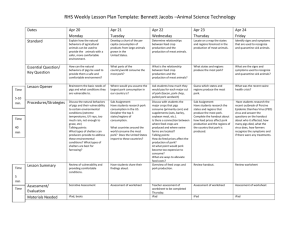
Quality Assurance III A Program of America’s
advertisement

® Quality Assurance A Program of America’s Pork Producers LEVEL III ® PQA Education To complete the course, you must: ■ Review the PQA Program GPPs with an educator. ■ Complete and sign the enclosed card and have your educator sign it. ■ Have your educator submit your information at http//pqa.porkboard.org or. . . ■ Mail or fax the card to the National Pork Board. National Pork Board PQA Department P.O. Box 9114 Des Moines, Iowa USA 50306 Ph: 800-456-PORK (7675) Fax: 515-223-2646 You may copy blank forms from the Pork Checkoff website: www.porkboard.org The National Pork Board will record you as a PQA Producer and will provide you with a PQA certificate, and a billfold PQA card. Information provided on the card is kept on record by National Pork Board and is strictly confidential. Continuing Education A checklist is included in the booklet for you to assess your operation’s compliance with the GPPs. On an annual basis, use the checklist to review, assess, and discuss with your educator any good production practices that may need attention. To maintain your PQA education status, complete and submit the enclosed registration card every three years. • Please print all information. • Complete all the blanks – missing information will cause delays. • Allow 3-4 weeks for processing. Table of Contents INTRODUCTION PRODUCER COMMENTS ABOUT THE PQA PROGRAM THE PORK INDUSTRY AND PQA 1 2 3 THE CURRENT REGULATORY SYSTEM 3 THE PORK QUALITY ASSURANCE® PROGRAM 4 HAZARD ANALYSIS AND CRITICAL CONTROL POINTS (HACCP) 5 FDA AND COMPLIANCE POLICY GUIDE 7125.37 11 PQA’S GOOD PRODUCTION PRACTICES (GPPs) 12 PQA EDUCATORS 13 THE PQA GOOD PRODUCTION PRACTICES (GPPs) 15 GPP #1: IDENTIFY AND TRACK ALL TREATED ANIMALS 17 GPP #2: MAINTAIN MEDICATION AND TREATMENT RECORDS 19 MINIMUM WITHDRAWAL TIMES GPP #3: PROPERLY STORE, LABEL, AND ACCOUNT FOR ALL DRUG PRODUCTS AND MEDICATED FEEDS 22 25 PREVENT CONTAMINATION OF DRUGS 27 INVENTORY CONTROL AND STORAGE 27 FEED ADDITIVES 28 GPP #4: USE A VALID VETERINARIAN/CLIENT/PATIENT RELATIONSHIP AS THE BASIS FOR MEDICATION DECISION-MAKING 29 VALID VETERINARIAN/CLIENT/PATIENT RELATIONSHIP (VCPR) 30 OVER-THE-COUNTER (OTC) DRUGS 32 PRESCRIPTION DRUGS 32 EXTRA-LABEL USE 32 VETERINARY FEED DIRECTIVE 34 ANTIMICROBIAL RESISTANCE AND THE JUDICIOUS USE OF ANTIMICROBIALS 35 I Table of Contents GPP #5: EDUCATE ALL EMPLOYEES AND FAMILY MEMBERS ON PROPER ADMINISTRATION TECHNIQUES 37 ADMINISTRATION OF INJECTABLE DRUGS 39 NEEDLE USAGE 40 DEVELOPING A STANDARD OPERATING PROCEDURE (SOP) 40 TESTING YOUR INJECTION TECHNIQUE 42 ADMINISTRATION OF WATER MEDICATIONS 43 ADMINISTRATION OF FEED MEDICATIONS 44 GPP #6: USE DRUG RESIDUE TESTS WHEN APPROPRIATE 45 GPP #7: ESTABLISH AND IMPLEMENT AN EFFICIENT AND EFFECTIVE HERD HEALTH MANAGEMENT PLAN 47 BIOSECURITY 48 DISEASE PREVENTION 51 ENVIRONMENTAL STEWARDSHIP 51 HERD HEALTH 52 RECORDS TO MEASURE PROGRESS 52 GPP #8: PROVIDE PROPER SWINE CARE 54 PORK PRODUCER CODE OF PRACTICE 54 SWINE HANDLING 55 HUMAN CONTACT 56 FACILITY CONSIDERATIONS 56 EQUIPMENT 57 LOADING AND TRANSPORT 58 EUTHANASIA 59 II Table of Contents GPP #9: FOLLOW APPROPRIATE ON-FARM FEED AND COMMERCIAL FEED PROCESSOR PROCEDURES 61 GOOD MANUFACTURING PRACTICES 62 BUILDINGS AND GROUNDS 62 EQUIPMENT 62 WORK SPACE AND STORAGE AREAS 63 PRODUCT QUALITY ASSURANCE 63 LABELING 63 RECORDKEEPING 63 ON-FARM FEED PROCESSING 64 PURCHASED FEED 68 GPP #10: COMPLETE THE QUALITY ASSURANCE CHECKLIST EVERY YEAR AND THE EDUCATION CARD EVERY THREE YEARS 71 APPENDIX 73 EUTHANASIA ACTION PLAN 75 VACCINATION AND MANAGEMENT SCHEDULE 77 FARM MEDICATION PLAN 79 PEN OR INDIVIDUAL PIG TREATMENT RECORDS 81 GILT / SOW / BOAR TREATMENT RECORDS 83 DRUG STORAGE RECORD – INVENTORY CONTROL SHEET 85 MEDICATED FEED MIXING RECORDS 87 BASIC GUIDELINES OF JUDICIOUS THERAPEUTIC USE OF ANTIMICROBIALS IN PORK PRODUCTION FOR PORK PRODUCERS 88 HACCP EXAMPLE SHEET 95 GLOSSARY 93 QUALITY ASSURANCE CHECKLIST 96 III NOTES _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ IV Introduction Perceived safety, wholesomeness, and nutritional value of food products influence consumer buying decisions. If consumers are not confident that a product is of the highest quality they have come to expect, they will not purchase it. In 1989, pork producers created the PORK QUALITY ASSURANCE® Program (PQA) to help American pork producers meet today’s consumer demands for quality and safety. Since that time, PQA materials have been revised periodically to provide producers timely, accurate information for producing safe, wholesome pork. Quality Assurance ® This revision builds on previous PQA materials to assist producers in developing a comprehensive management system to address the health and welfare of animals and the proper use of animal health products to prevent violative drug residues. In addition, this revision includes information about the Federal Government’s rules and regulations for pork producers and explains how the Pork Checkoff incorporates these and other scientific principles into the PQA program. As with previous materials, this manual outlines the ten Good Production Practices (GPPs) and offers suggestions about implementing each practice. The GPPs must be completed with a veterinarian, agricultural extension educator, or agricultural instructor as defined by the National Pork Board. Finally, this manual contains helpful sample forms and additional sources of information. The entire pork industry will benefit from widespread producer commitment to the PQA program. Implementing PQA on every farm will help the industry protect or even expand its markets by reducing the risk of incidents that could erode consumer confidence in the safety, quality, and wholesomeness of pork. 1. Producer Comments about the PQA Program Following are just a few comments from producers who have completed and use the PQA program. "The Pork Quality Assurance Program provides producers with the most current information on the use of medications and feed additives. Information that would affect the safety of the pork we produce is provided. This certification allows our consumers to feel confident that the person who raises their food strives to produce the safest food possible." Missouri producer "By participating in regular update meetings to re-certify, I get the latest information to take back home to my operation. PQA is more than just lip service to our operation; we have actually seen where it affects our bottom line and implemented changes due to the education program. PQA makes a positive, pro-active statement to our consumers and own family members that we care about the food they consume." Minnesota producer "We use PQA as an educational tool on our farm. It has helped us lower our cost of production through a better veterinarian-client working relationship, and it tells the packer and consumer that we are concerned about food safety and producing a quality product." South Dakota producer "The PQA program is a good update and refresher course for producers to keep the standard of their operation in tune with other producers. It is also a valuable tool for assurances in the international market. It gives our trading partners the basics of our commitment to food safety." Illinois producer "The PQA program is excellent training for employees or anyone who works with pigs." Iowa producer 2. Food and Drug Administration 5600 Fisher Lane Rockville, MD 20857 800-INFO-FDA (800-463-6332) www.fda.gov Environmental Protection Agency 1200 Pennsylvania Ave. NW Washington, D.C. 20460 202-260-2090 www.epa.gov USDA Food Safety and Inspection Service Washington, D.C. 20250-3700 www.fsis.usda.gov/ FSIS HACCP Hotline: 800-233-3935 (Press 2) or 402-221-7438 (Fax) FSIS Meat and Poultry Hotline: 800-535-4555 The Pork Industry and PQA The Current Regulatory System The current regulatory system to help provide safe, wholesome food to consumers is a combination of rules and enforcement procedures established by several state and federal government agencies. Producers ultimately are responsible for providing a residue-safe product. While many animal health products are available over the counter, it is critical for producers to seek veterinarian involvement in medication decisions. There are several federal agencies that help ensure the safety and wholesomeness of food products for consumers. They are the Food and Drug Administration (FDA), the Environmental Protection Agency (EPA), and the Food Safety and Inspection Service (FSIS). ■ The FDA, which is part of the Department of Health and Human Services, is responsible for regulating medicated animal feeds and most animal health products. It approves the product, sets residue tolerance or action levels in edible tissues, and determines how drugs are to be administered in animals. ■ The EPA sets tolerance levels for pesticides used in pork production. If a pesticide is applied to pigs or their immediate environment, then it is subject to regulations set by the EPA. ■ The FSIS, which is part of the U.S. Department of Agriculture, inspects all pigs at federally inspected packing plants and examines plant sanitation. It conducts both routine residue monitoring and targeted food safety surveillance activities. Routine testing is conducted on randomly selected carcasses to detect tissue residues that exceed maximum levels allowed. Targeted testing is directed toward herds with a history of violative tissue residues or where there is some reason to suspect that there could be violative tissue residues, such as visible injection sites in the muscle at marketing. Drug residues in pork can cause delays in marketing schedules and disruption of future production flow, which could result in direct financial loss for producers. Discuss with your educator the current FSIS policies regarding violative residues. 3. The Pork Quality Assurance® Program Pork producers introduced the PQA Program in 1989 as an educational program. The program emphasizes 10 management practices for handling pigs and using animal health products during production. The ultimate goal of the program is to help producers ensure that quality pork is delivered to consumers. The PQA Program is a voluntary program and is intended for all pork producers regardless of the size of their operation. The benefits of participating in the program include: ■ Improved management practices. ■ Use of systems and procedures that avoid violative drug residues. ■ Reduced production costs. ■ Increased awareness of food safety concerns. The 10 Good Production Practices in the PQA program are based upon: ■ The Hazard Analysis and Critical Control Point (HACCP) principles. ■ The Food and Drug Administration’s Compliance Policy Guide (CPG). CPG 7125.37 - “Proper Drug Use and Residue Avoidance by Non-veterinarians.” This will be explained in greater detail on page 11 of this manual. 4. Hazard Analysis and Critical Control Points (HACCP) The HACCP system focuses on identifying and preventing hazards in food and is based on sound scientific principles. HACCP is designed to be a preventative and systematic approach to food safety. An important aspect of the HACCP system is that all individuals involved understand their role and fulfill their responsibilities. One error can affect the entire system and its success. The HACCP system requires the producer to develop and record production information and use a recordkeeping system so that information may be accessed at a later date. Definitions: A control point: Any point, step, or procedure where you can control biological, physical, or chemical factors. A critical control point: A point, step, or procedure that you can control and which prevents, eliminates, or reduces a food safety hazard to an acceptable level. Critical limit: A limit that must be met for a certain preventive measure that is associated with a critical control point. For example, a certain temperature or time must be achieved to ensure the preventive measure is actually effective. Hazard: A biological, chemical, or physical property that may cause food to be unsafe for consumption. Preventive measure: Physical, chemical, or other factors that can be used to control an identified health hazard. 5. The seven basic principles of the HACCP system include: 1. Identify hazards. 2. Determine critical control points in the process. 3. Establish critical limits for each critical control point. 4. Establish monitoring procedures. 5. Establish corrective actions (to be used if monitoring shows there is a deviation from the critical limits). 6. Establish verification activities (to insure that the HACCP plan and system are working correctly). 7. Establish records and documentation (on critical control points, deviations, corrective actions, and disposition). The government requires meat packers to follow the HACCP system in their plants. In effect, HACCP has shifted the responsibility of safety from the government to meat packers. Meat packers are now responsible for controlling microbial contamination in their plants and have asked pork producers to help control antimicrobial residues, chemical residues, and physical hazards. In order for this approach to succeed, both the packer and the producer must understand his/her role and responsibilities under the HACCP system. Communication between packer and producer is vital! Packers are not able to hold treated animals until withdrawal is completed once the pig is at the plant. The producer must be responsible for properly observing drug withdrawal times to ensure that antimicrobial residues in swine tissues do not exceed acceptable limits. Using a scientific approach involves identification, testing, controlled procedures, and verification. The approach is formal, but it may not be as difficult as one might think. Chances are you are already using most, if not all of the elements of a scientific approach in your production practices. Following is an example of how you can incorporate the seven basic principles of the HACCP system to prevent physical hazards and violative chemical residues in pork products. 