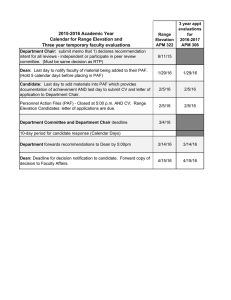
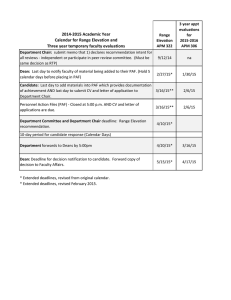
Role of Platelet-Activating Factor in Cardiovascular Pathophysiology
advertisement

PHYSIOLOGICAL REVIEWS Vol. 80, No. 4, October 2000 Printed in U.S.A. Role of Platelet-Activating Factor in Cardiovascular Pathophysiology GIUSEPPE MONTRUCCHIO, GIUSEPPE ALLOATTI, AND GIOVANNI CAMUSSI Laboratorio di Immunopatologia Renale, Dipartimento di Medicina Interna, Dipartimento di Biologia Animale e dell’Uomo e Istituto Nazionale di Fisica della Materia, Università di Torino, Torino, Italy I. Introduction II. Molecular Heterogeneity of Platelet-Activating Factor and Functional Implications III. Metabolism of Platelet-Activating Factor A. PAF biosynthetic pathways B. PAF catabolism IV. Platelet-Activating Factor Receptors and Signal Transduction V. Cardiovascular Responses to Platelet-Activating Factor A. Hemodynamic effect of PAF B. Effect of local administration of PAF on selected vascular districts VI. Effect of Platelet-Activating Factor on the Heart A. Coronary circulation: in vivo effects B. In vitro effects on isolated perfused heart C. Myocardial function D. Atrium and papillary muscle E. Effects on cardiomyocytes VII. Microvascular Effect of Platelet-Activating Factor In Vivo VIII. Effects of Platelet-Activating Factor on Endothelial Cells A. Effect of PAF on endothelial cell permeability B. Endothelium-leukocyte and -platelet interaction IX. Involvement of Platelet-Activating Factor in Cardiovascular Pathophysiological Processes A. Role of PAF in cardiac anaphylaxis and shock syndromes B. Role of PAF in ischemia-reperfusion injury of the heart C. Role of PAF in atherogenesis X. Role of Platelet-Activating Factor in Neoangiogenesis XI. Conclusions 1670 1670 1671 1671 1671 1673 1674 1674 1676 1677 1677 1678 1678 1679 1680 1681 1682 1682 1682 1683 1683 1684 1686 1686 1687 Montrucchio, Guiseppe, Guiseppe Alloatti, and Giovanni Camussi. Role of Platelet-Activating Factor in Cardiovascular Pathophysiology. Physiol Rev 80: 1669 –1699, 2000.—Platelet-activating factor (PAF) is a phospholipid mediator that belongs to a family of biologically active, structurally related alkyl phosphoglycerides. PAF acts via a specific receptor that is coupled with a G protein, which activates a phosphatidylinositol-specific phospholipase C. In this review we focus on the aspects that are more relevant for the cell biology of the cardiovascular system. The in vitro studies provided evidence for a role of PAF both as intercellular and intracellular messenger involved in cell-to-cell communication. In the cardiovascular system, PAF may have a role in embryogenesis because it stimulates endothelial cell migration and angiogenesis and may affect cardiac function because it exhibits mechanical and electrophysiological actions on cardiomyocytes. Moreover, PAF may contribute to modulation of blood pressure mainly by affecting the renal vascular circulation. In pathological conditions, PAF has been involved in the hypotension and cardiac dysfunctions occurring in various cardiovascular stress situations such as cardiac anaphylaxis and hemorrhagic, traumatic, and septic shock syndromes. In addition, experimental studies indicate that PAF has a critical role in the development of myocardial ischemia-reperfusion injury. Indeed, PAF cooperates in the recruitment of leukocytes in inflamed tissue by promoting adhesion to the endothelium and extravascular transmigration of leukocytes. The finding that human heart can produce PAF, expresses PAF receptor, and is sensitive to the negative inotropic action of PAF suggests that this mediator may have a role also in human cardiovascular pathophysiology. http://physrev.physiology.org 0031-9333/00 $15.00 Copyright © 2000 the American Physiological Society 1669 1670 MONTRUCCHIO, ALLOATTI, AND CAMUSSI I. INTRODUCTION Platelet-activating factor (PAF) is one of the most potent and versatile mediators found in mammals. It was originally described as “a soluble factor” involved in leukocyte-dependent histamine and serotonin release from platelets (177, 379). In 1972, Benveniste et al. (31) demonstrated that this soluble factor was released from rabbit basophils after IgE stimulation and coined the term PAF. Several reports followed describing the lipid nature of PAF (29, 32, 113, 332). Almost concomitantly, a factor with properties similar to PAF was isolated from the renal medulla by Muirhead and co-workers (44, 303, 305). This factor was named antihypertensive polar renal lipid because of its ability to lower blood pressure in the Goldblatt rat model of hypertension. In 1979, three independent groups (33, 44, 113) demonstrated that a semisynthetic phosphoacylglycerol, 1-O-alkyl-2-acetyl-sn-glycero3-phosphocholine, had physicochemical as well as biological properties indistinguishable from those of naturally occurring PAF/antihypertensive polar renal lipid. Hanahan et al. (164) characterized by gas-liquid chromatography and mass spectral analysis the chemical structure of PAF released by IgE-sensitized rabbit basophils as a 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine. Although PAF continues to be the common term used, it is a misnomer, because it identifies only the platelet effect of this mediator. PAF is now considered a phospholipid with diverse and potent physiological effects that belongs to a family of biologically active, structurally related alkyl phosphoglycerides (48, 79, 100, 163, 280, 337, 384, 385, 442, 446). PAF is thought to be a mediator of cell-to-cell communication, which may function either as an intercellular or an intracellular messenger (100). Some of its actions are achieved at concentrations as low as 10⫺12 M and include events relevant for the development of several pathological and physiological processes. Numerous cell types and tissues have been shown to produce PAF upon appropriate stimulation (47). In particular, PAF is produced by a variety of cells that may participate in the development of inflammatory reaction such as monocytes/ macrophages, polymorphonuclear neutrophils (PMN), eosinophils, basophils, and platelets (50, 79, 260, 428). In addition, human endothelial cells were found to produce PAF after stimulation by several inflammatory mediators (458) including thrombin (69, 179, 336, 474), angiotensin II (69), vasopressin (69), leukotrienes C4 and D4 (276), histamine (276), bradykinin (276), elastase (80), cathepsin G (80), hydrogen peroxide (145, 255), plasmin (289, 298), interleukin (IL)-8 (21) and IL-1␣, or tumor necrosis factor (TNF)-␣ (61, 64, 60, 73, 228, 229, 286). Cardiomyocytes have been also reported to synthesize PAF under appropriate stimulation (200). Most of the cells that produce PAF also possess PAF Volume 80 receptors (185, 232, 403, 470) and are target for PAF action. In vitro, PAF promotes the aggregation, chemotaxis, granule secretion, and oxygen radical generation from leukocytes and the adherence of leukocytes to the endothelium (51, 81, 322, 373, 480). PAF increases the permeability of endothelial cell monolayer (62), stimulates the contraction of smooth muscle (139, 209, 396) and myometrium (292, 414), and has negative inotropic, arrhythmogenic effects on cardiac muscle (11, 30, 71, 251, 352, 353). In this paper we review the molecular and cellular basis for the structural and functional diversity of PAF molecules, the different biosynthetic and catabolic pathways of PAF, and the signal transduction triggered by the engagement of PAF receptor. Moreover, we describe the systemic and local effects of PAF administration in vivo and the mechanical and electrophysiological effects of PAF on isolated perfused heart, atrium, and papillary muscle and on cultured cardiomyocytes. We also examine the microcirculatory effect of PAF and its role in the interaction between endothelial and inflammatory cells. Finally, the involvement of PAF in cardiovascular pathophysiological processes such as shock syndromes, ischemia-reperfusion injury, atherogenesis, and neoangiogenesis is discussed in the light of the biological properties of this mediator and of the effect of PAF-receptor antagonists. II. MOLECULAR HETEROGENEITY OF PLATELET-ACTIVATING FACTOR AND FUNCTIONAL IMPLICATIONS Although PAF is generally considered as a single molecular entity with a wide spectrum of diverse and potent biological properties, it is now clear that there are a variety of structurally related phospholipid molecules of biological origin that share many of the same physiological activities (for review, see Ref. 280). However, the chemical structure strictly influences the biological potency of PAF (384). The full expression of its biological potency requires an ether linkage at the sn-1 position of the glycerol backbone, a short acyl chain, usually an acetyl residue, at the sn-2 position, and the polar head group of choline or ethanolamine at the sn-3 position (280, 384). The length of the alkyl chain at the sn-1 position and the number of double bonds have modest effects on its biological potency. In contrast, the presence of an esther linkage at the sn-1 position of glycerol or a three-carbons longer acyl chain at sn-2 position significantly diminishes and changes the biological properties. Mass spectral analysis of PAF synthesized by human PMN showed the presence of multiple molecular species of alkyl PAF including 15:0-, 16:0-, 17:0-branched chain, 17:0-, 18:0-, 18:1-, 18:2-, and October 2000 PAF AND THE CARDIOVASCULAR SYSTEM 22:2-PAF (262, 333, 452, 453). Human PMN also produce acyl-PAF that represents 13–25% of total PAF synthesized by these cells (301, 426). More recently, it has been suggested that the acyl-PAF may be the major molecular species of PAF produced by several cell types including human pulmonary mastocytes (425, 427) and vascular endothelium (104, 428, 457). Perfused rat and guinea pig hearts (312) produce vinyl-PAF. Other species of biologically active acetylated glycerolipids are the plasmalogen analogs of PAF such as 1-alk-1⬘-enyl-2-acetyl-sn-glycero-3-phosphocholine and 1-alk-1⬘-enyl-2-acetyl-sn-glycero-3-phosphoethanolamine and the 1-alkyl-2-acetyl-sn-glycerols, the neutral lipid precursor of PAF. Such plasmalogens even if less active than PAF itself can act synergistically with PAF (262, 385). The biological activities of 1-alkyl-2-acetyl-snglycerols have been related to the conversion of these neutral lipids to PAF. However, 1-alkyl-2-acetyl-sn-glycerols and their metabolic products have been shown to possess intrinsic biological activities (437) including differentiation of HL-60 cells (281), attenuation of diacylglycerol-induced activation of protein kinase C (PKC), and activation of macrophages (466). Finally, the 1-alkyl-2-acetyl-sn-glycero-phosphate, an analog of phosphatidic acid, can act as a calcium ionophore (63). The different molecular species of PAF have different potency on platelet activation (280). However, platelet bioassay does not necessarily reflect the biological potential of a given molecular species on other cell types or tissues. For instance, it has been shown that the presence of a single double bond in the alkyl chain at the sn-1 position significantly alters the cardiac activity by increasing 50-fold the negative inotropism and 100fold coronary vasoconstriction but has little effect on the stimulation of platelets (280). McManus et al. (280) have recently reviewed the differences in the rank orders of potency of alkyl and acyl-PAF molecules in vitro on rabbit platelets, human PMN, and isolated guinea pig heart, and in vivo, when injected into rabbit on thrombocytopenia, leukopenia, and right ventricular hypertension. It has been suggested that the differences in the biological properties of various molecular species of PAF may depend on differences in the biophysical properties as critical micellar concentration or albumin binding affinity or on the existence of more than one receptor subtype for PAF. III. METABOLISM OF PLATELET-ACTIVATING FACTOR 1671 PAF is synthesized by two main pathways in a variety of tissues and cells. The “remodeling pathway” is mainly involved in the synthesis of PAF by stimulated inflammatory cells (14, 240, 319). This pathway requires a tightly coupled reaction of phospholipase (PL) A2 and acetyl CoA:1-alkyl-sn-glycero-3-phosphorylcholine 2-Oacetyltransferase (464). The activation of PLA2 (461) determines the hydrolysis of membrane phospholipids to generate a variety of 2-lysophospholipids (e.g., 1-alkyl-2-lyso-glycero-3-phosphocholine, lyso-PAF). These 2-lysophospholipids are the substrate of acetyl CoA:1alkyl-sn-glycero-3-phosphorylcholine 2-O-acetyltransferase, which catalyzes the transfer of the acetyl moiety from acetyl CoA to the free hydroxyl at sn-2 position. In addition to a direct deacylation of membrane glycerophospholipids, another pathway for the generation of 2-lysophospholipids has been recently described. Indeed, lyso-PAF can be obtained via a CoA-independent transacylation reaction between alkylacyl-glycerol-3phosphocholine and the lysophospholipid acceptor formed via the action of a putative PLA2 (25, 106, 317, 386, 434, 445). This CoA-independent transacylase route accounts for the simultaneous PAF synthesis and mobilization of arachidonic acid, since it is specific for the arachidonate linked species of alkyl choline phosphoglycerides (354, 460). Figure 1 shows the enzymatic steps of the remodeling pathway. The second biosynthetic pathway of PAF that is mainly operative in the kidney and in the central nervous system (65, 350, 384) has been termed “de novo pathway” (Fig. 2). This involves the synthesis of 1-O-alkyl-2-acetylglycerol, which is then converted to PAF by a specific dithiothreitol-insensitive CDP-choline:1-alkyl-2-acetyl-snglycerol cholinephosphotransferase. Except for the insensitivity of cholinephosphotransferase to dithiothreitol, this pathway is analogous to that involved in the biosynthesis of lecithins (350). The direct precursors of PAF in this pathway are 1-alkyl-2-acetyl-sn-glycerols, formed via an acetylation-dephosphorylation sequence, which is catalyzed by acetyl CoA:1-alkyl-2-lyso-sn-glycero-3-phosphate acetyltransferase and by 1-alkyl-2-acetyl-sn-glycero3-phosphate phosphohydrolase (239, 243, 244). The enzymes of the remodeling pathway and de novo pathway have relative broad substrate specificities that provide a basis for heterogeneity in the molecular species of PAF produced by a given cell or tissue in response to a specific stimulus (280, 384). B. PAF Catabolism A. PAF Biosynthetic Pathways The molecular heterogeneity of PAF possibly depends on the multiple enzymatic steps involved in its synthesis and degradation by different cell types. The synthesis and catabolism of this potent phospholipid autacoid are highly regulated. The final molecular composition of PAF in tissues and the expression of its biological activities depend also on the activation of cat- 1672 MONTRUCCHIO, ALLOATTI, AND CAMUSSI Volume 80 receptors (382) and have been implicated in the pathogenesis of atherosclerosis (174). The lyso-PAF is then reacylated by an acyl-CoA:1-radyl-sn-glycero-3-phosphorylcholine acyltransferase. The alkyl moiety of lyso-PAF is known to be cleaved to an aldehyde by a tetrahydropiridine-dependent alkyl monooxygenase (239). Alternatively, phospholipase D can hydrolyze phosphocholine moiety to produce an analog of phosphatidic acid or catalyze a phosphatase transfer by a transphosphatidylation reaction (7). The acyl PAF molecule can be also degraded by a PLA1, which hydrolyzes the long-chain fatty acyl residue esterified at the sn-1 position to produce 1-lyso-2-acetyl-glycero-3-phosphocholine (424). Recently, it has been shown that guinea pig hearts release acetyl hydrolase in the systemic circulation and that isolated ventricular myocytes are capable to take up PAF and catabolize it to inactive products (341, 423). The coronary artery bypass surgery has been shown to induce changes in serum PAF-acetyl hydrolase activity (314). FIG. 1. Remodeling pathways for synthesis of platelet-activating factor (PAF). PAF synthesis is initiated by the activation of phospholipase A2 (PLA2), which may act on 1-O-alkyl-2-arachidonoyl glycerophosphocholine to yield lyso-PAF and free arachidonic acid. The lysoPAF is acetylated by a specific lyso-PAF acetyltransferase using acetyl coenzyme A as a donor. Alternatively PLA2 may act on arachidonatecontaining plasmalogen phosphatidylethanolamine to release free arachidonate. The lyso-phosphatidylethanolamine may act as an acceptor for arachidonate in a transacylation reaction from the PAF precursor with formation of lyso-PAF. Therefore, the activation of PLA2 is essential for generation of lyso-PAF and triggering the remodeling pathways. The activation of lyso-PAF acetyltransferase, which is modulated by a phosphorylation/dephosphorylation cycle, is a limiting factor for generation of biologically active PAF. abolic pathways. The most important enzyme in the limitation the PAF bioactivity is a PAF-specific acetylhydrolase (PAF-AH), which cleaves the short acyl chain at sn-2 position and forms the biologically inactive lyso-PAF. This enzyme is present in plasma (130) and in various tissues (43, 186, 242, 318, 392). The molecular cloning and characterization of the human plasma PAF-AH have been recently reported (415, 418). The PAF-AH activity found in human plasma circulates as a complex with low- (LDL) and high-density lipoproteins (HDL) (203, 391, 429). In addition to the extracellular enzyme, the molecular characterization of two intracellular PAF-AH has been reported (171–173). PAF-AH degrades also PAF-like oxidized phospholipids that were shown to bind PAF FIG. 