the same process described by Lord Rayleigh and which accounts for the blue color of the sky. A change in the frequency (wavelength) of the light is called Raman scattering. Raman shifted photons of light can be either of higher or lower energy, depending upon the vibrational state of the molecule. Figure 2.15 Light scattered from a molecule The schematic diagram in Figure 2.16 represent the Raman spectrometer. Figure 2.16 Schematic representation of a Raman spectrometer 39 2.13 Fourier Transform Infrared (FTIR) Spectroscopy Fourier Transform Infrared Spectroscopy, or simply FTIR Analysis, is a failure analysis technique that provides information about the chemical bonding or molecular structure of materials, whether organic or inorganic. It is used in failure analysis to identify unknown materials present in a specimen. The technique works on the fact that bonds and groups of bonds vibrate at characteristic frequencies. A molecule that is exposed to infrared rays absorbs infrared energy at frequencies which are characteristic to that molecule. During FTIR analysis, a spot on the specimen is subjected to a modulated IR beam. The specimen's transmittance and reflectance of the infrared rays at different frequencies is translated into an IR absorption plot consisting of reverse peaks. The resulting FTIR spectral pattern is then analyzed and matched with known signatures of identified materials in the FTIR library. As shown in Figure 2.17, in the interferometer the light passes through a beam splitter, which sends the light in two directions at right angles. One beam goes to a stationary mirror then back to the beam splitter. The other goes to a moving mirror. The motion of the mirror makes the total path length variable versus that taken by the stationary-mirror beam. When the two meet up again at the beam splitter, they recombine, but the difference in path lengths creates constructive and destructive interference pattern called an interferogram. The recombined beam passes through the sample. The sample absorbs all the different wavelengths characteristic of its spectrum, and this subtracts specific wavelengths from the interferogram. The detector now reports variation in energy versus time for all wavelengths simultaneously. A laser beam is superimposed to provide a reference for the instrument operation. 40 Figure 2.17 Schematic sketch of the essential features of a Fourier transform infrared (FTIR) spectrometer. The infrared spectrum can be divided into two regions, one called the functional group region and the other the fingerprint region. The functional group region is generally considered to range from 4000 to 1500 cm-1 and all frequencies below 1500 cm-1 are considered characteristic of the fingerprint region. The fingerprint region involves molecular vibrations, usually bending and vibration motions that are characteristic of the entire molecule or large fragments of the molecule. Thus these are used for identification. The functional group region tends to include motions, generally stretching vibrations, which are more localised and characteristic of the typical functional groups, found in organic molecules. While these bands are not very useful in confirming identity, they do provide some very useful information about the nature of the components that make up the molecule. The major types of molecular vibrations are stretching and bending. The various types of vibrations are illustrated in Figure 2.18 and the example of IR spectra is shown in Figure 2.19. 41 Figure 2.18 Major types of vibrational modes a) Stretching vibrations b) Bending vibrations Figure 2.19 An example of an FTIR spectrum According to the earlier study by Um and Kumazawa (2000), in their study a broad absorption band in the range of 2700 to 3600 cm-1 suggests the presence of considerable amounts of H2O and OH- in the particle. According to Yan et al. (2009), from the FT-IR spectrum of TiO2, the peaks of 3420 and 1630cm−1 correspond to the stretching vibrations of O-H and bending vibrations of adsorbed water molecules 42 (δH2O), respectively, indicating the existence of O-H on the surface of TiO2. All studies on carbonate surface species including a recent work on iron oxide studied by IR spectroscopy showed that peak for CO32 appears at 1490–1450 cm−1 (Chang et al. 2008). It was confirmed by Kumar and Buddhudu (2009) that bands at 1426 and 866 cm-1 are due to the vibration of CO32 anions. In particular, the sharp absorption band observed at 568 cm-1 characteristic for an octahedrally coordinated Ti-O stretching mode, which was assigned by Park et al. (2001). Last in his study found that cubic SrTiO3 shows two strong infrared bands around 395 and 610 cm-1. He considered the vibrations of this material in terms of the TiO3 octahedra and suggested that these two bands could be assigned to the O-Ti-O bending and Ti-O stretching modes, respectively (Last, 1957). According to Monica Popa et al. (2001) on his studies about praseodymium oxide, band at 380, 420, 440, 450, 590, 660 cm-1 are due to praseodymium oxide, in accordance with Hussein study (1994). In his paper, Monica Popa also stated that absorption bands due to bending and stretching vibrations of the bond in CO32 appear at 855, 878 and 1455 cm-1. 2.14 Photoluminescence Studies Photoluminescence spectroscopy is a contactless, nondestructive method of probing the electronic structure of materials. Light is directed onto a sample, where it is absorbed and imparts excess energy into the material in a process called photo-excitation. One way this excess energy can be dissipated by the sample is through the emission of light, or luminescence. In the case of photo-excitation, this luminescence is called 43 photoluminescence. The photoluminescence spectrum provides the transition energies, which can be used to determine electronic energy levels (Gfroerer, 2000). Photo-excitation causes electrons within the material to move into permissible excited states. When these electrons return to their equilibrium states, the excess energy is released and may include the emission of light (a radiative process) or may not (a nonradiative process). The energy of the emitted light (photoluminescence) relates to the difference in energy levels between the two electron states involved in the transition between the excited state and the equilibrium state. The quantity of the emitted light is related to the relative contribution of the radiative process. This process is shown in Figure 2.20 Figure 2.20 Process of luminescence CHAPTER 3 EXPERIMENTAL AND CHARACTERIZATION 3.1 Introduction A proper understanding of the experimental techniques and the instruments used is imperative before they are subjected to fruitful research. This chapter deals with a brief account of the tools and techniques employed to carry out this work. The synthesis of nano-crystalline phosphors imposes a challenge on the traditional solid-state synthesis methods, which fail to offer a low temperature and desired homogeneity at the nanometer level. Preparation of definite size is very difficult task, but is essential since in the nano world the properties are size dependent. Nowadays, numerous methods are employed for the preparation of phosphors of desired quality and size. In this study, the simplest but most precise methods of preparation and characterization of samples are employed. Low temperature calcinations can be achieved using sol-gel method. Visual observations on morphology and grain size were done with scanning electron microscopes and EDAX. The samples were identified and characterized by X-ray diffractometry, Infrared and Raman Spectroscopy. Photoluminescence Spectroscopy was used to study the luminescence properties. 