6. Seven Principles of HACCP: ■ Identify hazards Scenario: You are having a problem with broken needles in pigs on your farm. What are the steps that you and your family members or employees can take to eliminate these physical hazards in your pigs? Here are the seven HACCP principles. Fill in the blanks to offer suggestions on how to handle this hazard. An example is offered on the next page. ■ Find critical points in the process ■ Establish critical limits for each critical control point ■ Monitor each critical control point 1. Identify the hazard ______________________________________________________________________ 2. Find critical points in the process ■ Take corrective action if there is a problem ______________________________________________________________________ ■ Keep records on each critical control point 3. Establish critical limits for each critical control point ■ Verify that the HACCP plan is working correctly ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ 4. Monitor each critical control point ______________________________________________________________________ ______________________________________________________________________ 5. Take corrective action if there is a problem ______________________________________________________________________ ______________________________________________________________________ 6. Keep records on each critical control point ______________________________________________________________________ ______________________________________________________________________ 7. Verify that the HACCP plan is working correctly ______________________________________________________________________ ______________________________________________________________________ 7. Scenario: You are having a problem with broken needles in pigs on your farm. What are the steps that you and your family members or employees can take to eliminate these physical hazards in your pigs? Here are the seven HACCP principles. Fill in the blanks to offer suggestions on how to handle this hazard. 1. Identify the hazard Broken needleS in pigs ______________________________________________________________________ 2. Find critical points in the process ______________________________________________________________________ Needle use, injection technique ______________________________________________________________________ 3. Establish critical limits for each critical control point Never re-use bent needles, use correct needle size, ______________________________________________________________________ and use proper site and technique ______________________________________________________________________ 4. Monitor each critical control point ______________________________________________________________________ Identify pigs with broken needles, record number of pigs with broken needles ______________________________________________________________________ 5. Take corrective action if there is a problem ______________________________________________________________________ Account for all needles, provide training for employees and family members on using correct needle sizes, ______________________________________________________________________ proper injection sites and techniques, and not straightening bent needles 6. Keep records on each critical control point Keep records current, complete, and reviewed ______________________________________________________________________ ______________________________________________________________________ 7. Verify that the HACCP plan is working correctly Review farm records and reconcile those with ______________________________________________________________________ packer detection information ______________________________________________________________________ 8. Seven Principles of HACCP: ■ Identify hazards ■ Find critical points in the process ■ Establish critical limits for each critical control point ■ Monitor each critical control point ■ Take corrective action if there is a problem ■ Keep records on each critical control point ■ Verify that the HACCP plan is working correctly Scenario: You have been notified of a violative drug residue in your pigs. What are the steps that you and your family members or employees can take to eliminate this hazard in your pigs? Here are the seven HACCP principles. Fill in the blanks to offer suggestions on how to handle this hazard. An example is offered on the next page. 1. Identify the hazard ______________________________________________________________________ 2. Find critical points in the process ______________________________________________________________________ ______________________________________________________________________ 3. Establish critical limits for each critical control point ______________________________________________________________________ ______________________________________________________________________ 4. Monitor each critical control point ______________________________________________________________________ ______________________________________________________________________ 5. Take corrective action if there is a problem ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ 6. Keep records on each critical control point ______________________________________________________________________ ______________________________________________________________________ 7. Verify that the HACCP plan is working correctly ______________________________________________________________________ ______________________________________________________________________ 9. Scenario: You have been notified of a violative drug residue in your pigs. What are the steps that you and your family members or employees can take to eliminate this hazard in your pigs? Here are the seven HACCP principles. Fill in the blanks to offer suggestions on how to handle this hazard. 1. Identify the hazard Violative drug residues ______________________________________________________________________ 2. Find critical points in the processt Proper withdrawal times have been met ______________________________________________________________________ ______________________________________________________________________ 3. Establish critical limits for each critical control point No violative drug residues ______________________________________________________________________ ______________________________________________________________________ 4. Monitor each critical control point Use medication records ______________________________________________________________________ ______________________________________________________________________ 5. Take corrective action if there is a problem ______________________________________________________________________ Follow current Good Manufacturing Practices (cGMPs) for medicated feeds, follow label directions, use _____________________________________________________________________ medication appropriately and judicously , train and education personnel, and follow veterinarian recommendations ______________________________________________________________________ 6. Keep records on each critical control point Keep records current, complete, and reviewed ______________________________________________________________________ ______________________________________________________________________ 7. Verify that the HACCP plan is working correctly ______________________________________________________________________ Review farm records and reconcile those with packer detection information ______________________________________________________________________ 10. FDA and Compliance Policy Guide 7125.37 Pork producers have long been required to follow the guidelines set forth in the Food and Drug Administration’s published Compliance Policy Guide (CPG) 7125.37: "Proper Drug Use and Residue Avoidance by Non-veterinarians." The guidelines in the CPG form the basis for the producer responsibilities of tissue residue avoidance that the government and meat packers expect under packer HACCP plans. They are required to be observed anytime an animal health product is used. The CPG states that, for responsible use of animal health products, the producer must: 1. Identify and track animals that were administered drugs. Animals can be tracked by: a. Individual b. Pen c. Group (building or site) 2. Maintain medication and treatment records that identify: a. The animals treated. b. The date(s) of treatment. c. The drug(s) administered. d. The person who administered each drug. e. The amount of each drug administered. f. The withdrawal time prior to slaughter. 3. Properly store, label, and account for all drug products and medicated feeds. 4. Obtain and use only veterinary prescription drugs through a licensed veterinarian based on a valid veterinarian/client/patient relationship (VCPR). 5. Educate all employees and family members involved in treating, hauling, and selling animals about: a. Proper administration techniques. b. Observance of withdrawal times. c. Methods used in production to avoid marketing adulterated products for human food. Current FDA regulations hold producers responsible for providing residue-safe products. While many animal health products are available as over-the-counter, it is critical for producers to seek involvement of a veterinarian in making medication decisions. 11. The PQA Good Production Practices (GPPs) The guidelines of CPG 7125.37 are incorporated into the PQA program. GPP #1 Identify and track all treated animals. GPP #2 Maintain medication and treatment records. GPP #3 Properly store, label, and account for all drug products and medicated feeds. GPP #4 Use a valid veterinarian/client/patient relationship as the basis for medication decision-making. GPP #5 Educate all employees and family members on proper administration techniques. GPP #6 Use drug residue tests when appropriate. GPP #7 Establish and implement an efficient and effective herd health management plan. GPP #8 Provide proper swine care. GPP #9 Follow appropriate on-farm feed processing and commercial feed processor procedures. GPP #10 Complete the Quality Assurance Checklist every year and the Education Card every three years. Completing the PQA educational program with an educator will use a HACCP-like approach to supply the best quality animal in the most efficient manner. 12. PQA Educators Experts such as veterinarians, agricultural extension personnel, and agricultural educators may serve as PQA educators. To ensure that you fully and successfully implement each Good Production Practice, review these practices with your educator. Handy Forms This program provides sample recordkeeping and management forms to help you keep the necessary records to validate that a system for animal identification, drug use, and proper withdrawal is in place. They are designed for easy separation from the booklet and may be copied as necessary for use on your farm. 13. NOTES _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ 14. Pork Quality Assurance Level III ® ® Good Production Practices The PQA program consists of ten Good Production Practices. You will find explanations of each practice and suggestions for their implementation. Several example recordkeeping forms with the type of information that should be tracked, a checklist that can be used for a self-assessment, and additional information sources about specific practices can be found in the Appendix. Upon completion of the PQA program, submit the enclosed card to the National Pork Board for registration as a PQA producer. For the fastest submission have your educator submit your information at http://pqa.porkboard.org. For additional blank forms, visit the website www.porkboard.org. You must complete and mail or fax your registration card to the National Pork Board every three years to maintain your PQA education status. 15. 16. Pork Quality Assurance Level III ® Good Production Practice #1: Identify and track all treated animals. 