2. De novo pathway for synthesis of PAF. In this pathway, 1-O-alchyl-sn-glycero-3-phosphate is acetylated by 1-O-alkyl-sn-glycero3-phosphate:acetyl CoA:acetyltranferase and is transformed by a specific phosphohydrolase into 1-O-alkyl-2-acetyl-sn-glycerol. The later is then converted to PAF by a dithiothreitol-insensitive CDP-cholinephosphotransferase. This pathway is mainly involved in the constitutive synthesis of PAF and is regulated only by the availability of substrates. October 2000 PAF AND THE CARDIOVASCULAR SYSTEM IV. PLATELET-ACTIVATING FACTOR RECEPTORS AND SIGNAL TRANSDUCTION PAF acts via specific receptors on the membranes of responsive cells (38, 374) (Fig. 3). Binding studies revealed two distinct types of binding sites on human platelets (439). One binding site for PAF exhibited high affinity with a dissociation constant (Kd) value of 37 ⫾ 13 nM and had a low capacity of 1,399 ⫾ 498 sites/platelet. The second binding site showed nearly infinite binding capacity with a low affinity for PAF. The activation of platelets was due to the interaction of PAF with the high-affinity binding sites. Rabbit platelets showed, as human platelets, high-affinity binding sites for PAF with a Kd of 0.9 ⫾ 0.5 nM (184, 372, 435). In contrast, rat platelets that are insensitive to PAF action exhibited only the lowaffinity binding site for PAF (196, 313). It was subsequently shown that specific binding sites for PAF are present in smooth muscle cells (193), cardiomyocytes (403), neutrophils (321, 441), monocytes-macrophages (257, 438), eosinophils (436), endothelial cells (223), and Kupffer cells (94, 95, 97). Moreover, PAF specific binding sites were identified in cells of the central nervous system. Three distinct classes of PAF binding sites have been detected in synaptic plasma membranes and intracellular membranes of rat cerebral cortex (121, 266, 416). Recently, it has been shown that PAF receptor in endothelial cells is expressed not only on the cell surface but also in the large endosomal compartment (195). The significance of intracellular receptors has not yet been clarified. However, it has been suggested that intracellular PAF receptors may mediate a PAF-dependent signal transduction pathway, as postulated for PAF-induced protooncogene 1673 expression (416). A cDNA for a PAF receptor from guinea pig lung has been cloned (185). The strategy used involved the construction of a cDNA library from sidefractionated poly(A) RNA, the synthesis of a transcript of the cDNA using phage DNA as template, and the expression of the transcript in the Xenopus oocytes (185). The analysis of PAF receptor cDNA indicated that PAF receptor belongs to the family of “serpentine receptors,” which contain seven ␣-helical domains that wave in and out of the plasma membrane seven times. Surprisingly, PAF receptor contains only 342 amino acids and has a molecular mass of 38,982 Da (185). The third intracellular loop and the carboxyl tail, which is thought to bind G proteins in the serpentine receptor family, are very short in PAF receptor. It has been also found that there are nine potential phosphorylation sites on the carboxy end of the receptor. Phosphorylation of the sites may modulate the binding of G proteins to the receptors and may account for the rapid desensitization of PAF receptors (310). Subsequently, PAF receptor was cloned from human leukocytes (232, 310) and HL-60 granulocytes (470). PAF receptor cloned from human leukocytes revealed 83% identity in the amino acid sequence with that of guinea pig lung. Recently, it has been shown that the PAF receptor protein expressed by human cardiomyocytes is exactly the same as that of human leukocytes (403). However, the 5⬘-noncoding region of cDNA encoding for cardiac PAF receptor is different from that of leukocytes, suggesting the presence of a tissue-specific regulatory mechanism (403). The induction of PAF receptor expression in Xenopus laevis oocytes and in COS-7 cells shows that PAF receptor is functionally linked to phosphoinositide metabolism by a G protein (310). Although researchers believe that PAF is FIG. 3. Schematic representation of PAF receptor-signal transduction mechanisms. PLA2, phospholipase A2; G protein, GTP-binding protein; PLC, phospholipase C; PtdinsP2, phosphatidylinositol 4,5-bisphosphate; inositol-P2, inositol 4,5-bisphosphate; InsP3, inositol 1,4,5-trisphosphate; DAG, diacylglycerol; PKC, protein kinase C. 1674 MONTRUCCHIO, ALLOATTI, AND CAMUSSI coupled to PLC and PLA2 through a G protein, the G protein involved has not yet been fully characterized. It has been suggested that PAF binds to receptor and activates the associated G protein by exchanging guanosine triphosphate for guanosine diphosphate. In turn, the G protein activates a phosphatidylinositol-specific PLC (100). Therefore, stimulation of PAF receptors leads to the transient production of diacylglycerol, which activates PKC, and of inositol trisphosphate, which mediates the release of internal calcium stores. It has been proposed that the basic components of this pathway operate in all cells bearing PAF receptors and that the different responses to PAF depend on the function of target cells. Moreover, PAF has been found to stimulate the release of arachidonic acid in various cell types by different mechanisms (309, 311, 334, 449, 472). For instance, in neutrophils, PAF induces the activation of PLA2 by a mechanism requiring only the mobilization of intracellular calcium stores while in Kupffer cells PLA2 activation is dependent on extracellular calcium influx (95). In addition, the activation of PLA2 by PAF occurs through a PKC-dependent mechanism (96, 277, 320) and is prevented by pertussis toxin, suggesting a G protein involvement (311, 365, 411). PAF-induced activation of PLA2 is also regulated by the intracellular levels of cAMP (88, 96). The arachidonic acid metabolites have been shown to mediate several biological activities of PAF. In the heart, PAF-induced coronary vasoconstriction and reduced contractility are affected both by cyclooxygenase- and lipoxygenase-derived arachidonic metabolites (334, 335). Furthermore, PAF induces an elevation of cytosolic free calcium in several cell types including vascular smooth muscle cells (241, 369). The two main mechanisms involved in PAF-induced increase in cytosolic free calcium are 1) the mobilization of calcium from the intracellular stores as a result of inositol trisphosphate generation and 2) the influx of extracellular calcium through a membrane-associated channel regulated either directly by PAF or indirectly by intracellular second messenger such as lipoxygenase-derived metabolites of arachidonic acid (100). Calmodulin inhibition (254) and calcium channels antagonists, such as verapamil (105), were shown to block the influx of 45Ca2⫹ in rabbit platelets; moreover, verapamil prevents several in vitro and in vivo biological effects of PAF (12). Recently, it has been shown that PAF stimulates tyrosine phosphorylation of several proteins in platelets (117), neutrophils (153), and macrophages (98, 99). Moreover, it was found that PAF is capable of inducing the stimulation of NFB activation (469) and the transcription of c-fos and c-jun genes in inflammatory cells (367). Because the PAF receptor contains several tyrosine residues in its intracellular loops and tail, it was suggested that tyrosine phosphorylation may be involved in the downregulation of the receptor (99). Recently, it has been shown that PAF may activate a mitogen-activated protein kinase (MAPK) (26, Volume 80 375) and may induce the early tyrosine phosphorylation of focal adhesion kinase (p125FAK) in human endothelial cells (387). Moreover, in human neutrophils, PAF activates MAPK kinase-3, a known activator of p38 MAPK (315). Many antagonists of the PAF receptor have been described including compounds without any structural relationship with PAF and structural analogs (for review, see Refs. 49, 86, 87, 166, 218, 362, 442). The development of potent and selective PAF receptor antagonists has been particularly valuable for studies on the pathophysiology of PAF (136). A number of natural PAF antagonists have been identified, including BN 52021 and kadsurenone, isolated from Ginkgo biloba and Piper futokadsura, respectively. More recently, several synthetic PAF receptor antagonists were also developed. They include 1) phospholipid analogs such as FR 72112, CV 3988, CV 6209, SRI 63441, ONO 6240, and RP 48740; 2) tetrahydrofuran derivatives such as L 652731; and 3) triazolobenzodiazepine derivatives such as WEB 2086, WEB 2170, BN 50726, and BN 50739. These chemically different PAF receptor antagonists share the ability to inhibit PAF binding to its receptor and to antagonize specific PAF-induced responses on target cells. V. CARDIOVASCULAR RESPONSES TO PLATELET-ACTIVATING FACTOR A. Hemodynamic Effect of PAF The potential regulatory role of PAF on hemodynamics has been extensively studied. This line of research was initiated by the observation that one of the two antihypertensive lipids isolated from renal medulla and from renal venous effluent after unclipping one-kidney, oneclip hypertensive rats, the polar renomedullary lipid, has the same chemical structure of PAF (303, 304, 338). Early hemodynamic studies emphasized that antihypertensive polar renal lipid as well as synthetic PAF, in microgram doses, lowered arterial pressure in guinea pigs (135), rats (44, 67, 148, 233, 273, 344, 347, 360, 406, 430), and rabbits (12, 160, 161, 245, 290, 303) in the normal and hypertensive states after intravenous or oral administrations. The potential role of PAF in the modulation of blood pressure was inferred from the observation of decreased levels of PAF (275) and increased activity of plasma acetyl hydrolase (42) in hypertensive rats. Indeed, when administered intravenously, PAF is hypotensive in all species studied (134). Despite sensitivity variation among the species, several characteristics of hemodynamic response to PAF are common: the extent of hypotension is dose dependent, the onset is very rapid, the maximum effect is reached within 30 – 60 s, and the recovery time is also dose dependent. The basal mean arterial pressure (MAP) is obtained within 5–10 min after intravenous injection. In animal species such as the rat, where platelets are insensitive to PAF action, no tachyphylaxis was observed (92, 120) In contrast, in the rabbit, where platelets are highly sensitive to PAF action, tachyphylaxis is present, suggesting that physiological alterations induced by PAF may at least in part occur via platelet activation and secretion of other mediators (160). Therefore, the rat model was used to differentiate direct vascular effects of PAF from those dependent on the activation of circulating platelets. Table 1 shows hemodynamic alterations induced by PAF infusion in different animal species. Although the mechanisms of PAF-induced hypotension are not completely understood, some data indicate that the action of PAF on the heart, peripheral vasculature, and microcirculation may account, at least in part, for the reduction of systemic blood pressure. PAF decreases the cardiac output through several mechanisms: 1) it reduces venous blood return as the consequence of peripheral vasodilatation or vasoconstriction and increased microvascular permeability; 2) it produces a right ventricular overload with an increased right atrial pressure and a reduced filling of left ventricle as the result of an increase in pulmonary vascular resistance; 3) it may impair cardiac function by a direct negative inotropic effect and by reduction of coronary blood flow (CBF); and 4) finally, PAF was shown to affect cardiac electrical activity and the conducting system. The vascular effects of PAF on blood vessels vary depending on doses of PAF used, on different districts, and on animal species. In conscious rats (380), low doses of PAF increase blood flow and decrease vascular resistances in all vascular districts. High doses of PAF, in contrast, produced a sustained decrease of cardiac index, tachycardic response, and transient fall followed by sustained increase of total peripheral resistances (380, 404). In the mesenteric district, a marked reduction of blood flow consequent to the increase in mesenteric resistance was observed (380). These dose-dependent and regional TABLE Rat Guinea pig Rabbit Pig hemodynamic effects of PAF mimic that obtained by infusion of antihypertensive polar renal lipid (129). Moreover, it was found that PAF-induced hypotension is not mediated through the central nervous system, renin-angiotensin system, muscarinic, -adrenergic, dopaminergic, eicosanoids, calcium influx, thyrotropin releasing hormone, steroids, or histaminergic mechanisms (67, 202, 233, 383). In contrast, it was found that delayed and persistent but not early hypotension induced by PAF is inhibited by N-nitro-L-arginine, which inhibits the production of nitric oxide from endothelial cells, thus suggesting a role for the “endothelium-derived relaxing factor” (406). In vitro studies demonstrate the involvement of the nitric oxide pathway in PAF-induced relaxation of rat thoracic aorta (45, 299). The tachycardic effect of PAF was inhibited by the -adrenoreceptor blocker propranolol, suggesting a baroreceptor reflex activation consequent to systemic hypotension (326). In rabbit, dog, pig, and baboon, PAF is a potent activator of platelets and of leukocytes; therefore, vasoactive substances released from these cells contribute to the hemodynamic effect of PAF. The intravenous administration of relatively high doses of PAF (0.6 – 0.8 g/kg) in rabbits produces bradycardia, reduction of cardiac output, hypotension, decrease of lung compliance, and increase in peripheral vascular and lung resistances (Fig. 4) (160, 290). The hemodynamic alterations developed in three sequential phases: 1) a transient bradycardia (15 s) with reduction in left ventricular systolic pressure (LVSP), MAP, and cardiac output occurred; 2) a rise in cardiac frequency, which developed within 30 s, an increase in LVSP, MAP, total peripheral resistances (TPR), and cardiac output was observed; and 3) ⬃90 s after PAF infusion, LVSP and MAP were decreased, whereas TPR and right arterial systolic pressure persisted elevated. These alterations were reversed in 30 – 60 min (290). When low doses of PAF (0.2 g/kg) were used the hemodynamic response was similar, but the intensity was attenuated (unpublished observations). H1 and H2 histamine receptor antag- 1. Effects of PAF on hemodynamic parameters in different animal species Species Dog 1675 PAF AND THE CARDIOVASCULAR SYSTEM October 2000 Dose MAP HR CO TPR MPAP PVR Reference No. 0.3–1 3 0.2 0.38 1.14–1.52 0.038 0.19–0.95 8–36 0.04–0.28 D D I/D D I/D D D D D I ND ND D I/D S ND D I D ND ND ND D I I/D D D D ND ND ND I D D/I I I ND D ND I ND ND I/D I/D I ND ND ND ND ND ND I I I 380 92 110 160, 161 290 468 208 35 236 Doses are expressed as nmol platelet-activating factor (PAF)/kg intravenous bolus injection. MAP, mean arterial pressure; HR, heart rate; CO, cardiac output; TPR, total peripheral resistance; MPAP, mean pulmonary artery pressure; PVR, pulmonary vascular resistances; I, increase; D, decrease; S, stable; I/D, increase followed by decrease; D/I, decrease followed by increase; ND, not determined. 