Problem set # 2
advertisement

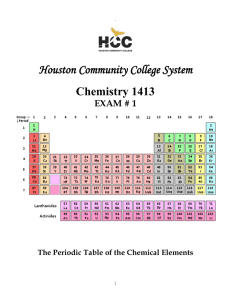
Problem set # 2 Chemistry 100 Name: 1. What is the mass number and Atomic number of these unknown elements? a) 12 protons, 12 electrons, and 12 neutrons. b) 35 protons, 35 electrons, and 45 neutrons. c) 94 protons, 94 electrons, and 130 neutrons. 2. Find the names and symbol of elements in question 1. 3. The two most abundant naturally occurring isotopes of carbon are carbon-12 (98.90%, 12.000 amu) and crbon-13 (1.10%, 13.003 amu). Calculate the atomic weight of carbon. 4. Circle the metals and draw a box around the nonmetals: Fe Rb Ar Ag N2 Br2 Al Xe Sr At As 5. Verify the charge of these ions and correct them: Na2+ Cl3+ O3- Be2- P4- Ca4+ 6. Fine the number of protons, electrons, and neutrons for the following atoms/ions: a) 208 Pb 82 b) 33 15 P c) 32 2S 16 7. How many electrons are contained in each of the ions? a) Fe2+ b) Cl- c) K+ d) O2- e) Mg2+ f) S2- Dr. Behrang Madani Chemistry 100 IVC