17. Good Production Practice #1 Identify and Track All Treated Animals Before treating any animal, you should decide what method of identification is appropriate for your operation. It is important to use an identification and tracking system that can reliably identify medicated pigs from the time of drug administration completely through the proper withdrawal for the medication. To satisfy requirements of the Food and Drug Administration (FDA) you should keep written medication records for at least 12 months following marketing of any medicated livestock. Tools to identify pigs: You can identify medicated animals: ■ Individually ■ By pen ■ By group (building or site) ■ Sow Many producers will use some form of sow card or building record for tracking animals. When individual identification of treated pigs in a pen is impractical, you can withhold the entire pen until the proper withdrawal period has been completed. Each nursery, grower, and finisher pen should be uniquely identified. Consider the system you use in your operation to identify medicated animals. ■ What is your system for identification and tracking? _______________________ __________________________________________________________________________ ■ Do all persons administering medication either by injection, water, or feed understand and use your appropriate identification practices? ❒ Yes ❒ No ■ How are all treatments recorded? ________________________________________ __________________________________________________________________________ ■ Where is the central location for gathering and storing records? _____________ __________________________________________________________________________ ■ How do the records follow the pig if the pig is moved to another location? __________________________________________________________________________ __________________________________________________________________________ 18. ■ Ear Card Notch ■ Paint ■ Tattoo ■ Ear Tag Pork Quality Assurance Level III ® Good Production Practice #2: Maintain medication and treatment records. 19. Good Production Practice #2 Maintain Medication and Treatment Records All food animal producers are required to keep medication and treatment records according to the FDA Compliance Policy Guide, "Proper Drug Use and Residue Avoidance by Non-veterinarians" (CPG 7125.37). The Farm Medication Plan contains information that the FDA would use to evaluate your safe use of animal health products. This form is provided in the Appendix. It will help you organize your system and provide a useful tool for discussion with your educator. Discuss with your educator the records used if: You have a routine vaccination protocol. You use medication to help prevent disease during times when you know your animals will be subjected to the stress of mixing or shipping. You routinely use animal health products in the breeding herd or other groups of pigs. You use a feed additive to enhance efficiency in the finisher unit. You have a routine treatment plan. During your educator’s visit, be sure to show him/her your treatment records. Are the records: ■ Complete? ■ Accurate? ■ Useful? Are there any suggestions for improvement? ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ 20. Good Production Practice #2 The minimum standards for your medication and treatment records are listed in the CPG 7125.37. All Records Should: ■ ■ ■ ■ ■ ■ ■ Identify the animal(s) treated. Specify the date(s) of treatment. Name the drug(s) administered. Include who administered the drug(s). Give the amount of drug(s) administered. Show the withdrawal time prior to slaughter. Identify the veterinarian directing medication use. Recordkeeping There are forms for a Farm Medication Plan, Gilt/Boar Treatment Records, and Pen or Individual Pig Treatment Records in the Appendix. Please feel free to copy the forms and use them, with your veterinarian, to write your farm’s medication plan and treatment records. Remember when writing treatment records, note animal or pen identification along with the other information. Maintaining the Gilt/Sow/Boar and Pen or Individual Pig Treatment charts at the back of this book, or other equivalent farm specific records, will show that a system for animal identification, drug use, and withdrawal is in place. A Feed Mixing Records Form is also included for your use. Copy this form and keep it on a clipboard near your feed mixing area or in your feed medication room. Filling out this form will not only help you keep the records needed regarding feed mixing, but it will also help you track medicated premix use. Keeping written records will help you comply with proper withdrawal times. 21. Good Production Practice #2 Minimum Withdrawal Times As with lists of this nature, it is accurate as best we can complete as of 1/1/02. This is only a partial list of approved medications that have preslaughter withdrawal times in swine. There are other approved medications, some of which have a zero day withdrawal. Withdrawal times may change. Consult the label for directions. For complete information on all animal health products approved for use in swine (including those with zero day withdrawal to market) visit the Food and Drug Administration’s (FDA) website at www.fda.gov/cvm. Drinking Water Application Preslaughter Withdrawal (days) Drug Trade Name (example) Apramycin Sulfate Chlortetracycline Chlortetracycline bisulfate and Sulfamethazine Chlortetracycline hydrochloride Gentamicin sulfate Levamisole hydrochloride Apralan Soluble Powder Aureomycin Soluble Powder 28 1 Aureomycin Sulmet Soluble Powder 15 Fermycin Soluble Garacin Soluble Powder Levasole Soluble Tramisol Soluble Lincomix Soluble Powder Lincomycin Soluble Neomix 325 Soluble Powder Terramycin Soluble Terramycin 343 Sulmet Drinking Water Solution Tetra 324 Polyotic Denagard 3.5 mg/lb 10.5 mg/lb Tylan Soluble 5 10 Lincomycin hydrochloride Neomycin sulfate Oxytetracycline Sulfamethazine Tetracycline hydrochloride Tiamulin Tylosin tartrate 22. 3 6 3 13 15 4 7 3 7 2 Oral Application Preslaughter Withdrawal (days) Drug Trade Name (example) Gentamicin sulfate (solution) Neomycin sulfate Sulfachlorpyridazine Spectinomycin dihydrochloride Garacin Pig Pump Biosol Liquid Vetisulid Powder 14 20 4 Spectam Scour Halt 21 Feed Application Drug Trade Name (example) Apramycin Carbadox Hygromycin B Ivermectin Levamisole hydrochloride Lincomycin hydrochloride Apralan Mecadox Hygromix 8 Ivomec premix for Swine Tramisol Hog Dewormer Mix Lincomix (20 g/ton) (40 g/ton) (100 g/ton) (200 g/ton) Terramycin 10-50 g/ton 10 mg/lb body wt. Oxytetracycline Oxytetracycline plus Neomycin Pyrantel tartrate Roxarsone Sulfamethazine Sulfathiazole Tiamulin Tilmicosin Neo-Terramycin 20/20 (neomycin level<140 g/ton) (neomycin level = 140g/ton) Banminth Premix – 48 3-Nitro 20 Aureomix 500; Aureo SP-250 Tylan 40 Sulfa-G Premix CSP-250, CSP-500 Aureosol Denagard 10 g/ton 35 g/ton 200 g/ton Pulmotil 18 Preslaughter Withdrawal (days) 28 42 15 5 3 0 0 6 6 0 5 5 10 1 5 15 7 0 2 7 7 23. Minimum Withdrawal Times Continued... Injectable Application Preslaughter Withdrawal (days) Drug Trade Name (example) Erythromycin Erythro 200 Gallimycin 100 Garacin Piglet Injections Predef 2x Ivomec 0.27% or 1.0% Lincomix Injectable LA-200 Oxy-Mycin 200 7 2 40 7 18 2 28 Oxy-tet 100 Penicillin G Procaine BO-SE Tylan 50 or 200 Injectable Vitamin ADB12 26 7 14 14 60 Gentamicin sulfate Isoflupredone acetate Ivermectin Lincomycin hydrochloride Oxytetracycline Oxytetracycline hydrochloride Procaine penicillin Sodium selenite Tylosin Vitamin Injectables 24. Pork Quality Assurance Level III ® Good Production Practice #3: Properly store, label, and account for all drug products and medicated feeds. 25. Good Production Practice #3 Properly Store, Label, and Account For All Drug Products and Medicated Feeds Always follow the label’s directions for storage and use. The effectiveness of a stored drug may quickly diminish based on its storage temperature, exposure to sunlight, and other factors as listed on the medication’s label. For example, if the label direction of a vaccine says, "Use the entire contents", do so once it is opened. Discard the unused portion because the drug or vaccine will rapidly lose its effectiveness. Check labels and identify those requiring refrigeration. As a general rule, vaccines and certain antibiotics need to be refrigerated at about 40° - 45° F. Store in the refrigerator all injectable medications that have been opened. Read the Label Drug labels contain the following information: ■ Trade name ■ Active ingredient ■ Indications ■ Dosage and administration or directions for use ■ Precautions ■ Cautions ■ Warnings - withdrawal time to market ■ Lot number ■ Expiration date Do you read and understand the labels on medications before giving them to your animals? ❒ Yes ❒ No Do you have any drugs or vaccines in storage that violate the "Use the entire contents" rule? ❒ Yes ❒ No Pay close attention to expiration dates. Are there any outdated drugs in your storage area? ❒ Yes ❒ No Are all drugs that require refrigeration kept in a refrigerator? ❒ Yes ❒ No What is the temperature of your refrigerator? _____________________________ Discuss with your educator the importance of understanding label instructions. 26. Good Production Practice #3 Prevent Contamination of Drugs Use medications with closer expiration dates first. Avoid bacterial or fungal contamination of drugs with a long shelf life by using clean needles to draw contents from multi-dose bottles. Ask your educator about the proper procedure for withdrawing medication from multi-dose bottles. Inventory Control and Storage Do not store medication in syringes. Syringes should be thoroughly cleaned or sterilized after use. ■ If you use soap or disinfectant and some residue remains in the syringe even after cleaning it, the residue could inactivate a modified live virus (MLV) vaccine. ■ Syringes must be rinsed thoroughly to remove residual soaps or disinfectants. Keep stored water medication and feed additives dry until use. Store leftover medications properly. This means they are not accessible by children or people not authorized to use them and they are separated from each other and labeled to prevent mistaking one medication for another. Inventory control is extremely important in any well-run business. At the back of the book, you will find a drug storage record and inventory control sheet. This form is designed to help you keep track of purchases and uses in your drug inventory supply. You will need to copy the form for each drug you use. Do not use oral medications if caked or clumped. Separate and store medications from farm chemicals. Most medications require a cool, dark, dry storage place. For example, the dashboard in your car or truck, or your office window, is not an acceptable place to store medication. Describe your storage practices for: 1. Injectable medication______________________________________ ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ 2. Water medication__________________________________________ ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ 3. Feed additives_____________________________________________ ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ 27. Good Production Practice #3 Feed Additives Feed additives are used in swine rations to improve feed efficiency and promote faster gain or to prevent or treat disease. They are not a substitute for good management and sanitation practices, a sound preventive health program, a good nutrition program, or proper environmental conditions. The FDA requires minimum production standards called current Good Manufacturing Practices (cGMPs) for all medicated feed manufacturers including pork producers with on-farm mixing operations. Observance of cGMPs in your feed mill will help assure that pork produced from your operation will not have violative drug tissue residues. The cGMPs will be discussed in more detail in GPP #9, Follow Appropriate On-Farm Feed Processing and Commercial Feed Processor Procedures. Licensed veterinarians, feed manufacturers, and producers may order, produce, or use drugs in medicated feeds ONLY: ■ If the drug is approved by FDA. ■ When the drug is used in the manner for which it was labeled and approved. ■ As provided by a FDA feed mill license, where applicable. Note: No person (licensed veterinarian, feed manufacturer, or producer) may use drugs in medicated feeds in an extra-label manner. Refer to GPP #4 for definition of extra-label. 28. Pork Quality Assurance Level III ® Good Production Practice #4: Use a valid veterinarian/client/ patient relationship as the basis for medication decision-making. 29. Good Production Practice #4 Use a Valid Veterinarian/Client/Patient Relationship as the Basis for Medication Decision-Making Valid Veterinarian/Client/Patient Relationship (VCPR) FDA regulations make the following statement about a valid Veterinarian/Client/Patient Relationship (VCPR): "An appropriate veterinarian/client/patient relationship will exist when: 1. The veterinarian has assumed the responsibility for making medical judgments regarding the health of the animal(s) and the need for medical treatment, and the client (owner or other caretaker) has agreed to follow the instructions of the veterinarian; and when 2. There is sufficient knowledge of the animal(s) by the veterinarian to initiate at least a general or preliminary diagnosis of the medical condition of the animal(s). This means that the veterinarian has recently seen and is personally acquainted with the keeping and care of the animal(s), and/or by medically appropriate and timely visits to the premises where the animal(s) are kept; and when 3. The practicing veterinarian is readily available for follow-up in case of adverse reactions or failure of the regimen of therapy." Let’s examine the three points more closely. Number One Who is responsible for making medical judgments on your farm? If you can’t easily answer, it may be because too many individuals – you, your feed suppliers, your equipment salesperson, your neighbor, etc.- make the decisions. The list could be even longer. It is strongly recommended that you and your veterinarian make medical decisions. Number Two The second point emphasizes the word valid. Much of this responsibility is on your veterinarian. He or she must visit your facilities regularly to have "sufficient knowledge" of the animals. Visits do not necessarily have to occur monthly or on a time-based schedule, but should at least occur when medically appropriate. 