Cloned Cattle Fetuses with the Same Nuclear Genetics Are More... Contemporary Half-Siblings Resulting from Artificial Insemination and Exhibit Fetal
advertisement

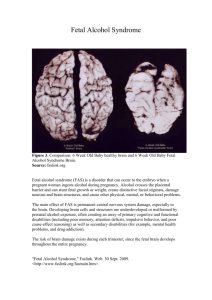
BIOLOGY OF REPRODUCTION 70, 1–11 (2004) Published online before print 17 September 2003. DOI 10.1095/biolreprod.103.020982 Cloned Cattle Fetuses with the Same Nuclear Genetics Are More Variable Than Contemporary Half-Siblings Resulting from Artificial Insemination and Exhibit Fetal and Placental Growth Deregulation Even in the First Trimester1 Rita S.F. Lee,2,3 A. James Peterson,3 Martyn J. Donnison,3 Susan Ravelich,4 Anita M. Ledgard,3 Ning Li,3 Jan E. Oliver,3 Andria L. Miller,3 Fleur C. Tucker,3 Bernhard Breier,4 and David N. Wells3 Reproductive Technologies Group,3 AgResearch, Ruakura Research Centre, Hamilton 2001, New Zealand The Liggins Institute,4 University of Auckland, Grafton, Auckland, New Zealand ABSTRACT 12] and rabbit [13]; and in a carnivore, the cat [14]. To date, the technology remains very inefficient and technically demanding. The biggest challenge facing the somatic cell NT technology is the high rate of pregnancy failure as well as fetal morbidity and mortality. Pregnancy failure occurs throughout the entire gestation, from embryo transfer to parturition [15–19]. Cloned offspring tend to have increased birth weight and poorer postnatal survival and health. However, those that do survive to maturity are fertile and produce normal offspring [15]. The predominant cause of pregnancy failure associated with NT in cattle and sheep is believed to be either failure of the placenta to form or abnormal placental development and function. Abnormal placental development has also been reported in the cloning of mice [12, 20, 21]. The failure of placental formation may account for most first-trimester losses in cattle, because fetal development is halted in the absence of the placenta. The absence of vascularization of the chorioallantoic membranes was implicated as a possible cause for the failure of placentation [16]. Abnormal placental development and function probably accounts for a high proportion of postimplantation fetal losses and may contribute to inadequate mammary gland development in the surrogate recipients and the failure of signaling in preparation for parturition [1, 2, 19]. In cattle, the major cause of fetal mortality is the acute, excessive accumulation of allantoic and, to a lesser extent, amniotic fluid, which is collectively referred to as the hydrops syndrome or hydropsy. In the experience of this group, approximately 60% of the fetal losses between Day 120 and full gestation may be attributed to or associated with this syndrome. The aim of the present study was to compare placental and fetal development in NT cattle pregnancies with closely matched control pregnancies generated either by artificial insemination (AI) or after embryo transfer of in vitro-produced (IVP) embryos. Pregnancies were assessed at the start of placentome formation (Day 50), when the placentomes were completely formed (Day 100), and during the period when hydropsy frequently occurs (Day 150). Contributing factors, such as the genetic background of the fetuses, age of the surrogate recipients and their reproductive history, and nutrition during the pregnancy, were controlled in the experimental design. Use of the same sire allowed comparison of fetal and placental development in the offspring from one sire, although it was not possible to control for maternal gene contribution. The cell line chosen to generate NT embryos had been demonstrated previously to be totipotent, with the production of 20 viable cloned calves. However, previous pregnancies from NT embryos derived The cloning of cattle by somatic cell nuclear transfer (NT) is associated with a high incidence of abnormal placentation, excessive fluid accumulation in the fetal sacs (hydrops syndrome), and fetal overgrowth. Fetal and placental development was investigated at Day 50, during placentome formation; at Day 100, when placentation was completed; and at Day 150, when the hydrops syndrome frequently develops. The NT fetuses were compared with contemporary half-siblings generated from in vitro-produced embryos or by artificial insemination (AI). Fetal cotyledon formation and vascularization of the chorioallantoic membranes was initiated normally in NT conceptuses, but fewer cotyledons successfully formed placentomes. By Day 100, the mean number of placentomes was significantly lower in surviving NT fetuses. Only those with normal placentome numbers were represented in surviving NT pregnancies at Day 150. The mean total caruncle tissue weight of the placentomes was significantly higher in the surviving NT groups at Days 100 and 150, irrespective of the placentome numbers, indicating that increased NT placental weight was caused by excessive uterine tissue growth. By Day 100, NT fetuses exhibited growth deregulation, and those that survived to Day 150 were 17% heavier than contemporary AI controls. Placentome, liver, and kidney overgrowth accompanied the hydrops syndrome at Day 150. The NT fetal overgrowth was not a consequence of in vitro embryo culture and showed no correlation with placental overgrowth. However, in vitro culture and incomplete reprogramming of the donor genome are epigenetic effects that may override genetic traits and contribute to the greater variability in placental and fetal development in the NT group compared with AI half-siblings. early development, embryo, placenta, pregnancy, uterus INTRODUCTION The successful production of viable offspring by cloning with somatic cell nuclear transfer (NT) has been achieved in ungulates, such as cattle [1–3], sheep [4], goat [5–7], pig [8, 9], and mouflon [10]; in rodents, such as the mouse [11, Supported by the Foundation for Research, Science and Technology, New Zealand, grant C10X0018. 2 Correspondence: Rita S.F. Lee, Reproductive Technologies Group, AgResearch, Ruakura Research Centre, East St., Private Bag 3123, Hamilton 2001, New Zealand. FAX: 64 7 838 5628; e-mail: rita.lee@agresearch.co.nz 1 Received: 7 July 2003. First decision: 29 July 2003. Accepted: 29 August 2003. Q 2004 by the Society for the Study of Reproduction, Inc. ISSN: 0006-3363. http://www.biolreprod.org 1 2 LEE ET AL. from this cell line showed many of the problems associated with clone pregnancies. The present experiment thus allowed us to assess mainly the effect of the NT technology itself on fetal and placental development. MATERIALS AND METHODS All manipulations and treatments of animals involved in the present study were conducted in accordance with the regulations of the New Zealand Animal Welfare Act of 1999. The generation of embryos for transfer to recipients or AI of the heifers was carried out in two separate experiments, conducted approximately 2 mo apart, with equivalent numbers of animals in each experiment. All dams or recipients were 2-yr-old heifers of predominantly dairy or dairy cross-breeds. Oocytes were obtained