45 3.2 Sample preparation The phosphors were prepared by sol-gel method. The process for preparing SrTiO3 phosphor is schematically indicated in Figure 3.1. Titanium tetraisopropoxide (TTIP) was used as starting material and strontium nitrate, Sr(NO3)2 as modifier. Ethanol was selected as solvents for TTIP and hydrochloric acid (HCl) as catalyst. Praseodymium nitrate, Pr(NO3)3.6H2O and aluminium nitrate, Al(NO3)3.9H2O were used as dopant. The range of the system is SrTiO3; xPr, yAl where 0 mol % x 1mol % 0 mol % y 1mol % 25 ml of ethanol dropwise in 5 ml TTIP and stir during the process. The white colour sol obtained was labeled as SOL 1. Then, 0.5 ml distilled water and 0.5 ml of HCl were added to 25 ml of ethanol which then assume as SOL 2. SOL 2 was dropwised into SOL 1. The obtained solutions were vigorously stirred at 80⁰C for about 15 minutes. A stoichiometric amounts of Sr(NO3)2 was added to the obtained sol. The praseodymium and aluminum salt were dissolved in a small amount of water and added slowly to the obtained sol with different molar ratios of Pr3+ and Al3+. The sols obtained were heated at 120⁰C for 24 hours to form gels. The samples of crushed gels were heated at 450⁰C, 600⁰C, 700⁰C and 800⁰C for 2 hours to form nanocrystalline SrTiO3 powders doped with Pr3+ and Al3+. The phosphors thus obtained were finely powdered and used for the studies. A number of SrTiO3 phosphor doped with Pr3+ and Al3+ ions were prepared as shown in Table 3.1. 46 Ethanol Distilled water TTIP HCl Ethanol Transparent solution White color sol SOL 1 SOL 2 SOL 1 Dropwise SOL 2 White color sol Stirr at 80⁰C (15 min) Dropwise White color gel Sr(NO3)2 Pr(NO3)3.6H2O Al(NO3)3.9H2 O Evaporation and drying at 1200C Powder Calcinations Figure 3.1 Schematic steps involved in the sol-gel process used for the preparation of SrTiO3: Pr, Al 47 Table 3.1: Concentration of praseodymium and aluminum in the prepared samples of strontium titanate phosphor Sample Code Concentration (mol %) Praseodymium(x) Aluminium(y) SrTiO3 3.3 0 0 SrTiO3:x1Pr 0.1 0 SrTiO3:x2Pr 0.2 0 SrTiO3:x3Pr 0.5 0 SrTiO3:x4Pr 1 0 SrTiO3: x4Pr ,y1Al 1 0.1 SrTiO3: x4Pr ,y2Al 1 0.2 SrTiO3: x4Pr ,y3Al 1 0.5 SrTiO3: x4Pr ,y4Al 1 1 Experimental Characterizations There are few measurement were used to characterize the samples. The morphologies of the phosphors were observed using scanning electron microscopy (SEM). Phase identification of the obtained powders was performed by X-ray diffraction (XRD) analysis. Average particle sizes were evaluated from the XRD patterns using the Scherrer‟s equation (Deren, 2008) and from scanning electron micrographs. The photoluminescent properties were investigated by measuring the emission spectra employing photoluminescent spectrum. For the structural characterization of the system Raman and Infrared spectroscopy were used. 48 3.3.1 X-ray Diffraction (XRD) studies X-Ray Diffraction (XRD) is a high-tech, non-destructive technique for analyzing a wide range of materials, including fluids, metals, minerals, polymers, catalysts, plastics, pharmaceuticals, thin-film coatings, ceramics, solar cells and semiconductors. Throughout industry and research institutions, XRD has become an indispensable method for materials investigation, characterization and quality control. Example areas of application include qualitative and quantitative phase analysis, crystallography, structure and relaxation determination, texture and residual stress investigations, controlled sample environment, micro-diffraction, nanomaterials, lab- and process automation, and high-throughput polymorph screening. In this research, the phase purity and phase structure of powder samples were characterized by the X-ray powder diffraction (patterns) with Cu-K radiation operating at 40 kV, 30 mA at room temperature using Siemens Diffractometer D5000, equipped with diffraction software analysis. Identification of the diffraction peaks of the XRD patterns was carried out by using the International Centre for Diffraction Data (ICDD) database. Scherrer equation was used to determine the particle size. Figure 3.2 shows a XRD that has been used. Figure 3.2 X-ray Diffractometer (Siemens Diffractometer D5000) at Faculty of Mechanical Engineering, Universiti Teknologi Malaysia, Skudai. 49 3.3.2 Scanning Electron Microscope (SEM) Scanning Electron Microscopy (SEM) is a high resolution imaging technique, with a great depth of field. It shows topographical, structural and elemental information at magnifications of 10X to 200,000X. In this study, morphology of the phosphor powder was examined on a JEOL JSM-6701F Field Emission Scanning Electron Microscopy as in Figure 3.3. An electron beam with 2.0 kV acceleration voltages was used for imaging. Samples were coated with platinum to avoid charging effects. The compositions of the powders were investigated using EDX JED 2300F. Figure 3.3 Scanning Electron Microscopy (JEOL JSM-6701F) at Institute of Ibnu Sina, Universiti Teknologi Malaysia, Skudai. 50 3.3.3 Raman Spectroscopy Raman is an invaluable technique for characterisation of materials. In the field of semiconductor characterisation, the use of Raman microscopes is now widespread because it offers a non-destructive and quantitative microanalysis of structures and electronic properties. Because of its higher resolution with respect to FTIR spectroscopy and its versatility and simplicity in terms of sample handling and the possibility of acquisition of the whole spectra (4000 to 10 cm-1) with the same instrument. Measurement of Raman spectra were obtained on a Jobin Yvon HR 800 UV as shown in Figure 3.4. Raman spectra were excited with argon ion laser emission at 514.5 nm and recorded by monocromators. The spectra were monitored in the range of 0-4000 cm-1 with the laser power on the sample of 20 mW. Figure 3.4 Equipment used for Raman spectroscopy at N.O.R laboratory, School of Physics, Universiti Sains Malaysia. 51 3.3.4 Fourier Transform Infrared (FTIR) Spectroscopy Fourier Transform Infrared Spectroscopy, or simply FTIR Analysis, is a failure analysis technique that provides information about the chemical bonding or molecular structure of materials, whether organic or inorganic. It is used in failure analysis to identify unknown materials present in a specimen. FTIR spectra were recorded with a Perkin-Elmer (Spectrum One FT-IR) spectrometer in the frequency range 400-4000 cm-1 at room temperature as shown in Figure 3.5. Pellets were prepared for FTIR measurements by mixing and grinding a small quantity of powder sample with spectroscopic grade dry KBr powder and then compressing the mixtures to form pellets for measurements. All measurements were at 4 cm-1 resolution. Figure 3.5 FTIR spectroscopy at Chemistry Department, Universiti Teknologi Malaysia, Skudai. 