Chapter 7--Reading Quiz--PHYS 151--S16
advertisement
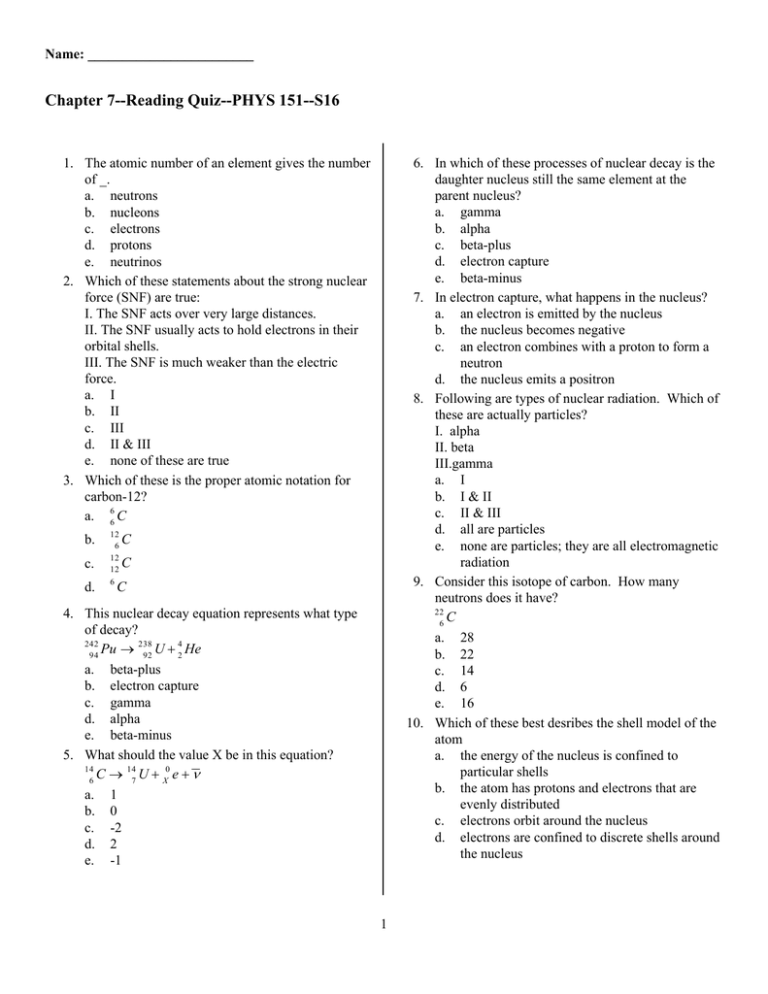
Name: ________________________ Chapter 7--Reading Quiz--PHYS 151--S16 1. The atomic number of an element gives the number of _. a. neutrons b. nucleons c. electrons d. protons e. neutrinos 2. Which of these statements about the strong nuclear force (SNF) are true: I. The SNF acts over very large distances. II. The SNF usually acts to hold electrons in their orbital shells. III. The SNF is much weaker than the electric force. a. I b. II c. III d. II & III e. none of these are true 3. Which of these is the proper atomic notation for carbon-12? a. 66 C b. c. d. 12 6 12 12 6 6. In which of these processes of nuclear decay is the daughter nucleus still the same element at the parent nucleus? a. gamma b. alpha c. beta-plus d. electron capture e. beta-minus 7. In electron capture, what happens in the nucleus? a. an electron is emitted by the nucleus b. the nucleus becomes negative c. an electron combines with a proton to form a neutron d. the nucleus emits a positron 8. Following are types of nuclear radiation. Which of these are actually particles? I. alpha II. beta III.gamma a. I b. I & II c. II & III d. all are particles e. none are particles; they are all electromagnetic radiation 9. Consider this isotope of carbon. How many neutrons does it have? 22 C 6 a. 28 b. 22 c. 14 d. 6 e. 16 10. Which of these best desribes the shell model of the atom a. the energy of the nucleus is confined to particular shells b. the atom has protons and electrons that are evenly distributed c. electrons orbit around the nucleus d. electrons are confined to discrete shells around the nucleus C C C 4. This nuclear decay equation represents what type of decay? 242 Pu → 238 U + 42 He 94 92 a. beta-plus b. electron capture c. gamma d. alpha e. beta-minus 5. What should the value X be in this equation? 14 C → 147 U + X0 e + ν 6 a. 1 b. 0 c. -2 d. 2 e. -1 1 ID: A Chapter 7--Reading Quiz--PHYS 151--S16 Answer Section MULTIPLE CHOICE 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: ANS: D E B D E A C B E D PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: PTS: 1 1 1 1 1 1 1 1 1 1 1