from ovaries of Friesian cows collected at the slaughterhouse, and the same pool was used as a source of recipient cytoplasts for NT or was in vitro fertilized to generate the IVP embryos. In vitro maturation of oocytes was carried out as described previously [2]. To minimize the potential effect of maternal breed on fetal growth and survival, the breed of the recipients was kept as similar as possible for all three groups; however, it was not possible to obtain enough animals from the same breed for these experiments. Only Friesian heifers were inseminated to obtain Friesian fetuses. NT Embryos The NT embryos were generated essentially as described previously [2] using the same ovarian follicular cell line (EFC) from a Friesian cow in that study. The cells used for NT in these studies had been passaged at least nine times and previously cryopreserved. Cells were cultured in a 1: 1 mixture of Dulbecco modified Eagle medium and Ham F12 (Gibco, Life Technologies, Gaithersburg, MA) supplemented with 10% (v/v) fetal calf serum (FCS; Life Technologies) and 1 mM sodium pyruvate. Cells were induced into a quiescent state by lowering the FCS concentration in the medium to 0.5% and culturing for a further 9 days (experiment 1) or 11 days (experiment 2) before NT. After injection of the donor cells into the perivitelline space, fusion was carried out at 22–24 h after the start of maturation, and fused donor cell/cytoplast couplets were activated 27–28 h after the start of maturation. After activation, in vitro culture was carried out as described by Wells et al. [22] in a biphasic AgResearch Synthetic Oviduct Fluid medium (AgR SOF; AgResearch, Hamilton, New Zealand). This medium was replaced on Day 4 of culture with fresh medium containing 10 mM 2,4-dinitrophenol as an uncoupler of oxidative phosphorylation [23]. The fatty acid-free bovine albumin (8 mg/ml) in the AgR SOF was ABIVP (ICP Bio, Auckland, New Zealand). Forty-nine NT embryos judged to be of sufficiently good quality by a subjective grading system were selected for transfer into recipients on Day 7 after fusion. Of the heifers that received NT embryos, 20 of 25 in the first experiment and 11 of 24 in the second experiment were Friesian or Friesian cross-breeds. The remainder were either Jersey cross-breeds or beef cattle. IVP Embryos In vitro-matured oocytes were fertilized with frozen-thawed spermatozoa in 50-ml drops under oil for 24 h as described previously [23]. The semen used was from the same Friesian bull that sired the cow from which the EFC cells were isolated. Zygotes were cultured as described for NT embryos, and at Day 7 after fertilization, the embryos were graded, also as described for the NT embryos. Of those receiving IVP embryos, 8 of 10 from the first experiment and six of nine from the second experiment were Friesian or Friesian-cross. The remainder were beef breeds. Artificial Insemination Twenty-one Friesian heifers were synchronized for estrus using intravaginal controlled progesterone release devices (CIDR; Pharmacia, Ltd., Auckland, New Zealand) inserted for 12 days. On Day 8, all heifers were injected with 1 ml of estrumate (Schering-Plough, Union, NJ), and the devices were withdrawn after a further 4 days. The mean onset of estrus was approximately 48 h later. Approximately 12 h after the onset of estrus, the heifers were inseminated with frozen semen from the above-described bull used for IVP. Recipients of IVP or NT Embryos Seventy healthy heifers were synchronized for estrus concurrent with those heifers used for AI. Single IVP (n 5 19) or NT (n 5 49) embryos were transferred nonsurgically into the uterine lumen ipsilateral to the corpus luteum of each heifer on Day 7 after estrus was observed. The transfer of single embryos avoided the complications of multiple pregnancies. Pregnancy Monitoring and Morphometric Measurements After embryo transfer or AI, all heifers were grazed on pasture together. Between Days 40 and 50 of gestation, all heifers were scanned by transrectal ultrasonography using a Piemed 200 scanner with a linear 3.5to 5-MHz rectal probe (Philip-sweg, Maastricht, The Netherlands); pregnant animals were identified and subsequently scanned at monthly intervals until slaughter. Only the presence of a gestational sac was recorded at the first scan. Subsequently, the presence of a fetal heartbeat was regarded as a sign of a viable pregnancy. A sample of pregnant animals from each group was slaughtered at Days 50, 100, and 150 of gestation, and the reproductive tracts were collected and transported back to the laboratory within 1 h. Conceptuses were recovered from the uterus, and morphometric measurements were carried out. The volumes of both allantoic and amniotic fluids were measured, and both fetal weights and crownrump (C-R) lengths were recorded. The fetal membranes with the cotyledons were weighed wet after all fluids were drained and the membranes separated from the uterus and fetus. At Days 100 and 150, the heart, brain, kidneys, and liver were weighed separately. The appearance of the fetus, organs, uteroplacental units, and fetal membranes were noted. At Days 100 and 150, all caruncles of the uteroplacental units were cut from the uterus after removal of the fetal cotyledons, combined, and weighed, and the numbers were recorded. Statistical Analyses At each stage, the treatments were compared for each variable using least significant differences calculated from analyses of variance. Certain variables (fetal brain, heart, liver, and kidney weights) were also expressed as a ratio to the fetal weight. An analysis of variance across stages was done on log-transformed variables to check for treatment by stage interaction. Pairwise comparison of variances between treatment groups was carried out with the F-test, assuming unequal variance. Differences were considered to be significant at P # 0.05. RESULTS In Vitro Development of Postfusion NT Embryos In the first experiment, 81% (105/129) of donor cell/ cytoplast couplets were fused successfully, and 104 reconstructed embryos were placed in culture, from which 45 blastocysts (43%) were judged subjectively to be of grades 1 and 2 and, thus, suitable for transfer to recipients. In the second experiment, 83% (108/130) of donor cell/cytoplast couplets were fused successfully. From the 105 of these placed in culture, 45 developed to blastocysts (43%) of grades 1 and 2. Thus, the development rate to grade 1 and 2 blastocysts for the NT embryos was similar in both experiments. Pregnancy Rates The pregnancy rate, as assessed by ultrasonography or at slaughter with the recovery of a fetus, was similar between experiments 1 and 2 for all three treatment groups. Therefore, the data were combined for simplicity of analyses. Figure 1 shows the pregnancy rates of the three treatment groups at different stages of gestation. Pregnancy rates after the first slaughter were calculated by taking into account the number that were pregnant at the previous slaughter and adding that to the remaining number that were