30. Use only FDA approved over-thecounter (OTC), Veterinary Feed Directive (VFD) or prescription (Rx) drugs with the advice or on the order of a licensed veterinarian. How can this be part of a preventive medicine approach to drug use and herd health? With your veterinarian, discuss the appropriate schedule for visits. ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ Do you have a written medical plan to explain when to use injectable drugs, water medications, and feed additives? ❒ Yes ❒ No Are you following this plan? ❒ Yes ❒ No If not, why? ___________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ Does your plan list withdrawal times? ❒ Yes ❒ No Number Three The third point of the VCPR is a commitment by your veterinarian. Is a veterinarian available for follow-up consultation when necessary? ❒ Yes ❒ No Do you have a valid Veterinarian/Client/Patient Relationship? ❒ Yes ❒ No Use only FDA approved over-the-counter (OTC), Veterinary Feed Directive (VFD) or prescription (Rx) drugs with the advice or on the order of a licensed veterinarian. 31. Good Production Practice #4 Over-the-Counter (OTC) Drugs When label directions can be written so that the drug can be safely and correctly used by a non-veterinarian, a drug can be sold OTC for its labeled uses. When deciding whether a drug can be OTC or prescription only, the FDA considers these factors: ■ The margin of safety for the animal ■ The effects on the animal of accidental overdose ■ The difficulty of identifying the disease or condition for which the drug is labeled ■ The safety of the person handling and administering the drug OTC drugs can be purchased from veterinary clinics, feed stores, and animal health suppliers. They do not require a prescription. However, any time you use animal health products, even OTC drugs, you should consult with your veterinarian. OTC drug labels will have exact instructions on dosage, administration, withdrawal times, handling, and storage. According to the law, you must exactly follow these labeled instructions or those of your veterinarian. Prescription Drugs Prescription drugs can only be used by or on the order of a licensed veterinarian. Prescription product labels will contain this statement: "Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian." Extra-label Use When labeled drugs are not available to maintain adequate animal care, your veterinarian has the ability to prescribe extra-label drug use. Extra-label use means using an animal drug in a manner not in accordance with the approved drug labeling. Only a veterinarian with a valid VCPR for your operation can direct extra-label use. There is no extra-label use in medicated animal feeds. The following are examples of extra-label use: ■ ■ ■ ■ ■ Increasing the dosage Changing the frequency or the route of administration Changing the duration of treatment Treating for a disease not listed on the label Changing the animal species to be treated 32. You and your veterinarian accept these added responsibilities when drugs are used in an extra-label manner: ■ Make sure a careful medical diagnosis has been made by your veterinarian. ■ Verify that adequate directions for use have been provided and will be followed. ■ Follow extended drug withdrawal times so that no violative levels of residues remain in the animal. ■ Maintain identity of all treated animals for the extended withdrawal time. Do you ever use drugs in an extra-label manner? Remember, extra-label usage is only allowed under a valid VCPR. ❒ Yes ❒ No Do you keep a copy of the extra-label order provided from your veterinarian? ❒ Yes ❒ No Do you keep written records of these events? ❒ Yes ❒ No Do you observe the pre-slaughter withdrawal times recommended by your veterinarian when extra-label drug usage occurs? ❒ Yes ❒ No There are some drugs that are not to be used even in an extra-label manner in pork production. They are specifically banned by the FDA. These compounds are: chloramphenicol ipronidazole diethylstilbestrol ronidazole dimetridazole clenbuterol oral nitrofurans fluoroquinolones streptogramins glycopeptides 33. Good Production Practice #4 Veterinary Feed Directive The Veterinary Feed Directive (VFD) is a category of animal drugs created by the Animal Drug Availability Act of 1996. Previously, animal drugs were available either over-the-counter or by prescription from a veterinarian. The VFD is a category specifically for new antimicrobial drugs that are to be used in the feed to treat disease. A producer may not buy VFD products and store them on the farm unless he or she meets one of the following criteria: ■ Holds a valid feed mill license ■ Is a distributor of VFD feeds (and has complied with all requirements for distributors) ■ Has a valid VFD issued by a veterinarian In all cases, VFD feeds are not intended for routine use. They may be fed to animals only in accordance with a valid VFD issued by a veterinarian with whom you have a valid veterinarian/client/patient relationship. To obtain a VFD, contact a veterinarian with whom you have a valid veterinarian/client/patient relationship to ensure you have an accurate diagnosis. If appropriate, the veterinarian will write the VFD, keeping a copy and giving you two copies. When you buy the product at a VFD retailer or feed supplier, he/she will keep one copy of the VFD as a record of your purchase. If the drug is to be mixed on the farm, you can buy the appropriate premix. Alternatively, a feed processor may be able to supply a complete, ready to use feed that contains the VFD product. Keep your copy of the VFD for two years. The National Pork Board has VFD Fact Sheets and Q&A sheets that will help explain more about the VFD process. There is also a video in the PQA Video Series “Mixing Medicated Feed for Pigs” that explains the Good Manufacturing Practices (GMPs) and the VFD process in more detail. 34. PORK QUALITY ASSURANCE® is based upon the fact that you are responsible for the production and care of your pigs. Remember, you have a vested interest in the pork you produce. Good Production Practice #4 Antimicrobial Resistance and the Judicious Use of Antimicrobials USE J UDICIOUS U SE G UIDELINES Physicians and their patients and veterinarians and their clients share responsibility to properly use antimicrobials. Whether you use these products for therapeutic or production purposes, you and your veterinarian need to carefully consider if they are really needed for your particular situation. If they are, then make sure to follow the product’s labeled directions or the directions of your veterinarian. TO CONTAIN ANTIBIOTIC RESISTANCE Why is this so important? Although bacterial resistance to antimicrobials may be an important consideration during treatment decisions in your operation, the issue also has further-reaching implications. Some antimicrobials used in food animals are also used for human therapy. The pork industry has been a leader in providing the educational efforts that ensure antimicrobial use does not compromise food safety. Current and future availability of safe and effective animal health products are important to your ability to maintain healthy and productive animals, prevent animal suffering and ensure the consumer a safe and wholesome pork product. Antibiotics have been used to treat and prevent disease or promote growth in animals for 50 years. The issue of antibiotic resistance centers on the theory that antibiotics used in meat production causes the bacteria in the animal’s intestine to develop resistance to that antibiotic, with the potential for the resistant bacteria to be transferred to people through food. These bacteria could then make a person ill, and if treatment is necessary, the treatment may be compromised because of the bacteria’s resistance to antibiotics. 35. Antimicrobial Resistance and the Judicious Use of Antimicrobials Continued... The American Association of Swine Veterinarians (AASV) has developed guidelines for the judicious use of antimicrobials when treating disease. In part, they say: "When a condition exists that threatens or impairs animal health and well being, it is essential that an accurate clinical diagnosis be obtained. Appropriate diagnostic techniques and clinical experience should substantiate a presumptive diagnosis. Once the decision is reached to use antimicrobials for therapy, veterinarians strive to optimize therapeutic efficacy, minimize resistance to antimicrobials, and protect public and animal health.” The American Association of Swine Veterinarians supports and is committed to the following objectives as developed by the American Veterinary Medical Association’s Steering Committee on Judicious Therapeutic Antimicrobial Use: ■ Support development of a scientific knowledge base that provides the basis for judicious therapeutic antimicrobial use. ■ Support educational efforts that promote judicious therapeutic antimicrobial use. ■ Preserve therapeutic efficacy of antimicrobials. ■ Ensure current and future availability of veterinary antimicrobials. How do these guidelines apply to how you use antimicrobials in your operation? The National Pork Board has prepared a checklist for Judicious Use Guidelines (JUGS) – Pork Safety Fact Sheet, Vol. 2, No. 4, July 2000. It is included in the Appendix in the back of this book and can be used by you and your veterinarian to review your operation’s use of antimicrobials. 36. Pork Quality Assurance Level III ® Good Production Practice #5: Educate all employees and family members on proper administration techniques. 37. Good Production Practice #5 Educate All Employees and Family Members on Proper Administration Techniques If your production relies on employees or family members, then you need a plan that educates everyone about proper drug use. Remember, an education requirement is part of the FDA’s Compliance Policy Guide and the PQA Program satisfies this condition. You need to determine how best to educate family and employees. Include them in your discussion with your educator or discuss the Good Production Practices and other production issues during periodic farm meetings. Document that this educational process has occurred with your family members and your employees. 3 Ways to Administer Medications: Making sound decisions on drug use depends on understanding the importance of: •Injection 1. Following the proper guidelines when using medications and animal health products in pork production 2. Avoiding an on-farm quarantine that prevents pigs from being sold 3. Carefully following all label directions or your veterinarian’s instructions for use 4. Protecting and expanding our pork markets by emphasizing quality and safety Briefly explain your plan for including family and/or employees in producing quality assured pork: ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ 38. •Water •Feed Talk with your veterinarian regarding appropriate selection and method of delivery. When in question, ask and follow the advice of a veterinarian. Good Production Practice #5 Administration of Injectable Drugs Improper injection procedures cost the pork industry thousands of dollars every year. Failure to inject an antimicrobial or vaccine in the proper site could reduce the product’s effectiveness. In addition, packers expect that there will be no physical hazards, such as broken needles, in your pigs. There are five ways to give injectable medication to pigs: 1. In the muscle (Intramuscular - IM) ■ Use a spot on the neck just behind and below the ear, but in front of the shoulder. ■ Never inject in the ham or loin. There may be some bleeding and bruising of the muscle followed by scarring. This scar can stay in the muscle for the life of the pig and decrease the value of the meat. ■ Use the proper size and length needle to ensure the medication is deposited in the muscle, not in other tissues. 2. ■ ■ ■ ■ ■ Under the skin (Subcutaneous – SQ) Inject only into clean, dry areas. For small pigs use the loose flaps of skin in the flank or elbow. For larger pigs use proper needle length and technique. Use the proper length needle and angle to avoid injecting into the muscle. Slide the needle under the skin away from the site of skin puncture before depositing the product. ■ This technique should be used only upon veterinary instruction and guidance as serious injury to the pig can occur. 3. In the abdominal cavity (Intraperitoneal – IP) ■ This technique should be used only upon veterinary instruction and guidance as serious injury to the pig can occur. 4. In the vein (Intravenous – IV) ■ This technique should be used only upon veterinary instruction and guidance as serious injury to the pig can occur. 5. In the nasal passages (Intranasal – IN) ■ Withdraw the product from the bottle using a needle. Remove the needle from the syringe. Use the recommended application tip for administering the product. ■ Keep the pig’s head tilted upward during and immediately following administration to help the product reach the deep nasal passages. 