1676 MONTRUCCHIO, ALLOATTI, AND CAMUSSI Volume 80 FIG. 4. Typical hemodynamic changes occurring in a rabbit injected intravenously with PAF. ECG, electrocardiogram; LVP, left ventricular pressure; dP/dt, derivative pressure/derivative time; FAP, femoral arterial pressure; CVP, central venous pressure. onists markedly prevented the development of the second phase, namely, the rise in cardiac frequency, LVSP, MAP, and TPR, but did not significantly modify the first and third phases (290). Moreover, the generation of thromboxane A2 from platelets may contribute to the development of hemodynamic alterations in rabbit. Indomethacin indeed determined an overall reduction in the extent of PAF-induced hemodynamic changes (290). These results suggest that the release of histamine and thromboxane A2 from platelets may in part account for hemodynamic alterations induced by PAF. This was confirmed by the evidence of entrapment of platelets and leukocytes, particularly in the pulmonary microvasculature (279), and by experiments of platelet depletion (161). A calcium channel blocker, verapamil, was found to prevent all the hemodynamic and electric alterations induced by PAF (12). Administration of chronic intravenous PAF induces pulmonary arterial atrophy and hypertension with persistent increase in pulmonary resistances and reduction in cardiac output (323). When the effects of PAF infusion were studied in the anesthetized dogs, a triphasic response was observed (35, 208). In the first phase (15–30 s), hypotension was attributed to a decrease in systemic vascular resistances that was associated with a rise in cardiac output. The second phase (30 –90 s) consisted of sustained hypotension caused by a reduction in cardiac output associated with an increase in pulmonary and systemic resistances. The third phase was characterized by a gradual recovery of MAP, associated with a sevenfold rise in systemic vascular resistances and a persistent low cardiac output. Blockade of leukotriene receptors substantially inhibited the rise in systemic vascular resistances in the third phase, suggesting the role of leukotrienes as secondary mediators (208). The vasodilation observed in the first phase was independent from prostaglandin generation (208, 468). In contrast, the reduction of cardiac output observed in the second phase was shown to depend on generation of cyclooxygenase metabolites of arachidonic acid (468). Low doses of PAF caused only the first vasodilatory phase with hypotension (468). Studies performed in domestic pigs demonstrate an early pulmonary vasoconstriction with a right ventricular failure as the first determinant of PAF-induced shock. The subsequent decline in cardiac output, underfilling of the left ventricle, and systemic hypotension were interpreted as the consequence of right-sided events (149, 235, 236). B. Effect of Local Administration of PAF on Selected Vascular Districts In vivo the effects of PAF on selected blood vessels are masked by the sympathetic vasoconstrictor reflex and by mediators released from platelets and leukocytes. The effects of PAF depend on the doses and animal species (23, 46, 101, 109, 128, 133, 138, 165, 199, 210, 235, 282, 327, 363, 405, 444, 451). In rats, systemic administration of picomolar amounts of PAF, unable to induce changes of MAP, reduce vascular resistance and increase blood flow in the hindquarter, the mesenteric vessels, and the kidney (211, 380). In contrast, nanomolar concentrations of PAF injected into abdominal aorta proximal to the superior mesenteric artery or into the carotid artery induce vaso- PAF AND THE CARDIOVASCULAR SYSTEM October 2000 constriction (Table 2) (108, 148, 217). The injection of PAF into renal artery determines the following dose-dependent alterations: vasodilatation at 1 pmol/kg (165); initial vasodilatation followed by vasoconstriction at 4 –19 pmol/kg (165), and vasoconstriction at 30 –90 pmol/kg (23). In dogs, the prominent effect of intrarenal administration of PAF is vasoconstriction for all tested concentrations (24, 363). In contrast, in this animal species, PAF produces vasodilatation in gastric, mesenteric, and femoral arterial circulation (101). The vasodilator effect on femoral but not gastric and mesenteric vascular districts was dependent on prostaglandin synthesis. Moreover, the block of prostaglandin synthesis enhanced the vasoconstrictor effect of PAF on the renal vascular district (101). When selected organs are perfused under constant pressure with low doses of PAF, where blood and autonomic nervous system control were absent, the common response was vasodilation. In these experimental conditions, PAF induces vasodilation in the kidney (368), but still produces vasoconstriction in the isolated lung (162, 175) and heart (30, 251, 335). The question arises whether these experiments reflect only a pharmacological effect or a pathophysiological or a physiological role of PAF. It is hard to answer this question since it is difficult to measure the actual concentration of PAF in relevant fluids, cells, and tissues because this mediator is readily metabolized. The concentration of PAF in cells or tissues depends on the balance between its synthesis and degradation. The levels of PAF detected in blood and/or tissues in pathological conditions are in the range of nanograms and are therefore consistent with the doses used in experiments of exogenous administration. Therefore, it is conceivable that PAF may mediate hemo- 2. Effects of PAF on blood flow in selected vascular districts TABLE Species Rat Vascular District Renal Cerebral Mesenteric Dog Pig Renal Gastric Mesenteric Coronary Femoral Coronary Blood Flow Reference No. 0.001 nM 0.004–0.019 nM 0.02–0.1 nM/min 0.03 nM/min 0.2–2 nM/min 7.6 nM I I/D D D D D 0.01–0.38 nM/min 0.04–0.19 nM/min 0.04–0.19 nM/min 0.5–2 nM 0.61–610 nM 0.38–3 nM 0.57 nM/min 0.61–610 nM 0.1–10 nM 1–9 nM/min D I I I D I/D I I I/D I/D 165 165 23 451 148 108 109 363 101 101 199 405 138 210 405 133 128 Doses I, increase; D, decrease; S, stable; I/D, increase followed by decrease; ND, not determined. 1677 dynamic changes occurring in pathophysiological conditions such as anaphylaxis and endotoxic shock or acute and chronic inflammation. Pharmacological agents that antagonize the binding of PAF to its receptors have been used to support this contention when it was found that they attenuate or reverse certain pathological processes. It is more difficult to evaluate a modulatory role of PAF on blood pressure in physiological conditions. The fact that PAF can act as vasodilator at very low concentrations supports the hypothesis that endogenous PAF may be a regulator of blood pressure. The levels of PAF in the blood of normal subjects are in the range of picograms per milliliter (85, 359). In early studies it has been shown that PAF was not detectable in anephric hypertensive patients, suggesting a role of PAF synthesized in renal medulla in the regulation of blood pressure (85). This contention was not supported by subsequent studies, showing that the mean circulating PAF levels in patients with essential hypertension were not significantly different from those in normotensive subjects (359). However, it was found that high salt intake significantly increased the circulating levels of PAF, suggesting the synthesis of PAF to counteract the hypertensive effect of high dietary salt intake (359). Recently, it has been shown that there is an enhanced intracellular PAF-triggered signal transduction in approximately one-third of immortalized lymphoblasts derived from patients with essential hypertension (157). Moreover, it has been reported that PAF-acetylhydrolase activity in maternal and umbilical venous plasma was significantly lower in normotensive pregnant women than in nonpregnant women or in pregnancy-induced hypertension (215). This finding suggests that the inactivation of PAF by acetylhydrolase is decreased during normal pregnancy. Such modulation apparently does not occur in pregnant women that developed hypertension, suggesting that the catabolism of PAF plays a relevant role in the regulation of blood pressure in this contest (215). VI. EFFECT OF PLATELET-ACTIVATING FACTOR ON THE HEART A. Coronary Circulation: In Vivo Effects PAF administration into the coronary circulation induced variations in the coronary vascular tone depending on the doses and the animal species used. In the pig, intracoronary bolus injection of PAF produced a transient dose-dependent increase (up to 50%; ED50 ⫽ 0.38 nM) in CBF followed by a second phase characterized by decrease (up to 92%) in CBF (ED50 ⫽ 0.92 nM) (133). In this study it was shown that at low doses of PAF (0.03– 0.3 nM), both the increment and the decrement of CBF was present in the absence of significant changes in systemic 1678 MONTRUCCHIO, ALLOATTI, AND CAMUSSI blood pressure. In contrast, the reduction in CBF caused by high doses of PAF was accompanied by significant decrease in systemic blood pressure and by electrocardiogram signs of ischemia such as S-T segment elevation or depression when flow decreases by more than 75% of control values. Similar results were reported after continuous intracoronary infusion of PAF in the pig (128, 134). Pharmacological studies demonstrated that the early increase in CBF is independent from the generation of cyclo- and lipoxygenase-derived metabolites, while the subsequent vasoconstriction is primarily due to the production of thromboxane A2 (133). PAF induces coronary vasoconstriction and S-T depression also in rabbits (290). Conflicting effects were observed in dogs (199, 282, 405). In one study it was shown that intracoronary injection of PAF reduced CBF concomitantly with a marked and rapid reduction in systemic arterial pressure and a negative inotropic response, effects that could have obscured the direct action of PAF on coronary artery (405). In other studies, similar doses of PAF are reported to produce a platelet-dependent coronary vasodilation (199) or a biphasic vasodilator/vasoconstrictor effect (282). In subsequent studies, it was found that PAF is vasodilator when the endothelium of coronary arteries is intact, whereas it induces vasoconstriction when the endothelium is injured as it may occur after ischemia (210). In vivo studies provided evidence that PAF induced a significant attenuation of endothelium-dependent dilation to intracoronary infusion of acetylcholine and serotonin, further suggesting an endothelial effect of PAF (111). B. In Vitro Effects on Isolated Perfused Heart In the isolated guinea pig heart perfused at constant pressure, PAF produced a dose-dependent increase in coronary vascular resistances (30, 149, 208, 251, 335, 398). This vasoconstrictor effect of PAF was completely blocked by verapamil, a calcium antagonist (12). Pharmacological inhibition of cyclooxygenase and blockade of leukotrienes receptors were reported ineffective by Levi et al. (251) and Stahl et al. (398), while PAF receptor antagonists reduce the coronary vasoactive effect of PAF (335, 410, 447, 448). Similar coronary vasoconstriction was obtained with high doses of PAF in isolated perfused rat heart (334, 397). However, when low doses were used, vasodilation alone or vasodilation followed by vasoconstriction was observed (187, 188). Pharmacological studies on the action of PAF on isolated rat heart suggest that both prostaglandins and leukotrienes are involved in the vasoconstrictor effect of PAF. However, it has been shown that lipoxygenase products are mainly responsible for the vasodilator and vasoconstrictor effects of PAF on coronary vasculature, whereas cyclooxygenase products play only a partial role (147, 265, 334, 397). In the isolated Volume 80 perfused rabbit heart, the coronary vasculature was apparently insensitive to PAF (208, 283, 291). However, when isolated rabbit heart was perfused with blood, PAF markedly reduced coronary flow (208). The isolated rabbit heart was used as a model to study in vitro interaction between platelets, leukocytes, and endothelial cells in the coronary circulation. In the rabbit heart perfused with platelets, the infusion of PAF induced a dose-dependent decrease of coronary flow, which was prevented by pretreatment of the heart with H1 and H2 histamine receptor antagonists and a leukotriene receptor antagonist (291). A similar protective effect was obtained after treatment of platelets with prostacyclin, which inhibits the activation of platelets by PAF (8). Perfusion of rabbit heart with PMN followed by PAF stimulation did not alter coronary tone (10). However, PMN may influence the effect of PAF on coronary vessels as a result of cooperation with platelets. The reduction of coronary flow induced by PAF in rabbit heart perfused with platelets and PMN was completely blocked by a leukotriene receptor antagonist, suggesting that leukotrienes released by PMN as a consequence of a PAF-induced cooperation with platelets are the main mediators of coronary constriction (10). This evidence is also supported by experiments of perfusion of rabbit heart with N-formyl-L-methionyl-L-leucyl-L-phenylalanine-activated PMN (417). In this experimental condition, PAF antagonist inhibited the neutrophil-dependent increase in coronary resistances (417). Similar results were obtained in studies on the PMN-induced contraction of the isolated rings of cat coronary artery (307). In humans, in basal conditions isolated coronary artery rings did not react to PAF challenge. However, Soloviev and Brachet (388) have shown that isolated human coronary artery strips after hypoxia undergo to a PAF-dependent biphasic contraction: an initial short phase of contraction, followed by a longer tonic shortening inhibited by treatment with a PAF receptor antagonist. C. Myocardial Function The alterations in cardiac function, including the reduction in cardiac output observed in vivo after infusion of PAF, can result either from a direct action on the heart or from indirect effects such as systemic changes and variations in pre- and afterload pressures. Furthermore, alterations in cardiac performance may depend on the effect of PAF on the coronary circulation, on the conduction system, and on the contractile properties of myocardium (150). A direct effect of PAF on cardiac contractility was suggested by experiments of intracoronary infusion of low doses of PAF in pig, that induced a marked reduction in cardiac output as well as in regional shortening fraction in the absence of significant effects on systemic blood October 2000 PAF AND THE CARDIOVASCULAR SYSTEM pressure (126, 128, 133, 134). In in vitro experiments on the isolated coronary perfused heart, the effect of PAF is quite different, depending on the animal species studied. In guinea pig isolated heart perfused at constant pressure, infusion of PAF reduces the force of contraction and the coronary flow in a dose-dependent manner. Moreover, PAF markedly alters the electrical activity of the heart (351); the severity and duration of these alterations are related to PAF dosage. PAF induces conduction arrhythmias, ranging from second degree atrioventricular conduction block to complete atrioventricular dissociation and ventricular extrasystoles, disappearance of the T wave, and depression of the S-T segment, a sign of myocardial ischemia (12, 30, 253, 357, 398, 410). The studies of ventricular action potentials by means of intracellular electrodes have shown that after the infusion of PAF the action potential duration progressively shortens, and the resting membrane potential, the overshoot, and the maximum rate of depolarization, which are initially unchanged, progressively reduce 10 min after the challenge (12). The response to PAF is prompt and long lasting; the maximal effect is reached within 2 min after the challenge. At 10 nM, PAF causes irreversible conduction arrhythmias so that the normal sinus rhythm and cardiac contractility do not return even after a prolonged perfusion with physiological solution without PAF. These effects seem to be independent from the release of acetylcholine from the nerve endings, since pretreatment with atropine does not affect the action of PAF (30). Moreover, the effects of PAF are independent from the generation of secondary mediators derived from the arachidonate metabolism, since neither the cyclooxygenase inhibitor indomethacin, nor the thromboxane synthetase inhibitors, nor the leukotriene receptor antagonists significantly modify the response to PAF (251, 253). In these experiments, peptide leukotrienes or thromboxane B2 were not detected by radioimmunoassay in the coronary effluent (398). However, pretreatment of the heart with the calcium antagonist verapamil significantly reduces the entity of PAF-induced coronary spasm and completely abrogates both the electrical and mechanical alterations (12). The entity of cardiac alterations induced by PAF is related to the molecular structure for different species of PAF. In particular, the most potent species of PAF in inducing coronary constriction in the guinea pig heart was 18:1 PAF, whereas 16:0 and 18:0 had minor effects. The order of potency in reducing contractile force was 16:0 ⬎ 18:1 ⬎ 18:0. Therefore, the presence of a single double bond in the alkyl chain at the sn-1 position markedly alters the cardiac activity of alkyl-PAF (252, 280). In contrast to PAF, its deacetylated derivative lyso-PAF causes no significant variation in contractile force, coronary flow, and cardiac rhythm (251). The effects of PAF are also present in the isolated heart perfused at constant flow; in this case, a dose-dependent increase in coronary 1679 resistance is observed (251). Because a reduction of cardiac inotropism and alteration of cardiac rhythm induced by an intracoronary infusion of PAF are also present when the guinea pig isolated heart is perfused at constant pressure, it is unlikely that the effects of PAF depend on an ischemic state consequent to a reduction of coronary flow. The effects induced by PAF in the isolated rat heart are similar to that observed in guinea pig heart (e.g., reduction in coronary flow and developed pressure, increase in diastolic pressure and conduction arrhythmias) (188, 189, 334, 339, 340). In this animal, however, it has been shown that the PAF-induced coronary vasoconstriction is mediated by the peptide leukotrienes (leukotriene C4 and leukotriene D4) (188, 189, 334). This finding indicates that in the rat heart PAF may have a small direct negative effect, whereas the reduction of coronary flow consequent to production and release of endogenous leukotrienes appears to induce a major depression of cardiac contractile force. Arachidonic acid metabolites released by PAF may stimulate the release of atrial natriuretic factor in rat heart (348, 349). Interestingly, comparison of the effect of PAF in adult and senescent rats shows that all the electrical and mechanical alterations induced by PAF are more marked in the senescent hearts (1). Isolated rabbit heart is responsive to PAF only if blood (208), platelets, and platelets plus leukocytes are present in the coronary vessels (283, 291). In these conditions, PAF induces mechanical and electrical alterations similar to those observed in the isolated guinea pig heart. D. Atrium and Papillary Muscle The isolated perfused heart preparation does not differentiate the direct negative inotropic effect of PAF from that consequent to changes in coronary flow and O2 delivery to the heart. Therefore, the isolated atrium and papillary muscles were used to address the direct effect of PAF on cardiac muscle. In isolated rat atrium, PAF does not elicit significant effects on chronotropy and inotropy at concentration up to 3 ⫻ 10⫺5 M (89). At the concentration 1 ⫻ 10⫺4 M, PAF induces positive chronotropic and inotropic effects on isolated spontaneously beating right and electrically driven left atria. Both effects were blocked by propranolol, whereas reserpine pretreatment antagonized only the chronotropic response. Studies on isolated guinea pig atrium and