52 3.3.5 Photoluminescence Spectroscopy Photoluminescence spectroscopy is a contactless, nondestructive method of probing the electronic structure of materials. Photoluminescence spectra were also recorded with a Jobin Yvon HR 800 UV by HeCd laser excitation at wavelength of 325 nm which is attached together with Raman Spectroscopy as shown in Figure 3.4. CHAPTER 4 RESULTS AND DISCUSSIONS 4.1 Introduction A structural study is an essential part in this research in order to study the characteristic of phosphor material. Knowledge of the structure of the compounds involved is essential to understand the processes taking place during a chemical reaction. Chemical and physical properties of a substance can only be understood when its structure is known. The structure determination is mainly performed by Xray diffraction (XRD). Besides, spectroscopy is also used as a necessary tool for structure determination. In this research, Infrared (IR) Spectroscopy and Raman Spectroscopy are used. Their bands were analyzed to identify the functional groups. This chapter reports the analysis of phosphor system in terms of structural studies. In term of photoluminescence studies, SrTiO3 doped with Pr,Al was successfully synthesis using sol-gel technique with the addition of Al together with Pr to SrTiO3 as a luminescent center. The dopant may act as trapping/recombination/luminescent centers in the host materials. In this chapter, the doping effects of Pr3+ and Al3+ on the luminescence properties of SrTiO3 will be discussed. 54 4.2 Structural Studies 4.2.1 Phase Formation 4.2.1.1 The effect of calcined temperature on host structure XRD was taken to examine the crystal structure and phase purity of the products, and the typical XRD patterns of the SrTiO3 host structure prepared using sol-gel technique at various temperatures ranging from 450℃ to 800℃ for 2 h were shown in Figure 4.1. When considering 450⁰C, it is obvious that only the Sr(NO3)3 phase is present, no diffraction pattern of other phases were identified. When the calcination temperature was increased to 600⁰C, peaks of new phases, SrTiO3 and TiO2 (anatase) are identified from the XRD pattern, which can be indexed to the cubic phase structure of SrTiO3 (ICDD card no. 84-0444) and ICDD card no 711169 for TiO2 (anatase). As a result, the creation of the perovskite SrTiO3 phase occurs at 600⁰C. From this point of view, this method can form SrTiO3 at low temperature in comparison with the conventional solid state method. The same pattern was observed for phosphor at calcinations 700⁰C which are SrTiO3 and TiO2 (anatase). However, with the calcinations temperature increasing to 800⁰C, almost all the diffraction peaks can be indexed to the cubic SrTiO3 phase. It is evident from the sharpness of the peaks that the crystallinity and uniformity is continuously increasing with increasing calcination temperature. The formation of SrTiO3 phases after calcinations at 600⁰C is almost similar to the results reported by Park et al. (2001) in SrTiO3: Al, Pr phosphor from a complex precursor polymer. In their study, after calcinations at 600⁰C, the crystalline SrTiO3 and minor amounts of SrCO3 have formed. 55 800oC SrTiO3 TiO2 - Anatase Intensity (a.u) 700oC 600oC 450oC 10 Sr(NO3)3 20 30 40 50 60 70 2 Figure 4.1 XRD pattern of SrTiO3 at various calcination temperatures 80 56 For further analysis, calcinations temperature at 800⁰C was chosen as the optimum synthesis temperatures of the sample because of the phases are well matched and it‟s highly crystalline. Figure 4.2 shows the XRD pattern of SrTiO3 at 800⁰C. The patterns show the main peaks at 2θ~32.4⁰, 39.9⁰, 37.4⁰, 57.8⁰, 67.8⁰ and 77.2⁰ which correspond to the (110), (111), (200), (211), (220) and (320) respectively. Our data are in good agreement with the reported work by Potdar et al. (1992). Intensity (a.u) (110) (111) 10 20 30 40 (200) (211) 50 60 (220) 70 (310) 80 2 Figure 4.2 XRD pattern of SrTiO3 at 800⁰C. 4.2.1.2 The influence of dopant to the host structure The XRD patterns for doped and undoped sample are shown in Figure 4.3. The patterns show well-defined peaks, which indicate the crystalline and phase formation of the synthesized compounds. The diffraction patterns of SrTiO3: Pr, Al and SrTiO3:Pr phase are found to be in agreement with that of the host material SrTiO3 from the ICDD card no 84-0444 and also those are in coincidence with the 57 reported data (Kakihana et al., 1998). Comprising of these three patterns, it is found that they are in cubic structure with a space group Pm3m. Pr3+ ions could easily be substituted (in the place Sr2+) in the host matrix without any change in the host matrix structure. As for that, a change is not observed in the XRD pattern by the addition of Pr and Al ions. This correspond to the research done by Kim et al. (2004) which reported that the addition of group-IIIb ions, resulted in no changes being observed. Comparing the XRD patterns of the samples, there are also no identifiable differences of diffraction peaks even by addition of Al3+. Due to the closed ionic radius between Al3+ (0.535 Å) and Ti4+ (0.605 Å), it can be considered that Al3+ has substituted for Ti4+ site in the SrTiO3: Pr, Al sample. Tang et al. (2006) also reported that no changes were observed when Al was added to Al–CaTiO3:Pr sample which is consistent with our observation. SrTiO3 Intensity (a.u) (c) SrTiO3:1mol%Pr, 1mol%Al (b) SrTiO3:1mol%Pr (a) SrTiO3 10 20 30 40 50 60 2 Figure 4.3 XRD pattern for (a) SrTiO3 (b) SrTiO3:1mol%Pr (c) SrTiO3:1mol%Pr,1mol%Al 70 80 58 4.2.1.3 The influence of dopant concentration Figure 4.4 (a) showed the XRD patterns for various concentration up to 1 mol % of Pr3+ which was calcined at 800⁰C while Figure 4.4(b) showed patterns for various concentration up to 1 mol % of Al3+ when Pr3+ was fixed at 1 mol%. The diffraction peak positions and the relative intensities of the prepared sample were well matched with the standard powder diffraction file (ICDD card no. 84-0444). It is evident that the phosphor powders show all the peaks attributed to the SrTiO3 phase. In the XRD studies, it can be conclude that the results proved that all phosphor samples prepared in this work are almost single SrTiO3 phase. It also indicates that the little amount of co-doped Pr3+ ions and Al3+ have almost no effect on the SrTiO3 phase composition. There are no identifiable differences of diffraction peaks even addition of Pr and Al. Due to the closed ionic radius between Pr3+ and Sr2+ and Al3+ with Ti4+, it can be considered that Pr3+ substituted for Sr2+ and Al3+ substituted for Ti4+ in the SrTiO3: Pr, Al sample. This process is illustrated in Figure 4.5. This is corresponding to the research that has been done by Tang et al. (2006) which was mentioned in previous section. A small quantity of doped rare earth active ions Al3+and Pr3+ has negligible effect on the basic crystal structure. 