pregnant at the next ultrasound scanning or slaughter. The data in Figure 1 did not take into account the health and potential viability of the conceptus, only that a conceptus was detected at scanning or slaughter. The pregnancy rates were similar for all three groups at Day 50 (AI, 3 GROWTH DEREGULATION IN CLONED CATTLE FETUSES going AI was 66.7% (14/21), which is consistent with the reported mean conception rate of 64% for New Zealand dairy herds after AI [24]. The conception rates for Friesian heifers after embryo transfer was similar for NT (53.6%, 15/28) and IVP embryos (61.5%, 8/13). Conception rate for IVP embryos in cross-bred heifers was 50% (3/6), which is similar to the rate for Friesian heifers. The conception rate for cross-bred heifers after transfer of NT embryos was 81% (17/21), compared with 53.6% for Friesian heifers; however, this difference was not significant (P 5 0.09, 2 3 2 exact test). Morphometric Analysis FIG. 1. Percentage of animals pregnant after AI (C) or embryo transfer with single IVP (#) or NT (D) embryos. Pregnancy was determined either by ultrasound scanning or with the recovery of a fetus at slaughter. 67%; IVP, 58%; NT, 65%). From then onward, NT pregnancies were continually lost, until only 40% of the recipients that received an NT embryo were still pregnant by Day 150. No fetal losses were recorded with either the AI or the IVP group after Day 50. Maternal Breeds and Pregnancy Losses To determine if maternal breed had any effect on embryonic survival, the conception rates were compared between the groups. At the first ultrasound scan between Days 40 and 50, the conception rate for Friesian heifers under- The numbers of AI, IVP, and NT fetuses from which data were obtained at Days 50, 100, and 150 are shown in Table 1. Fetal fluid volumes at Days 100 and 150 sometimes were not recorded, because the tracts had ruptured during slaughter and fluid from the amniotic and allantoic compartments were mixed or, in some cases, partially lost. During estimation of the mean fetal membrane weights, those fetal membranes with large amounts of a gelatinous substance were excluded from the analysis. The mean 6 SEM at each stage is presented in Table 1. The data are also graphically represented (see Figs. 2 and 4–6) to display the variability with stage and treatment, with the means for each treatment being joined by a line to show how they relate between the treatment groups. C-R length and fetal weight. The C-R length of the fetus is significantly correlated with gestation and is frequently TABLE 1. Mean 6 SEM values for the morphometric measurements made at Days 50, 100 and 150.a C-R length (cm) Fetal weight (g) Fetal membrane weight (g) Total fluid volume (ml) Allantoic fluid volume (ml) Amniotic fluid volume (ml) Total caruncle weight (g) Caruncle numbers Mean weight per caruncle (g) Brain weight (g) Heart weight (g) Liver weight (g) Kidney weight (g) a b 50 100 150 50 100 150 50 100 150 50 100 150 50 100 150 50 100 150 100 150 100 150 100 150 100 150 100 150 100 150 100 150 Significance of comparisonsb Treatment Gestation (days) AI 3.97 18.78 39.99 4.43 283.4 2589 37.2 328 893 164 1655 6500 143 480 3866 20.7 1025 2477 120 802 103 109 1.17 7.37 7.43 37.2 2.55 22.6 19.8 113 1.98 19.7 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 0.10 (n 5 5) 0.43 (n 5 4) 0.33 (n 5 5) 0.24 2.4 149 2.1 36 117 13 196 444 11 213 542 1.8 38 315 11 28 15 10 0.15 0.92 0.51 3.3 0.08 1.53 0.9 9 0.05 0.9 IVP 3.94 18.79 38.93 4.02 302.8 2726 46.1 387 774 174 1609 5088 155 616 1981 18.1 744 2621 120 811 99 108 1.21 7.48 8.03 39.6 2.73 22.6 20.2 105 2.10 18.8 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 0.45 0.27 0.73 0.75 12.2 196 10.1 17 57 18 204 698 13 259 751 4.9 178 265 11 109 16 7 0.17 0.90 0.80 0.7 0.15 2.11 0.5 5 0.22 1.8 NT (n 5 3) (n 5 4) (n 5 4) (n 5 3) (n 5 3) 3.83 18.59 38.36 4.45 320.6 3042 63.0 304 1360 213 1206 8033 194 235 6433 18.9 935 3985 214 1133 58 98 3.39 11.52 7.07 36.4 3.33 27.1 17.6 159 2.39 27.0 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 6 0.10 0.75 0.92 0.25 31.5 170 6.5 51 271 13 228 1800 13 146 1531 1.2 96 1021 44 86 9 10 0.67 1.52 0.26 1.5 0.44 2.11 1.2 10 0.42 2.8 (n 5 10) (n 5 6) (n 5 8) (n 5 5) (n 5 6) (n 5 5) (n 5 5) (n 5 5) (n 5 5) (n 5 5) NT:AI NT:IVP IVP:AI — — — — — — * — * — — — — — — — — — * * * — * * — — — — — * — * — — — — — — — — * — — * — — — — — — * * * — * * — — — — — * — * — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — — Sample numbers for the variables at each stage of gestation and treatement are the same as that stated for C-R lengths, unless indicated otherwise. —, Means are not significantly different; *, means are significantly different at P # 0.05. 4 LEE ET AL. FIG. 2. Crown-rump (C-R) length, fetal weight, and weight of combined fetal membranes at Days 50, 100, and 150. The means for each treatment (AI, IVP or NT) are joined by a line. used an indicator for the size of the fetal skeletal frame and to estimate the age of the fetus. No significant difference was found in the mean C-R length between treatments at all stages of gestation (Fig. 2 and Table 1). By itself, the C-R length did not indicate fetal overgrowth in either the NT or IVP group compared with the AI group. No skeletal abnormalities were observed. The mean fetal weight was similar among all three groups at Day 50 (Fig. 2 and Table 1). One NT fetus appeared to be slightly anemic, and the cotyledon-caruncle attachment was poor, suggesting imminent pregnancy failure. One IVP fetus had both low fetal weight and C-R length. An example of a Day 50 NT conceptus with exFIG. 3. A) Example of a Day 50 NT fetus together with all its fetal membranes. The chorion over the amniotic sac has been peeled back to better show the fetus (amnion with fetus [A1F]). Note the absence of fetal cotyledons at the tips of the chorioallantoic membranes (arrows). B) Closeup of the fetus and the membranes showing good vascularization of the cotyledons (arrows). C) Head of a normal Day 100 AI fetus. D) A Day 100 NT fetus with a shortened snout and thick, short neck. Bar 5 1 cm. cellent vascular development is shown in Figure 3, A and B. A positive linear correlation was found between the fetal weight and (C-R length)3 at Day 50 in the AI (r 5 0.79, P 5 0.11) and IVP (r 5 0.99, P 5 0.09) groups, but the correlation in the NT group was poorer (r 5 0.55, P 5 0.10). At Day 100, the mean fetal weight (Table 1) in the NT group was 320 6 32 g, compared with 303 6 12 g for the IVP fetuses and 283 6 2 g for the AI group. Although the means between the groups were not significantly different, five of the six NT fetuses were more than 2 SD heavier than the mean weight of AI fetuses, whereas only one in four IVP fetuses was above this weight. Gross malforma- GROWTH DEREGULATION IN CLONED CATTLE FETUSES 5 FIG. 4. Total fluid, allantoic fluid, and amniotic fluid volumes at Days 50, 100, and 150. The means for each treatment (AI, IVP, or NT) are joined by a line. tions were absent in all but one NT fetus, which had a shortened