D.3: OPIATES INTRO:
advertisement

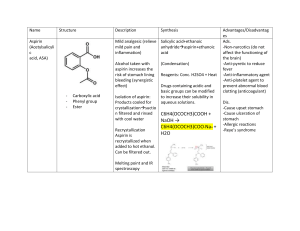
D.3: OPIATES INTRO: https://www.youtube.com/watch?v=8iaYksFXoZQ (OPIATES – PERIODIC TABLE OF VIDEOS) UNDERSTANDINGS - The ability of a drug to cross the blood-brain barrier depends on it chemical structure and solubility in ________ and ________. - Opiates are natural narcotic ___________ that are derived from the opium __________. - Morphine and codeine are used as _____________ analgesics. Strong analgesics work by temporarily binding to receptor sites in the _____________, preventing the transmission of pain impulses without depressing the central nervous system. - Medical use and addictive properties of opiates are related to the presence of opioid receptors in the brain. APPLICATION AND SKILLS - Explanation of the synthesis of codeine and diamorphine from morphine. - Description and explanation of the use of strong analgesics. - Comparison of the structures of morphine, codeine, and diamorphine (heroin). - Discussion of the advantages and disadvantages of using morphine and its derivatives as strong analgesics. - Discussion of side effects and addiction to opiate compounds. - Explanation of the increased potency of diamorphine compared to morphine based on their chemical structure and solubility. NATURE OF SCIENCE - Data and its subsequent relationships – opium and its many derivatives have been used as a painkiller in a variety of forms for thousands of years. One of these derivatives is diamorphine. 1. INTRO: STRONG ANALGESICS - OPIUM AND OPIATES (e.g. codeine, morphine, heroin) - Opium is harvested from the naturally occurring opium ____________. - The primary active ingredients in opium, _____________ and _____________, occur naturally in the opium poppy. - Morphine, codeine, and heroin are examples of _____________. - Semi-synthetic _______________ is a derivative (i.e. synthesized from) ________________. - ______________ is a totally synthetic opiate. - Opiates are also known as ______________ analgesics or strong analgesics. - Strong analgesics block the transmission of _______ impulses by temporarily binding to opioid receptors in the ____________. - Opiates are used to treat ____________ pain. (e.g., severe injuries, surgical procedures, heart attack, chronic disease) OPIATE ADDICTION: - Highly addictive - especially _____________. - Addicts develop ______________ / “chasing the dragon” scenario - Heroin is responsible for nearly _____% of all drug-related deaths (e.g. via overdose) around the globe. - Legal use is banned, or restricted to use by terminally-ill patients, in most countries. - Withdrawal symptoms occur within 6 to 24 hours for addicts if supply of drug is stopped. - _______________ is a milder analgesic used to wean addicts off heroin use. 2. EFFECTS OF OPIATES SHORT-TERM EFFECTS - induced feeling of ______________ - dulling of _______________ - depress _______________ system - slow _____________ and ___________ rate - ____________ reflex inhibited - ____________ and __________ (first-time users) - high doses lead to ______ and / or __________ LONG-TERM EFFECTS - constipation - loss of _____ drive - disrupts ____________ cycle - poor ____________ habits - risk of AIDS, hepatitis etc. through ___________ needles - ____________ problems (e.g. theft, prostitution, job loss) 3. STRUCTURES OF MORPHINE, DIAMORPHINE (HEROIN) & CODEINE 4. POTENCY OF HEROIN COMPARED WITH MORPHINE CROSSING THE BLOOD-BRAIN BARRIER -Nonpolar lipophilic cell membranes coat the blood vessels in the brain. Polar molecules do not readily enter the CNS, as a result. *____ POLARITY OF THE OPIATE = ____ POTENCY OPIATE RELATIVE POLARITY EXPLANATION / FACTS *MORPHINE - ______________ and two ______________ groups make it sufficiently soluble in water - low solubility in _____________, lipid-based environment of the _____________ limits its ability to reach ____________ receptors *CODEINE (“________-morphine”) - polarity ______________by replacing the phenolic _____________ group with the less polar _____________ group (-OCH3) - readily crosses blood-brain barrier BUT does not bind to opioid receptor because of the steric effect of the ‘bulky’ ether group - codeine, however, is __________ metabolized into ______________ - approximately _____ times less potent an analgesic than morphine - _________ therapeutic window / ____________ potential for abuse - most widely used opiate in the world *HEROIN (“diamorphine”) - polarity greatly decreased by the substitution of both hydroxyls on the morphine molecule with ____________ groups - heroin is _____________ metabolized into morphine - approximately ____ times more potent than morphine - synthesized in the same way that aspirin is prepared from salicylic acid and ethanoic anhydride