59 (a) (b) SrTiO3 doped with Pr3+ SrTiO3 doped with Al3+ 1mol% 1 mol % 0.5mol% 0.5 mol % Intensity (a.u) 0.2mol% Intensity (a.u) 0.2 mol % 0.1mol% 0.1 mol % 0mol% 0 mol % 10 20 30 40 50 60 70 80 10 20 30 2 40 50 60 2 Figure 4.4 XRD pattern of SrTiO3 doped with various concentration of (a) Pr3+ and (b)doped with Al3+ when Pr3+ was fixed at 1 mol % 70 80 60 Sr2+ addition Substitution of Sr2+ by Pr3+ Substitution of Ti4+ by Al3+ Figure 4.5 Illustration of substitution process in SrTiO3 sample as addition of Pr and Al 4.3 Grain Size and Morphology Analysis On the basis of XRD patterns, full-width at half-maximum (FWHM) data could be analyzed by Scherrer formula (Equation 2.2) to determine the average crystallite sizes Figure 4.6 shows the grain size obtained by Scherrer‟s equation for (110) cubic direction as a function of the dopant concentration. We observe that the grain are nanosize, ranging approximately from 25 to 55 nm and is dependent on the dopant concentration, which has also been reported by Buscaglia et al. (2000). In this research, increasing of Pr concentration increased the grain size which is show in 61 Figure 4.6 (a) while increasing Al concentrations with Pr fixed causes a decrease in the grain size which is shown in Figure 4.6 (b). SEM study was carried out to investigate the surface morphology and the crystallite sizes of the synthesized phosphor powder. The powder samples calcined at temperature 800⁰C for 2 hours were used for these experiments. Figure 4.7 (a-c) shows the representative SEM micrographs taken for SrTiO3, SrTiO3:1mol%Pr and SrTiO3:1mol%Pr, 1mol%Al, respectively. It can be seen that the crystallite morphology of phosphors varied with addition of dopant. This indicated that the addition of dopant and co-activator influence the texture and morphology of SrTiO3. (a) (b) Figure 4.6 Grain size obtained using the Scherrer‟s equation as a function of (a) Pr concentration (b) Al concentration when Pr3+ fixed at 1 mol % 62 (a) (b) (c) Figure 4.7 Scanning electron micrographs of (a) SrTiO3 (b) SrTiO3:1mol%Pr (c) SrTiO3:1mol%Pr, 1mol%Al calcined at 800⁰C 4.4 Compositional analyses Figure 4.8 represent the elemental analysis of the synthesized products was performed using the energy dispersive X-ray analysis (EDAX) technique. Figure 4.8(a)–(c) present the EDAX spectra of SrTiO3, SrTiO3:1mol%Pr, SrTiO3:1mol%Pr, 1mol%Al phosphor. Respectively, it shows that the compositions of phosphor samples mainly consist of Sr, Ti and O with a trace of Al and Pr elements. EDAX analysis was carried out mainly to confirm the presence of rare-earth ions in the phosphor prepared and the results confirm their presence. 63 Figure 4.8(a) shows the EDAX result for undoped SrTiO3. In this figure it clearly shows that Sr become a major element in this sample. Cl elements which origin from the raw material (Hydrochloric acid, HCl) was also observed in this spectrum. As Pr was added to the host structure, Figure 4.8(b) shows their presence although the concentration of Pr is small. The same phenomena was observed when small amounts of Al concentration was added and Figure 4.8(c) confirmed the Al presence in the sample. Pr and Al elements trace by EDAX confirm that this instrument can be used to detect doping although in small amount which is difficult to detect using XRD. 4.5 Infrared (IR) spectra 4.5.1 The influence of calcined temperature on host structure FTIR spectra of SrTiO3 host matrix were recorded and studied in the wave number range 400-4000 cm-1. FTIR spectra of SrTiO3 samples at different calcinations temperatures which are 600⁰C, 700⁰C and 800⁰C are shown in Figure 4.9. Phase sample calcined at 600⁰C for 2 hours shows prominent absorption peaks obtained at 3405,1625, 1462, 861, 560 and 456 cm-1. In the case of SrTiO3 with calcined at temperature 700⁰C absorption peaks were obtained at 3414, 1633, 1468, 861, 576 and 439 cm-1. At a higher temperature of 800⁰C, five peaks also develop at 3407, 1627, 1469, 861, 565 and 433 cm-1. 64 Counts (a) 4400 4000 3600 3200 2800 2400 2000 1600 1200 800 400 0 Sr 0.00 Counts (b) Ti TiO Ti C 6000 5500 5000 4500 4000 3500 3000 2500 2000 1500 1000 500 0 Sr 0.80 ClCl 1.60 2.40 Ti 3.20 4.00 Energy (keV) 4.80 5.60 6.40 7.20 S r C T i O T i T i 0.00 Pr Pr T Pr Pr i S r 1.00 2.00 3.00 4.00 5.00 6.00 7.00 8.00 9.00 10.00 7.00 8.00 9.00 10.00 Energy (keV) Counts (c) 4400 4000 3600 3200 2800 2400 2000 1600 1200 800 400 0 S r T Ti iO C 0.00 C Cl l S A Pr r l 1.00 2.00 3.00 T i Pr T Pr Pr i 4.00 5.00 Energy (keV) 6.00 Figure 4.8 EDAX spectra of (a) SrTiO3 (b) SrTiO3:1mol%Pr (c) SrTiO3:1mol%Pr, 1mol%Al phosphor 65 In the case of this SrTiO3, the vibrational peaks at 3405, 3414 and 3407 cm-1 corresponding to calcinations at 600⁰C, 700⁰C and 800⁰C are due to hydrogen bonded water. The area and intensity of the bands which accounts the presence of water diminishes with rise in calcinations temperature. The peak at 3405 cm-1 corresponding to calcinations temperature 600⁰C has maximum area and intensity. The peak at 3407 cm-1 developed during calcined at 800⁰C has the least area and intensity. The bands at 1625, 1633 and 1627 cm-1 are due to the deformation vibrations of water molecules (δH2O). The appearance of this mode is probably due to the adsorption of water during the compaction of the powder specimens with KBr. The peaks at 861, 1462, 1468 and 1469 cm-1 are due to cation coordination by carboxylic groups, indicating the absorption of CO2 molecules on the surface of the samples, tend to disappears with increasing the calcinations temperature. Absorption peaks at 560, 576 and 565 cm-1 characteristic for an octahedral coordinated Ti-O stretching mode is observed which correspond to Last (1957) and Park et al. (2001) studies. Band around 400 cm-1 can be assigned as O-Ti-O bending mode. Our data are in good agreement with the reported work on poly crystalline SrTiO3 by Last (1957). The most important bands and their assignments are listed in Table 4.1. Table 4.1: Position (cm-1) and Assignment of IR Bands IR bands (cm-1) Assignments Our work Literature 3444 3420 υ(O-H) 1627 1630 δ(H2O) 1468 1490–1450 υ(CO3)2- 563 568 υ(Ti-O) 395 δ(O-Ti-O) 380, 420, 440, 450, 590, 660 Pr2O3 445, 425,540 δ(O–Al–O) 399-450 Intensity (a.u) v[Ti-O] 565 1469 1627 3407 861 800C 576 456 1468 1633 861 439 700C 3414 433 v[CO3]2v[CO3]2- δ[HOH] v[OH] δ[O-Ti-O] 66 4000 3600 3200 2800 2400 2000 1800 1600 560 1462 1625 3405 861 600C 1400 1200 1000 800 Wavenumber (cm-1) Figure 4.9 FTIR spectra for undoped SrTiO3 at different calcinations temperatures 600 400 67 4.5.2 The influence of dopant addition to the host structure Figure 4.10 shows the FTIR spectra for undoped SrTiO3, SrTiO3: Pr and SrTiO3: Pr, Al which were calcined at 800⁰C. Undoped SrTiO3 show prominent peaks at 3407, 1627, 1469, 861, 565 and 433 cm-1. When Pr was added, absorption peaks were obtained at 3425, 1633, 1465, 861, 559 and 438 cm-1. Four peaks existed when Al was added which is at 3434, 1632, 556 and 436 cm-1. Referring to the discussion on infrared studies in the previous chapter, the bands at 3407, 3425 and 3434 cm-1 show the presence of considerable amounts of H2O (Um and Kumazawa, 2000). The absorption peaks around 1600 cm-1 correspond to the bending vibrations of adsorbed water molecules (δH2O) (Yan et al., 2009). According to Kumar and Buddhudu (2009), bands at 1400 and 861 cm-1 are due to the vibrations of CO32 anions. The width and intensity of 1400 cm-1 band decrease with addition of Pr and vanishes when Al was added while band at 861 cm-1 vanishes with the addition of the Pr and Al, showing removal of organics. With SrTiO3: Pr and SrTiO3: Pr, Al patterns, there appears to be no difference in the finger print region (400-600 cm-1) with respect to SrTiO3 host matrix which does not allow us to determine the influence of dopant addition to the host matrix. 