snout and a thick neck in comparison with other fetuses (Fig. 3, C and D). A strong, positive linear correlation was found between fetal weight and (C-R length)3 in the AI (r 5 0.99, P 5 0.014) and NT (r 5 0.94, P 5 0.005) groups but not in the IVP group (r 5 0.48, P 5 0.68). The slope for this relationship was 7.5-fold higher in the NT group compared with the AI group, indicating that weight increase with C-R length was greater in the NT group. By Day 150, the mean fetal weight had increased 9- to 10-fold in all groups (NT, 3042 6 170 g; IVP, 2726 6 196 g; AI, 2589 6 149 g) Although the mean fetal weight of the NT group was only 17% and 11% higher than those of the AI and IVP groups, respectively (not significantly different), five of the eight fetuses were greater than 2 SD above the mean weight of the AI fetuses, whereas only one in five and one in four were greater than this weight for the AI and IVP groups, respectively. No gross fetal abnormalities were detected in any Day 150 fetus. At this stage of gestation, the skin pigmentation was established, and it was possible to tell that all fetuses were Friesian. No linear correlation between the fetal weight and (C-R length)3 was evident in any of the three groups at Day 150. Fetal membrane weight. The combined wet weights of the amniotic and chorioallantoic membranes containing the fetal cotyledons are shown in Figure 2 and Table 1. These fetal membranes are derived entirely from the extraembryonic tissues. At Day 50, all chorioallantoic membranes were vascularized, and the fetal cotyledons were tenuously attached to the uterine caruncles of the uterus. In all cases, the vascularization and attachment of the cotyledonary burrs to the caruncles were more advanced on the amniochorion and the allantochorion adjacent to the fetus than they were toward the tips of the chorioallantoic sacs. The number of visible cotyledonary burrs was similar in all three groups, with an overall mean of 68 6 3. The mean fetal membrane weight was significantly greater in NT conceptuses (63.0 6 6.5 g) compared with the AI group (37.2 6 2.1 g, P , 0.05) but not compared with the IVP group (46.1 6 10.1 g). The highest membrane weight recorded (101.5 g) was from the anemic NT fetus, in which attachment of the fetal cotyledons to the caruncles was poor. The total membrane weight at Day 100 was highly var- iable, but it was not significantly different between the groups. In two cases in the NT group (one involving the fetus shown in Fig. 3D), weights were not recorded because of the extremely gelatinous nature; such gelatinous membranes were absent from either the AI or IVP group. All fetal membranes were well vascularized and the fetal cotyledons firmly attached the caruncles. Two developmentally retarded NT fetuses had correspondingly lower membrane weights. Amniotic pustules, which were located on the inner surface of the amnion facing the fetus, were flat, whitish plaques in the AI group, whereas in several cases in the NT group, they were small, yellow spikes. By Day 150, the mean fetal membrane weight was significantly higher (P , 0.05) in the NT group (1360 6 271 g) compared with either the AI (893 6 117 g) or IVP (774 6 57 g) group. This increased weight may be caused, in part, by a greater total fetal fluid volume in several of the NT pregnancies. Fetal fluids. The total fetal fluid volume represented the combined volumes of the allantoic and amniotic fluids. At all three stages of gestation, no significant difference was found in the mean allantoic, amniotic, or total fluid volume between the treatment groups (Fig. 4 and Table 1). However, 3 of the 10 NT cases at Day 50 and three of the eight cases at Day 150 had total fluid volumes greater than 2 SD above the mean for the AI group at the respective stages of gestation. The ratio of allantoic to amniotic fluid volume varied with gestation. At Day 50, the allantoic fluid volume was 9- to 10-fold higher than the amniotic fluid volume. Increased total fluid volume in all three of the above-described Day 50 NT cases was caused by increased allantoic fluid volume, which was greater than 2 SD above the mean for the AI group. At Day 100, allantoic fluid volumes were highly variable in all three groups, ranging from 60 to 1300 ml (Fig. 5). The volumes for two NT cases were not recorded, because the tract had ruptured after slaughter in one case and the allantoic sac was full of a jelly-like substance in the other. Amniotic fluid volumes were less variable between individuals. One small NT fetus with only 36 placentomes had low allantoic and amniotic fluid volumes, suggesting a loss of ability to maintain appropriate fetal fluid volume. At Day 150, three NT and one IVP allantoic and am- 6 LEE ET AL. FIG. 5. Fetal brain, liver, heart, and kidney weights at Days 100 and 150 expressed as a percentage of the fetal weight. The means for each treatment (AI, IVP, or NT) are joined by a line. At Days 100 and 150, the means for the NT relative liver weight were significantly different from the AI and IVP groups (P , 0.05). niotic fluid volumes were not recorded, because the fetal membranes had ruptured. Hydropsy was evident in two of eight NT pregnancies and was likely in a third. All three cases had total fluid volumes greater than 2 SD above the AI mean, and two cases also had allantoic fluid volumes that were 2 SD above the AI mean (20 and 12 L compared with 6.6 6 0.4 L in the AI group). In the animal with 20 L of fluid, the amniotic fluid accounted for 8 L (AI, 2.4 6 0.3 L), an indication of polyhydramnios, which is far less prevalent than hydrallantois in the hydrops syndrome associated with NT. Increased total fluid volume was associated with increased fetal and placental growth. In two of the above-described cases, fetal weights and heart weights were greater than 2 SD above the AI mean, and the kidney and liver weights in all three cases were 2 SD above the AI mean. All three cases had total caruncle weights greater than 2 SD above the AI mean but normal caruncle numbers. These three cases had the highest kidney and total caruncle weights of any group at this gestation, but the fetuses were not edematous. Because the allantoic and amniotic fluids are contained within the fetal membranes, we determined if a correlation exists between the fetal membrane weight and the total fetal fluid volume. A positive linear correlation was detected in several instances, but no consistency was observed in this relationship with either treatment or stage of gestation. Thus, it was not always possible to attribute the increase in fetal membrane weight to increased fluid accumulation. Fetal organ development. The weights of the brain, liver, heart, and kidney were compared among the three groups, both with (Fig. 5, expressed as a percentage of fetal weight) and without (Table 1) correction for the weight of the fetus. No significant difference was observed in the mean brain weight between groups at either Day 100 or 150. Mean heart weights averaged over Days 100 and 150 were