Document 14306137
advertisement

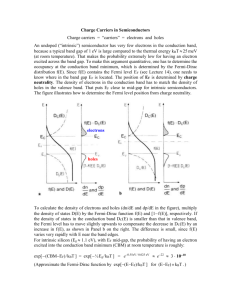
Semiconductor Conductivity Ch. 1, S • It is well-known that in semiconductors, there are Two charge carriers! Electrons e- & Holes e+ What is a hole? We’ll use a qualitative definition for now! A quantitative definition will come later! • Holes are often treated as “positively charged electrons”. How is this possible? Are holes really particles? We’ll eventually answer both of these questions as the course proceeds. A Qualitative Picture of Holes (from Seeger’s book) An idealized, 2 dimensional, “diamond” lattice for e- & e+ conduction “Thought Experiment”# 1 • Add an extra e- (“conduction electron”) & apply an electric field E (the material is n-type: negative charge carriers) e- E Field Direction e- Motion Direction (“almost free”) “Thought Experiment”# 2 • Remove an e- leaving, a “hole” e+ & apply an electric field E. (the material is p-type: positive charge carriers) + e E Field Direction e- Motion Direction e+ Motion Direction Crude Analogy: CO2 Bubbles in Beer! Beer Glass Bubbles g (gravity) Bubble Motion • We could develop a formal theory of bubble motion in the earth’s gravitational field. Since the bubbles move vertically upward, in this theory, the Bubbles would need “negative mass”! Thermal Pair Generation & Annihilation • Now: A classical Treatment. Simple, classical, statistical analysis. Later: Quantum Treatment • Define: Eg Binding energy of a valence electron. (In the Band Picture: This is the band gap energy). • Apply an energy Eg to an atom (from thermal or other excitation). • An e- is promoted out of a valence level (band) into a conduction level (band). Leaves a hole (e+) behind. • Later: e- - e+ pair recombine, releasing energy Eg (in terms of heat, lattice vibrations, …) • Schematically: e- + e+ Eg e-, e+ Pair Generation Recombination This chemical “reaction” can go both ways. As the temperature T increases, more e- - e+ pairs are generated & the electrical conductivity increases & the conductivity σ increases with increasing T. T Dependences of e- & e+ Concentrations • Define: n concentration (cm-3) of ep concentration (cm-3) of e+ • Can show (& we will): np = CT3 exp[- Eg /(kBT)] (C = material dependent constant) From the “Law of mass action” from statistical physics • In a pure material: n = p ni (np = ni2) ni “Intrinsic carrier concentration” ni = C1/2T3/2exp[- Eg /(2kBT)] At T = 300K Si : Eg= 1.2 eV, ni =~ 1.5 x 1010 cm-3 Ge : Eg = 0.67 eV, ni =~ 3.0 x 1013 cm-3 Also: Band Gaps are (slightly) T dependent! • It can be shown that: Eg(T) = Eg(0) - αT Si : α = 2.8 x 10-4 eV/K Ge : α = 3.9 x 10-4 eV/K But this doesn’t affect the T dependence of ni! ni2 = CT3exp[- Eg(T)/(kBT)] = Cexp(α/kB)T3exp[- Eg(0)/(kBT)] = BT3exp[- Eg(0)/(kBT)] where B = Cexp(α/kB) is a new constant prefactor Intrinsic Concentration vs. T Measurements/Predictions Note the different scales on the right & left figures! Doped Materials: Materials with Impurities! These are more interesting & useful! • Consider an idealized carbon (diamond) lattice (we could do the following for any Group IV material). C : (Group IV) valence = 4 • Replace one C with a phosphorous. P : (Group V) valence = 5 4 e- go to the 4 bonds 5th e- ~ is “almost free” to move in the lattice (goes to the conduction band; is weakly bound). • P donates 1 e- to the material P is a DONOR (D) impurity Doped Materials • The 5th e- is really not free, but is loosely bound with energy We’ll show later how to calculate this! ΔED << Eg The 5th e- moves when an E field is applied! It becomes a conduction e• Let: D any donor, DX neutral donor D+ ionized donor (e- to the conduction band) • Consider the chemical “reaction”: e- + D+ DX + ΔED As T increases, this “reaction” goes to the left. But, it works both directions • Consider very high T All donors are ionized n = ND concentration of donor atoms (constant, independent of T) • It is still true that np = ni2 = CT3 exp[- Eg /(kBT)] p = (CT3/ND)exp[- Eg /(kBT)] “Minority carrier concentration” • All donors are ionized The minority carrier concentration is T dependent. • At still higher T, n >>> ND, n ~ ni The range of T where n = ND the “Extrinsic” Conduction region. n vs. 1/T Almost no ionized donors & no intrinsic carriers lllll High T Low T n vs. T • Again, consider an idealized C (diamond) lattice. (or any Group IV material). • • • • C : (Group IV) valence = 4 Replace one C with a boron. B : (Group III) valence = 3 B needs one e- to bond to 4 neighbors. B can capture e- from a C e+ moves to C (a mobile hole is created) B accepts 1 e- from the material B is an ACCEPTOR (A) impurity • The hole e+ is really not free. It is loosely bound by energy ΔEA << Eg Δ EA = Energy released when B captures e e+ moves when an E field is applied! • NA Acceptor Concentration • Let A any acceptor, AX neutral acceptor A- ionized acceptor (e+ in the valence band) • Chemical “reaction”: e++A- AX + ΔEA As T increases, this “reaction” goes to the left. But, it works both directions Just switch n & p in the previous discussion! Terminology “Compensated Material” ND = NA “n-Type Material” ND > NA (n dominates p: n > p) “p-Type Material” NA > ND (p dominates n: p > n) Doping in Compound Semiconductors • This is MUCH more complicated! • Semiconductor compound constituents can act as donors and / or acceptors! • Example: CdS, with a S vacancy (One S-2 “ion” is missing) • The excess Cd+2 “ion” will be neutralized by 2 conduction e-. So, Cd+2 acts as a double acceptor, even though it is not an impurity! CdS with S vacancies is a p-type material, even with no doping with impurities!