438 433 559 4000 3600 3200 2800 2400 2000 1800 1600 1400 1200 1000 800 565 861 1469 1627 436 v[Ti-O] 556 861 1465 1633 (a) SrTiO3 3407 v[CO3]2- v[CO3]2- (b) SrTiO3: Pr 3425 Intensity a.u δ[HOH] 1632 (c) SrTiO3: Pr, Al 3434 v[OH] 68 600 400 Wavenumber cm-1 Figure 4.10 FTIR spectra of (a) undoped SrTiO3 (b) SrTiO3: Pr (c) SrTiO3: Pr, Al which calcined at 800⁰C 69 4.5.3 The influence of dopant concentration 4.5.3.1 Pr addition FTIR spectra of powder sample SrTiO3: xPr (0 ≤ x ≤ 1 mol %) in the frequency range 399–1500 cm−1 is shown in Figure 4.11. As can be seen from the figure, main bands appearing along the spectrum are in the region of 600-400 cm-1. The effect of Pr doping on the infrared spectral feature of local structure of SrTiO3 is clearly shown in the low-frequency regions. In this study, it can be seen that band around 400-450 cm-1 clearly appear with increasing Pr content. These bands are due to praseodymium oxide but by Last (1957) band around 400 cm-1 also can be assigned as O-Ti-O bending mode. It can be assume that there are overlapping band in these region. The bending vibration band is still considered as a scattering effect, but not a real absorption band. 4.5.3.2 Al addition The FTIR pattern for SrTiO3: 1mol%Pr, yAl (0 ≤ y ≤ 1 mol %) in the frequency range 399–1500 cm−1 is illustrated in Figure 4.12. With increasing Al3+ doping concentration (y=0.1-1 mol %), there are only have slight changes in the finger print region especially in the low region. δ[O-Ti-O] / Pr2O3 v[Ti-O] v[CO3]2- 70 455 435 555 1468 v[CO3]2- 455 435 x = 1 mol % 576 1457 858 x = 0.2 mol % 435 559 1465 Intensity (a.u) 858 x = 0.5 mol % 427 861 x = 0.1 mol % 861 1400 565 1469 1500 433 568 x = 0 mol % 1300 1200 1100 1000 900 800 700 600 500 399.2 Wavenumber (cm-1) Figure 4.11 FTIR spectra of the SrTiO3: xPr with 0 ≤ x ≤ 1 mol % which calcined at 800⁰C 71 Study that have been done by Mikhailov (2001) et al stated that the band due to symmetric bending vibrations of O–Al–O or Al-O-Al bonds or deformation of tetrahedral and octahedral units, appears at 445, 425 and 540 cm−1. From the literature, it can be assumed that Al vibration also occurred at the same frequencies either with Ti or Pr in the region 400- 600 cm-1. Al, Pr and Ti vibration mode at low wavenumber cannot be measured, because the spectrum was recorded starting at 400 cm-1. Further study is really necessary to index the Al, Pr and Ti peak. In IR studies the vibration of Sr2+ cannot be detected because when Sr enter the TiO6, it formed ionic bonding. IR spectroscopy only can detect covalent bonding. Raman spectroscopy has in general better frequency resolution for ceramic material than IR spectroscopy and is worthy to stress here is that unlike IR spectroscopy it is sensitive to the presence of TiO2. 4.6 Raman Spectra 4.6.1 The influence of calcined temperature on Host Structure According to factor group analysis, anatase TiO2 has six Raman active modes (A1g+ 2B1g + 3Eg). The Raman spectrum of an anatase single crystal has been investigated by Ohsaka (1980), who concluded that the six allowed modes appear at 144 cm-1 (Eg), 197 cm-1 (Eg), 399 cm-1 (B1g), 513 cm-1 (A1g), 519 cm-1 (B1g), and 639 72 cm-1 (Eg). In this study, we assigned and interpreted the Raman bands of the SrTiO3 563 y = 1 mol % 440 v[Ti-O] δ[O-Ti-O] / Pr2O3 /Al203 using earlier results obtained for the bulk phase. 566 y = 0.2 mol % 444 561 Intensity (a.u) 433 y = 0.5 mol % 550 441 y = 0.1 mol % 555 435 y = 0 mol % 1500 1400 1300 1200 1100 1000 900 800 700 600 500 Wavenumber (cm-1) Figure 4.12 FTIR spectra of the SrTiO3: 1mol%Pr, yAl with 0 ≤ y ≤ 1 mol% which calcined at 800⁰C 399.2 73 Figure 4.13 shows the Raman spectrum of the SrTiO3 samples calcined at 600-800⁰C for 2 hrs. The Raman bands are assigned based on the literature data on the wave number ranges related to the corresponding vibrations of the structural units in various glassy and crystalline titanate. The position and the relative intensity of each component of Raman spectra related to the vibrational mode of titanate units were determined. All of the Raman spectrums in Figure 4.13 have five peaks which are located at 146, 251, 406, 526 and 641 cm−1 identical with the feature of anatase type TiO2, and a weak peak observed at 1072 cm-1. Peaks 146, 251and 406 cm-1 is attributed to the O-Ti- bending mode while 526 and 641 cm−1 are attributed to Ti-O vibration mode, υ(Ti-O). Weak peak at 1072 cm-1 is assigned to assigned to the υ(CO3)2symmetric stretching mode (Frost Ray, 2007). This results correspond with Hyun Chun Choi et al. (2005) studied on size effects in the raman spectra of TiO2 nanoparticles while according to Federico et al. (2008), SrCO3 displays a strong peak at 148 cm-1. In our experimental evidence suggests that the peak at 146 cm-1 observed in SrTiO3 raman spectra overlapped between SrCO3 and O-Ti-O bending mode, δ(O-Ti-O). From the literature and review that have been done, the Raman vibration for our sample can be concluded as in Table 4.2. Table 4.2 Raman modes comparison with the literature values Raman Shift (cm-1) Assignments Our work Literature 146 144 Eg / δ(O-Ti-O) 146 148 SrCO3 251 197 Eg / δ(O-Ti-O) 406 399 B1g/ δ(O-Ti-O) 526 513,519 A1g, B1g / υ(Ti-O) 641 639 Eg / υ(Ti-O) 1072 1072 υ(CO3)2- 74 δ(O-Ti-O) υ(Ti-O) Intensity (a.u) υ(CO3)2800C 251 1072 641 526 600C 406 146 700C 100 200 300 400 500 600 700 800 900 1000 1100 1200 1300 1400 1500 1600 1700 1800 1900 2000 Raman Shift (cm-1) Figure 4.13 Raman spectrum of the SrTiO3 samples calcined at 600-800⁰C for 2 h. 75 4.6.2 The influence of dopant addition to the host structure The doping dependence of Raman spectra of SrTiO3 is shown in Figure 4.13. The low-frequency modes below 900 cm−1 show marked doping dependence while the change of higher frequency modes is not remarkable as dopants were added. As dopants were added to the SrTiO3 systems, according to Nilsen et al, we find that the Raman spectrum to be entirely attributed to second-order Raman scattering which is show by Figure 4.14(b) and (c). Both figures consist of two second-order broad bands centered in the 200-400 and 600-800 cm-1 region. The assignments proposed by previous authors which are Nilsen et al. (1968) and Perry et al. (1967) for the two broad bands are in good agreement with our data. When Al is added to the SrTiO3:1mol%Pr system, there are new bands appearing at lowfrequency which could be assume the band of SrCO3 overlapped with O-Ti-O bending mode. There are also slight changes in 200-400 and 600-800 cm-1 region as Al added to the system. The intensities of band at 303 cm-1 decrease with addition of Al causing a reduction of the band contour around 300-400 cm-1. Same phenomenon also happened for the 600-800 cm-1 region, which decrease by Al addition. Unfortunately, there are few reports on the vibrational study of second-order Raman scattering. The exact mechanism for this observation is not clearly understood as of yet. 76 υ(CO3)2- Intensity (a.u) (c) SrTiO3:1%Pr, 1%Al 303 (b) SrTiO3:1%Pr (a)SrTiO3 100 200 300 400 500 600 700 800 900 1000 1100 1200 1300 1400 1500 1600 1700 1800 1900 2000 Raman Shift (cm-1) Figure 4.14 Raman spectra of SrTiO3 sample by addition of dopant which calcined at 800⁰C 4.6.3 The Influence of dopiant concentration 4.6.3.1 Pr addition Figure 4.15 presents the Raman spectra of SrTiO3:xPr with different doping concentrations (0≤ x ≤1 mol%).When the concentration of Pr is increase from 0.1 mol% to 1 mol%, it exhibits second-order Raman spectrum of SrTiO3 and all the Raman spectra show similar spectra. 