significantly higher (P , 0.05) in the NT group compared with either control group; no difference was found between the AI and IVP groups. However, when adjusted for gestation and fetal weight, the difference between the NT group and either control group was not significant. No difference was observed in the mean liver weights between the groups at Day 100 (Table 1). When corrected for the fetal weight, the livers of NT fetuses were disproportionately lighter (P , 0.05) compared with either the AI or IVP group. This trend was reversed by Day 150, when the mean for the surviving NT group (159 6 10 g) was significantly higher (P , 0.05) than that in either the AI (113 6 9 g) or the IVP (105 6 5 g) group, even when adjusted for fetal weight (P , 0.05). The mean NT liver weight increased 9-fold between Days 100 and 150, compared with only 6.6- and 5-fold in the AI and IVP groups, respectively, suggesting a ‘‘catch-up’’ growth, whereas the fetal weight increase was similar in all three groups (9- to 10-fold). If this rate of growth was maintained for the rest of gestation, the liver would be significantly enlarged by the time these NT fetuses reached full term. The mean kidney weights were not significantly different between the groups at Day 100, with and without correction for the fetal weight. At Day 150, kidneys from the NT groups were significantly larger (P , 0.05) than those in either control group (Table 1). However, when expressed as a percentage of fetal weight (Fig. 5), the mean value for the NT group (0.90% 6 0.06%) was still higher than that for either the AI (0.77% 6 0.10%) or IVP (0.70% 6 0.14%) control group, although the difference was not significant. No gross malformations were seen in the brain, liver, heart, or kidneys at Day 100 or 150. However, livers from one NT fetus at Day 100 and from another three NT fetuses at Day 150 showed small, pale foci on the surface, giving these livers a mottled appearance. The mottling may be caused by fatty accumulation or hepatic congestion. In all except one Day 150 case, mottling of the livers was associated with hepatic enlargement. An association between liver mottling and cardiac enlargement was also seen in one Day 100 and one Day 150 case. Three Day 150 NT kidneys had dispersed clusters of fat cells on the capsules, and one had a slightly misshapen kidney. Placentome development. At the initiation of placentome formation at Day 50, we could detect no difference between the three groups in the number of cotyledonary burrs formed on the chorion. Five of the 10 NT conceptuses had very red fetal cotyledons, suggestive of good vascularization (Fig. 3), compared with two from the five AI and none from the IVP group (paler cotyledons). The number of caruncles and their weights at Days 100 and 150 (Fig. 6) were used as indicators of placentome numbers and weights, because the caruncles are the maternal tissues that, together with the fetal cotyledons, form the placentomes. In both the AI and IVP groups, the numbers of caruncles at Days 100 and 150 were very similar (Table 1), indicating that placentome numbers were fixed by Day 100. No difference was observed in the caruncle numbers between the AI and IVP groups at either Day 100 or 150. In contrast, the mean number of caruncles at Day 100 in the surviving NT group (58 6 9) was significantly lower (P , 0.05) than in either the AI (103 6 15) or IVP (99 6 16) group. Only 36 and 39 caruncles were recorded in two GROWTH DEREGULATION IN CLONED CATTLE FETUSES 7 FIG. 6. Total caruncle weights (g), caruncle numbers, and average caruncle weights (g) at Days 100 and 150. The means for each treatment (AI, IVP, or NT) are joined by a line. NT pregnancies, and both these fetuses showed signs of growth retardation accompanied by decreased fetal fluid volume and membrane weight. Caruncle numbers in the surviving NT group at Day 150 were similar to those in the two control groups. Despite lower caruncle numbers at Day 100, the mean weight of all caruncles added together (Fig. 6 and Table 1) was significantly higher (P , 0.05) in the NT pregnancies (NT . AI 5 IVP). Together with the lower mean caruncle numbers, this resulted in significantly increased (P , 0.05) average caruncle weights in this group (NT . IVP 5 AI). Figure 7 shows examples of uterine caruncles from an AI, FIG. 7. A–D) Examples of Day 100 uteri from an AI (A) with 66 caruncles, an IVP (B) with 84 caruncles, an NT with only 36 caruncles (C), and an NT with 80 caruncles (D) showing the considerably larger NT caruncles. The fetal membranes together with the fetal cotyledons have been removed. E) Sagittal cross-sections of a Day 150 NT and an IVP placentome showing the increased thickening of the NT placentome. The white arrows indicate the fetal side. Fetal villi growing into the caruncle (‘‘streaks’’) are indicated by the black arrows. Bar 5 1 cm. 8 LEE ET AL. TABLE 2. SD and significance of pairwise comparisons of within-group variances between treatment groups. Significance of pairwise comparisonsa SD Day Day Day Day Day Day Day Day Day Day Day Day Day Day Day Day a 100 C-R length (cm) 100 fetal weight (g) 100 kidney weight (g) 100 heart weight (g) 150 liver weight (g) 150 kidney weight (g) 50 fetal membrane weight (g) 100 fetal membrane weight (g) 150 fetal membrane weight (g) 100 total caruncle weight (g) 100 ave. caruncle weight (g) 150 total caruncle weight (g) 100 amniotic fluid (ml) 150 amniotic fluid (L) 150 allantioc fluid (L) 150 total fluid (L) AI IVP NT NT:AI NT:IVP IVP:AI 0.85 4.9 0.10 0.16 19.6 2.05 4.7 70.1 262 21.1 0.30 63 76 0.70 1.21 0.99 0.54 24.4 0.44 0.30 28.2 8.03 17.6 33.2 114 21.0 0.34 217 357 0.46 1.30 1.40 1.83 77.1 1.04 1.08 50.4 11.5 20.6 124 767 108 1.34 243 215 2.28 3.42 5.09 NS A B B C B A NS C C C C NS C C B C C NS C NS NS NS C B C C NS NS C NS C NS C C NS NS C C NS NS NS NS C C NS NS NS NS, not significant; A, P # 0.001; B, P # 0.01; C, P # 0.05. an IVP, and two NT pregnancies after removal of the fetal membranes and associated cotyledons. The large, flat caruncles in Figure 7C were from a uterus in which only 36 were detected, whereas Figure 7D shows an NT example with 80 caruncles and increased total and average caruncle weights. A negative linear correlation was found between the caruncle numbers and the average caruncle weight in the AI (r 5 0.91, P 5 0.09) and IVP (r 5 0.81, P 5 0.19) groups but not in the NT group (r 5 0.26, P 5 0.62). Although the NT caruncle numbers were ‘‘normal’’ at Day 150, the mean total caruncle weight was still significantly higher (NT . IVP 5 AI; P , 0.05), resulting in significantly higher (P , 0.05) average caruncle weight (NT . IVP 5 AI). A negative linear correlation between the average caruncle weight and caruncle number was evident in both the AI (r 5 0.98, P 5 0.002) and NT (r 5 0.70, P 5 0.05) groups but not in the IVP group (r 5 0.11, P 5 0.88). The larger of the NT placentomes assumed fistlike structures and were thicker in the sagittal cross-section compared with AI or IVP placentomes (Fig. 7E), which were