39. Good Production Practice #5 Needle Usage The following are recommended needle sizes and lengths: Intramuscular Injection Baby Pigs Nursery Finisher Breeding Stock Gauge 18 or 20 16 or 18 16 14, 15, or 16 Length 5/8" or 1/2" 3/4" or 5/8" 1" 1" or 1 1/2" Gauge 16 or 18 16 14 or 16 Length 1/2" 3/4" 1" Subcutaneous Injection Nursery Finisher Breeding Stock Pork check-off funded research on needle strength shows that disposable needles will seldomly break during initial use. However, the needle shaft will break if it is bent during an injection, straightened, and used again. Developing A Standard Operating Procedure (SOP) for Needle Usage Writing a Standard Operating Procedure (SOP) will help you address this important part of your operation in a thoughtful, consistent way. It will also help you educate employees and family about how you would like these issues to be handled. If needle breakage does occur, also encourage honesty, proper identification, and reporting. An SOP for preventing physical hazards needs to include your needle handling, injection technique, animal identification, and packer notification procedures specifically developed for your operation. Here are some points to consider including in your SOP: I. Prevention 1. Evaluate the strength and detectability characteristics of the needles you are using. (Information about needles can be seen on the Internet at www.porkscience.org.) 2. Provide needle use guidelines that address: ■ Ensuring proper animal restraint ■ Selecting the proper site for injection ■ Selecting the proper size and length of needle according to the pig’s age, the injection site and the characteristics of the product to be injected ■ Changing the needle as appropriate to maintain cleanliness and sharpness. ■ Retrieving dropped needles ■ Changing bent needles – NEVER STRAIGHTEN A BENT NEEDLE, ALWAYS CAREFULLY REMOVE AND REPLACE IT ■ Considering the appropriate number of needles that would be reasonable to use for a particular job 40. A PQA Series Video explains the needle strength and breaking research. "Needle Strength Evaluation" can be ordered by calling the National Pork Board’s PQA Department at 800-456-PORK (7675). I. Prevention, Continued... Additionally, it is essential that you communicate with your packer. You can ask about the packer’s payment policies and request fair market value for at-risk animal(s). You can also talk with your packer about his wishes for a useable identification scheme for pigs that could be carrying a broken needle and their policy for notification before or at delivery. II. Identification of At Risk Animals Write a standard procedure to help everyone understand what to do if a needle breaks while giving an injection. Consider: ■ Stopping injections ■ Attempting to remove the needle ■ Temporarily identifying the pig if the needle is not retrieved ■ Permanently identifying the pig(s) according to packer specifications III. Notification Follow packer recommendations about notification before or upon delivery of at-risk animals. IV. Training Communicate farm policy and procedures with all employees or persons responsible for giving injections. An SOP will only be fully effective if every one is aware of it. Document family and employee education and training. Do you have a standard procedure for handling needles and giving injections? ❒ Yes ❒ No What do you do if a needle happens to break during the injection process? ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ 41. Good Production Practice #5 Testing Your Injection Technique Do you follow label directions for the quantity of medicine injected at one site and for site selection? Failure to follow these directions may lead to prolonged drug levels at the site of injection or failure of therapy. ❒ Yes ❒ No Before injection, do you properly adjust syringes to deliver the correct dose? ❒ Yes ❒ No Do you restrain animals properly to avoid bending the needle shaft and to prevent administration of an inappropriate dose? ❒ Yes ❒ No Do you use the appropriate recommended needle size to minimize stress, minimize tissue and skin damage, and reduce leakage at the injection site? ❒ Yes ❒ No Do you use a sharps container for used needles and knife blades? ❒ Yes ❒ No Where do you give intramuscular injections? ____________________________________________________________ ____________________________________________________________ Where do you give subcutaneous injections? ____________________________________________________________ ____________________________________________________________ Proper disposal of used syringes and needles is important to the safety of yourself and the people around you. Write your syringe and needle disposal plan in the space below. The disposal plan should follow local or state regulations for proper needle, sharps, and biological product disposal methods. ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ ____________________________________________________________ 42. You may want to view the National Pork Board video “Injection Techniques For Swine” for more information. Good Production Practice #5 Administration of Water Medications Do you have water medicators? ❒ Yes ❒ No When are they used? ____________________________________________________________ ____________________________________________________________ Have you calibrated your medicators according to the manufacturer’s directions? Make sure the non-medicated water supply is turned back on after the treatment regimen is finished. It is very important that pigs have adequate access to water. ❒ Yes ❒ No When was the last time the medicators were calibrated? ____________________________________________________________ ____________________________________________________________ How often do you clean the medicator? ____________________________________________________________ ____________________________________________________________ Where do you store your water medications? ____________________________________________________________ ____________________________________________________________ Do you flush your water lines after a medication is used or do you have separate water lines for medications? ____________________________________________________________ ____________________________________________________________ Do you mix water medications fresh daily and monitor consumption? ❒ Yes ❒ No 43. Good Production Practice #5 Administration of Feed Medications For proper administration of feed medications, follow the current Good Manufacturing Practices for feed processing. These practices are presented in detail in Good Production Practice #9, Follow Appropriate On-Farm Feed Processing and Commercial Feed Processor Procedures. Following label directions is one of the best ways to avoid drug residues. To ensure this good practice, use your judgment as a professional pork producer to carefully select family members and/or employees to help with administering feed medications. Be certain that family members and/or employees understand the directions and translate written instructions if necessary. Document the educational training. You are responsible for your pigs and the quality of the pork you produce. Discuss your program with your veterinarian. You cannot excuse a violative drug level in your pigs by saying it was all a mistake made by a family member or employee. 44. Pork Quality Assurance Level III ® Good Production Practice #6: Use drug residue tests when appropriate. 45. Good Production Practice #6 Use Drug Residue Tests When Appropriate Testing for residues before marketing can help to identify unsafe animals and serve as a preventive measure that saves money, ensures quality, and enhances the pork industry’s reputation. Some importing countries test product individually and at multiple times (distribution, wholesale, and retail). If a violative residue is found, multiple costly tests at the packing plant of origin must be done before (and if) the plant is allowed to re-enter the export market. This is why some packing plants will do additional testing on pork products for export. If one pig not properly withdrawn from antimicrobials causes a freighter full of pork loins to be returned and a packer to be shut out of an international market, the U.S. pork industry pays a price by losing a market opportunity, which affects market prices. Consider testing when: 1. Sows are culled for sale directly from the farrowing house 2. Animals receive extra-label treatment as prescribed by your veterinarian 3. Any of your feeder pigs are sold as roasters 4. Exhibiting at stock shows and fairs 5. Other special situations occur Your best defense is to follow proper withdrawal time. Commercially available tests have limited detection ranges. Consult with your veterinarian for information about your specific situation. 46. Pork Quality Assurance Level III ® Good Production Practice #7: Establish and implement an efficient and effective herd health management plan. 47. Good Production Practice #7 Establish and Implement an Efficient and Effective Herd Health Management Plan Maintaining and improving herd health is one of the key issues in economical pork production. It is much less expensive and more efficient to prevent disease than it is to treat it. Because many health problems can be controlled by good management practices, producers with healthy herds use less drugs. This in turn lowers costs and reduces the potential for violative drug residues. The following procedures will help maintain and improve the health of your herd and assist with controlling and lowering costs: ■ Examine your herd for diseases. ■ Carefully observe the herd with your veterinarian. ■ Perform diagnostic tests of the herd for disease exposure as advised by your veterinarian. ■ Conduct routine post mortem examinations with the proper follow-up diagnostic procedures. ■ Conduct slaughter checks as necessary. ■ Periodically review production and financial records. Biosecurity Keep your herd safe from the introduction or further spread of diseases (viral, bacterial, fungal, or parasitic) from the environment (people, equipment, etc.) and other pigs. Use the checklist on the following page to help assess your herd biosecurity procedures. 48. For more information, the “Foreign Animal Disease Awareness” video can be ordered by calling the Pork Checkoff Service Center at 800-456-PORK (7675). BIOSECURITY PROCEDURES ❑ Know what diseases are now present in your herd or within different age groups in your herd. your herd veterinarian to contact the veterinarian of your potential source of new stock to ❑ Ask discuss their health monitoring procedures and their current health status. new stock (breeding stock, feeder pigs, etc.), test, vaccinate and/or medicate (if needed) ❑ Isolate new stock during the isolation period, and/or use other means to be sure you are not buying any health problems. the number of visitors to your facilities and control their contact with your pigs. ❑ Limit Do you question them about their last contact with other swine and the health level of the last herd contacted? each visitor with a complete set of clean coveralls, hairnet and boots or require showers ❑ Supply with a change of clothes. visitors from bringing cameras, equipment, or other items into the production areas unless ❑ Prohibit they have been properly disinfected. all visitors to completely wash their hands with a disinfectant soap before entering the ❑ Require swine production unit. a farm traffic pattern for both people and pigs that prevents exposure of young pigs to older ❑ Use pigs, sows or their manure. effective boot cleaning and disinfection stations and/or dedicated coveralls and boots at ❑ Provide strategic sites of your production facilities. ❑ Take additional precautions to prevent the introduction of a foreign animal disease to your herd. ■ Require international visitors to observe a "pig free time" according to the diseases present in the countries they are from or have visited and according to the risk of potential human transmission of these diseases to pigs. ■ Require international visitors or employees who have traveled internationally to shower and wear farm-supplied clothing. ■ Prohibit international visitors or employees from bringing imported food including meat products or drink onto your pork production site. ■ Prohibit visitors or employees who have been traveling internationally from bringing any articles of clothing worn on international pork production sites or equipment from these sites onto your pork production site. truckers from entering your facilities or loading chutes. Ensure that they follow proper ❑ Prohibit biosecurity measures, and that the truck (tractor and trailer) is clean and disinfected when it arrives at your farm. ❑ Change clothes and shower after visiting other farms, livestock markets, or livestock fairs and shows. animal movement restrictions to avoid pigs returning to the unit following exposure to other ❑ Provide animals or their manure. ❑ Provide a designated, protected area for rendering pick up outside the perimeter of the farm. ❑ Prohibit feed delivery and/or other trucks from entering lots or crossing animal traffic flow patterns. 