Document 14386897
advertisement

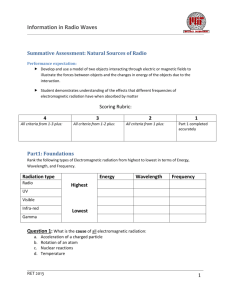
Information in Radio Waves Summative Assessment: Natural Sources of Radio Performance expectation: Develop and use a model of two objects interacting through electric or magnetic fields to illustrate the forces between objects and the changes in energy of the objects due to the interaction. Student demonstrates understanding of the effects that different frequencies of electromagnetic radiation have when absorbed by matter Scoring Rubric: 4 3 All criteria from 1-­‐3 plus: All criteria from 1-­‐2 plus: 2 1 All criteria from 1 plus: Part 1 completed accurately Part1: Foundations Rank the following types of Electromagnetic radiation from highest to lowest in terms of Energy, Wavelength, and Frequency. Radiation type Radio Highest UV Lowest Visible Infra-­‐red Gamma Energy Wavelength Frequency Question 1: What is the cause of all electromagnetic radiation: a. b. c. d. Acceleration of a charged particle Rotation of an atom Nuclear reactions Temperature RET 2013 1 Information in Radio Waves Question 2: The following model illustrates hydrogen in its ground state. Draw where the electron would be if it absorbed enough energy to raise it to the first excited state. Give 1 mechanism that could raise this electron to another energy level. N=1 __________________________________________________________ N=2 N=3 __________________________________________________________ __________________________________________________________ Question 3: Label each spectral distribution as being either thermal or non-­‐thermal Question 4: Describe the forces between the following charged particles Pair A Pair B Description: Description: RET 2013 2 Information in Radio Waves Part 2: Analyzing the Bohr model In neutral Hydrogen, electrons can jump from one energy state to another. If in a vacuum, then the only energy inputs or outputs will be from photons of light (EM radiation). Given the following relationships determine the frequency of the emission line from the H1 transition line (between n=1 and n=2) seen in the hydrogen energy level diagram. Important relationships: The change in energy, ΔE, from one state to another is: ∆𝐸 = 𝐸!"!#!$% − 𝐸!"#$% = 𝐸!!!"!# Energy of a photon : 𝐸!!!"!! = ℎ𝑣 Where: • • h is the Planck’s constant, h = 6.626 x 10-­‐34 Joule-­‐seconds (J-­‐s) v is the frequency of the radiation in Hertz (Hz, s-­‐1) Energy Level Diagram of Neutral Hydrogen Problem 1: Determine the frequency of the emission line N=4 from the H1 transition line (between n=1 and n=2) seen in the hydrogen energy level diagram to the left. Show all calculations and place a box around your final answer for the frequency. -­‐1.5 eV N=2 -­‐3.4 eV N=1 -­‐13.6 eV Energy à N=3 -­‐0.85 eV Principle quantum number RET 2013 3 Information in Radio Waves Question 1: You determined a specific frequency of EM radiation coming from an excited hydrogen atom. How could the process of absorption and emission of light be used to analyze the light from stars and other luminous objects in space? __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ RET 2013 4 Information in Radio Waves Extention: Deciphering a radio spectrum The graph on the right shows a generalized pattern for quasars observed over the frequencies labeled. Use the general patterns to answer whether this source of radiation contains thermal or non-­‐thermal emissions. ___________________________________________________ ___________________________________________________ ___________________________________________________ ___________________________________________________ ___________________________________________________ ___________________________________________________ ___________________________________________________ ___________________________________________________ ___________________________________________________ ___________________________________________________ ___________________________________________________ ___________________________________________________ ___________________________________________________ ___________________________________________________ ___________________________________________________ __________________________________________________ Quasars typically represent Active Galactic Nuclei. Is this one most likely radio ‘loud’ or radio ‘quiet’. Justify your answer with the trends in the graph. _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ RET 2013 5