more likely to be flat, discoid structures. Individual variability. Plots of the morphometric data in Figures 2 and 4–6 demonstrate considerably variability in every parameter examined, particularly within the NT group. We performed F-tests to compare the variances between the groups. Table 2 shows the SD for the variables and the significance of the pairwise comparisons of variances between treatment groups. Despite all NT fetuses having the same nuclear genetics of the cell line, a significantly greater variability was observed in the fetal weight at Day 100 than in the contemporary AI (P , 0.001) or IVP (P , 0.05) group. The IVP group was also more variable compared with the AI group (P , 0.05). In addition, the NT kidney weights at Day 100 showed greater variability compared with the AI group (P , 0.05); however, this may be caused, in part, by the greater variability in the NT fetal weight. The Day 150 NT liver and kidney weights were more variable compared with those in the AI, but not with those in the IVP, group. Greater variability in the NT group was most evident in the components forming the uteroplacental tissues. Day 50 fetal membrane weights were significantly more variable in the NT group compared with the AI group (P , 0.001) but not with the IVP group (P 5 0.30). Membrane weights of Day 50 IVP fetuses were also more variable compared with the AI group (P , 0.05). At later stages of gestation, some of the variability in fetal membrane weights may be caused, in part, by greater variability in fetal fluid volumes (Table 2). By the time placentation was complete at Day 100, the total caruncle and average caruncle weights in the NT group were significantly more variable compared with either the AI (P , 0.05) or IVP (P , 0.05) group. Total caruncle weights of both the NT (P , 0.05) and the IVP (P , 0.05) groups were significantly more variable than the weights of the AI group at Day 150. The analyses suggest that greater variability occurs in fetal membrane and placental development in the NT group and, to a certain extent, in the IVP group compared with the AI group. DISCUSSION A plethora of developmental abnormalities associated with cloning by NT have been reported in several species [16–20]. This considerable variation in abnormalities may be caused by the different cell types and lines used, the different methods of producing and culturing NT embryos, and species differences in the mode of placentation and gestational physiology. The in vitro culture of bovine embryos itself has been associated with abnormal phenotypes [25] and the ‘‘large offspring syndrome’’ frequently associated with NT. In the present study, the number of confounding factors was minimized to allow comparison of NT fetal development with contemporary half-siblings produced by AI or from embryos after in vitro fertilization. The inclusion of IVP embryos allowed us to evaluate any potential effect of embryo culture on subsequent in utero development. Failure of extraembryonic membrane development has been postulated to be one of the potential causes of pregnancy failure before or around the time of placentome formation with cattle IVP and NT embryos. In our previous experience, approximately 20%–25% of IVP conceptuses were defective in allantoic development [26]. In the present study, we did not see a single case of allantoic aplasia or failure of allantoic vascularization at Day 50. It is unclear whether this resulted from a change in the source of the bovine albumin added to the embryo culture medium, from a product obtained from Sigma (St. Louis, MO) to the current source [27], because these were the first embryos produced with this modified medium that had been transferred GROWTH DEREGULATION IN CLONED CATTLE FETUSES back to recipients and allowed to develop further. However, we cannot exclude failure of allantoic development as one of the causes of IVP and NT embryonic losses (30%–40%) before Day 50. Clearly, vasculogenesis and the initiation of fetal cotyledon development were unimpaired in the surviving Day 50 NT conceptuses in the present study. Abnormal placental development and overgrowth are hallmark pathologies associated with cloning by NT. The significantly lower number of caruncles and, hence, placentomes in the NT group at Day 100 is consistent with the report of Hill et al. [18]. Cotyledonary burr formation and vascularization were unimpaired at Day 50, which suggests that a high proportion of NT fetal cotyledons fail to interact appropriately with the uterine caruncles to form placentomes. Some NT pregnancies in which no placentomes were found by Day 70 have been encountered (unpublished data). Placentome numbers in surviving Day 150 NT pregnancies were similar to those in the control groups, an indication that, perhaps, those with unusually low placentome numbers were lost before Day 150 (3/5 and 0/6 were lost in experiments 1 and 2, respectively). Despite the decrease in caruncle numbers at Day 100, total caruncle weight in the NT group was significantly higher, suggesting growth overcompensation. The loss of an inverse correlation between the average caruncle weight and the caruncle number in the NT group suggests that mechanisms normally regulating the total weight of placentomes are defective in NT pregnancies at Day 100. Even when the caruncle numbers were normal in surviving Day 150 NT pregnancies, the total caruncle weights remained significantly higher, suggesting a loss of placental growth regulation even in apparently ‘‘normal’’ NT pregnancies. The increased caruncle weights suggest that NT placental overgrowth in cattle involved excessive uterine tissue growth, perhaps in response to factors secreted by the fetal cotyledons. Whereas the mouse placenta is made predominantly of conceptus-derived tissues, the maternal tissues in the bovine placentome form an equivalent or greater part of the structure. The ratio of maternal to fetal tissue weight in the bovine placentome is 1–1.2 between Days 70 and 130 and 1.5–1.8 from Day 130 onward [28]. The larger, thicker NT placentomes resembled those of later stages of gestation in normal cattle pregnancy, indicating that NT placentome growth was advanced for gestation. No correlation was found between total caruncle and fetal weight, suggesting that placental overgrowth in NT is neither a cause nor an indicator of fetal overgrowth nor a compensatory mechanism to overcome placental insufficiency in fetal intrauterine growth retardation. Fetal and placental overgrowth were closely associated in mouse cloning [20]. Whether this was caused, in part, by the smaller litter sizes in clone pregnancies is unknown. In polytocous species, such as the rat, mouse, rabbit, and guinea pig, the placental and fetal weights are inversely related to the number of fetuses in the litter. Fetal overgrowth and the ‘‘large offspring syndrome’’ in sheep and cattle have been associated with in vitro culture of embryos [29–32]. Recently, an association between in vitro fertilization in humans and the