49. Biosecurity Continued... Take these precautions, if you deliver your own pigs to market: ■ Keep separate boots and coveralls in your truck to wear while unloading at the market, then put them in a plastic bag until washed and disinfected. ■ At the market, don't let pigs run off the trailer and then back on. ■ Thoroughly wash and disinfect your truck before returning to your facilities. Your facilities and the level of herd biosecurity you maintain can directly impact your herd’s health and affect the safety of your product, all the way to the consumer’s table. ■ Maintain an effective rodent control program. Rodents can serve as a source of infection to your herd for over 45 swine diseases. Cats are not effective at controlling rodents and they and their feces may even transmit zoonotic and/or pig diseases to your herd. ■ Proven effective methods of rodent control include: • Working with your verifier or a rodent control specialist to develop an effective rodent control program for your farm • Cleaning up feed spills promptly to prevent attracting rodents and/or wildlife to the facility • Replacing lids and covers of feeders. All lids and covers of feeders must fit tightly • Plugging holes and gaps in building walls and doors and around pipes and conduits • Placing bait stations strategically throughout the facility, using fresh bait on a rotational basis (using baits with different modes of action) • Maintaining a 3-foot "sterile zone" of either well-maintained residential height grass, 1-1.5 inch diameter gravel at a depth of 2-3 inches around the hog facility, or leveled or slightly graded bare dirt devoid of vegetation. • Preventing potential rodent harborage within 100 feet of the pig buildings, harborage includes all removable debris that is not necessary in the day-to-day operation of the facility • Controlling access of birds, wildlife, and pets to your swine, facilities, and feed stuffs, as with rodents, these animals may also serve as sources of infections affecting the health of your pigs and the quality as well as safety of their meat 50. For further specific information refer to the National Pork Board’s “Rodent Control for the Pork Industry" educational video, and the other rodent control educational materials available from National Pork Board. Good Production Practice #7 Disease Prevention ❑ Use "all in - all out" by air space. Completely clean and disinfect each room or building between groups of ❑ pigs, including groups of finishing pigs. selection is governed by many factors including: ❑ Disinfectant • Type of surface to be disinfected. • Temperature and other weather conditions. • Effectiveness against specific diseases. • Time required for the disinfectant to inactivate the organisms present. The efficiency of all disinfectants is impaired by the presence of organic ❑ material. Thorough cleaning is required prior to the use of any disinfectant. Consult with your educator, veterinarian or disinfectant company ❑ technician to determine what type of disinfectant is best for your operation. Consult with your veterinarian to develop your herd's vaccination and ❑ parasite prevention programs. (Use the Vaccination and Management Schedule provided in this manual.) Environmental Stewardship Familiarize yourself with the state regulations that govern your operation ❑ and conduct a self-assessment to minimize compliance risks. You can view your state regulations by viewing the state agencies links at www.porkenvironment.org. Conduct adequate sampling of both manure and soil to assure field ❑ agronomic balance is achieved when applying manure as a fertilizer. In addition, utilize and adhere to the Comprehensive Nutrient Management Plan (CNMP) for your facility. Familiarize yourself with the setback distances that are associated with ❑ your state when planning a new facility and when land applying manure. Maintain organized records of all activities related to compliance ❑ (i.e. nutrient analysis, field application). frequently with your conservation representatives regarding ❑ Consult "Best Management Practices" for your particular operation. Manage mortality and solid waste disposal in accordance with state ❑ regulatory standards. Develop an Emergency Response Plan and have it updated per the state ❑ agency requirements. ❑ Avoid any discharge to waters of the state. 51. Good Production Practice #7 Herd Health Discuss what actions need to be taken to address your herd’s diseases or health conditions: Production Loss Example: Death loss in nursery too high. _____________________________ _____________________________ _____________________________ _____________________________ _____________________________ _____________________________ _____________________________ _____________________________ Corrective Action Necropsy dead pigs; send feed for analysis. _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ Records to Measure Progress What records will you need to measure your herd’s progress? Do you have a method to collect and keep these records? Production Loss Example: Death loss in nursery. _____________________________ _____________________________ _____________________________ _____________________________ _____________________________ _____________________________ _____________________________ _____________________________ 52. Records Needed Percent mortality: cause of death, age at death. _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ Pork Quality Assurance Level III ® Good Production Practice #8: Provide proper swine care. 53. Good Production Practice #8 Provide Proper Swine Care PORK PRODUCER CODE OF PRACTICE Producers take pride in providing proper care to the swine on their farms. They consider management and husbandry practices for good swine care to include the following: ■ Providing facilities to protect and shelter pigs from weather extremes while protecting air and water quality in the natural environment ■ Providing well-kept facilities to allow safe, humane, and efficient movement of pigs ■ Providing personnel with training to properly care for and handle each stage of production for which they are responsible with no tolerance for mistreatment of swine in their care ■ Providing access to good quality water and nutritionally balanced diets for each class of swine ■ Observing pigs to make sure basic needs for food and water are being met and to detect illness or injury ■ Developing herd health programs with veterinary advice ■ Providing prompt veterinary medical care when required ■ Using humane methods to euthanize sick or injured swine not responding to care and treatment and disposing of them properly ■ Providing transportation that avoids undue stress caused by overcrowding, excess time in transit, or improper handling during loading and unloading 54. Good Production Practice #8 All producers are encouraged to keep themselves updated on advancements in the industry that can impact the animal’s welfare. Make decisions based on sound production practices that consider the welfare of the pig and are founded on good scientific principles. Look carefully at every animal every day. The welfare of your pigs directly impacts their health, productivity, and product quality. Swine Handling When the animal’s well-being is properly addressed, studies have shown that reproductive performance can increase, resulting in 1 to 2 more pigs per sow per year and improved rate of gain. Pork Checkoff videos ‘Swine Handling for Pork Producers’, and ‘Swine Handling for Transporters’, are excellent resources for learning more about handling and transporting pigs. In addition, there are other factors on the farm, in transport, and at the packing plant that affect pork quality. The booklet “A System for Assuring Pork Quality” is available from the National Pork Board to give you more detail about how your control of genetics, nutrition, on-farm pig handling, and handling during transport affects pork quality. It includes specific recommendations that impact both the animal well-being and meat quality. Improper handling can affect the carcass, causing: ■ Bruising: Estimates show that bruising alone attributes to over $48 million dollars a year in trim loss. ■ Pale, Soft, Exudative (PSE) Meat: PSE is caused by factors such as pigs with stress-susceptible genes, improper handling shortly before slaughter and poor carcass chilling. Consumers dislike the pale appearance, dry taste, and shrinkage during cooking. ■ Dark, Firm, Dry (DFD) Meat: DFD occurs when pigs are stressed for long periods of time and fatigued when they reach slaughter. DFD meat is not desired nor purchased by customers, and can cost you and our industry money. 55. Good Production Practice #8 Provide Proper Swine Care Human Contact It is important that everyone who has responsibility for caring for your pigs is adequately trained and has the necessary animal husbandry skills to work effectively with your animals. Following are some helpful tips: ■ When moving, processing, or examining baby pigs, support them under the belly. Holding baby pigs close to your chest can make them feel more secure and will minimize struggling. ■ When sorting in the barn, keep in mind that pigs don’t understand the objective of your work. Remain calm, quiet, patient, and organized. Use the proper equipment for your safety. ■ Spend time observing the animals every day to get them accustomed to human contact. This will help get the pigs used to your presence and will make movement and loading easier on them and on you. Facility Considerations There are a variety of production facilities in use in the industry today. The important point is that the management of the facility, regardless of its type, is sufficient to address the well-being of the pigs. The Swine Care Handbook from the National Pork Board provides pork producers with the latest information available on proper swine care, including recommendations about facility specifications. Following are just some of the swine care issues it addresses. Refer to it for more specific information when you talk with your educator. ■ Provide proper diets and adequate feeder space. ■ Check the water flow daily. This nutrient is too often overlooked. Flow rates and average daily water needs are listed below. 56. Production Stage Flow Rate Weanling Grower/Finisher Sow 70 sec/pint 50 sec/pint 35 sec/pint Category of Pig Daily Water Needed 1 wk old/3.3 lb 5 -9 wk old/22-57 lb 2.9 oz per lb 1.6 oz per lb Facility Considerations Continued... ■ Maintain comfortable environments in barns with respect to the age and weight of housed pigs. Maintain good air quality. ■ Have an area, at each stage of production, designated for sick or injured pigs and sows. Hospital pens can aid in recovery and provide easier follow-up treatment. ■ Provide non-slip flooring for pigs and sows in all housing and loading facilities. ■ Remove sharp and rough edges on loading chutes, which may cause bruising that results in trim loss. ■ Use ramps that are level with the trailer for loading pigs. If you have to use an incline, ramps should not exceed 20 degrees (about 48 in. height increase for each 11 ft. in length). ■ If you are building a concrete step ramp, make the steps less than 2.5 inches high and at least 10 inches deep. ■ Cleat steps for finisher pigs should not be more than 8 inches apart. Cleat steps for baby pigs should be 3 inches apart or less. Equipment A panel is the most effective tool for moving a pig because it blocks its path and vision while protecting the person holding the panel. Brooms or paddles are effective as a smaller version of a panel and are good for moving sows out of farrowing stalls. Slappers, buzzers, and prods should be eliminated or significantly curtailed because they can cause unnecessary stress, pain, and injury. Negative behaviors by handlers will also create stress and fear in the pigs. All these factors can have negative effects on meat quality. Rattles, shakers, and similar tools are quite effective in moving pigs. When an animal is put in a fearful situation, you risk both your health and the health of the animal. A large number of human injuries to the back and legs result from improper equipment and/or handling. Are sorting panels easily accessible in appropriate areas of your facility? ❒ Yes ❒ No What equipment is routinely used to move pigs in all sections of your operation? ______________________________________________________________________ ______________________________________________________________________ ______________________________________________________________________ 57. Good Production Practice #8 Loading and Transport ■ You are ultimately responsible for how your animals are handled. If a truck driver is abusive to your animals, stop the loading and correct the action or pursue other trucking options. ■ Move three to five finisher pigs at one time. If your alley way is narrower than 3 feet, reduce the number of pigs you are moving at one time. ■ When moving pigs, make sure objects and people do not block the flow of the animals. ■ When loading in hot weather, early morning loading can keep the animals cool and more comfortable. Wet shavings or sand in the bottom of the truck or trailer and spraying the pigs before or during transport can also reduce heat stress. ■ In cold weather, bed down the truck or trailer with straw to keep the animals comfortable. The following chart can help you make decisions when transporting pigs in inclement weather. LIVESTOCK WEATHER SAFETY INDEX DRY BULB TEMP RELATIVE HUMIDITY INTERVALS 10 20 30 40 50 60 70 80 90 100 75 80 85 A L E RT ALERT DANGER DANGER 90 95 100 58. EMERGENCY EMERGENCY Good Production Practice #8 Euthanasia The National Pork Board has stated that pigs unable to walk or sick pigs that obviously will not recover should be humanely euthanized on the farm and not transported to market. Euthanasia is defined as a humane death occurring without pain or distress. Even with our best efforts in every swine production system, animals will become ill or injured in such a way that euthanasia may need to be considered. If euthanasia is necessary, the following must be considered when choosing the best methods for humane euthanasia: 1. Human safety 2. Pig welfare 3. Practicality/technical skill required Pigs unable to walk or sick pigs that obviously will not recover should be humanely euthanized on the farm and not transported to market. 