Nomenclature and Structure of Carbohydrates
advertisement

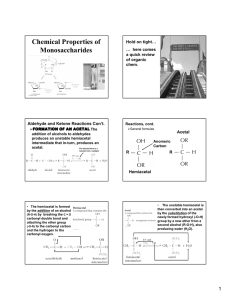
Nomenclature and Structure of Carbohydrates AILEEN ZAYDEL Carbohydrate Nomenclature “carbon-hydrates” (CH2O)n Monosaccharides, disaccharides, oligosaccharides, polysaccharides Complex carbohydrate: more than one monosaccharide building unit, extended branching polymer, a sugar attached to another biomolecule that forms a glycoconjugate glycoconjugate: glycoproteins, glycolipids, proteoglycans Basic Structure Emil Fischer • Polyhydroxy aldehydes and ketones • (CH2O)n where n can be 3-9 • 2 classes: aldoses and ketoses •Subclasses based on number of C atoms Aldose carbons are numbered with the carbonyl group dubbed as C-1 Ketose carbons are numbered with the carbonyl group dubbed as C-2 Aldose Ketose Sterioisomers All monosaccharides (except dihydroxy acetone) have one or more chiral C atoms Depending on the orientation of the CHOH group on the chiral C, a monosaccharide can have a “D” or “L” configuration. If there is more than one chiral C, choose the one furthest from the aldehyde or ketone group Any sugar that differs only in the orientation of 1 chiral C is called an epimer Total # sterioisomers = 2k Where k = # of chiral Carbons Common Monosaccharides Cyclization In solution, carbonyl group condenses with –OH group to form rings of hemiacetals/ hemiketals Cyclic Sterioisomers Generates new chiral center at the original carbonyl carbon atom called anomeric carbon 2 possible isomers: α or β depends on position above or below the plane of the ring Haworth projection Ring Conformations Because of the flexibilty of the tetrahedral bonds, the planar ring model is actually inaccurate. Chair, boat, twist, envelope, crown, tub The 2 conformations are labeled 1C4 or 4C1 1st numeral = number of the C atom above the seat of the chair 2nd numeral = number of the C atom below the seat. The pyranose ring, for example, can have 2 chair conformations. Saccharide Reactions- Mutarotation The conversion between the α and β forms of a ring structure Acidic/basic environment In solution at equilibrium Equilibrium reaction between alpha and beta pyranose and furanose rings of glucose Reaction- Oxidation Oxidation of the terminal groups to –COOH makes acid Free aldehyde group functions as the reducing terminus Reaction- Reduction Reduction of terminal groups with sodium borohydride (NaBH4) Makes polyhydroxy alcohols and sugar alcohols like alditol Conversion of a monosaccharide to a tritium-labeled alditol by reduction with NaB3H4 Reaction- Schiff Base Formation Carbonyl groups can react with amines and hydrazides to form imines and hydrazones Attaches monosaccharides to protein via amino group of lysine residue through glycation High levels of products of glycation found in diabetics. Reaction- Glycosidic Bond Formation When an anomeric center of one monosaccharide reacts with an –OH group in a second sugar Fundamental linkage of sugars hemiacetal + alcohol = full acetal Glycosidic linkage When 2 monosaccharides combine through a glycosidic linkage, a disaccharide results. Maltose formation When a monosaccharide and a hydroxyl group of an amino acid react, a glycoprotein results Gycoprotein Linkage Protein- bound glycans can be divided into two classes 1. 2. N-linked: bound to the nitrogen atom of asparagine side chains O-linked: bound to the oxygen atom of serine or threonine side chains Thy-1 glycoprotein Chemistry of Hydroxyl Groups Methylation: used to cap hydroxyl groups of saccharides to generate methyl ethers used in structural analysis Esterification: hydroxyl groups esterified by carboxylic acid. Required to allow interaction with certain biomolecules Deoxygenation: hydroxyl groups replaced by hydrogen atoms to form deoxysugars. E.g. DNA biosynthesis Reducing/non-reducing Ends Reducing end bears a free anomeric center available to form linkages Structures written from the non-reducing end to the reducing end Some structures are non-reducing Why are glycans so complex? One monosaccharide already has two steriosomers because of the anomeric C atom 2. The second monosacchaide can use any of its free hydroxyl groups to bind to the anomeric carbon 3. A single monosaccharide can form a glycosidic linkage with more than one other monosaccharide, effectively forming a branching point 4. A glycosidic linkage is strong but flexible, allowing for several conformations 5. A glycosidic linkage can form between any other biomolecule with a hydroxyl group , effectively forming a glycoconjugate 1. References http://rpi.edu/dept/bcbp/molbiochem/MBWeb/mb 1/part2/sugar.htm