papillary muscle (71, 118, 251, 407) showed a direct negative inotropic effect of PAF. Lyso-PAF caused only minimal effect (⬃5%) on these preparations. We studied the effect of PAF on the electrical activity of guinea pig papillary muscle by means of intracellular electrodes (71). PAF at 2 ⫻ 10⫺10 M induces a biphasic effect with an increase of 20% of contractile force within 2–3 min after the challenge, followed 1680 MONTRUCCHIO, ALLOATTI, AND CAMUSSI by a progressive decrease of developed tension, which was reduced by 50 – 60% with respect to control values. The positive inotropic effect was preceded by a slight augmentation of action potential duration (APD); subsequently, APD decreased concomitantly with the negative inotropic effect. Pretreatment with propranolol prevented the positive inotropic effect and increase of APD. Similar biphasic dose-dependent effect of PAF was observed in guinea pig papillary muscle by Tamargo et al. (407). Moreover, these authors showed that at high concentrations of PAF (10⫺9 to 10⫺7 M), reduction of APD was accompanied by increase of action potential amplitude, maximum rate of depolarization (MRD), and resting membrane potential (Er) of the action potential. Short runs of spontaneous discharges of ventricular muscle fibers accompanied the electrophysiological effects of PAF at all concentrations tested. A similar biphasic dose-dependent effect was described by Gollasch et al. (152), Kecskemeti (206), and Kecskemeti and Braquet (207) in guinea pig auricles: 10⫺10 M PAF induces transient positive inotropic effect followed by a negative one; 10⫺7 M PAF induces negative inotropism accompanied by decrease in amplitude, duration, and MRD of the action potential without changes in Er. The effect of PAF was reversed after washout of PAF. The study on the slow action potential was performed in these preparations to obtain insight into the mechanism of PAF-induced negative inotropism and decrease of APD. However, the results of these studies are partially conflicting. For instance, we reported that PAF induces a transient positive inotropic effect and enhancement of the slow action potential, followed by a profound depression of both the electrical and mechanical activities, suggesting a biphasic effect of PAF, i.e., an initial stimulation followed by depression of slow calcium channels (71). In contrast, Tamargo et al. (407) and Robertson et al. (353) found that PAF induces only a dose-dependent increase in amplitude and MRD of slow action potentials. These discrepancies are probably due to the different methods used. In our study, slow action potentials were obtained by elevating both K⫹ and Ca2⫹ extracellular concentrations to 22 and 6 mM, respectively, to inactivate the fast Na⫹ current and increase the driving force for Ca2⫹, while in the case of Tamargo et al. (407) and Robertson et al. (353) papillary muscles were bathed in high-K⫹ solution with the addition of isoproterenol or histamine to obtain slow action potentials. These drugs are known to stimulate adenylate cyclase via specific receptors, to increase the intracellular levels of cAMP, and to activate protein kinase A, leading to phophorylation not only of the Ca2⫹ channel protein but also of a large number of other substrates within the cell. It has been reported that after pretreatment of papillary muscle with isoproterenol, which per se has a positive inotropic effect, PAF further enhances action potential amplitude Volume 80 and duration, as well as the force of contraction, while the second phase (depression of action potential and contractility) is absent. These results are consistent with that observed in the isolated rat heart by Bensard et al. (28), that PAF depresses basal myocardial function and enhances the functional response to -adrenergic stimulation. Finally, the direct demonstration of the involvement of calcium current comes from voltage-clamp experiments on frog and guinea pig atrial fibers, in which Gollasch et al. (152) showed that the negative inotropic effect of PAF is accompanied by a significant reduction of this current. The mechanical and electrical changes induced by PAF were studied in human cardiac preparations such as the isolated papillary muscle (11) and atrial tissue (352). Challenge of human papillary muscles excised from the left ventricle by open heart surgery with various doses of PAF (1 ⫻ 10⫺10 to 1 ⫻ 10⫺6 M) induced a biphasic dose-dependent effect, characterized by a transient positive effect on inotropism and APD, followed by a marked prolonged negative effect on force of contraction and APD. No changes in Er, overshoot, and MRD of the action potential were detected after PAF challenge (11). Robertson et al. (352) reported a similar dose-dependent negative inotropic effect of PAF in human atrial muscle. However, lyso-PAF has no effect on contractile activity in both cardiac preparations, even at concentration up to 100-fold greater than those of PAF. In human papillary muscle, propranolol blocked the transient inotropic effect of PAF, suggesting a stimulation of -receptors by endogenous catecholamines; pretreatment with indomethacin did not modify the initial positive effect, but markedly reduced the negative effect of PAF (11). In contrast, in human atrial tissue, the effect of PAF was blocked by antagonists of PAF receptor, but not modified by atropine, indomethacin, and the leukotriene receptor antagonist FPL 55712, suggesting a direct effect of this mediator (352). Moreover, it was shown that the decrease of contractile force caused by PAF in guinea pig papillary muscle may depend on the reduction of intracellular sodium activity, which may affect sodium/calcium exchange, finally causing reduction of intracellular calcium and contractility (353). E. Effects on Cardiomyocytes The direct effects of PAF on cardiac inotropism and chronotropism have been studied in isolated adult or cultured neonatal cardiomyocytes (112, 272). In this experimental condition, PAF was shown to decrease myocardial twitch tension and velocity of contraction and relaxation as well as to increase spontaneous beating frequency (272). Recently, it has been shown that PAFinduced negative inotropic effect correlated with a decrease in systolic intracellular calcium concentration October 2000 PAF AND THE CARDIOVASCULAR SYSTEM (331). Parallel biochemical studies demonstrated that PAF stimulates phosphoinositide pathway, leading to accumulation of [3H]inositol phosphate and activation of PKC in cardiomyocytes (102). Because it has been shown that blockade of PAF receptors prevented both mechanical and biochemical changes induced by PAF, the presence of PAF receptors on cardiomyocytes may be suggested. The PAF receptor gene in human cardiomyocytes (403) has been recently cloned and characterized. Moreover, the patch-clamp technique has been applied to the study of electrophysiological effects of PAF on isolated guinea pig ventricular and atrial cells. Single-channel studies on cell-attached patches have shown that PAF affects inwardly rectifying background potassium channels (IK1) (450). PAF initially induces a flickering of the channel, followed by a gradual prolonged depression of the channel activity. Because these potassium channels have a prominent role in determining the resting potential and excitability of the cardiac cells, it has been suggested that the effect of PAF on IK1 may play a major role in the electrophysiological action of PAF in the heart. Moreover, PAF was shown to stimulate cardiac muscarinic potassium channels in isolated guinea pig atrial cells. The effect of PAF was prevented by specific PAF receptor antagonists, lipoxygenase and PLA2 inhibitors, but not by cyclooxygenase antagonists. The opening of the channel was shown to be dependent on the activation of a G protein (309). Similar results were obtained using isolated bullfrog atrial myocytes (346). In contrast, in human and chick ventricular myocytes, PAF was found to stimulate T- and L-type calcium currents in a dose-dependent manner, whereas no effect on fast sodium current or delayed outward potassium current was observed. The effect of PAF on calcium currents was receptor dependent, because it was inhibited by PAF receptor blockade with WEB 2170 (41). The different response to PAF of cardiomyocytes derived from atrium or ventriculum suggests a functional differentiation of these cells. Moreover, it as been shown that PAF induces secretion of atrial natriuretic peptide (103, 348, 349) and eicosanoids (27) in spontaneously beating neonatal rat cardiomyocytes. VII. MICROVASCULAR EFFECT OF PLATELET-ACTIVATING FACTOR IN VIVO The potent vasoactive and leukotactic properties of PAF were initially studied in the rat cremaster muscle and skin after infusion of colloidal carbon and local injection of PAF (191). PAF was shown to be 1,000 –10,000 times more potent than histamine on molar basis in inducing vascular permeability (191). Ultrastructural studies demonstrated subendothelial carbon accumulation in the 1681 postcapillary venules. A concomitant leukocyte margination was observed. However, the vasoactive properties of PAF in the rat appeared to be neutrophil and platelet independent. A direct stimulation of venular and endothelial cells was suggested. The effect of PAF on microcirculation has been studied also on the hamster cheek pouch (89, 119). Vasoconstriction was the predominant vasomotor response to PAF. This biological effect was dependent on the dose of PAF and the size of the vessel and was mediated by PAF receptor interaction and production of thromboxane A2. In addition, PAF increases vascular permeability both by a direct mechanism and platelet- and leukocyte-mediated mechanisms (119). It was found that PKC activation is an in vivo biochemical pathway in the signal transduction of PAF-stimulated microvascular cellular responses, leading to increases in the transport of the macromolecules. Indeed, PKC inhibitors significantly blocked the increase in intravascular permeability (214). In contrast, they did not interfere with the PAF-induced arteriolar constriction (214). Studies on intestinal microvasculature have shown that PAF promotes the filtration of fluid and protein across intestinal capillaries in cats (226). These microvascular effects of PAF are mediated in part by adherent leukocytes (226, 227). In the guinea pig, PAF causes a dose-dependent increase in airway vascular permeability, as measured by extravasation of Evans blue dye, at concentrations as low as 1 ng/kg (168). The site of leakage, as for other mediators, is in the postcapillary venules. This effect of PAF is receptor mediated and platelet independent, as it is inhibited by receptor antagonists, but not by platelet depletion. PAF also affects mean renal vascular resistance (23) and glomerular permeability (82, 364) by a dual mechanism: 1) it enhances size permeability of glomerular basal membrane by a direct action on the glomerular capillary wall (330), and 2) it modifies perme-selectivity owing to a loss of fixed negative charges of the glomerular capillary wall due to the release of cationic proteins from PAF-activated platelets and neutrophils (82). Intradermal injection of PAF into the skin of human volunteers has been reported to produce an immediate vasoconstriction followed by a vasodilatation and an increase in vascular permeability (19). The increase in plasma protein extravasation elicited by PAF was enhanced by concomitant intradermal injections of prostaglandin E2 or prostaglandin E1 and inhibited by a -adrenoceptor agonist (isoprenaline) or an ␣-adrenoceptor agonist (phenylephrine) (300). The effect of PAF on vascular permeability was independent from the stimulation of H1 histamine receptor (191). Recently, it has been shown that the synthesis of PAF mediates the increase in vascular permeability induced by vascular endothelial growth factor (VEGF) in certain organs, such as stomach, duodenum, and pancreas (381), but not in skin (308). 1682 MONTRUCCHIO, ALLOATTI, AND CAMUSSI Volume 80 VIII. EFFECTS OF PLATELET-ACTIVATING FACTOR ON ENDOTHELIAL CELLS B. Endothelium-Leukocyte and -Platelet Interaction Endothelium modulates the microenvironement homeostasis by affecting the traffic of macromolecules and cells from the bloodstream to the tissue. The molecules involved in the control of such traffic are soluble mediators, surface receptors that translate external signals and adhesion molecules. In this setting, PAF acts as an autocrine (176) and paracrine mediator that may modulate endothelial functions. Several stimuli are capable of inducing the synthesis of PAF from endothelium including thrombin, vasoactive mediators, and proinflammatory cytokines, suggesting that PAF may transduce or amplify the signals delivered by these mediators. The two main endothelial cell functions regulated by the synthesis of PAF are the endothelial cell barrier function and the adhesion of leukocytes to the endothelial layer, which precedes their transmigration. Three subsequent steps are thought to be involved in neutrophil migration from bloodstream to inflamed tissues (66). The first step requires a transient interaction of leukocytes with the endothelial cells mediated by surface molecule known as selectins. This interaction induces the rolling of leukocytes along the vessel wall, but it is not strong enough to completely stop them. The second step involves the activation of the leukocytes brought, by selectins, into contact with endothelium. This activation leads to a stable adhesion dependent on the interaction of integrins expressed on the surface of leukocytes with the endothelial counter receptors belonging to the superfamily immunoglobulins. In the third step, chemoattractants stimulate the transmigration of leukocytes across the vessel wall (390). PAF is considered a mediator of leukocyteendothelium interaction, which may participate in the cell activation phase (479, 480). Prescott et al. (336) correlated the adhesion of neutrophils to thrombin-activated endothelium with the PAF synthesized and expressed on the surface of endothelial cells. By using specific PAF receptor antagonists, they demonstrated that PAF produced by stimulated endothelial cells was a crucial determinant in neutrophil adhesion to endothelium. PAF produced after thrombin stimulation is coexpressed with P-selectin on the endothelial cell surface. It has been suggested that P-selectin triggers and PAF activates neutrophils by interacting with its specific receptor (259, 376). This leads to an influx of calcium ions in neutrophils associated with the upfunctional regulation of CD11/ CD18 integrin complex and the cell polarization (259). Moreover, PAF was shown to play a different role when endothelial cells are stimulated by cytokines such as IL-1 (61, 228, 229) or TNF-␣ (64, 228). Cytokines promoted the neosynthesis and coexpression on the endothelial cell surface of a selectin (E-selectin) (390) and of PAF. The latter promoted a calcium influx in adhering neutrophils (228, 229) that enhanced their response to the IL-8 produced by endothelial cells (228). PAF was not essential in the delayed cytokine-induced PMN adhesion (228, 229) but was involved in neutrophil emigration, because PAF receptor antagonists were shown to block the neutrophil migration across monolayers of cytokine-pretreated endothelial cells (228). The difference in the role of PAF exposed on endothelial cell surface after thrombin or cytokines treatment may depend either on differences on molecules coexpressed on the endothelial cell surface or on the amount of PAF exposed. Finally, exogenously added PAF increases the endothelial cell proadhesive properties for leukocytes (336, 376, 476, 477) and promotes their transendothelial migration (88). In this experimental condition, PAF stimulates the endothelial cell receptor, promoting the expression of P-selectin and a A. Effect of PAF on Endothelial Cell Permeability The endothelium was shown to express PAF-binding sites (223) and to be a target for PAF (62). Therefore, PAF produced by endothelial or inflammatory cells may stimulate endothelial cell functions. In vitro PAF enhances the permeability of cultured human endothelial cell monolayer and induces changes of the cell cytoskeleton leading to cell retraction and formation of intercellular gaps (62). Specific PAF receptor antagonists (62) inhibit these effects. Shape change of bovine pulmonary endothelial cells was also obtained in the presence of PAF (57, 155). Moreover, PAF induces the production from endothelial cells of several vasoactive mediators. PAF stimulation of cultured human endothelial cells (117a) induces a dose-dependent synthesis of prostacyclin and thromboxane A2, or release of plasminogen activator (124). In addition, the changes in shape of endothelial cells were associated with activation of calcium-dependent K⫹ channels and hyperpolarization of cell membrane. PAF induced an increase in cytosolic free calcium through the production of inositol trisphosphate (41, 54, 58, 155), and possibly the opening of receptor-operated calcium channels may serve as a signal for increasing macromolecular transport and activation of PLA2. The stimulation of PLA2 may lead to the synthesis and release of leukotrienes and thromboxane A2, which are involved in PAF-induced permeability changes and arteriolar constriction, respectively. Recent studies on the basic mechanism by which leukocyte-endothelial cell adhesion mediates PAF-induced increases in capillary permeability demonstrate a correlation between the extent of arteriolar pairing to venules and the PAF-induced increase in capillary fluid filtration rate (170). October 2000 