4. Cost 5. Aesthetics (degree of unpleasantness for the observer) 6. Limitations (size of pig, location, etc.) The National Pork Board and the American Association of Swine Veterinarians (AASV) have published a brochure “‘On-farm Euthanasia of Swine: Options for the Producer” with detailed information about methods of humane euthanasia. It can be ordered at no charge by contacting the Pork Checkoff at 800-456-PORK. (7675) Discuss this brochure with your veterinarian and determine the options appropriate for you to use. With your veterinarian, complete the Euthanasia Action Plan found in the Appendix. 59. NOTES _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ 60. Pork Quality Assurance Level III ® Good Production Practice #9: Follow appropriate on-farm feed and commercial feed processor procedures. 61. Good Production Practice #9 Follow Appropriate On-Farm Feed and Commercial Feed Processor Procedures The importance of quality feed to a livestock operation cannot be over emphasized. The goal of feed manufacturing is to produce feed that: ■ Meets specifications for nutritional composition ■ Meets the desired medication level, if appropriate ■ Is free of contaminants A set of guidelines for processing feed, referred to as current Good Manufacturing Practices (cGMPs), is designed to prevent feed contamination and to provide reasonable assurance that the feed is manufactured accurately. The cGMPs must be followed to ensure safe, wholesome meat products for human consumption. Talk with your educator about how each of the following cGMPs that apply to feed mills is addressed in your operation. Good Manufacturing Practices 1. Buildings and Grounds ■ Maintain good housekeeping. Prevent accumulation of dust that could contaminate finished feeds. ■ Ensure adequate space exists for equipment, processing, and storage of medicated feeds. ■ Provide access for preventive maintenance and equipment cleaning. ■ Implement vermin and pest control procedures. 2. Equipment: ■ Check equipment to be sure it can produce medicated feeds of intended potency, safety, and purity. ■ Pick up spills, plug leaks in equipment, and prevent build-up of feed ingredients. ■ Regularly check scales and metering devices to ensure they are accurate, functioning properly, and are suitable for their intended purpose. ■ The equipment must be of suitable size and construction to facilitate cleaning and adjustments when needed. 62. 3. Work Space and Storage Areas: ■ Design workspaces and storage areas to avoid accidental contamination of feed with toxic or other non-feed substances. ■ Ensure that feed work areas, equipment, and storage areas for animal drugs and manufactured feeds are physically separated from other work areas. Feed work areas should also be separated from equipment used for pesticides, fertilizers, and toxic substances. 4. Product Quality Assurance Laboratory Assays: ■ It is advisable to periodically analyze feeds for both their nutritive and drug content, or to ask your feed supplier for this information. Equipment Cleanout Procedures: ■ Establish equipment cleanout procedures (physical cleanout, flushing, sequencing of production, and delivery sequencing) to prevent unsafe cross-contamination of feeds or carryover of medicated feed products. 5. Labeling ■ Receive, handle, and store medications and their labels in a way that prevents confusion. ■ Make sure that the correct label is fixed to all medicated feeds you receive. The label should accompany bulk feed shipments and deliveries, identify the product and contents, provide directions about use, and state withdrawal times (for medicated feeds). 6. Recordkeeping ■ Visually inspect received feed ingredients for quality or defects. Written records that record the delivery date, method, and carrier, and any observations about color, weight, or other quality measurements will be very useful if a question of feed quality or contamination is ever raised. ■ Keep written records of medicated feed production. The Medicated Feed Mixing Records chart at the back of the book includes the minimum information that must be kept. ■ Retain records for one year after feed is used. ■ Samples of ingredients and finished feeds should be taken and stored for six months. 63. Good Production Practice #9 On-Farm Feed Processing The following questions are designed to help you avoid drug residue problems by reviewing your on-farm mixing operations. What type of mixer do you use? ❒ Vertical ❒ Horizontal Do you know and follow the manufacturer’s guidelines for recommended mixing time? ❒ Yes ❒ No What is its measuring capability? Volumetric _______ Scales _______ How often does your operator’s manual recommend calibrating your volumetric mill? __________________________________________________________________________ When was the last time you calibrated your mixer or scales? __________________________________________________________________________ Do you keep a written record of calibration dates? ❒ Yes ❒ No Do you recalibrate when you use new sources of feed ingredients? ❒ Yes ❒ No When was the last time you did a feed analysis to check for mixing accuracy? (Your feed equipment manufacturer or feed ingredient supplier can help you with the specific procedure for checking the mixing accuracy of your particular feed mixer.) __________________________________________________________________________ 64. On-Farm Feed Processing Continued... What were the results and what were your actions? __________________________________________________________________________ __________________________________________________________________________ Have you checked for wear on the key mixer parts? ❒ Yes ❒ No Research has shown the particle size of feed is important in feed efficiency. Have you analyzed your feed for fineness of grind? ❒ Yes ❒ No What events trigger the cleaning of the: Mill? ___________________________________________________________________ Mixer? (This is particularly important if liquid ingredients are used in your feed.) __________________________________________________________________________ Mill area? ________________________________________________________________ Hauling wagon or delivery truck? ___________________________________________ Bulk bins? ____________________________________________________________ Augers? ____________________________________________________________ Feeders? ____________________________________________________________ Do you have a separate auger system for delivering medicated feeds with a withdrawal time to finishing animals? ❒ Yes ❒ No Do you ever mix non-medicated feeds with medicated feeds in bins or feeders? ❒ Yes ❒ No Medicated feeds can contaminate across pens or between groups of pigs. Research has shown that when pens are not thoroughly cleaned, pigs can pick up sulfa residues if moved into pens where other pigs had been fed sulfamethazine. 65. On-Farm Feed Processing Continued... Do you clean your pens between batches? ❒ Yes ❒ No Do you clean your feeders between batches? ❒ Yes ❒ No Do you know the labeled uses, mixing instructions, and withdrawal times for all feed medications used? ❒ Yes ❒ No Exceeding mixer capacity has been shown to severely decrease mixing ability. What is the designed capacity of your mixer? __________________________________________________________________________ Mixing feed in a sequence, such as mixing nursery feed followed by lactation, gestation, grower, and finishing feeds, is one way to help ensure antimicrobials won’t accidentally contaminate finishing feeds. Have you established a sequencing pattern? ❒ Yes ❒ No Flushing the mixer means preparing non-medicated feed to "flush" through your system, helping to clean out residual medicated feed. It is another way to protect finishing feeds from cross-contamination and is especially important with stationary mixers. Since this flushed feed will contain medication, it should be added to the previously prepared medicated feed or labeled and stored seperately for future batch of the same medicated feed. Do you flush your mixer after processing your medicated feeds? ❒ Yes ❒ No Cleaning out equipment is especially important with portable grinder/mixers. For example, 20 pounds of feed with 100 grams of sulfamethazine per ton left in the mixer is enough to contaminate the next ton of finishing feed. Two pounds of medicated feed left in the distribution system is enough to contaminate the first 200 pounds of finishing ration. Where are the clean-out ports on your feed processing equipment? __________________________________________________________________________ 66. The Pork Checkoff offers the video “Mixing Medicated Feed For Pigs” for more specific information about preparing and delivering medicated feeds. On-Farm Feed Processing Continued... A violative residue in your pigs may cause an investigative visit to your farm by the FDA. Keeping samples from feed ingredients and mixed batches will help you find if and where a mistake could have been made. Do you keep labeled samples of purchased premixes, supplements, and mixed medicated feeds for 6 months after marketing the livestock to which they were fed? ❒ Yes ❒ No Do you store feed additives in a clean, orderly area in original or properly labeled packages? ❒ Yes ❒ No Are farm chemicals stored separately? ❒ Yes ❒ No Are feed additives and ingredients properly labeled? ❒ Yes ❒ No Are feed bins appropriately identified? ❒ Yes ❒ No Are feeders numbered for accuracy of delivery? ❒ Yes ❒ No According to cGMPs, records of medicated feeds must be kept for one year after feeding. This includes records of medication purchases and on-farm mixing. Can you document your use of all medications purchased for at least the last 12 months? ❒ Yes ❒ No Do you keep records of where and when you used purchased feed grade medications? ❒ Yes ❒ No The Kansas State University Cooperative Extension Service has developed a guide for on-farm feed processors, “The Feed Quality Assurance Handbook”. A series of bulletins collected into one manual, this handbook gives complete up-to-date information about producing quality feeds on your farm. 67. Good Production Practice #9 Purchased Feed The feed industry has developed its own quality assurance program to help supply you with a quality feed product. If you purchase feed, it is advisable to ask your feed supplier the following questions: ■ What feed labeling information will be provided with the bag or bulk feed purchased from your firm? ■ Does your firm routinely use some type of clean-out method for the mixer, bulk truck, and pellet mill to prevent feed cross-contamination? ■ Are scales and metering devices, used during the manufacture of livestock feeds, tested for accuracy? ■ Will I receive a scale ticket and feed tag upon feed delivery? ■ Does your firm take and retain, for at least six months, a sample of each customer’s formula feed that is manufactured? ■ What precautions are taken to assure feeds do not come in contact with substances such as pesticides, fertilizers, or industrial chemicals? ■ Do employees who are routinely involved in feed manufacturing attend on-going training programs? ■ Do you have a quality control or quality assurance program for your facility? ■ Do your cGMPs include pest management practice? If so, what are they? 68. Purchased Feed Continued... ■ How often do you review your quality control or quality assurance program? ■ What are your visitor policies? ■ When did you pass your last FDA inspection (if registered) or state inspection (if non-registered)? If you did not pass, what actions have you taken to correct this situation? ■ Do you have a policy on biosecurity between delivery sites? ■ Have you analyzed your feed for fineness of grind? ■ Do you ever mix non-medicated feeds with medicated feeds in your storage bins? ■ Are your feed storage bins appropriately identified? ■ Can you document your use of all medications for at least the last 12 months? 69. NOTES _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ 70. Pork Quality Assurance Level III ® Good Production Practice #10: Complete the Quality Assurance Checklist every year and the education card every three years. 71. Good Production Practice #10 Complete the Quality Assurance Checklist Every Year and the Education Card Every Three Years An annual review of these Good Production Practices will help you efficiently manage production and maintain the highest quality product. A checklist is provided in the Appendix to evaluate your implementation of the Good Production Practices. The PQA manual is designed to make that review process easier. Benefits of an annual review of your pork production practices include: ■ Receiving a periodic, objective, professional assessment of your pork production practices ■ Examining your production process for possible cost-saving areas ■ Discussing newly available animal health care products with your veterinarian ■ Reviewing and updating your production facility design and repair needs ■ Learning about new technology and developments to improve your production systems, nutrition program, and swine health Three years after you complete the PQA program contact National Pork Board or your educator to receive another copy of this booklet. Use the booklet to review your production practices with your educator and submit your education card. For a complete list of educational materials, brochures, and audio-visual aids, contact the Pork Checkoff Service Center at 800-456-PORK. 72. Pork Quality Assurance Level III ® Appendix 73. 