77 4.6.3.2 Al addition Figure 4.16 represent the Raman spectra for the phosphor sample as concentration of Al change when concentration of Pr fixed at 1 mol%. As concentration of Al increase from 0.1 mol% to 1 mol%, a change in the second-order Raman spectrum was observed. When Al concentrations are 0.5 and 1 mol %, new band is observed at 120 cm-1.A weak peak at 171 cm-1 show up at y=0.2 mol % and clearly seen at y=0.5 and 1 mol %. The intensities of band 238 and 400 cm -1 decrease with the increasing of Al3+ concentration causing a reduction of the band contour around 200-610 cm-1.Also, shoulder at 780 cm-1 becoming more intense as concentration of Al3+ increase. A weak peak at 1072 cm-1 is attributed to the υ(CO3)2. . The vibrational studies using IR confirmed the vibration of Ti but cannot detect the vibration of dopant because it overlapped with the host vibration. Raman analysis shows by addition of dopant, the patterns become second-order Raman scattering of SrTiO3. It can be observed that by addition of dopant, there are changes on the patterns but cannot be explained clearly because of lack of references on second-order Raman scattering of SrTiO3. This phenomenon is interesting to study but there are a few reports in this area and need much more time to make it clear. 78 Intensity (a.u) υ(CO3)2- x = 1 mol % x = 0.5 mol % x = 0.2 mol % x = 0.1 mol % x = 0 mol % 100 200 300 400 500 600 700 800 900 1000 1100 1200 1300 1400 1500 1600 1700 1800 1900 2000 Raman Shift (cm-1) Figure 4.15 Raman spectra of SrTiO3:xPr with various Pr concentrations (0≤ x ≤1 mol %) which calcined at 800⁰C 120 cm-1 171 cm-1 79 υ(CO3)2- y = 0.5 mol % 780 cm-1 606 cm-1 y = 0.2 mol % 400 cm-1 238 cm-1 Intensity (a.u) y = 1 mol % y = 0.1 mol % y = 0 mol % 100 200 300 400 500 600 700 800 900 1000 1100 1200 1300 1400 1500 1600 1700 1800 1900 2000 Raman Shift (cm-1) Figure 4.16 Raman spectra of SrTiO3:1 mol% Pr, yAl with 0≤ y≤1 mol % which calcined at 800⁰C 80 4.7 Photoluminescence Spectra Figure 4.17, 4.19 and 4.20 represent emission spectra for the representative samples. The samples were excited with 325 nm wavelength and the emission spectra were recorded. 4.7.1 The influence of dopant addition Effect of dopant on luminescence properties of SrTiO3 are show in Figure 4.17. Undoped SrTiO3 showed that bands in the region of 330 to 430 nm were attributed to the host emission which corresponds with Pr and Al doping spectrum. As the Pr3+ is added, the region of higher wavelength (430-730nm) exhibit the emission spectrum consisted of four emission peaks, originating from the 3P0 →3Hj (j=6, 5, 4) and 1D2→3H4 transitions of Pr3+,respectively.This was assigned based on energy level of Pr3+ (Figure 4.18). From the literature, had the emission originated from the 3P0 level, it would be green as in Gd2O2S:Pr; however, if the emission originated from 1D2 level, it would be red (Blasse, 1994). In the presence of Al3+ addition, the intensity of 1D2→3H4 tends to be stronger with an unchanged position. This agrees with Yamamoto and Okamoto (2000) study on efficiency enhancement by aluminum addition to some oxide phosphors for field emission displays. SrTiO3:Pr,Al 3 P0 →3H6 P0 →3H5 SrTiO3:Pr 3 3 SrTiO3 P0 →3H4 1 D2→3H4 81 Figure 4.17 Photoluminescence (PL) spectra of SrTiO3, SrTiO3:Pr3+ and SrTiO3:Pr, Al Figure 4.18 Energy level of Pr3+ 82 4.7.2 The influence of Pr concentration Figure 4.19 presents the photoluminescence emission spectra of SrTiO3 with different Pr3+ concentrations (0-1 mol% of Pr) under 325 nm excitation at room temperature. Figure shows that by addition of Pr, the 3P0 →3H4 band dominates the spectrum. Apart from this, three other emission bands corresponding to the radiative 4f → 4f transitions from some excited states of the Pr3+ also observed. The bands in the blue-green region 480-500 nm and 517-536 nm are assigned to the transition 3P0 →3H4 and 3P0 →3H5 of Pr3+, respectively, while the band in the red region 600-630 nm is associated with the 1D2→3H4 transition of Pr3+. Refer to the dominant peak of the spectrum which is at 490 nm, the maximum emission intensity is at 0.5 mol% Pr3+. Beyond this doping level, emission intensity decreased with increasing Pr3+ contents, presumably due to concentrations quenching. 4.7.3 The influence of Al concentration In this section we investigated Al addition effect on photoluminescence (PL) properties of Pr-doped SrTiO3. Figure 4.20 shows the PL spectra for SrTiO3:Pr, yAl in the range 0≤y≤1 mol % and Pr3+ was kept constant at 1 mol% under the 325 nm laser excitation at room temperature. The PL spectrum have peak at 490, 530, 615 and 650 nm, which is attributed to transition from excited states 3 3 P0 →3H4 , P0→3H5, 1D2→3H4 and 3P0 →3H6 of Pr3+, respectively. As the addition of Al, band at 615 nm which emit red emission dominates the spectrum which is attributed to the intra-4f transition from the excited state 1D2 to P0 →3H6 D2→3H4 1 3 P0 →3H5 3 3 P0 →3H4 83 Figure 4.19 Photoluminescence spectra of the SrTiO3:xPr (0≤x≤1 mol %) phosphors under 325 nm excitation the ground state 3H4 of Pr3+(1D2→3H4 emission), instead of by Pr addition, the domain band is at 3P0 →3H4 transition. It can be noticed from Figure 4.20 that the emission intensity increases with doping of the second ion Al3+. In fact the intensity of emission increases with the increase in Al3+ concentration up to 0.2 mol%, beyond which the emission intensity decreases.Leverenz has proposed an explanation of this concentration quenching phenomenon. According to his results, optimum concentration of Mn in ZnSiO4 is approximately 0.3 wt.%. At above 0.5 Mn wt.% in 84 the host material, it is generally found that increases in chemical complexity and structural heterogeneity of phosphors decreased their relative emission intensity (Leverenz, 1968). These phenomena may be attributed to concentration quenching, 3 P0 →3H6 P0 →3H5 3 3 P0 →3H4 1 D2→3H4 though the mechanism of it is not yet clearly understood. Figure 4.20 PL spectra of SrTiO3:Pr, yAl (Pr3+:1 mol%) with various molar ratios of Al (0≤y≤1 mol %) under the 325 nm laser excitation at room temperature The results described above indicates that SrTiO3:Pr exhibits the typical emission profile with a prominent luminescence in the greenish-blue region arising from 3 P0 level, while SrTiO3:Pr,Al shows its characteristic 1 D2→3H4 red luminescence. We are focus on the red emission of the sample which are assign by 85 1 D2→3H4 transition. The red emission of SrTiO3: Pr3+ with addition of Al3+ was greatly intensified compared to the undoped Al samples. Pr3+ substitutes for the Sr2+ site in the SrTiO3 lattices and a positive charge defect is formed. Such charge defects hamper the process of energy