A 639 substitution of Asp for Tyr in 1S0V (T7... Polymerase) results in a dramatic drop of enzymatic Hydroxyl
advertisement

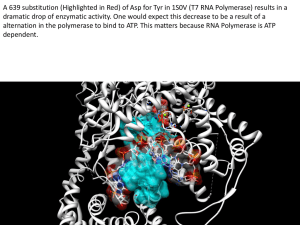
A 639 substitution of Asp for Tyr in 1S0V (T7 RNA Polymerase) results in a dramatic drop of enzymatic activity. One would expect this decrease to be a result of an alternation in the polymerase to bind to RNA and loose selectivity. The active site’s Hydroxyl group will bond with RNA’s addition oxygen on the Ribose 2C. Hydroxyl RNA polymerase Tyrosine RNA DNA Heme Oxygen Coordinating Histidine Of these four polypeptide chain units, two are alpha and two are beta units. These different units are complementary to one another. Although the alpha and beta chains have different sequences of amino acids, they fold to form a similar 3D structure. Only noncovalent interactions hold the four chains together. Myoglobins unit does not have a shape that is unique in this fashion, thus it cannot associate with other forms to have a larger tetrameric structure. Myoglobin is a globular protein, suggesting that it is more de Noncovalent interactions hold the hemoglobin’s chains together. Hemoglobin has a greater number of hydrophilic regions, shown in red. While Myoglobin is more hydrophobic, suggesting it is less likely to interact with other surrounding proteins.