1683 PAF AND THE CARDIOVASCULAR SYSTEM rapid and selective loss of sulfated proteoglycans, thus reducing the charged repealing forces between cells (376). Indeed, it has been shown that PAF plays a critical role in monocytes as well as neutrophil migration across monolayers of cytokine-prestimulated endothelial cells (228). Recently, it has been shown that integrins are upregulated by PAF and that the 1-integrins are critically involved in PAF-induced leukocyte locomotion in extravascular tissue (454). Moreover, platelet/endothelial cell adhesion molecule-1 has been involved in PAF-induced cell activation, suggesting that platelet/endothelial cell adhesion molecule-1 may serve as a costimulatory agonist receptor capable of modulating integrin function in human platelets during adhesion and aggregation (443). These mechanisms of leukocyte recruitment were shown to play a critical role in the ischemia-reperfusion injury (Fig. 5) (261). Indeed, it was found that cultured human endothelial cells exposed to sublethal anoxia followed by reoxygenation induced PAF synthesis (84) and a leukocyte adhesion and transmigration that was inhibited by PAF receptor antagonists (20, 194, 201, 284, 345, 471). It was found that cultured human endothelial cells and neonatal rat heart myocytes synthesize PAF after prolonged hypoxia (56). Moreover, several studies demonstrated that monoclonal antibodies against adhesion molecules as well as antagonists of selectins or of PAF significantly prevented the recruitment of leukocytes in ischemic heart and reduced the necrotic area (261, 287, 307). The role of PAF as mediator of direct interaction between platelets and endothelium is controversial. In vitro studies have shown that exogenously added PAF did not promote platelet adhesion to an endothelial monolayer. However, it has been recently shown that PAF, but not leukotriene B4, induces the adhesion of platelets to the endothelium in the presence of activated PMN (180 – 182). The inhibitory effect of PAF receptor antagonists suggests that PAF mediate a PMN-platelet interaction. The generation of oxygen radicals from activated PMN was shown to stimulate the subsequent adhesion of platelets to the endothelium (182). IX. INVOLVEMENT OF PLATELET-ACTIVATING FACTOR IN CARDIOVASCULAR PATHOPHYSIOLOGICAL PROCESSES A. Role of PAF in Cardiac Anaphylaxis and Shock Syndromes Cardiac anaphylaxis defines the involvement of the heart as a target organ of immediate hypersensitivity reaction. The in vitro studies on isolated guinea pig heart passively sensitized to dinitrophenol demonstrated a coronary vasoconstriction, impaired myocardial contractility, and arrhythmias following the antigen challenge (251). A hallmark of cardiac anaphylaxis is the release of mediators such as histamine, thromboxane A2, prostacyclin, and leukotrienes (5, 6, 251, 334). During anaphylaxis PAF is released in the coronary effluent of the isolated guinea pig heart (251). Moreover, when administered to isolated perfused heart, PAF reproduces the mechanical and electrical changes typically encountered during allergic reactions (e.g., rightward shifts in the QRS axis, ischemic S-T segment changes, and brady- and tachyarrhythmias) (30, 251, 334, 335). In vivo, an intravascular release of PAF occurs during experimentally induced anaphylaxis in rab- FIG. 5. Role of PAF in leukocyte recruitment and transmigration in injured myocardium. P-selectin contained in the bodies of Weibel-Palade (WP) is coexpressed with PAF on the surface of endothelium activated by hypoxia/reoxygenation. P-selectin by recognizing its counterreceptor sialyl Lewis X (sLex) allows a transient adhesion of leukocytes leading to interaction of leukocyte PAF receptor (PAF-Rc) with PAF expressed on the endothelial cell surface. PAF stimulates an upfunctional regulation of CD11/CD18 integrin complex, making it competent to bind specific endothelial ligands such as intercellular adhesion molecule-1 (ICAM-1), leading to a firm adhesion. Moreover, PAF acts in concert with interleukin-8 (IL-8) in promoting the transmigration of leukocytes in the extravascular space. LTB4, leukotriene B4; PMN, polymorphonuclear neutrophils; EC, endothelial cells. 1684 MONTRUCCHIO, ALLOATTI, AND CAMUSSI bits and implies a relationship between PAF and other mediators (110, 132, 160, 161, 332). It has been shown that the action of PAF on isolated rat heart was dependent on the release of leukotrienes and of cyclooxygenase products with vasoconstrictor action (334). A partial protective effect of PAF antagonists in anaphylactic reactions further supports the role of PAF. BN 52021 and WEB 2086 were shown to inhibit cardiac anaphylactic responses such as coronary constriction and decreased contractility of passively sensitized guinea pig hearts (219, 264). Moreover, the PAF receptor antagonists were shown to reduce significantly the sustained vasoconstriction induced by challenge with ovalbumin of presensitized animals (220). Even though indomethacin suppresses the production of 6-keto-prostaglandin F1␣ and thromboxane B2, it did not affect vasoconstriction during anaphylaxis (401). These observations suggest that PAF-induced coronary constriction depends on leukotriene production rather than thromboxane A2. In addition, PAF has been involved in the pathogenesis of immune complex-induced and septic shock. PAF was implicated in the hemoconcentration and the systemic enhancement of vascular permeability induced by infusion of immune complexes or IgG aggregates (167, 361). In this experimental condition, PAF also mediates leukopenia and thrombocytopenia induced by the immune complexes (81). Moreover, PAF has been considered a mediator of septic shock (75, 78, 137) on the basis of the following evidence: 1) when administered in experimental animals PAF reproduces several aspects of the lipopolysaccharides (LPS)- or TNF-␣-induced shock (70, 134, 306, 344, 412). 2) PAF is synthesized during septic shock (120) by several cell types including monocytes, PMN, Kupffer cells, splenic cells, and endothelial cells stimulated either by LPS (74, 159, 198, 238), bacterial exotoxins (55, 225), porins (432), or TNF-␣ (64, 73, 440). Recently, we demonstrated that LPS-binding protein and CD14 (463) modulate the synthesis of PAF induced by LPS (74). 3) Transgenic mice overexpressing PAF receptor show an increased mortality when exposed to bacterial endotoxin (197). 4) PAF receptor antagonists inhibit or reverse endotoxin/TNF-␣-induced hypotension and reduce mortality (4, 86, 93, 120, 140, 166, 205, 324, 325, 343, 370, 406, 412, 422, 462, 465, 467, 473). It has been shown that a variety of chemically unrelated PAF antagonists inhibit and/or reverse also the endotoxin-induced leukopenia, thrombocytopenia, and hemoconcentration (4, 107, 234, 422). Moreover, PAF receptor blockade has been shown to improve cardiovascular function in nonhypotensive sepsis (2, 125). Further support for the role of PAF was provided by the observation that a PAF receptor antagonist attenuates the induction of the cytokine network in experimental endotoxemia in pigs (462) and chimpanzees (230). Therefore, it has been suggested that PAF is the Volume 80 most proximal mediator in the cytokine cascade triggered by endotoxin or sepsis (230). There is evidence suggesting that PAF contributes to the pathogenesis of cardiac, lung, and renal complications of septic shock (328). Several negative inotropic substances such as TNF-␣ and IL-1 are present in the circulation (231). Because PAF was shown to mediate several biological effects of TNF-␣ (68), it is therefore a potential candidate for mediation of cardiac function depression in septic shock (11, 13, 30, 178, 205). In the guinea pig papillary muscle, cardiac alterations induced by TNF-␣ are mediated by PAF and nitric oxide, the production of which is downstream to the synthesis of PAF (13). Recently, it has been shown that PAF mediates the action of LPS on coronary microcirculation in isolated perfused rat heart (83). Moreover, PAF antagonists were shown to prevent systemic and pulmonary hemodynamic changes as well as acute lung and renal vascular injury in endotoxin-treated rats (92, 328, 420, 451). In the kidney, the synthesis of PAF from glomerular mesangial cells and endothelial cells may be triggered either directly by LPS or other bacterial products such as porins or by LPS-induced cytokines such as TNF-␣ and IL-1 (36, 72, 432). PAF extracted from kidney is increased in endotoxic shock, and PAF receptor antagonists not only prevent but also revert the normotensive LPS-induced hemodynamic insufficiency in rats (420, 451). In humans, thrombocytopenia and reduction of PAF free receptors were observed in septic shock, and the involvement of platelets was correlated with development of adult respiratory distress syndrome (ARDS) (258, 274, 389). Moreover, PAF has been detected also in bronchoalveolar lavage of patients with ARDS (274). A preliminary trial in which a PAF receptor antagonist has been used in the treatment of patients with septic shock has been recently published. This study suggests that mortality is reduced in gramnegative but not in gram-positive septic shock (116). Furthermore, studies on anesthetized rats support the role of PAF in mediating traumatic shock. An increased content of PAF in the peritoneal fluid of traumatized rats and a partial protection from shock of several PAF receptor antagonists has been reported (393, 395, 413). On the basis of experiments with PAF receptor antagonists, PAF has been also implicated in hemorragic shock (3, 271, 394) and in postischemic shock reaction (358), although no direct evidence is available on an enhanced synthesis of this mediator. B. Role of PAF in Ischemia-Reperfusion Injury of the Heart The role of PAF in ischemia and reperfusion injury of the heart is supported by experiments aimed at evaluating October 2000 PAF AND THE CARDIOVASCULAR SYSTEM the synthesis of PAF and the protective effect of PAF receptor antagonists in these physiopathological conditions (47). Myocardial synthesis of PAF occurs in baboons following myocardial infarction (17), and an intravascular release of this mediator was detected in blood of patients with coronary artery disease undergoing atrial pacing to evaluate the severity of ischemia (294). Moreover, PAF was detected in the coronary sinus after occlusion and reperfusion injury in sheep (212). Indirect evidence for PAF biosynthesis during myocardial ischemia was obtained measuring the lyso-PAF, a metabolite of PAF, in canine myocardium subjected to permanent ligation of the left anterior descending coronary branch (247). Experiments on isolated perfused hearts demonstrated the cardiac origin of PAF released in significant amounts during ischemic reperfusion injury. In rabbits, PAF was detected in the coronary effluent during the initial reperfusion of ischemic heart (34, 204, 291). The release of PAF was concomitant with that of 6-keto-prostaglandin F1␣ (34). The precise cellular source of PAF was not identified in this model; however, likely candidates are endothelial cells (69, 276, 278, 336, 478) and cardiomyocytes (56). Indeed, it has been reported that cultured endothelial cells (20, 471) as well as cultured neonatal rat heart myocytes synthesize PAF after prolonged hypoxia (56). In the isolated rabbit heart, the effects of PAF were platelet dependent (291). When reperfusion was performed in the presence of autologous platelets, there was a significant worsening of left ventricular function and an increased rate of ventricular arrhythmias, which were prevented by a PAF receptor antagonist (90). The effect of platelets was due to the release of histamine, thromboxane A2, and leukotrienes (291). It was shown that a PAF-dependent PMN-platelet cooperation significantly worsens reperfusion injury (10). The vasoactive effect of PAF and its PMN-dependent mechanism have been directly studied in coronary resistance vessels using an isolated and perfused microvessel preparation (190). In this study topical application of PAF to the vessels induced a dose-dependent decrease in the diameter but an increase in the apparent permeability coefficient of albumin. Disruption of the endothelium abolished the vasomotor response to PAF, and perfusion of PMN significantly augmented PAF-induced changes in vasomotor tone and permeability. Furthermore, administration of PAF caused PMN adhesion to the endothelium of coronary arterioles at low-flow perfusion velocities. These results suggest that PAF induces vasoconstriction and hyperpermeability in coronary arterioles via an endothelium-dependent and PMN-mediated mechanism and that PAF is able to stimulate PMN adhesion in coronary arterioles under a condition of low flow rate (190). Moreover, a link exists between the well-established role of oxygen radicals and that of PAF in ischemia reperfusion injury. In fact, it was found that generation of oxygen 1685 radicals stimulates the synthesis of PAF by endothelium in isolated perfused guinea pig heart and that a PAF receptor antagonist blunts the mechanical and electrical alterations induced by oxygen radicals (9). Additional support for the role of PAF in myocardial ischemia was obtained in experiments of PAF administration after induction of cardiac ischemia (250, 283, 393). In this experimental condition, the infusion of PAF significantly enhanced the ischemic injury by a mechanism dependent on thromboxane A2 generation (283). Finally, the effect of PAF receptor antagonists was studied in four experimental conditions: 1) the isolated perfused heart under conditions of ischemia followed by reperfusion, 2) the experimental myocardial infarction by coronary occlusion and reperfusion, 3) in a model of low-flow high-demand ischemia and reperfusion (366), and 4) in the cyclic constriction of a coronary artery to mimic the clinical situation of unstable angina. In the isolated perfused heart, PAF antagonists prevented both the platelet-dependent and platelet-independent mechanical and electrical alterations that occurred, respectively, in rabbit (10, 34, 291), guinea pig (141), and rat heart (127, 221, 222) after ischemia-reperfusion. In the experimental myocardial infarction by coronary occlusion, PAF receptor antagonists reduced the hematological and hemodynamic alterations as well as the size of necrotic area and the accumulation of platelets and leukocytes observed in rabbit (287, 288) and sheep (213) hearts but did not affect plasma protein leakage (91, 287, 459). In rats, PAF antagonists reduced infarct size and arrhythmias (144, 158, 263, 265). In dogs, conflicting results were obtained with different PAF receptor antagonists. With the use of BN 52021, SRI 63441, and TCV-309, a protective effect was observed (156, 267–270, 408), whereas WEB 2086 was uneffective (248, 249). Moreover, it was found that CV 6209 prevented pulmonary edema following coronary ligation in dogs (409). With the use of PAF antagonists in the cyclic constriction of a coronary artery to mimic the clinical situation of unstable angina, it was found that PAF contribute to platelet activation involved in the cyclic flow variations at the site of arterial stenosis and endothelial injury (151). It was shown that the cyclic flow variations depend on the release of mediators from platelets (18). In humans, an increase of PAF concentration in blood was observed in patients with defined unstable angina (371). Moreover, acute myocardial infarction is associated with a depression of plasma acetyl hydrolase activity that may allow a prolonged half-life of newly synthesized PAF (316, 400). Plateles from patients with acute myocardial infarction exhibit an increased sensitivity to the aggregatory effect of PAF in vitro in the first 48 h after the onset of the symptoms (431). We (293) and Graham et al. (154) were unable to find an increased synthesis of PAF in peripheral blood of patients with myocardial infarction. Whether a local synthesis of PAF occurs is unknown. However, suc- 1686 MONTRUCCHIO, ALLOATTI, AND CAMUSSI cessful thrombolytic therapy with streptokinase was associated with intravascular release of PAF. Streptokinase as well as plasmin were shown to stimulate PAF synthesis by endothelium, an event that may limit the beneficial effect of thrombolytic therapy by promoting platelet and leukocyte adhesion and activation on the endothelial cell surface as well as transmigration into ischemic tissue (289, 293, 298). Indeed, it has been shown that PAF receptor antagonists prevent thrombotic reocclusion in dogs treated with recombinant-tissue-type plasminogen activator (421). Moreover, blockade of PAF receptors abrogated hypotension and platelet activation in rabbits treated with streptokinase and recombinant-tissue-type plasminogen activator (289). Recently, it has been reported that PAF released during angioplasty in humans (123) mediates the neutrophil stimulation seen in this clinical setting (377, 378). C. Role of PAF in Atherogenesis PAF may play a role in atherogenesis and atherosclerosis (183, 402). The possible involvement of PAF in cholesterol deposition in the arterial wall has been investigated in rabbits fed a hypercholesterolemic diet (131). The administration of a PAF receptor antagonist to these rabbits significantly reduced the amount of estherified cholesterol in the aorta without affecting the plasma levels of cholesterol (131). Clinical studies show higher levels of PAF in coronary artery samples from patients with severe atherosclerosis (302). It has been suggested that PAF synthesized by endothelial cells and exposed on the cell surface may, together with P selectin, promote leukocyte adhesion to