Beckwith-Wiedemann syndrome (BWS), a human fetal overgrowth syndrome, has been reported [33]. The IVP fetuses reported in the present study were not significantly heavier than the AI fetuses at all three stages of gestation. Therefore, in vitro culture is unlikely to be a significant contributor to the NT fetal overgrowth in five of the six Day 100 fetuses and in five of the eight Day 150 fetuses in the present study. However, in 9 vitro culture did have subtle effects on the development of IVP fetuses, such as the greater variability in fetal weight and C-R lengths, and it may contribute to the greater variability of NT fetal growth. Increased NT fetal weight at Days 100 and 150 was not accompanied by greater C-R length. Because most of the fetal weight gain normally occurs during the few weeks before parturition, a modest, 11%–17% gain in mean weight just past midgestation could result in considerably heavier NT calves at birth. The mean live birth weight of singleton NT calves produced from this cell line was 40.4 6 1.3 kg (n 5 16), compared with the mean live birth weight of 34.4 6 0.7 kg (n 5 74) for Friesian heifers born through AI on various farms around the region (Dr. Susanne Meier, Dexcel, New Zealand; personal communication), a weight increase of 17%. The unusually high incidence of hydropsy and its associated fetal mortality in the NT cloning of cattle emphasizes the need to understand the underlying mechanisms of hydropsy. Fetal fluid homeostasis in cattle is still not fully understood. In the present study, perturbed fluid homeostasis was evident as early as Day 50, when the placentomes were not yet fully formed. At Day 150, the hydrops syndrome was associated with fetal, placental, and organ overgrowth; whether this association holds true for NT fetuses generated with other cell lines needs further investigation. Organomegaly involving the liver, kidney, and heart and an increased incidence of polyhydramnios are also seen with BWS in humans and in mouse models of the syndrome [34]. The degree of penetrance of the growth deregulation phenotype in NT fetuses is reminiscent of the BWS phenotype: It is highly variable, and not all organs are affected in the same way in every individual. The association between excessive fetal fluid accumulation and renal and placental growth deregulation may indicate impairment of renal and placental function. Although the placenta is the major organ regulating the fetal environment, the fetal kidney also plays an important role in the regulation of fetal arterial pressure, fluid and electrolyte homeostasis, acidbase balance, and hormone synthesis [35]. In ruminants, fetal urine contributes to the allantoic and amniotic fluid. Reports have appeared of kidney defects [36] and impaired renal function in cloned offspring [15] as well as impaired liver function in cloned mice at 3 and 14 mo of age [37]. The growth deregulation in the liver and kidney is of particular concern, because these two organs play critical roles in the health of an animal. An interesting observation in the present study was the greater variability within the cohort of NT fetuses with the same nuclear genetics than within the cohorts of AI and IVP fetuses, given that sexual dimorphism in the growth of AI and IVP fetuses is expected to increase the variability within these two control groups. This suggests that nuclear genetic background alone does not account entirely for the phenotype. The cause of this variability is unknown, but heterogeneity in epigenetic modifications in donor nuclei and incomplete epigenetic reprogramming after NT could affect the expression of key regulatory genes during embryonic development. This may lead to greater ‘‘noise’’ in the expression of other genes further downstream, resulting in greater variability. It is, however, impossible to know if the somatic cells have acquired mutations either in vivo or during culture before NT. Such mutations could conceivably result in phenotypic variability between cloned offspring and the animal from which the cells were isolated or between individual cloned offspring. The NT cloned animals also differ from monozygotic 10 LEE ET AL. twins in that the mitochondrial genes are often different between individuals. However, conflicting evidence exists regarding whether the mitochondrial DNA is derived solely from the recipient cytoplast, as in the case of cloned sheep [38], or from both the cytoplast and donor somatic cell, as is common in NT cattle [39]. Therefore, even though the nuclear genome may be ‘‘identical,’’ NT animals from the same cell line may have different mitochondrial genotypes and, in cattle, may be mosaic for mitochondrial DNA subtypes. Because mitochondrial genes play a key role regulating the energy threshold and metabolic activity of all cells, they may have a significant effect on offspring performance. Loss of imprinting of IGF2 [40], paternal uniparental disomy involving the cluster of imprinted genes containing IGF2 [41], and mutations in the p57kip2 gene [42], also in this cluster, have been implicated in BWS, but the underlying mechanism of growth deregulation in this syndrome remains unknown. In cloned mouse fetuses, widespread misexpression of genes, including those that are imprinted, has been reported [43]. Many of these genes, including those affected in BWS, are excellent candidates for future investigations comparing gene expression profiles using the material collected in the present study. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. ACKNOWLEDGMENTS We thank Harold Henderson, David Duganzich, and Neil Cox for their invaluable help with data analyses; Martin Berg and Jacqui Forsyth for embryo transfer and AI of the heifers; Ljiljana Popovic and Don Saywell for generating the IVP embryos; John Lange for animal husbandry; and Alan Julian and staff of the former Animal Health Laboratories, Hamilton, for the use of their pathology laboratory for the sample collection. REFERENCES 1. Kato Y, Tani T, Sotomaru Y, Kurokawa K, Kato J, Doguchi H, Yasue H, Tsunoda Y. Eight calves cloned from somatic cells of a single adult. Science 1998; 282:2095–2098. 2. Wells DN, Misica PM, Tervit HR. Production of cloned calves following nuclear transfer with cultured adult mural granulosa cells. Biol Reprod 1999; 60:996–1005. 3. Kato Y, Tani T, Tsunoda Y. Cloning of calves from various somatic cell types of male and female adult, newborn and fetal cows. J Reprod Fertil 2000; 120:231–237. 4. Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature 1997; 385:810–813. 5. Baguisi A, Behboodi E, Melican DT, Pollock JS, Destrempes MM, Cammuso C, Williams JL, Nims SD, Porter CA, Midura P, Palacios MJ, Ayres SL, Denniston RS, Hayes ML, Ziomek CA, Meade HM, Godke RA, Gavin WG, Overstrom EW, Echelard Y. Production of goats by somatic cell nuclear transfer. Nat Biotechnol 1999; 17:456– 461. 6. Zou X, Chen Y, Wang Y, Luo J, Zhang Q, Zhang X, Yang Y, Ju H, Shen Y, Lao W, Xu S, Du M. Production of cloned goats from enucleated oocytes injected with cumulus cell nuclei or fused with cumulus cells. Cloning 2001; 3:31–37. 