transfer from the host to Pr3+, but it can be compensated by the negative charge defect when Ti4+ ion is substituted by Al3+ ions and producing a vacancy in the process. This vacancy ultimately plays an important role in enhancement of the luminescence efficiency Pr3+ ions, which act as an activator and emit the red luminescence, cannot directly absorb the radiation used for excitation. The excitation radiation is absorbed by the lattice defects associated with Al3+ ions, which act as sensitizers, and the Al3+ ions subsequently transfer it to the Pr3+ ions. After this, the Pr3+ ions emit the radiation. Figure 4.21 shows a tentative scheme of the Pr3+ energy levels and defect levels with respect to the conduction and valence bands of SrTiO3.The band gap for pure SrTiO3 is 3.5 eV (Kim et al. 2007); it is very close to the one for GaN (about 3.8 eV) (Dorenbos and van der Kolk, 2006). Since energy levels of rare earth ions vary little from host to host, we assume that the relative positions of the Pr3+ energy levels in both hosts are close to each other. This means that the 3P0,1,2 energy levels are located close to the bottom of the conduction band and even more closer to the defect‟s energy levels located in the forbidden gap below the conduction band. Such a location of energy levels favors energy transfer from Al3+ ions to Pr3+ ions, as shown in Figure 4.21. Approximate location of the Pr3+ energy levels with respect to the valence band (VB) and conduction band (CB) of SrTiO3 was obtained in analogy with Dorenbos et al. (2006) 86 CB Defect levels 25000 3 P2 P1, 1I6 3 P0 3 20000 Energy, cm-1 1 D2 15000 1 10000 G4 3 3 5000 3.5 eV=28229 cm-1 F4 F3 3 F2 3 H6 3 H5 3 H4 VB Figure 4.21 Proposed scheme of energy transfer from the defects created around Al3+ levels to the (3P0, 1, 2 , 1I6, 1D2) energy levels of Pr3+. CHAPTER 5 CONCLUSIONS AND RECOMMENDATIONS 5.1 Conclusions 5.1.1 Structural Studies Using sol-gel method, fine powders of SrTiO3: Pr, Al phosphor was prepared. This method yielded SrTiO3 formation lower temperatures than required for solid state reaction method which is at 600⁰C. Te structural studies of the samples have been investigated using X-Ray Diffraction, Infrared and Raman Spectroscopy. XRD patterns show the phase formation of SrTiO3 phase which can be indexed to the cubic phase structure of SrTiO3. No changes in phase formation were observed on the host lattice as addition of Pr and Al dopant. This means that the structure retains the host perovskite phase. Grain size was obtained using the Scherrer‟s equation as a function of Pr concentration and Al concentration. We observe that the grain size have nanoscale range, ranging approximately from 25 to 55 nm. 88 IR spectra exhibit the stretching and bending vibration of sample. The band at 527 cm-1 is due to the Ti-O stretching vibrations. Furthermore, the bands in the region 400-500 cm-1 are attributed to the Ti-O-Ti bending mode which overlapped with Pr2O3 and also Al2O3 vibration. As for that, FTIR cannot be used as local probe of doping rare-earth in the host material SrTiO3. Raman spectroscopy has in general better frequency resolution for ceramic material than IR spectroscopy and worthy to stress here is that unlike IR spectroscopy it is sensitive to the presence of TiO2. SrTiO3 host material has five peaks which is located at 146, 251, 406, 526 and 641 cm−1 which is almost similar with anatase TiO2. Peak at 146 cm-1 was overlapped between SrCO3 and O-Ti-O bending mode. With addition of dopants, it is found that the Raman spectrum to be entirely attributed to second-order Raman scattering. When the sample are doped, a slight shift was observed on the characteristic position of Raman modes that can be related to the degree of crystallization, interaction force between ions, structural defects and influence of doping. 5.1.2 Luminescence studies From the findings, addition of Pr doping make the 3P0 →3H4 transition has a larger intensity than the 1D2→3H4 transition of Pr3+ but with addition of Al, 1 D2→3H4 transition became dominant. As for that, SrTiO3:Pr has a blue-green emission between 480-500 nm red emission for SrTiO3: Pr,Al. The SrTiO3: Pr,Al phosphor has a red emission band between 580 and 640 nm peaking at 617 nm. This is due to the radiative decay of the 1D2 states (1D2→3H4 transition). Emission intensity increased with the increasing activator concentration up to maximum at 0.5 mol% of Pr3+ without addition of Al. As the Pr fixed at 1 89 mol%, the emission intensity up to a maximum at 0.2 mol% of Al3+. Beyond these doping level, emission intensity decreased with increasing doping contents. This decrease is understood to be due to concentration quenching. In conclusion from this research, the blue-green color emission of SrTiO3:Pr can be tuned to red by simply introducing a small amount of Al ions into the host to disturb the microstructure. Consequently, this color tunable phosphors offer a flexible choice for any potential application of phosphor material either the blue-green or red emission. 5.2 Recommendations Although it has been shown that the sol-gel method can synthesize SrTiO3, the processes have not been well controlled. Hence, further investigations in the controlled environment are needed to synthesize a quality samples.This experiment is done in open air, so, for future work; it is suggested to run this experiment under a controlled environment like in the glove box. In this work, we just used small amount of dopant concentration. So, for future work, we propose that the dopant concentration is varied up to 20 mol% or more to observe the effect on structural and luminescence properties. Moreover, it is an interesting observation when using raman spectroscopy, it showed that the spectrum become second-order Raman scattering. More thorough and systematic studies should be performed in order to understand the formation of second-order Raman scattering of SrTiO3.The mechanisms of this formation also requires further investigation in order to make it clear. 90 Since this material has potential application in field emission display (FED), more measurement are needed in order to understand the influence of doping addition on luminescence properties such as by using Transmission Electron Microscopy(TEM) which can detect the defect in samples. Besides that, because of only few researches have been done on the structural studies of this material; it is recommended that for future work a detail study should be done focusing on the local structure. As for that, Nuclear Magnetic Resonance (NMR) could be a great technique to use besides other measurements. 91 REFERENCES Blasse, G. and Grabmaier, B.C. (1994). Luminescent Materials. Berlin: SpringerVerlag. Bol, A. A., Ferwerda, J., Bergwerff, J. A., and Meijerink, A. (2002). Luminescence of nanocrystalline ZnS:Cu2+. Journal of Luminescence, 99(4), 325-334. Bose, H. N. (1992). Luminescence and allied phenomena. Indian Journal of History Science 27(4). Buscaglia, M. T., Buscaglia, V., Viviani, M., Nanni, P., & Hanuskova, M. (2000). Influence of foreign ions on the crystal structure of BaTiO3. Journal of the European Ceramic Society, 20, 1997-2007. Byong Kee Moon, I.