endothelial cells (479, 480). This interaction may be important for the activation and the subsequent infiltration of monocytes-macrophages, for the production of proliferative cytokines, and eventually for the accumulation of lipids within the cells (355). It has been shown that PAF and P-selectin cooperate in the nuclear translocation of a transcription factor NFB and in the secretion of NFB-dependent cytokines by monocytes. PAF has a weak agonist effect for NFB-dependent actions in nonadherent monocytes (456). In contrast, the adhesion to P-selectin expressed on the endothelial surface amplifies or integrates signals triggered by PAF receptor, leading to the activation of NFB-dependent functions (224, 455, 456). Furthermore, PAF stimulates, in monocytes, transcription of a heparin binding epidermal growth factor, and in vascular smooth muscle, synthesis of IL-6 that may act as potent mitogen for vascular smooth muscle cells (146, 329). Moreover, it has been shown that PAF mediates at least in part the adhesion of monocytes to endothelium induced by LDL and oxydized LDL (246). Cigarette smoking, a factor associated with the pathogenesis of atherosclerosis, causes platelet activation, LDL Volume 80 oxidative changes, and increased levels of PAF (285). The latter alteration was associated with a compensatory increase of PAF-AH activity. However, in vitro studies demonstrated that cigarette-derived products as well as oxidative changes of LDL, that physiologically carry PAF specific acetyl hydrolase, inhibit the activity of the enzyme that catabolizes PAF (285). Furthermore, PAF may also oxidize LDL, via stimulation of human monocytes/ macrophages and neutrophils to produce superoxide anions and hydrogen peroxide (356, 419). PAF may also induce a release of proteases such as elastase from leukocytes that may degradate components of the extracellular matrix of the intima (356). This may favor the fissuration of the plaque (256). Indeed, an enhanced concentration of PAF was detected in endarterectomy samples of patients with complicated coronary plaques (302). It has been also shown that PAF is transiently produced by macrophages and cholesterol-loaded macrophage foam cells activated by phagocytosis, suggesting that PAF of macrophage origin may exert potent proinflammatory, proatherogenic, and prothrombotic effects (114). X. ROLE OF PLATELET-ACTIVATING FACTOR IN NEOANGIOGENESIS Neoangiogenesis has an important role in the embryogenesis of the heart and in the repair of myocardial infarction. The angiogenic process is, in physiological conditions, highly regulated to direct the organ development or to limit the growth of new blood vessels to the repaired tissue. An unregulated growth of blood vessels may be involved in pathological processes such as chronic inflammation, rupture of coronary plaques and intraplaque hemorrhage, and growth of most solid tumors (142, 143). In these conditions, angiogenesis may contribute to the development of tissue injury. Several angiogenic factors have been shown to modulate angiogenesis (142, 143). Endothelial cells are the primary target for these mediators and are stimulated to degradate extracellular matrix, migrate, and proliferate. These events are required to initiate a capillary sprout and the formation of new vessels. In this complex process, endothelium is actively involved and is capable of producing autocrine mediators such as the vascular endothelial growth factor (142, 143) and IL-8 (216). The relevance of angiogenesis in the recovery from myocardial infarction is supported by the recent observations that the administration of basic fibroblast growth factor and heparin significantly improves the collateral formation (169, 237, 342, 433). There is evidence indicating that PAF may act as a mediator of angiogenesis (15, 16, 59, 76, 295). Whereas in vitro PAF has only a chemotactic effect on endothelial cells but does not stimulate endothelial cell proliferation (76), in October 2000 PAF AND THE CARDIOVASCULAR SYSTEM 1687 vivo PAF can induce an angiogenic response. The angiogenic effect of PAF is either a heparin-independent or heparin-dependent mechanism according to concentration (76). At micromolar concentrations, PAF induces an angiogenesis independent from addition of exogenous heparin, possibly because the inflammatory reaction elicited may determine heparin release from mastocytes and endothelial cells (15, 16). In contrast, at nanomolar concentrations, PAF requires the addition of exogenous heparin for its angiogenic activity, suggesting the production of heparin binding growth factors (59, 76). PAF may directly provide the signal for migration but not the signal for endothelial cell proliferation, which is induced by heparin-binding growth factors, produced by PAF-stimulated endothelial cells. Indeed, PAF induced the expression of several angiogenic factors and chemokines including acid and basic fibroblast growth factor, vascular endothelial growth factor and its specific receptor flk-1, hepatocyte growth factor, and macrophage inflammatory protein 2 (59, 475). Moreover, we recently observed that PAF-induced neoangiogenesis was dependent on the production of nitric oxide (297). In vivo and in vitro experiments, performed with a panel of different PAF receptor antagonists, suggest that the synthesis of PAF induced by several polypeptide mediators, such as TNF-␣, hepatocyte growth factor, VEGF, thrombopoietin, and IL-3, accounts for the endothelial migration required for the development of the new vessels (53, 77, 115, 295, 296). In contrast, the neoangiogenic effect of basic fibroblast growth factor appears to be independent from the expression of the PAF bioactivity (297). the physiological modulation of blood pressure, mainly by affecting the renal vascular circulation. However, most of the available studies have been performed using nanomolar concentration of the mediator, which are reached only in physiopathological conditions. In the cardiovascular system, PAF has been involved in the hypotension and cardiac dysfunctions occurring in cardiac anaphylaxis and in various cardiovascular stress situations such as septic, hemorragic, and traumatic shocks. Moreover, PAF cooperate in the recruitment of leukocytes in inflamed tissue, promoting the activation of cells ensuing in adhesion to the endothelium and extravascular transmigration of leukocytes. The autocrine and paracrine effects of PAF are also involved in the enhancement of endothelial cell permeability and regulation of macro- and microvascular tone. Moreover, the angiogenic properties of PAF may contribute either to the development of chronic inflammatory angiogenesis or to restoration of the collateral blood flow in ischemic tissue. The finding that PAF is present in complicated atherosclerotic plaques, where neoangiogenesis has been implicated in the fissuration, suggests that PAF may have a role in the evolution of atherosclerotic lesion. Finally, studies based on measurement of the local production of PAF and on the action of PAF receptor antagonists have indicated that this mediator is critical in the development of myocardial ischemiareperfusion injury and of adverse effects of thrombolytic therapy. In particular, the finding that the human heart can produce PAF, expresses PAF receptor, and is sensitive to the negative inotropic action of PAF suggests that this mediator may have a role in a local response of the heart to injury. XI. CONCLUSIONS This work was supported by the National Research Council (Consiglio Nazionale delle Ricerche), Targeted Project “Biotechnology,” and by Ministero dell’Universitá della Ricerca Scientifica e Technologica cofin98 (to G. Camussi). Address for reprint requests and other correspondence: G. Camussi, Laboratorio di Immunopatologia, Cattedra di Nefrologia, Dipartimento di Medicina Interna, Ospedale Maggiore S. Giovanni Battista, Corso Dogliotti, 14, 10126 Torino, Italy (Email: giovanni.camussi@unito.it). Despite growing evidence indicating a role of PAF in several pathological conditions, this mediator is still in search of a defined physiological role. The main difficulties in studying the physiological functions of PAF are related to technical hindrance in dosing the mediator, which in normal cells and tissues is synthesized in picomolar concentrations. Significant progress was achieved after cloning the receptor and the main catabolic enzyme PAF-AH. Moreover, functional information was derived from the use of several chemically unrelated PAF receptor antagonists. The in vitro studies provided evidence for a role of PAF both as intercellular and intracellular messenger involved in cell-to-cell communication. Triggering of PAF receptors was shown to elicit different responses, depending on cell type, PAF concentration, and cooperation with other intercellular mediators or intracellular messengers. In the cardiovascular system, PAF may have a role in embryogenesis, because it possesses angiogenic properties and acts by amplifying the effect of defined polypeptide mediators. PAF has been also implicated in REFERENCES 1. ABETE P, FERRARA N, LEOSCO D, CACCESE P, LANDINO P, SEDERINO S, BALBI R, AND RENGO F. Age-related effects of platelet activating factor (PAF) in the isolated perfused rat heart. J Moll Cell Cardiol 24: 1399 –1407, 1992. 2. ABU-ZIDAN FM AND WALTER S. Platelet-activating factor antagonism improves cardiovascular function in non-hypotensive sepsis in pigs. Eur J Surg 162: 499 –504, 1996. 3. ABU-ZIDAN FM, WALTER S, AND LENNQUIST S. Role of platelet antagonist factor in hemorrhagic shock in pigs. Eur Surg Res 27: 379 – 388, 1995. 4. ADNOT S, LEFORT J, BRAQUET P, AND VARGAFTIG BB. Interference of PAF-acether antagonist BN 52021 with endotoxin-induced hypotension in the guinea pig. Prostaglandins 32: 791– 802, 1986. 5. AEHRINGHAUS U, DEMBINSKA-KIEC A, AND PESKAR BA. Effects of exog- 1688 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. MONTRUCCHIO, ALLOATTI, AND CAMUSSI enous prostaglandins on the release of leukotriene C4-like activity and on coronary flow in indomethacin-treated anaphylatic guineapig hearts. Arch Pharmacol 326: 368 –374, 1984. AEHRINGHAUS U, PEAKAR BA, WITTEMBERG HR, AND WOBLING RH. Effect of inhibition of synthesis and receptor antagonism of SRS-A in cardiac anaphylaxis. Br J Pharmacol 80: 73– 80, 1983. AGWU DE, MCPHAIL LC, CHABOT MC, DANIEL LW, WYKLE RL, AND MCCALL CE. Choline-linked phosphoglycerides. A source of phosphatidic acid and diglycerides in stimulated neutrophils. J Biol Chem 264: 1405–1413, 1989. ALLOATTI G, MONTRUCCHIO G, AND CAMUSSI G. Prostacyclin inhibits the platelet-dependent effects of platelet-activating factor in the rabbit isolated heart. J Cardiovasc Pharmacol 15: 745–751, 1990. ALLOATTI G, MONTRUCCHIO G, AND CAMUSSI G. Role of platelet-activating factor (PAF) in oxygen radical-induced cardiac dysfunction. J Pharmacol Exp Ther 269: 766 –771, 1994. ALLOATTI G, MONTRUCCHIO G, EMANUELLI G, AND CAMUSSI G. Plateletactivating factor induced platelet/neutrophil co-operation during myocardial reperfusion. J Moll Cell Cardiol 24: 163–171, 1992. ALLOATTI G, MONTRUCCHIO G, MARIANO F, TETTA C, DE PAULIS R, MOREA M, EMANUELLI G, AND CAMUSSI G. Effect of platelet-activating factor (PAF) on human cardiac muscle. Int Arch Allergy Appl Immunol 79: 108 –112, 1986. ALLOATTI G, MONTRUCCHIO G, MARIANO F, TETTA C, EMANUELLI G, AND CAMUSSI G. Protective effect of verapamil on the cardiac and circulatory alterations induced by platelet-activating factor. J Cardiovasc Pharmacol 9: 181–186, 1987. ALLOATTI G, PENNA C, DE MARTINO A, MONTRUCCHIO G, AND CAMUSSI G. Role of nitric oxide and platelet-activating factor in cardiac alterations induced by tumor necrosis factor-␣ in the guinea-pig papillary muscle. Cardiovasc Res 41: 611– 619, 1999. ALONSO F, GILL MG, SANCHEZ-CRESPO M, AND MATO JM. Activation of 1-alkyl-2-lyso-glycero-3-phosphocholine-acetyl-CoA transferase during phagocytosis in human polymorphonuclear leukocytes. J Biol Chem 257: 3376 –3378, 1982. ANDRADE SP, VIEIRA LBGB, BAKHLE YS, AND PIPER PJ. Effects of platelet activating factor (PAF) and other vasoconstriction on a model of angiogenesis in the mouse. Int J Exp Pathol 73: 503–513, 1992. ANDRADE SP, VIERIA LBGB, BAKHLE YS, AND PIPER PJ. Effect of platelet activating factor (PAF) on the formation of blood vessels in subcutaneous implants in mice. J Lipid Med Cell Signal 9: 117–121, 1994. ANNABLE CR, MCMANUS LM, CAREY KD, AND PINCKARD RN. Isolation of platelet-activating factor (PAF) from ischemic baboon myocardium (Abstract). Federation Proc 44: 1271, 1985. APPRIL P, SCHMITZ JM, CAMPBELL WB, TILTON G, ASHTON J, RAHEJA S, BUJA LM, AND WILLERSON JT. Cyclic blood flow variations induced by platelet-activating factor in stenosed canine coronary arteries despite inhibition of thromboxane synthetase, serotonin receptors, and adrenergic receptors. Circulation 72: 397– 405, 1985. ARCHER CB, PAGE CP, PAUL W, MORLEY J, AND MCDONALD DM. Inflammatory characteristics of platelet activating factor (PAFacheter) in human skin. Br J Dermatol 110: 45–52, 1984. ARNOULD T, MICHIELS C, AND REMACLE J. Increased PMN adherence on endothelial cells after hypoxia: involvement of PAF, CD 18/CD 11b, and ICAM-1. Am J Physiol Cell Physiol 264: C1102–C1110, 1993. AU BT, WILLIAMS TJ, AND COLLINS PD. Zymosan-induced IL-8 release from human neutrophils involves activation via the CD11b/CD18 receptor and endogenous platelet activating factor as an autocrine modulator. J Immunol 152: 5411–5419, 1994. BACHELET M, ADOLFS MPJ, MASLIAH J, BEREZIAT G, VARGAFTIG BB, AND BONTA IL. Interaction between PAF-acether and drugs that stimulate cyclic AMP in guinea-pig alveolar macrophage. Eur J Pharmacol 149: 73–78, 1988. BADR KF, DEBOER DK, TAKAHASHI K, HARRIS RC, FOGO A, AND JACOBSON HR. Glomerular responses to platelet-activating factor in the rat: role of thromboxane A2. Am J Physiol Renal Fluid Electrolyte Physiol 256: F35–F43, 1987. BAER PG AND CAGEN LM. Platelet activating factor vasoconstriction of dog kidney: inhibition by alprazolam. Hypertension 9: 253–260, 1987. Volume 80 25. BALESTRIERI ML, SERVILLO L, AND LEE T. The role of platelet-activating factor-dependent transacetylase in the biosynthesis of 1-acyl-2acetyl-sn-glycero-3-phosphocholine by stimulated endothelial cells. J Biol Chem 272: 17431–17437, 1997. 26. BAZAN HE AND VARNER L. A mitogen-activated protein kinase (MAPkinase) cascade is stimulated by platelet activating factor (PAF) in corneal epithelium. Curr Eye Res 16: 372–379, 1997. 27. BECKER K, HEINROTH-HOFFMANN I, GIESSLER C, PONICKE K, AND BRODDE OE. PAF effects on eicosanoid release in neonatal rat cardiomyocytes. Prostaglandins Leukotrienes Essent Fatty Acids 53: 197– 200, 1995. 28. BENSARD DD, ANDERSON BO, BENERJEE A, NELSON DW, POGGETTI RS, BERENS RL, AND HARKEN AH. Platelet activating factor alters receptor-coupled function in the isolated perfused rat heart. J Surg Res 53: 321–325, 1992. 29. BENVENISTE J. Platelet activating factor, a new mediator of anaphylaxis and immune complex deposition from rabbit and human basophils. Nature 249: 581–582, 1974. 30. BENVENISTE J, BOULLET C, BRINK C, AND LABAT C. The actions of PAF-acether (platelet-activating factor) on guinea-pig isolated heart preparations. Br J Pharmacol 80: 81– 83, 1983. 31. BENVENISTE J, HENSON PM, AND COCHRANE CG. Leukocyte-dependent histamine release from rabbit platelets: the role of IgE, basophils, and platelet-activating factor. J Exp Med 136: 1356 –1377, 1972. 32. BENVENISTE J, LE COUEDIC JP, POLONSKY J, AND TENCE M. Structural analysis of purified platelet activating factor by lipases. Nature 269: 170 –171, 1977. 33. BENVENISTE J, TENCE M, VARENNE P, BIDAULT J, BOULLET C, AND POLONSKY J. Semisynthese et structure proposee du facteur activant les plaquettes (PAF): PAF-acether un alkyl ether analogue de la lysophosphatidilcholine. CR Acad Sci Paris 289: 1037–1040, 1979. 34. BERTI F, MAGNI F, ROSSONI G, DE ANGELIS L, AND GALLI G. Production and biologic interactions of prostacyclin and platelet activating factor in acute myocardial ischemia in the perfused rabbit heart. J Cardiovasc Pharmacol 16: 727–732, 1990. 35. BESSIN P, BONNET J, APFFEL D, SOULARD C, DESGROUX L, PELAS I, AND BENVENISTE J. Acute circulatory collapse caused by platelet-activating factor (PAF-acether) in dogs. Eur J Pharmacol 86: 403– 413, 1983. 36. BIANCONE L, TETTA C, TURELLO E, MONTRUCCHIO G, IORIO EL, SERVILLO L, BALESTRIERI C, AND CAMUSSI G. Platelet activating factor biosynthesis by cultured mesangial cells is modulated by proteinase inhibitors. J Am Soc Nephrol 2: 1251- 1261, 1992. 37. BIENVENU K AND GRANGER DN. Leukocyte adhesion in ischemia/ reperfusion. Blood Cells 19: 279 –289, 1993. 38. BITO H AND SHIMIZU T. Molecular characterization and physiological functions of PAF receptor. Adv Exp Biol 400: 215–21, 1997. 39. BJORK J AND SMEDEGARD G. Acute microvascular effects of PAFacether, as studied by intravital microscopy. Eur J Pharmacol 96: 87–94, 1983. 40. BKAILY G, D’ORLEANS-JUSTE P, NAIK R, PERODIN J, STANKOVA J, ABDULNOUR E, AND ROLA-PLESZCZYNSKI M. PAF activation of a voltage-gated R-type Ca2⫹ channel in human and canine aortic endothelial cells. Br J Pharmacol 110: 519 –520, 1993. 41. BKAILY G, WANG S, BUI M, STANKOVA J, AND ROLA-PLESZCZYNSKI M. Modulation of cardiac cell Ca2⫹ currents by PAF. Blood Press Suppl 3: 59 – 62, 1996. 42. BLANK ML, HALL MN, CRESS EA, AND SNYDER F. Inactivation of 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine by a plasma acetylhydrolase: higher activities in hypertensive rats. Biochem Biophys Res Commun 113: 666 – 671, 1983. 