7. Keefer CL, Keyston R, Lazaris A, Bhatia B, Begin I, Bilodeau AS, Zhou FJ, Kafidi N, Wang B, Baldassarre H, Karatzas CN. Production of cloned goats after nuclear transfer using adult somatic cells. Biol Reprod 2002; 66:199–203. 8. Polejaeva IA, Chen SH, Vaught TD, Page RL, Mullins J, Ball S, Dai Y, Boone J, Walker S, Ayares DL, Colman A, Campbell KH. Cloned pigs produced by nuclear transfer from adult somatic cells. Nature 2000; 407:86–90. 9. Boquest AC, Grupen CG, Harrison SJ, McIlfatrick SM, Ashman RJ, d’Apice AJ, Nottle MB. Production of cloned pigs from cultured fetal fibroblast cells. Biol Reprod 2002; 66:1283–1287. 10. Loi P, Ptak G, Barboni B, Fulka J Jr, Cappai P, Clinton M. Genetic rescue of an endangered mammal by cross-species nuclear transfer using post-mortem somatic cells. Nat Biotechnol 2001; 19:962–964. 11. Wakayama T, Perry AC, Zuccotti M, Johnson KR, Yanagimachi R. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature 1998; 394:369–374. Wakayama T, Yanagimachi R. Cloning of male mice from adult tailtip cells. Nat Genet 1999; 22:127–128. Chesne P, Adenot PG, Viglietta C, Baratte M, Boulanger L, Renard JP. Cloned rabbits produced by nuclear transfer from adult somatic cells. Nat Biotechnol 2002; 20:366–369. Shin T, Kraemer D, Pryor J, Liu L, Rugila J, Howe L, Buck S, Murphy K, Lyons L, Westhusin ME. A cat cloned by nuclear transplantation. Nature 2002; 415:859. Wells D, Misica P, Day A, Peterson A, Tervit H. Cloning sheep from cultured embryonic cells. Reprod Fertil Dev 1998; 10:615–626. Hill JR, Burghardt RC, Jones K, Long CR, Looney CR, Shin T, Spencer TE, Thompson JA, Winger QA, Westhusin ME. Evidence for placental abnormality as the major cause of mortality in first-trimester somatic cell cloned bovine fetuses. Biol Reprod 2000; 63:1787–1794. De Sousa PA, King T, Harkness L, Young LE, Walker SK, Wilmut I. Evaluation of gestational deficiencies in cloned sheep fetuses and placentae. Biol Reprod 2001; 65:23–30. Hill JR, Edwards JF, Sawyer N, Blackwell C, Cibelli JB. Placental anomalies in a viable cloned calf. Cloning 2001; 3:83–88. Heyman Y, Chavatte-Palmer P, LeBourhis D, Camous S, Vignon X, Renard JP. Frequency and occurrence of late-gestation losses from cattle cloned embryos. Biol Reprod 2002; 66:6–13. Eggan K, Akutsu H, Loring J, Jackson-Grusby L, Klemm M, Rideout WM III, Yanagimachi R, Jaenisch R. Hybrid vigor, fetal overgrowth, and viability of mice derived by nuclear cloning and tetraploid embryo complementation. Proc Natl Acad Sci U S A 2001; 98:6209–6214. Inoue K, Kohda T, Lee J, Ogonuki N, Mochida K, Noguchi Y, Tanemura K, Kaneko-Ishino T, Ishino F, Ogura A. Faithful expression of imprinted genes in cloned mice. Science 2002; 295:297. Wells DN, Laible G, Tucker FC, Miller AL, Oliver JE, Xiang T, Forsyth JT, Berg MC, Cockrem K, L’Huillier PJ, Tervit HR, Oback B. Co-ordination between donor cell type and cell cycle stage improves nuclear cloning efficiency in cattle. Theriogenology 2003; 59:45–59. Thompson J, McNaughton C, Gasparrini B, McGowan L, Tervit H. Effect of inhibitors and uncouplers of oxidative phosphorylation during compaction and blastulation of bovine embryos cultured in vitro. J Reprod Fertil 2000; 118:47–55. Xu ZZ, Burton JR, MacMillan KL. Reproductive performance of synchronized lactating dairy cows. Proc N Z Soc Anim Prod 1995; 55: 242–244. Farin PW, Crosier AE, Farin CE. Influence of in vitro systems on embryo survival and fetal development in cattle. Theriogenology 2001; 55:151–170. Peterson AJ, McMillan WH. Allantoic aplasia—a consequence of in vitro production of bovine embryos and the major cause of late gestation embryo loss. Proc Aust Soc Reprod Biol 1998; 29:4. Peterson AJ, Lee RS-F. Improving successful pregnancies after embryo transfer. Theriogenology 2003; 59:687–697. Laven RA, Peters AR. Gross morphometry of the bovine placentome during gestation. Reprod Domest Anim 2001; 36:289–296. Young LE, Sinclair KD, Wilmut I. Large offspring syndrome in cattle and sheep. Rev Reprod 1998; 3:155–163. Van Wagtendonk-de Leeuw AM, Aerts BJ, den Daas JH. Abnormal offspring following in vitro production of bovine preimplantation embryos: a field study. Theriogenology 1998; 49:883–894. Jacobsen H, Schmidt M, Holm P, Sangild PT, Vajta G, Greve T, Callesen H. Body dimensions and birth and organ weights of calves derived from in vitro produced embryos cultured with or without serum and oviduct epithelium cells. Theriogenology 2000; 53:1761–1769. Van Wagtendonk-de Leeuw AM, Mullaart E, de Roos AP, Merton JS, den Daas JH, Kemp B, de Ruigh L. Effects of different reproduction techniques: AI MOET or IVP, on health and welfare of bovine offspring. Theriogenology 2000; 53:575–597. DeBaun MR, Niemitz EL, Feinberg AP. Association of in vitro fertilization with Beckwith-Wiedemann syndrome and epigenetic alterations of LIT1 and H19. Am J Hum Genet 2003; 72:156–160. Sun FL, Dean WL, Kelsey G, Allen ND, Reik W. Transactivation of Igf2 in a mouse model of Beckwith-Wiedemann syndrome. Nature 1997; 389:809–815. Robillard JE, Smith FG, Segar JL, Guillery EW, Jose PA. Renal function in the fetus. In: Thorburn GD, Harding R (eds.), Textbook of Fetal Physiology. New York: Oxford University Press; 1994:196–204. McCreath KJ, Howcroft J, Campbell KHS, Colman A, Schnieke AE, Kind AJ. Production of gene-targeted sheep by nuclear transfer from cultured somatic cells. Nature 2002; 405:1066–1069. GROWTH DEREGULATION IN CLONED CATTLE FETUSES 37. Ogura A, Inoue K, Ogonuki N, Lee J, Kohda T, Ishino F. Phenotype effects of somatic cell cloning in the mouse. Cloning Stem Cells 2002; 4:397–405. 38. Evans MJ, Gurer C, Loike JD, Wilmut I, Schnieke AE, Schon EA. Mitochondrial DNA genotypes in nuclear transfer-derived cloned sheep. Nat Genet 1999; 23:90–93. 39. Steinborn R, Schinogl P, Wells DN, Bergthaler A, Muller M, Brem G. Coexistence of Bos taurus and B. indicus mitochondrial DNAs in nuclear transfer-derived somatic cattle clones. Genetics 2002; 162: 823–829. 40. Weksberg R, Shen DR, Fei YL, Song QL, Squire J. Disruption of insulin-like growth factor 2 imprinting in Beckwith-Wiedemann syndrome. Nat Genet 1993; 5:143–150. 11 41. Weksberg R, Teshima I, Williams BR, Greenberg CR, Pueschel SM, Chernos JE, Fowlow SB, Hoyme E, Anderson IJ, Whiteman DA, Fisher N, Squire J. Molecular characterization of cytogenetic alterations associated with the Beckwith-Wiedemann syndrome (BWS) phenotype refines the localization and suggests the gene for BWS is imprinted. Hum Mol Genet 1993; 2:549–556. 42. Hatada I, Ohashi H, Fukushima Y, Kaneko Y, Inoue M, Komoto Y, Okada A, Ohishi S, Nabetani A, Morisaki H, Nakayama M, Niikawa N, Mukai T. An imprinted gene p57KIP2 is mutated in BeckwithWiedemann syndrome. Nat Genet 1996; 14:171–173. 43. Humpherys D, Eggan K, Akutsu H, Friedman A, Hochedlinger K, Yanagimachi R, Lander ES, Golub TR, Jaenisch R. Abnormal gene expression in cloned mice derived from embryonic stem cell and cumulus cell nuclei. Proc Natl Acad Sci U S A 2002; 99:12889–12894.