-M. K., Hyun Kyoung Yang, Hyo Jin Seo, Jung Hyun Jeong, Soung Soo Yi and Jung Hwan Kim. (2008). Spectroscopy of nanocrystalline TiO2:Eu3+ phosphors. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 313-314, 5. Chang, C. and Mao, D. (2004). Long lasting phosphorescence of Sr4Al14O25:Eu2+, Dy3+ thin films by magnetron sputtering. Thin Solid Films, 460(1-2), 48-52. Chang, C.A., Ray, B., Paul, D. K., Demydov, D., & Klabunde, K. J. (2008). Photocatalytic reaction of acetaldehyde over SrTiO3 nanoparticles. Journal of Molecular Catalysis A: Chemical, 281(1-2), 99-106. Cullity, B. D. (1978). Elements of X-Ray diffraction (2nd ed ed.): Addison-Wesley Publishing Company, Inc., Reading, MA. Deren, P.J., R. P., W. Strek. Ph. Boutinaud, R. Mahiou. (2008). Synthesis and spectroscopic properties of CaTiO3 nanocrystals doped with Pr3+ ions. Journal of Alloys and Compounds, 451, 595-599. Diallo, P., Boutinaud, P., Mahiou, R. and Cousseins, J.(1997). Red Luminescence in Pr3+- Doped Calcium Titanates. phys. stat. sol. (a)(160), 255. 92 Dorenbos, P., & van der Kolk, E. (2006). Location of lanthanide impurity levels in the III-V semiconductor GaN. Applied Physics Letters, 89(6), 061122061123. Federico A. Rabuffetti, H.-S. K., James A. Enterkin, Yingmin Wang,Courtney H. Lanier, Laurence D. Marks, Kenneth R. Poeppelmeier and, and Stair, P. C. (2008). Synthesis-Dependent First-Order Raman Scattering in SrTiO3 Nanocubes at Room Temperature. Chem. Mater., 20, 8. Fouassier, C. (1984). Luminescence-Encyclopedia of Inorganic Chemistry Academic Press New York Frost Ray L, H. M. C., Jagannadha Reddy B. (2007). Aurichalcite - : An SEM and Raman spectroscopic study. Polyhedron, 26(13), 10. Gfroerer, T. H. (2000). Photoluminescence in Analysis of Surfaces and Interfaces., Encyclopedia of Analytical Chemistry. Chichester: John Wiley & Sons Ltd. Guo, H., Dong, N., Yin, M., Zhang, W., Lou, L., and Xia, S. (2006). Green and red upconversion luminescence in Er3+-doped and Er3+/Yb3+-codoped SrTiO3 ultrafine powders. Journal of Alloys and Compounds, 415(1-2), 280-283. Hussein, G. A. M. (1994). Formation of praseodymium oxide from the thermal decomposition of hydrated praseodymium acetate and oxalate. Journal of Analytical and Applied Pyrolysis, 29(1), 13. Hyun Chul Choi, Y. M. J., Seung Bin Kim. (2005). Size effects in the Raman spectra of TiO2 nanoparticles. Vibrational Spectroscopy, 37, 6. Jiang, L., Chang, C., Mao, D., and Zhang, B. (2004). A new long persistent blueemitting Sr2ZnSi2O7:Eu2+, Dy3+ prepared by sol-gel method. Materials Letters, 58(12-13), 1825-1829. Joseph, J. (2001). Luminescence Study on Geological Samples and Doped Phosphors. Mahatma Gandhi University, Kottayam. Kakihana, M., Okubo, T., Arima, M., Nakamura, Y., Yashima, M., & Yoshimura, M. (1998). Polymerized Complex Route to the Synthesis of Pure SrTiO3 at Reduced Temperatures: Implication for Formation of Sr-Ti Heterometallic Citric Acid Complex. Journal of Sol-Gel Science and Technology, 12(2), 95109. Kim, J. Y., You, Y. C., Kang, J. H., Jeon, D. Y., and Weber, J. (2004). New perspective in degradation mechanism of SrTiO3:Pr,Al,Ga phosphors: Materials Research Society. Kim, J.D., T. M., and Itaru Honma. (2007). SrTiO3 Thin Films with Visible-Light Band Gap Fabricated by Nitrogen Reactive Sputtering. Japanese Journal of Applied Physics, 46, 2. 93 Kumar, G. B., and Buddhudu, S. (2009). Synthesis and emission analysis of RE3+ (Eu3+ or Dy3+):Li2TiO3 ceramics. Ceramics International, 35(1), 521-525. Last, J. T. (1957). Infrared-Absorption Studies on Barium Titanate and Related Materials. Physical Review, 105(6), 1740. Leverenz, H. W. (1968). An Introduction to Luminescence of Solids. New York: Dover. Lucas Alonso Rocha, L. R. A., Bruna Leonardo Caetano, Eduardo Ferreira Molina, Herica Cristina Sacco, Katia Jorge Ciuffi, Paulo Sergio Calefi, Eduardo Jose Nassar. (2005). Europium incorporated into titanium oxide by the sol-gel method. Material Research, 8(3), 8. Mikhailov, D. A. J. a. G. G. (2001). Phase diagram of CaO–Al2O3 system. Ceramics International, 27(1), 4 Monica Popa, M. K. (2001). Praseodymium oxide formation by thermal decomposition of a praseodymium complex. Solid State Ionics, 141-142, 8. Nilsen, W. G., and Skinner, J. G. (1968). Raman Spectrum of Strontium Titanate. The Journal of Chemical Physics, 48(5), 2240-2248. Ohsaka, T. (1980). Journal of The Physical Society of Japan, 48, 1661. Park, J. K., Hojin Ryu., Hee Dong Park and Se-Young Choi. (2001). Synthesis of SrTiO3:Al, Pr phosphors from a complex precursor polymer and their luminescent properties. Journal of the European Ceramic Society, 21(4), 8. Perry, C. H., Fertel, J. H., and McNelly, T. F. (1967). Temperature Dependence of the Raman Spectrum of SrTiO3 and KTaO3. The Journal of Chemical Physics, 47(5), 1619-1625. Potdar, H.S., Deshpande, S.B., Godbole, P.D., Gunjikar, V.G., Date, S.K.. (1992). Low temperature synthesis of ultrafine strontium titanate (SrTiO3) powders. J. Mater. Res, 7(2). Qin Fei, C. C. a. D. M. (2005). Luminescent properties of Sr2MgSi2O7 and Ca2MgSi2O7 long lasting phosphors activated by Eu2+, Dy3+. Journal of Alloys and Compounds, 390(1-2), 5. Ronda, C.R., Justel, T. and Nikol, H. (1998). Rare earth phosphors: fundamentals and applications. Journal of Alloys and Compounds, 275-277, 7. Tang, J., Yu, X., Yang, L., Zhou, C., and Peng, X. (2006). Preparation and Al3+ enhanced photoluminescence properties of CaTiO3:Pr3+. Materials Letters, 60(3), 326-329. 94 Um, M. H., & Kumazawa, H. (2000). Hydrothermal Synthesis of Ferroelectric Barium And Strontium Titanate Extremely Fine Particles. Journal of Materials Science, 35(5), 1295-1300. Vij, D. R. (1998). Luminescence of Solids. New York: Plenum Publishing Corporation Wang, N., Lin, H., Li, J., Yang, X., and Zhang, L. (2007). Photoluminescence of TiO2:Eu nanotubes prepared by a two-step approach. Journal of Luminescence, 122-123, 889-891. William M. Yen, Marvin J. Weber. (2004). Inorganic Phophors: Compositions, Preparation and Optical Properties: CRC Press LLC. William M. Yen, S. S. and Yamamoto Hajime. (2006). Phosphor Handbook (2nd edittion ed.): CRC Press. Xia, C.-T., and Shi, C.-S. (1997). BaLiF3(Eu2+): A promising X-ray storage phosphor. Materials Research Bulletin, 32(1), 107-112. Yamamoto, H., & Okamoto, S. (2000). Efficiency enhancement by aluminum addition to some oxide phosphors for field emission displays. Displays, 21(23), 93-98. Yamamoto, H., Okamoto, S., and Kobayashi, H. (2002). Luminescence of rare-earth ions in perovskite-type oxides: from basic research to applications. Journal of Luminescence, 100(1-4), 325-332. Yan, J.-H., Zhu, Y.-R., Tang, Y.-G., and Zheng, S.-Q. (2009). Nitrogen-doped SrTiO3/TiO2 composite photocatalysts for hydrogen production under visible light irradiation. Journal of Alloys and Compounds, 472(1-2), 429-433. Yong Gao, C. S. a. Y. W. (1996). Luminescence properties of SrB4O7: Eu, Tb phosphors. Materials Research Bulletin, 31(5), 6. Zhao, C., and Chen, D. (2007). Synthesis of CaAl2O4:Eu,Nd long persistent phosphor by combustion processes and its optical properties. Materials Letters, 61(17), 3673-3675. Zhimin Liu, J. Z., Buxing Han, Jimin Du, Tiancheng Mu, Yong Wang and Zhenyu Sun. (2005). Solvothermal synthesis of mesoporous Eu2O3–TiO2 composites. Microporous and Mesoporous Materials, 81(1-3), 6.