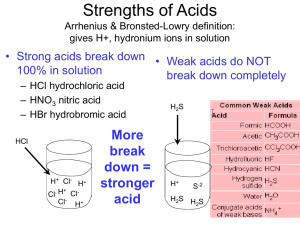
Preparing Solutions: Dilutions
advertisement
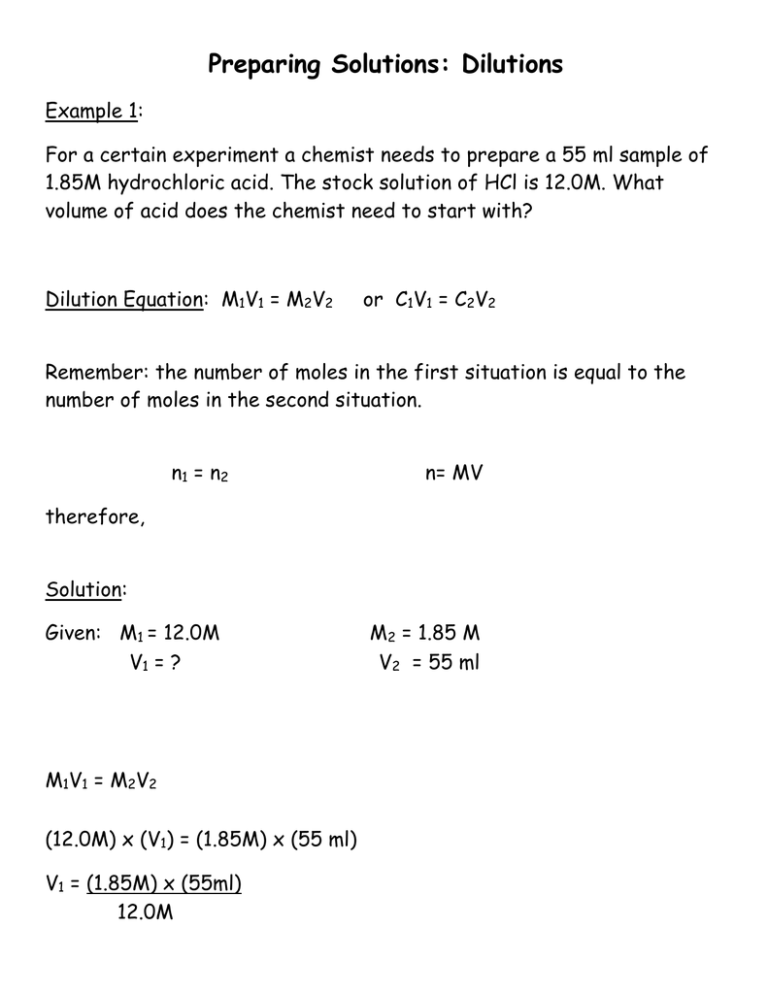
Preparing Solutions: Dilutions Example 1: For a certain experiment a chemist needs to prepare a 55 ml sample of 1.85M hydrochloric acid. The stock solution of HCl is 12.0M. What volume of acid does the chemist need to start with? Dilution Equation: M1V1 = M2V2 or C1V1 = C2V2 Remember: the number of moles in the first situation is equal to the number of moles in the second situation. n1 = n2 n= MV therefore, Solution: Given: M1 = 12.0M V1 = ? M1V1 = M2V2 (12.0M) x (V1) = (1.85M) x (55 ml) V1 = (1.85M) x (55ml) 12.0M M2 = 1.85 M V2 = 55 ml V1 = 8.48 ml of HCl Therefore the chemist will need to pour out 8.48 ml of HCl from the stock bottle. How much water will the chemist need to add to the 8.48ml to make the new 55ml solution?