74. 75. 76. 77. 78. 79. 80. 81. 82. 83. 84. 85. 86. 87. Basic Guidelines of Judicious Therapeutic Use of Antimicrobials in Pork Production for Pork Producers Follow Judicious Use Guidelines (JUGs) to Contain Antibiotic Resistance This checklist corresponds with the “Basic Guidelines of Judicious Therapeutic Use of Antimicrobials in Pork Production for Swine Practitioners” (Pork Safety Fact Sheet, Vol. 2, No. 4, July 2000). Use it as you consult with your veterinarian to ensure that your operation is using JUGs to contain antibiotic resistance and to help maintain the availability and effectiveness of these products. Putting all the guidelines into practice in all pork production operations is essential to maintaining public trust and the timely, cost-efficient availability of effective products. ❒ Everyone responsible for the care and husbandry of the operation’s pigs is a current Pork Quality Assurance® Level III producer. ❒ Preventive strategies, such as appropriate husbandry and hygiene, routine health monitoring, and immunization are in place and practiced (reference PQA GPP #7). ❒ With the advice of the operation’s veterinarian, other therapeutic options are considered prior to using antimicrobial therapy. ❒ When indicated, supportive care is used to replace or increase the effectiveness of antimicrobial treatment. ❒ A valid Veterinarian/Client/Patient Relationship (VCPR) is in place (reference PQA GPP #4). ❒ At some point in the decision-making process, the operation’s veterinarian is consulted about the use of antimicrobials, including those available as over-the-counter. ❒ Prescription, Veterinary Feed Directive (VFD), or extra-label use of antimicrobials is done only under the advice and direction of the operation’s veterinarian. 88. The use of antimicrobials is beneficial both for the health of the animal and for human health. There is an increased awareness about how the use of antimicrobials affects bacterial resistance to these products. Pork producers must properly handle and administer antimicrobials if they are to maintain public trust and the availability and effectiveness of these products. The industry’s Pork Quality Assurance® Program is an example of the proactive approach the pork industry has taken to enable the production of a safe, quality product. ❒ Written records of all treatments are kept for at least 12 months following the marketing of the medicated animal ❒ Written records of all treatments are used to evaluate the success of a treatment regimen and include: ____ Identity of the animal(s) medicated ____ Date(s) of treatment ____ Name of medication administered ____ Who administered the medication ____ Amount of medication administered ____ Withdrawal time prior to slaughter ____ Name of the veterinarian giving directions, if use is other than what is on the label ❒ Any antimicrobial used in the feed is only used according to labeled directions. ❒ Written feed mixing records are used to record feed medication use (reference PQA GPP #2). ❒ A written antimicrobial treatment action plan has been developed with the advice of the operation’s veterinarian (reference PQA GPP #2). ❒ The antimicrobial treatment action plan is regularly reviewed with the operation’s veterinarian to ensure it is up to date. ❒ When antimicrobials are used, as per the advice of the operation’s veterinarian, they are used only for as long as needed to reach the desired clinical outcome. ❒ Antimicrobial treatment is limited to ill or at risk animals. ❒ Feeders and waters are properly adjusted so that when antimicrobials are delivered by these routes environmental contamination is minimized. 89. 90. ® Pork Quality Assurance Level III ® Glossary 91. Glossary Administration techniques: Refers to proper delivery of medication by injection, water, or feed. Antibiotic: A drug used to treat bacterial infections. Antimicrobial: A drug used to treat a microbial infection. Biological hazard: These include microbiological or zoonotic agents, such as bacteria like Salmonella, decomposition, and parasites, such as Trichinae. Chemical hazard: These include natural toxins, drug residues, such as violative levels of sulfonamides or antibiotics, pesticides, and unapproved use of direct or indirect food or color aditives. Environmental Protection Agency (EPA): The government agency that sets tolerance levels for pesticides used in pork production. Extra-label use: Use of an animal drug in a manner that is not in accordance with the approved drug labeling. This type of use is done legally under the direction of a veterinarian under a valid VCPR. Exra-label use is not allowed in medicated feeds. Feed additive: A substance added to swine rations to improve feed efficiency, promote growth, and prevent or treat disease. Food and Drug Administration (FDA): Agency of the U.S. Department of Health and Human Services. The FDA is responsible for regulation of medicated animal feeds and most animal health products. Food Safety and Inspection Service (FSIS): A branch of the U.S. Department of Agriculture that is responsible for inspecting all pigs and sanitation levels at the packing plant. Current Good Management Practices (cGMPs): A set of guidelines for processing feed designed to prevent feed contamination and provide reasonable assurance that the feed is manufactured accurately. Good Production Practices (GPPs): A set of guidelines for the safe, healthy, efficient, and humane production of pork. Hazard Analysis and Critical Control Points (HACCP): A system, which identifies specific hazards and preventive measures for their control to minimize the risk of producing defective products and services. Intramuscular (IM): Injection given in the muscle tissue of the pig. 92. Intranasal (IN): Administration given in the pig’s nasal passages. Intraperitoneal (IP): Injection given in the pig’s abdominal cavity. This type of injection should only be used upon veterinary instruction and guidance as serious injury to the pig can occur. Intravenous (IV): Injection given in a pig’s vein. This type of injection should only be used upon veterinary instruction and guidance as serious injury to the pig can occur. Label use: Use of a drug as exactly specified on the label. Off-label use: Use of a drug by a producer in a manner other than what is stated on the label and without guidance from a veterinarian under the extra-label policy. This practice is illegal. Over-the-Counter (OTC): Drugs that can be purchased lawfully without a Veterinary Feed Directive or prescription. Physical hazard: These include glass, metal fragments, or needles fragments. Prescription drugs: Drugs that can be obtained only by the means of a veterinarian’s prescription. Subcutaneous (SQ): Injection given under a pig’s skin. Tissue tolerance level: Maximum amount of drug that may be allowed in the animal’s tissues at time of slaughter that has been demonstrated to be no-risk to public health and has been approved by the Food and Drug Administration. Veterinary/Client/Patient/Relationship (VCPR): A relationship that exists between a client and a veterinarian where the veterinarian has assumed the responsibility for making medical judgments regarding the health of the animals, has sufficient knowledge of the animals, and is readily available for follow-up consultations. Veterinary Feed Directive (VFD): A new category of animal drugs created by the Animal Drug Availability Act of 1996. This category is specific for new/approved antimicrobial drugs used in the feed to treat disease. These drugs must be ordered by your veterinarian. Violative drug residues: Drugs remaining in animal tissues after slaughter that exceeds the levels allowed by the FDA. Withdrawal time: Length of time between the last day animals were given an animal health product and their slaughter. 93. 94. Seven Principles of HACCP: ■ Identify hazards ■ Find critical points in the process ■ Establish critical limits for each critical control point ■ Monitor each critical control point ■ Take corrective action if there is a problem ■ Keep records on each critical control point ■ Verify that the HACCP plan is working correctly 1. Identify the hazard ______________________________________________________________________ 2. Find critical points in the process ______________________________________________________________________ ______________________________________________________________________ 3. Establish critical limits for each critical control point ______________________________________________________________________ ______________________________________________________________________ 4. Monitor each critical control point ______________________________________________________________________ ______________________________________________________________________ 5. Take corrective action if there is a problem ______________________________________________________________________ ______________________________________________________________________ 6. Keep records on each critical control point ______________________________________________________________________ ______________________________________________________________________ 7. Verify that the HACCP plan is working correctly ______________________________________________________________________ ______________________________________________________________________ 95. 96. 97. 1. Medications are stored in accordance with labeled instructions. 2. Medications are used prior to the expiration date or are disposed of properly following the expiration date. 3. Drug inventory purchases and uses are tracked. 4. Feed medications are used only according to labeled directions. GPP #3 Properly store, label, and account for all drug products and medicated feeds. 1. System for recording routine farm medication schedule is used. 2. There is a system for recording feed medication use. 3. System for recording treatment of individual pigs, pens, and barns is used. 4. Components of the system treatment records comply with the points of FDA CPG. 7125.37. GPP #2 Maintain medication and treatment records. 1. Medicated animals are identified. 2. Identification lasts through the withdrawal period. GPP #1 Identify and track all treated animals. Acceptable Needs Improvement Comments Producer Name _____________________________________________________________________________________ Educator Name _____________________________________________________________________________________ Date Completed _______________________ QUALITY ASSURANCE CHECKLIST QUALITY ASSURANCE CHECKLIST Continued... GPP #4 Use a valid veterinarian/client/patient relationship as the basis for medication decision-making. 1. A valid VCPR exists in the operation. 2. Only FDA approved drugs are used in the operation. 3. Extra-label drug use is done only under the direction of a veterinarian and with a valid VCPR. GPP #5 Educate all employees and family members on proper administration techniques. 1. All employees and family members responsible for administering animal health products are trained in proper administration techniques. 2. A written standard operating procedure for needle use is used. 3. A written standard operating procedure for the administration of water medications is used. GPP #6 Use drug residue tests when appropriate. 1. Feed or pre-market animal drug residue testing is done when appropriate. GPP #7 Establish and implement an efficient and effective herd health management plan. 1. A herd health management plan has been developed and is used. 2. An appropriate biosecurity program is used. 3. An appropriate rodent control program is used. Acceptable Needs Improvement Comments 98. 99. 1. At least an annual review of the implementation of the 10 GPPs in the operation is conducted. 2. All employees and family members responsible for the handling or administration of animal health products maintain PQA education status. GPP #10 Complete the Quality Assurance Checklist every year and the Education Card every three years. 1. Current Good Manufacturing Practices are implemented in the following areas: A. Facilities B. Equipment C. Product quality assurance D. Labeling and storage E. Record-keeping 2. Purchased feed comes from a supplier that has implemented current Good Manufacturing Practices. GPP #9 Follow appropriate on-farm feed processing and commercial feed processor procedures. 1. All employee and family members responsible for the care of the swine have been trained in following the Pork Producer Code of Practice. 2. Facilities for each stage of production are adequate and in good repair. 3. Swine are handled during loading an transporting to minimize stress. 4. A written standard operating procedure for euthanasia is used. GPP #8 Provide proper swine care. QUALITY ASSURANCE CHECKLIST Continued... Acceptable Needs Improvement Comments NOTES _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ 100. PORK QUALITY ASSURANCE® LEVEL III National Pork Board PHONE: FAX: E-MAIL: WORLD WIDE WEB: P.O. Box 9114 Des Moines, IA 50306 USA 515-223-2600 515-223-2646 porkboard@porkboard.org http://www.porkboard.org/ ©1997 National Pork Board #04279-4/2004