43. BLANK ML, LEE TC, FITZGERALD V, AND SNYDER F. A specific acetylhydrolase for 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine (a hypotensive and platelet activating lipid). J Biol Chem 256: 175–178, 1981. 44. BLANK ML, SNYDER F, BYERS LW, BROOHS B, AND MUIRHEAD EF. Antihypertensive activity of an alkyl ether analog of phosphatidylcholine. Biochem Biophys Res Commun 90: 1194 –1200, 1979. 45. BOURGAIN RH, MAES L, BRAQUET P, ANDRIES R, TOUQUI L, AND BRAQUET M. The effect of 1-O-alkyl-2-acetyl-sn-glycero-3- phosphocholine (PAF-acether) on the arterial wall. Prostaglandins 30: 185–195, 1985. 46. BRADLEY LM, GOLSTEIN RE, FEUERSTEIN GZ, AND CZAJA JF. Circulatory October 2000 47. 48. 49. 50. 51. 52. 53. 54. 55. 56. 57. 58. 59. 60. 61. 62. 63. 64. 65. PAF AND THE CARDIOVASCULAR SYSTEM effects of PAF-acether in the newborn piglets. Am J Physiol Heart Circ Physiol 256: H205–H212, 1989. BRAQUET P, PAULBERT-BRAQUET M, KOLTAY M, BOURGAIN R, BUSSOLINO F, AND HOSFORD D. Is there a case for PAF antagonists in the treatment of ischemic states? Trends Pharmacol Sci 10: 23–30, 1989. BRAQUET P AND ROLA-PLESCZCZYNSKI M. Platelet-activating factor and cellular immune response. Immunol Today 8: 345–350, 1987. BRAQUET P, TOUQUI L, SHEN TY, AND VARGAFTIG BB. Perspectives in platelet-activating factor research. Pharmacol Rev 39: 97–145, 1987. BRATTON D AND HENSON PM. Cellular origin of PAF. In: PlateletActivating Factor and Human Disease, edited by Barnes PJ, Page CP, and Henson PM. London: Blackwell, 1989, p. 23. BRATTON DL, DREYRE E, KAILEY JM, FADOK FV, CLAY KL, AND HENSON PM. The mechanism of internalization of platelet activating factor in activated human neutrophils. Enhanced transbilayer movement across the plasma membrane. J Immunol 148: 514 –523, 1992. BREVIARIO F, BERTOCCHI F, DEJANA E, AND BUSSOLINO F. Interleukin-1 induced adhesion of polymorphonuclear leukocytes to cultured human endothelial cells. Role of platelet activating factor. J Immunol 141: 3391–3399, 1988. BRIZZI MF, BATTAGLIA E, MONTRUCCHIO G, DENTELLI P, DEL SORBO L, GARBARINO G, PEGORARO L, AND CAMUSSI G. Thrombopoietin stimulates endothelial cell motility and neoangiogenesis by a plateletactivating factor-dependent mechanism. Circ Res 84: 785–796, 1999. BROCK TA AND GIMBRONE MA. Platelet activating factors alters calcium homeostasis in cultured vascular endothelial cells. Am J Physiol Heart Circ Physiol 250: H1086 –H1092, 1986. BUNTING M, LORANT DE, BRYANT AE, ZIMMERMAN GA, MCINTYRE TM, STEVENS DL, AND PRESCOTT SM. Alpha toxin from Clostridium perfrigens induces proinflammatory changes in endothelial cells. J Clin Invest 100: 565–574, 1997. BURGHARDT C AND JANERO DR. The anoxic rat-heart myocyte produces and release platelet-activating factor (PAF) as a component of its ischemia-like pathology. J Mol Cell Cardiol 19 Suppl IV: S69, 1987. BURHOP KE, GARCOA JC, SELIG WM, LO SK, VAN DER ZEE H, KAPLAN JE, AND MALIK AB. Platelet-activating factor increases lung vascular permeability to protein. J Appl Physiol 61: 2210 –2217, 1986. BUSSOLINO F, AGLIETTA M, SAVANIO F, STACCHINI A, LAURI D, AND CAMUSSI G. Alkyl-ether phosphoglycerides influence calcium fluxes into human endothelial cells. J Immunol 135: 2748 –2755, 1985. BUSSOLINO F, ARESE M, MONTRUCCHIO G, BARRA L, PRIMO L, BENELLI R, SANAVIO F, AGLIETTA M, GHIGO D, ROLA-PLESZCZYNSKI M, BOSIA A, ALBINI A, AND CAMUSSI G. Platelet activating factor produced in vitro by Kaposi’s sarcoma cells induces and sustains in vivo angiogenesis. J Clin Invest 96: 940 –952, 1995. BUSSOLINO F, ARESE M, SILVESTRO L, SOLDI R, BENFENATI E, SANAVIO F, AGLIETTA M, BOSIA A, AND CAMUSSI G. Involvement of a serine protease in the synthesis of platelet activating factor by endothelial cells stimulated by IL-1␣ or TNF-␣. Eur J Immunol 24: 3131–3139, 1995. BUSSOLINO F, BREVIARIO F, TETTA C, AGLIETTA M, MANTOVANI A, AND DEJANA E. Interleukin-1 stimulates platelet activating factor production in human endothelial cells. J Clin Invest 77: 2027–2033, 1986. BUSSOLINO F, CAMUSSI G, AGLIETTA M, BRAQUET P, BOSIA A, PESCARMONA G, SANAVIO F, D’URSO N, AND MARCHISIO PC. Endothelial cells are targets for platelet activating factor. Platelet activating factor induces changes in cytoskeleton structure. J Immunol 139: 2439 – 2446, 1987. BUSSOLINO F, CAMUSSI G, AND ARESE P. Platelet-activating factor phosphatidate, but not platelet-activating factor, is a powerful calcium ionophore in the human red cell. Cell Calcium 5: 463– 466, 1984. BUSSOLINO F, CAMUSSI G, AND BAGLIONI C. Synthesis and release of platelet activating factor by human vascular endothelial cells treated with tumor necrosis factor and interleukin-1␣. J Biol Chem 263: 11856 –11862, 1988. BUSSOLINO F, GREMO F, TETTA C, PESCARMONA GP, AND CAMUSSI G. Production of platelet activating factor by chick retina. J Biol Chem 261: 16502–16508, 1986. 1689 66. BUTCHER EC. Leukocyte-endothelial cells recognition: three (or more) steps to specificity and diversity. Cell 67: 1033–1036, 1991. 67. CAILLARD CG, MONDOT S, ZUNDEL JL, AND JULOU L. Hypotensive activity of PAF-acether in rats. Agents Actions 12: 725–730, 1983. 68. CAMUSSI G. Editorial: interactive effects of tumor necrosis factor and platelet activating factor in the pathogenesis of glomerular injury. Lab Invest 70: 435– 436, 1994. 69. CAMUSSI G, AGLIETTA M, MALAVASI F, TETTA C, SANAVIO F, PIACIBELLO W, AND BUSSOLINO F. The release of platelet activating factor from human endothelial cells in culture. J Immunol 131: 2397–2403, 1983. 70. CAMUSSI G, ALBANO E, TETTA C, AND BUSSOLINO F. The molecular action of tumor necrosis factor-␣. Eur J Biochem 202: 3–14, 1991. 71. CAMUSSI G, ALLOATTI G, MONTRUCCHIO G, MEDA M, AND EMANUELLI G. Effect of platelet activating factor on guinea-pig papillary muscle. Experientia 40: 697– 699, 1984. 72. CAMUSSI G, BIANCONE L, IORIO EL, SILVESTRO L, DA COL R, CAPASSO C, ROSSANO F, SERVILLO L, BALESTRIERI C, AND TUFANO MA. Porins and lipopolysaccharides stimulate platelet activating factor synthesis by human mesangial cells. Kidney Int 42: 1309 –1313, 1992. 73. CAMUSSI G, BUSSOLINO F, SALVIDIO G, AND BAGLIONI C. Tumor necrosis factor/cachectin stimulates peritoneal macrophages, polymorphonuclear neutrophils, and vascular endothelial cells to synthesize and release platelet activating factor. J Exp Med 166: 1390 –1404, 1987. 74. CAMUSSI G, MARIANO F, BIANCONE L, DE MARTINO A, BUSSOLATTI B, MONTRUCCHIO G, AND TOBIAS PS. Lipopolysaccharide binding protein and CD14 modulate the synthesis of platelet activating factor by human monocytes and mesangial and endothelial cells stimulated with lipopolysaccharide. J Immunol 155: 316 –324, 1995. 75. CAMUSSI G, MONTRUCCHIO G, DOMINIONI L, AND DIONIGI R. Septic shock: the unravelling of molecular mechanisms. Nephrol Dial Transplant 10: 1808 –1813, 1995. 76. CAMUSSI G, MONTRUCCHIO G, LUPIA E, DE MARTINO A, PERONA L, ARESE M, VERCELLONE A, TONIOLO A, AND BUSSOLINO F. Platelet activating factor directly stimulates in vitro migration of endothelial cells and promotes in vivo angiogenesis by a heparin-dependent mechanism. J Immunol 154: 6492– 6501, 1995. 77. CAMUSSI G, MONTRUCCHIO G, LUPIA E, SOLDI R, COMOGLIO PM, AND BUSSOLINO F. Angiogenesis induced in vivo by hepatocyte growth factor is mediated by platelet-activating factor synthesis from macrophages. J Immunol 158: 1302–1309, 1997. 78. CAMUSSI G, RONCO C, MONTRUCCHIO G, AND PICCOLI G. Role of soluble mediators in sepsis and renal failure. Kidney Int 53 Suppl 66: S38 –S42, 1998. 79. CAMUSSI G, TETTA C, AND BAGLIONI C. The role of platelet activating factor in inflammation. Clin Immun Immunopathol 57: 331–338, 1990. 80. CAMUSSI G, TETTA C, BUSSOLINO F, AND BAGLIONI C. Synthesis and release of platelet-activating factor is inhibited by plasma alpha-1proteinase inhibitor or alpha-1-antichymotrypsin and is stimulated by proteinases. J Exp Med 168: 1293–1306, 1988. 81. CAMUSSI G, TETTA C, BUSSOLINO F, CALIGARIS-CAPPIO F, MASERA C, AND SEGOLONI G. Mediators of immune complex induced aggregation of polymorphonuclear neutrophils. II. Platelet activating factor as the effector substance of immune-induced aggregation. Int Arch Allergy Appl Immunol 64: 25– 41, 1981. 82. CAMUSSI G, TETTA C, CODA R, SEGOLONI G, AND VERCELLONE A. Platelet-activating factor induced loss of glomerular anionic charges. Kidney Int 25: 73– 81, 1984. 83. CANNON TR, MANN GE, AND BAYDOUN AR. Mechanisms of acute vasodilator response to bacterial lipopolysaccharide in the rat coronary microcirculation. Br J Pharmacol 123: 637– 644, 1998. 84. CAPLAN MS, ADLER L, KELLY A, AND HSUEH W. Hypoxia increases stimulus-induced PAF production and release from human umbilical vein endothelial cells. Biochim Biophys Acta 1128: 205–210, 1992. 85. CARAMELLO C, FERNANDEZ-GALLARDO S, MARIN-CAO D, INARREA P, SANTOS JC, LEPEZ-NOVAA JM, AND SANCHEZ-CRESPO M. Presence of platelet activating factor in blood from human and experimental animals. Its absence in anephric individuals. Biochem Biophys Res Commun 120: 789 –796, 1984. 86. CASALS-STENZEL J. Protective effect of WEB 2086, a novel antagonist 1690 87. 88. 89. 90. 91. 92. 93. 94. 95. 96. 97. 98. 99. 100. 101. 102. 103. 104. 105. 106. MONTRUCCHIO, ALLOATTI, AND CAMUSSI of platelet activating factor, in endotoxin shock. Eur J Pharmacol 135: 117–122, 1987. CASALS-STENZEL J, MUACEVICS G., AND WEBER KH. Pharmacological actions of WEB 2086, a new specific antagonist of platelet activating factor. J Pharmacol Exp Ther 241: 974 –981, 1987. CEPINSKAS G, NOSEWORTHY R, AND KVIETYS PR. Transendothelial neutrophil migration. Role of neutrophil-derived proteases and relationship to transendothelial protein movement. Circ Res 81: 618 – 626, 1997. CERVONI P, HERZLINGER HE, LAI FM, AND TANIKELLA TK. Aortic vascular and atrial response to 1-O-octadecyl-2-acetyl-glyceryl-3-phosphocholine. Br J Pharmacol 79: 667– 671, 1983. CHAKRABARTY S, FLUCK DS, FLORES NA, AND SHERIDAN DJ. Effects of the PAF antagonist BN 50726 and BN 50739 on arrhythmogenesis and extent of necrosis during myocardial ischemia/reperfusion in rabbits. Br J Pharmacol 107: 705–709, 1992. CHAKRABARTY S, THOMAS P, AND SHERIDAN DJ. Contribution of platelets and platelet-activating factor (PAF) to the arrhythmogenic, haemodynamic and necrotic effects of acute myocardial ischemia. Eur Heart J 12: 583–589, 1991. CHANG SW, FEDDERSEN CO, HENSON PM, AND VOELKEL NF. Platelet activating factor mediates hemodynamic changes and lung injury in endotoxin-treated rats. J Clin Invest 79: 1498 –1509, 1987. CHANG SW, FERNYAK S, AND VOELKEL NF. Beneficial effect of a platelet-activating factor, WEB 2086, on endotoxin-induced lung injury in pigs. Am J Physiol Heart Circ Physiol 258: H153–H158, 1990. CHAO W, LIU H, DEBUYSERE MS, HANAHAN DJ, AND OLSON MS. Identification of receptors for platelet activating factor in rat Kupffer cells. J Biol Chem 264: 3591–3598, 1989. CHAO W, LIU H, HANAHAN DJ, AND OLSON MS. Regulation of platelet activating factor receptors in rat Kupffer cells. J Biol Chem 264: 448 – 457, 1989. CHAO W, LIU H, HANAHAN DJ, AND OLSON MS. Regulation of platelet activating factor receptor and PAF receptor-mediated arachidonic acid release by protein kinase C activation in rat Kupffer cells. Arch Biochem Biophys 282: 188 –197, 1990. CHAO W, LIU H, ZHOU W, HANAHAN DJ, AND OLSON MS. Regulation of platelet activating factor receptor and platelet activating factor receptor-mediated biological responses by cAMP in rat Kupffer cells. J Biol Chem 265: 17576 –17583, 1990. CHAO J, LIU H, HANAHAN DJ, AND OLSON MS. Platelet activating factor-stimulated protein tyrosine phosphorylation and eicosanoid synthesis in rat Kupffer cells. Evidence for calcium-dependent and protein-kinase C-dependent and C-independent pathways. J Biol Chem 267: 6725– 6735, 1992. CHAO W, LIU H, HANAHAN DJ, AND OLSON MS. Protein tyrosine phosphorylation and regulation of the receptor for platelet activating factor in rat Kupffer cells. Effect of sodium vanadate. Biochem J 288: 777–784, 1992. CHAO W AND OLSON MS. Platelet-activating factor: receptor and signal transduction. Biochem J 292: 617– 629, 1993. CHU KM, GERBER JG, AND NIES AS. Local vasodilator effect of platelet activating factor in the gastric, mesenteric and femoral arteries of the dog. J Pharmacol Exp Ther 246: 996 –1000, 1988. CHURCH DJ, BRACONI S, VALLOTTON MB, AND LANG U. Protein kinase C-mediated phospholipase A2 activation, platelet activating factor generation and prostacyclin release in spontaneously beating rat cardiomyocytes. Biochem J 290: 477– 482, 1993. CHURCH DJ, VAN DER BENT V, VALLOTTON MB, CAPPONI AM, AND LANG U. Calcium influx in platelet activating factor-induced atrial natriuretic peptide release in rat cardiomyocytes. Am J Physiol Endocrinol Metab 266: E403–E409, 1994. CLAY KL, JOHNSON C, AND WORTHEN GS. Biosynthesis of platelet activating factor and 1-O-acyl analogues by endothelial cells. Biochim Biophys Acta 1094: 43–50, 1991. COEFIER E, CERRINA J, JOUVIN-MARCHE E, AND BENVENISTE J. Inhibition of rabbit platelet aggregation by the Ca-antagonists verapamil and diltiazem and by trifluoperazine. Thromb Res 31: 565–576, 1983. COLARD O, BIDAULT J, BRETON M, AND NINIO E. Biosynthesis of platelet-activating factor in cultured mast cells: involvement of the CoA-independent transacylase demonstrated by analysis of the Volume 80 molecular species of platelet-activating factor. Eur J Biochem 216: 835– 840, 1993. 107. COUGHLAN AF, HAU H, DUNLOP LC, BERNDT MC, AND HANCOCK WW. P-selectin and platelet activating factor mediate initial endotoxininduced neutropenia. J Exp Med 179: 329 –334, 1994. 108. CRUSSI-GONZALES F AND HSUESH W. Experimental model of ischemic bowel necrosis: the role of platelet activating factor and endotoxin. Am J Pathol 112: 127–135, 1983. 109. CUEVA JP AND HSUEH W. Role of oxygen derived free radicals in platelet activating factor induced bowel necrosis. Gut 29: 1207– 1212, 1988. 110. DARIUS H, LEFER DJ, SMITH JB, AND LEFER AM. Role of plateletactivating factor-acether in mediating guinea pig anaphylaxis. Science 232: 58 – 60, 1986. 111. DEFILY DV, KUO L, AND CHILIAN WM. PAF attenuates endotheliumdependent coronary arteriolar vasodilation. Am J Physiol Heart Circ Physiol 270: H2094 –H2099, 1996. 112. DELBRIDGE LM, STEWART AG, GOULTER CM, MORGAN TO, AND HARRIS PJ. Platelet activating factor and WEB-2086 directly modulate rat cardiomyocyte contractility. J Mol Cell Cardiol 26: 185–193, 1994. 113. DEMOPOULOS CA, PINCKARD RN, AND HANAHAN DJ. Platelet activating factor. Evidence for 1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphorylcholine as the active component (a new class of lipid chemical mediators). J Biol Chem 254: 9355–9358, 1979. 114. DENTAN C, LESNIK P, CHAPMAN J, AND NINIO E. Phagocytic activation induces formation of platelet-activating factor in human monocytederived macrophages and in macrophage-derived foam cells. Relevance to the inflammatory reaction in atherogenesis. Eur J Biochem 236: 48 –55, 1996. 115. DENTELLI P, DEL SORBO L, ROSSO A, MOLINAR A, GARBARINO G, CAMUSSI G, PEGORARO L, AND BRIZZI MF. Human IL-3 stimulates endothelial cell motility and promotes in vivo new vessel formation. J Immunol 163: 2151–2159, 1999. 116. DHAINAUT JF, TENAILLON A, LE TULZO Y, SCHLEMMER B, SOLET JP, WOLFF M, HOLEPFER L, ZENI F, DREYFUSS D, AND MIRA JP. Plateletactivating factor receptor antagonist BN 52021 in the treatment of severe sepsis: a randomized, double-blind, placebo-controlled, multicenter clinical trial. Crit Care Med 22: 1720 –1728, 1994. 117. DHAR A, PAUL AK, AND SHUKLA SD. Platelet-activating factor stimulation of tyrosine kinase and its relationship to phospholipase C in rabbit platelets: studies with genistein and monoclonal antibody phosphotyrosine. Mol Pharmacol 37: 519 –525, 1990. 117a.D’HUMIERS S, RUSSO-MARIE F, AND VARGAFTIG BB. PAF-achetherinduced synthesis of prostacyclin by human endothelial cells. Eur J Pharmacol 13: 13–19, 1986. 118. DIEZ J, DELPON E, AND TAMARGO J. Effects of platelet activating factor on contractile force and Ca fluxes in guinea-pig isolated atria. Br J Pharmacol 100: 305–311, 1990. 119. DILLON PK, RITTER AB, AND DURAN WN. Vasoconstriction effects of platelet-activating factor in the hamster cheek pouch microcirculation: dose-related relations and pathways of action. Circ Res 62: 722–731, 1988. 120. DOEBBER T, WU M, ROBBINS J, CHOY B, CHANG M, AND SHEN T. Platelet activating factor (PAF) involvement in endotoxin-induced hypotension in rats. Studies with PAF-receptor antagonist Kadsurenone. Biochem Biophys Res Commun 127: 799 – 808, 1985. 121. DOMINGO MT, SPINNEWYN B, CHABRIER PE, AND BRAQUET P. Presence of specific binding sites for platelet activating factor (PAF) in brain. Biochem Biophys Res Commun 151: 730 –736, 1988. 123. ELDAR M, LYSKO PG, SCHULHOFF N, GAGNON RC, FEUERSTEIN G, AND SHANI J. Effects of coronary angioplasty on plasma platelet activating factor in man. J Lipid Mediat 5: 313–319, 1992. 124. EMEIS JJ AND KLUFT C. PAF-acether-induced release of tissue-type plasminogen activator from vessel walls. Blood 66: 86 –91, 1985. 125. EPHGRAVE K, KREMER T, BROADHURST K, AND CULLEN J. The role of platelet-activating factor in conscious, normotensive endotoxemia. J Surg Res 68: 170 –174, 1997. 126. EZRA D, FEUERSTEIN G, RAMWELL PW, HAYES E, AND GOLDSTEIN RE. Effects of platelet-activating factor on coronary hemodinamics and coronary venous plasma levels of TXB2, 6-keto-PGF1 alpha, and leukotriene C4 immunoreactivity in the intact domestic pig heart. Adv Prostaglandin Thromboxane Leukotrienes Res 13: 19 –21, 1985. October 2000 PAF AND THE CARDIOVASCULAR SYSTEM 127. EZRA D, GOLDSTEIN RE, CZAJA JF, AND FEUERSTEIN G. Inhibitor of platelet-activating factor diminishes dysfunction during reperfusion. Circulation 80: 402, 1989. 128. EZRA D, LAURINDO FR, CZAJA JF, SNYDER F, GOLDSTEIN RE, AND FEUERSTEIN G. Cardiac and coronary consequences of intracoronary platelet activating factor infusion in the domestic pig. Prostaglandins 34: 41–57, 1987. 129. FABER JE, BARRON KW, BONHAM AC, LAPPE R, MUIRHEAD EE, AND BRODY MJ. Regional hemodynamic effects of antihypertensive renomedullary lipids in conscious rats. Hypertension 6: 494 –502, 1984. 130. FARR RS, COX CP, WARDLOW ML, AND JORGENSEN R. Preliminary studies of an acid-labile factor (ALF) in human sera that inactivates platelet-activating factor (PAF). Clin Immunol Immunopathol 15: 318 –330, 1980. 131. FELISTE R, PERRET B, BRAQUET P, AND CHAP H. Protective effect of BN 52021, a specific antagonist of platelet activating factor (PAFacether) against diet-induced cholesteryl ester deposition in rabbit aorta. Atherosclerosis 78: 151–158, 1989. 132. FELIX SB, BAUMANN G, HAIEMCZYK T, AHMAD Z, AND BERDEL WE. Characterization of cardiovascular events mediated by platelet activating factor during systemic anaphylaxis. J Cardiovasc Pharmacol 15: 989 –997, 1990. 133. FEUERSTEIN G, BOYD LM, EZRA D, AND GOLDSTEIN RE. Effect of platelet-activating factor on coronary circulation of the domestic pig. Am J Physiol Heart Circ Physiol 246: H466 –H471, 1984. 134. FEUERSTEIN GZ AND GOLDSTEIN RE. Effect of PAF on the cardiovascular system. In: Platelet Activating Factor and Related Lipid Mediators, edited by Snyder F. New York: Plenum, 1987, p. 403. 135. FEUERSTEIN GZ, LUZ W, EZRA D, HAYES E, SNYDER F, AND FADEN A. Thyrotropin releasing hormone blocks the hypotensive effect of platelet activating factor in the unanesthetized guinea pig. J Cardiovasc Pharmacol 7: 335–340, 1985. 136. FEUERSTEIN GZ, RABINONOVICI R, LEOR J, WINKLER JD, AND VONHOF S. Platelet-activating factor and cardiac diseases: therapeutic potential for PAF inhibitors. J Lipid Mediat Cell Signal 15: 255–284, 1997. 137. FEUERSTEIN GZ AND SIREN AL. Platelet activating factor and shock. Prog Biochem Pharmacol 22: 181–190, 1988. 138. FIEDLER VB, MARDIN M, AND ABRAM TS. Comparison of cardiac and hemodynamic effects of platelet-activating factor-acether and leukotriene D4 in anesthetized dogs. Basic Res Cardiol 82: 197–208, 1987. 139. FINDLAY SR, LICHTENSTEIN LM, HANAHAN DJ, AND PINCKARD RN. The contraction of guinea pig ileal smooth muscle by acetyl glyceryl ether phosphorylcholine. Am J Physiol Cell Physiol 241: C130 – C133, 1981. 140. FLETCHER J, DISIMONE AG, AND EARNEST MA. Platelet activating factor improves survival and attenuates eicosanoid release in severe endotoxiemia. Ann Surg 211: 312–316, 1990. 141. FLORES NA AND SHERIDAN DJ. Electrophysiological and arrhythmogenic effects of platelet activating factor during normal perfusion, myocardial ischemia and reperfusion in the guinea-pig. Br J Pharmacol 101: 734 –738, 1990. 142. FOLKMAN J AND KLAGSBRUN M. Angiogenic factors. Science 235: 442– 447, 1987. 143. FOLKMAN J. Clinical application of research on angiogenesis. N Engl J Med 333: 1757–1763, 1995. 144. FONTALIRAN F, GUILLON JM, KOLTAI M, AND BRAQUET P. Reduction of infarct size by ginkgolide B (BN 52021) in coronary artery ligated rats. In: Ginkgolides—Chemistry, Biology, Pharmacology and Clinical Perspectives, edited by Braquet P. Barcelona, Spain: Prous Science, 1989, vol. 2, p. 405– 411. 145. GASIC AC, MCGUIRE G, RATER SK, FARHOOD AI, GOLDSTEIN MA, SMITH CW, LENTMAN M, AND TAYLOR AA. Hydrogen peroxide pretreatment of perfused canine vessels induced ICAM-1 and CD18-dependent neutrophil adherence. Circulation 84: 2154 –2166, 1991. 146. GAUMOND F, FORTIN D, STANKOVA J, AND ROLA-PLESZCZYNSKI M. Differential signaling pathways in platelet-activating factor-induced proliferation and interleukin-6 production by rat vascular smooth muscle cells. J Cardiovasc Pharmacol 30: 169 –175, 1997. 147. GIESSLER C, PONICKE K, STEINBORN C, AND BRODDE OE. Effects of PAF on cardiac function and eicosanoid release in the isolated perfused 148. 149. 150. 151. 152. 153. 154. 155. 156. 157. 158. 159. 160. 161. 162. 163. 164. 165. 166. 167. 1691 rat heart: comparison between normotensive and spontaneously hypertensive rats. Basic Res Cardiol 90: 337–347, 1995. GOLDSTEIN BM, GABEL FJ, CERVONI P, AND GRANDALL DI. Effects of platelet activating factor (PAF) on blood flow distribution in the spontaneously hypertensive rat. Life Sci 35: 1373–1378, 1984. GOLDSTEIN RE, EZRA D, LAURINDO FRM, AND FEUERSTEIN GZ. Coronary and pulmonary vascular effects of leukotrienes and PAFacether. Pharmacol Res Commun 18: 151–162, 1986. GOLDSTEIN RE, FEUERSTEIN GZ, BRADLEY LM, STAMBOULY JJ, LAURINDO FRM, AND DAVENPORT NJ. Cardiovascular effects of platelet activating factor. Lipids 26: 1250 –1256, 1991. GOLINO P, AMBROSIO G, RAGNI M, PASCUCCI I, TRIGGIANI M, ORIENTE A, MCNATT J, BUJIA LM, CONDORELLI M, CHIARIELLO M, AND WILLERSON JT. Short-term and long-term role of platelet activating factor as a mediator of in vivo platelet aggregation. Circulation 88: 1205–1214, 1993. GOLLASCH M, IGNATIEVA V, KOBRINSKY E, VORNOVITSKY E, AND ZABOROVSKAYA L. Electrophysiological mechanisms responsible for the action of PAF in guinea-pig myocardium. Relation to the putative membrane signalling processes of PAF. J Lipid Mediat 3: 139 –159, 1991. GOMEZ-CAMBRONERO J, WANG J, JOHNSON G, HUANG CK, AND SHA’AFI RI. Platelet activating factor induces tyrosine phosphorylation in human neutrophils. J Biol Chem 266: 6240 – 6245, 1991. GRAHAM RM, STRAHAN ME, NORMAN KW, WATKINS DN, STURM MJ, AND TAYLOR RR. Platelet and platelet activating factor in sepsis and myocardial infarction. J Lipid Mediat Cell Signal 9: 167–182, 1994. GRIGORIAN GY AND RYAN US. Platelet activating factor effects on bovine pulmonary artery endothelial cells. Circ Res 61: 389 –395, 1987. GROSS GJ, MARUYAMA M, VERCELLOTTI GM, JACOB HS, AND CHRISTENSEN CW. Effect of the PAF antagonist, BN 52021, on myocardial infarct size in dogs. In: Ginkgolides—Chemistry, Biology, Pharmacology and Clinical Perspectives, edited by Braquet P. Barcelona, Spain: Prous Science, 1989, vol. 2, p. 421– 425. GRUSKA S, IHRKE R, STOLPER S, KRAATZ G, AND SIFFERT W. Prevalence of increased intracellular signal transduction in immortalized lymphoblasts from patients with essential hypertension and normotensive subjects. J Hypertens 15: 29 –33, 1997. GUILLON JM, ROCHETTE L, AND BARANES J. Effects of ginkgo extract on two models of experimental myocardial ischemia. In: Rokan (Ginkgo Biloba). Recent Results in Pharmacology and Clinic, edited by Funfgeld EW. Berlin: Springer-Verlag, 1988, p. 153–161. HAGAR AF, DUSAPIN K, AND SPITZER JA. Endotoxin infusion primes elicited neutrophils and Kupffer cells for platelet-activating factorinduced and tripeptide formyl-methionine-leucine-phenylalanineinduced basal free intracellular calcium concentration responses. Crit Care Med 21: 1750 –1757, 1993. HALONEN M, PALMER JD, LOHMAN IC, MCMANUS LM, AND PINKARD RN. Respiratory and circulatory alterations induced by acetyl glyceryl ether phosphorylcholine, a mediator of IgE anaphylaxis in the rabbit. Am Rev Respir Dis 122: 915–924, 1980. HALONEN M, PALMER JD, LOHMAN IC, MCMANUS LM, AND PINCKARD RN. Differential effects of platelet depletion on the physiologic alteration of IgE anaphylaxis and acetyl glyceryl ether phosphorylcholine infusion in the rabbit. Am Rev Respir Dis 124: 416 – 421, 1981. HAMASAKI YM, MOJARAD M, SAGA T, TAI H, AND SAID SI. Platelet activating factor raises airway and vascular pressures and induces edema in lungs perfused with platelet-free solution. Am Rev Respir Dis 129: 742–746, 1984. HANAHAN D. Platelet-activating factor: a biologically phosphoglyceride. Annu Rev Biochem 55: 483–509, 1986. HANAHAN DJ, DEMOPOULOS CA, LIHER J, AND PINCKARD RN. Identification of platelet activating factor isolated from rabbit basophils as acetyl glyceryl ether phosphorylcholine. J Biol Chem 255: 5514 – 5516, 1980. HANDA RK, STRANDHOY JW, AND BUCKALEW VM. Platelet activating factor is a renal vasodilator in the anesthetized rat. Am J Physiol Renal Fluid Electrolyte Physiol 258: F1504- F1509, 1990. HANDLEY DA. Preclinical and clinical pharmacology of platelet activating factor receptor antagonist. Med Res Rev 10: 351–370, 1990. HANDLEY DA, ANDERSON RC, AND SAUNDERS RN. Inhibition by SRI 63 1692 168. 169. 170. 171. 172. 173. 174. 175. 176. 177. 178. 179. 180. 181. 182. 183. 184. 185. 186. 187. MONTRUCCHIO, ALLOATTI, AND CAMUSSI 072 and SRI 63 119 of PAF-acether and immune complex effects in the guinea pig. Eur J Pharmacol 141: 409 – 416, 1987. HANDLEY DA, ARBEENY CM, LEE ML, VAN VALEN RG, AND SAUNDERS RN. Effect of platelet activating factor on endothelial permeability to macromolecules. Immunopharmacology 8: 137–144, 1984. HARADA K, GROSSMAN W, FRIEDMAN M, EDELMAN ER, PRASAD PV, KEIGHLEY CS, MANNING WJ, SELLKE FW, AND SIMONS M. Basic fibroblast growth factor improves myocardial function in chronically ischemic porcine hearts. J Clin Invest 94: 623– 630, 1994. HARRIS NR, FIRST GA, AND SPECIAN RD. Influence of arteriovenular pairing on PAF-induced capillary filtration. Am J Physiol Heart Circ Physiol 276: H107–H114, 1999. HATTORI M, ADACHI H, AOKI J, TSUJIMOTO M, ARAI H, AND INOUE K. Cloning and expression of cDNA encoding the beta-subunit (30kDa subunit) of bovine brain platelet-activating factor acetylhydrolase. J Biol Chem 270: 31345–31352, 1995. HATTORI M, ADACHI H, MATSUZAWA A, YAMAMOTO K, TSUJIMOTO M, AOKI J, HATTORI M, ARAI H, AND INOUE K. cDNA cloning and expression of intracellular platelet-activating factor (PAF) acetylhydrolase II. Its omology with plasma PAF acetylhydrolase. J Biol Chem 271: 33032–33038, 1996. HATTORI M, ARAI H, AND INOUE K. Purification and characterization of bovine brain platelet-activating factor acetylhydrolase. J Biol Chem 268: 18748 –18753, 1993. HEERY JM, KOZAK M, STAFFORINI DM, JONES DA, ZIMMERMAN GA, MCINTYRE TM, AND PRESCOTT SM. Oxidatively modified LDL contains phospholipids with platelet activating factor-like activity and stimulates the growth of smooth muscle cells. J Clin Invest 96: 2322– 2330, 1995. HEFFNER JE, SHOEMAKER SA, CANHAM EM, PATEL M, MCMURTRY IF, AND MORRIS HG. Acetyl glyceryl ether phosphoryl-choline-stimulated human platelets cause pulmonary hypertension and edema in isolated rabbit lungs: role of thromboxane A2. J Clin Invest 71: 351–357, 1983. HELLER R, BUSSOLINO F, GHIGO D, GARBARINO G, PESCARMONA G, TILL U, AND BOSIA A. Human endothelial cells are target for platelet activating factor. Platelet activating factor induces platelet activating factor synthesis in human umbilical vein endothelial cells. J Immunol 149: 3682–3688, 1992. HENSON PM. Release of vasoactive amines from rabbit platelets induced by sensitized mononuclear leukocytes and antigen. J Exp Med 131: 287–306, 1970. HERBERTSON MJ, WERNER HA, AND WALLEY KR. Platelet-activating factor antagonism improves ventricular contractility in endotoxemia. Crit Care Med 25: 221–226, 1997. HIRAFUJI M, MENCIA-HUERTA JM, AND BENVENISTE J. Regulation of PAF-acether (platelet activating factor) biosynthesis in cultured human vascular endothelial cells stimulated with thrombin. Biochim Biophys Acta 930: 359 –369, 1987. HIRAFUJI M AND SHINODA H. Platelet-leukocyte interaction in adhesion to endothelial cells induced by platelet activating factor in vitro. Br J Pharmacol 136: 1356 –1377, 1991. HIRAFUJI M AND SHINODA H. PAF-mediated platelet adhesion to endothelial cells induced by FMLP-stimulated leukocytes. J Lipid Mediat 4: 347–352, 1991. HIRAFUJI M AND SHINODA H. Roles of prostacyclin, EDRF and active oxygens in leukocyte-dependent platelet adhesion to endothelial cells induced by platelet activating factor in vitro. Br J Pharmacol 109: 524 –529, 1993. HOLVOET P AND COLLEN D. Thrombosis and atherosclerosis. Curr Opin Lipidol 8: 320 –328, 1997. HOMMA H, TOKUMURA A, AND HANAHAN DJ. Binding and internalization of platelet activating factor 1-O-alkyl-2-acetyl-glycero-3-phosphocholine in washed rabbit platelets. J Biol Chem 262: 10582– 10587, 1987. HONDA Z, NAKAMURA M, MIKI I, MINAMI M, WATANABE T, SEYAMA Y, HOKADO H, TOH H, ITO K, MIYAMOTO T, AND SHIMIZU T. Cloning by functional expression of platelet-activating factor receptor from guinea-pig lung. Nature 349: 342–346, 1991. HOWARD KM, MILLER JE, MIRWA M, AND OLSON MS. Cell-specific regulation of expression of plasma-type platelet-activating factor acetylhydrolase in the liver. J Biol Chem 272: 27543–27548, 1997. HU W, CHOY PC, AND MAN RY. Characterization of the coronary 188. 189. 190. 191. 192. 193. 194. 195. 196. 197. 198. 199. 200. 201. 202. 203. 204. 205. 206. Volume 80 vascular responses to platelet-activating factor in the isolated perfused heart. Lipids 26: 700 –704, 1991. HU W, KINNAIRD AAA, AND MAN RYK. Mechanisms of the coronary vascular effects of platelet-activating factor in the rat perfused heart. Br J Pharmacol 103: 1097–1102, 1991. HU W AND MAN YK. Interaction of vasoactive substances released by platelet-activating factor in the rat perfused heart. Br J Pharmacol 104: 933–937, 1991. HUANG Q, WU M, MEININGER C, KELLY K, AND YUAN Y. Neutrophildependent augmentation of PAF-induced vasoconstriction and albumin flux in coronary arterioles. Am J Physiol Heart Circ Physiol 275: H1138 –H1147, 1998. HUMPHREY DM, MCMANUS LM, HANAHAN DJ, AND PINCKARD RN. Morphologic basis of increased vascular permeability induced by acetyl glyceryl ether phosphorylcholine. Lab Invest 50: 16 –25, 1984. HUMPHREY DM, MCMANUS LM, ATOUCHI KS, HANAHAN DJ, AND PINCKARD RN. Vasoactive properties of acetyl glyceryl ether phosphorylcholine and analogues. Lab Invest 46: 422– 427, 1982. HWANG SB, CHING-SHIN CL, CHEAH MJ, AND SHEN TY. Specific receptor sites for 1-O-alkyl-2-O-acetyl-sn-glycero-3-phosphocholine (platelet activating factor) on rabbit platelet and guinea pig smooth muscle membranes. Biochemistry 22: 4756 – 4763, 1983. ICHIKAWA H, FLORES S, KVIETYS PR, WOLF RE, YOSHIKAWA T, GRANGER DN, AND AW TY. Molecular mechanisms of anoxia/reoxygenationinduced neutrophil adherence to cultured endothelial cells. Circ Res 81: 922–931, 1997. IHIDA K, PREDESCU D, CZEKAY RP, AND PALADE GE. Platelet activating factor receptor is found in a large endosomal compartment in human umbilical vein endothelial cells. J Cell Sci 112: 285–295, 1999. INARREA P, GOMEZ-CAMBRONERO J, NIETO M, AND SANCHEZ-CRESPO M. Characteristic of the binding of platelet activating factor to platelets of different animal species. Eur J Pharmacol 105: 309 –315, 1984. ISHII S, NAGASE T, TASHIRO F, IKUTA K, SATO S, WAGA I, KUME K, MIYAZAKI J, AND SHIMIZU T. Bronchial hyperreactivity, increased endotoxin lethality and melanocytic tumorigenesis in transgenic mice overexpressing platelet-activating factor receptor. EMBO J 16: 133–142, 1997. ISOGAI E, HIROSE K, KIMURA K, HAYASHI S, KUBOTA T, FUGJII N, AND ISOGAI H. Role of platelet activating-factor (PAF) on cellular responses after stimulation with leptospire lipopolysaccharide. Microbiol Immunol 41: 271–275, 1997. JACKSON CV, SCHUMACHER WA, KUNKEL SL, DRISCOLL EM, AND LUCCHESI BR. Platelet activating factor and the release of plateletderived coronary artery vasodilator substance in the canine. Circ Res 58: 218 –229, 1986. JANERO DR AND BURGHARDT C. Production and release of plateletactivating factor by the injured heart-muscle cell (cardiomyocyte). Res Commun Chem Pathol Pharmacol 67: 201–218, 1990. KALRA VK, SHEN Y, SULTANA C, AND RATTAN V. Hypoxia induces PECAM-1 phosphorylation and transendothelial migration of monocytes. Am J Physiol Heart Circ Physiol 271: H2025–H2034, 1996. KAMITANI T, KATAMOTO M, TASUMI M, KATSUTA K, ONO T, KIKUCHI H, AND KUMADA S. Mechanisms of the hypotensive effect of 1-O-octadecyl-2-O-acetyl-glyceryl-3-phosphorylcholine. Eur J Pharmacol 98: 357–366, 1984. KARABINA SAP, LIAPIKOS TA, GREKAS G, GOUDEVENOS J, AND TSELEPIS AD. Distribution of PAF acetylhydrolase activity in human plasma low-density lipoprotein subfractions. Biochim Biophys Acta 1213: 34 –38, 1994. KATOH S, TOYAMA J, KODAMA I, KOIKE A, AND ABE T. Role of platelet activating factor in ischemia-reperfusion injury of isolated rabbit hearts: protective effect of a specific platelet activating factor antagonist, TCV-309. Cardiovasc Res 27: 1430 –1434, 1993. KAWAMURA M, KITAYOSHI T, TERASHITA Z, FUJIWARA S, TAKATANI M, AND NISHIKAWA K. Effects of TCV-309, a novel PAF antagonist, on circulatory shock and hematological abnormality induced by endotoxin in dogs. J Lipid Mediat Cell Signal 9: 255–265, 1994. KECSKEMETI V. Electrophysiological effects of platelet-activating factor (PAF) in guinea-pig cardiac preparations. Acta Physiol Hung 75 Suppl: 165–166, 1990. October 2000 PAF AND THE CARDIOVASCULAR SYSTEM 207. KECSKEMETI V AND BRAQUET P. Cellular electrophysiological effects of platelet-activating factor (PAF) and its antagonist BN 52021 in cardiac preparations. Drugs Exp Clin Res 18: 9 –16, 1992. 208. KENZORA JL, PEREZ JE, BERGMANN SR, AND LANGE LG. Effects of acetyl glyceryl ether phosphorylcholine (platelet activating factor) on ventricular preload, afterload and contractility in dogs. J Clin Invest 74: 1193–1203, 1984. 209. KESTER M, LEDVORA RF, AND BARANY M. The potentiation of arterial contraction with platelet activating factor. Pflügers Arch 400: 200 – 202, 1984. 210. KIM YD, DANCHAK RM, HEIM KF, LEES DE, AND MYERS AK. Constriction of canine coronary arteries by platelet activating factor after brief ischemia. Prostaglandins 46: 269 –276, 1993. 211. KING KA, LIM SL, AND PANG CC. Regional haemodynamic effects of platelet activating factor in the rat. Eur J Pharmacol 281: 187–193, 1995. 212. KO W, HAWES AS, DOUGLAS LAZENBY W, CALVANO SE, SHIN YT, ZELANO JA, ANTONACCI AC, WAYNE ISOM O, AND KRIEGER KH. Myocardial reperfusion injury: platelet activating factor stimulates polymorphonuclear leukocyte hydrogen peroxide production during myocardial reperfusion. J Thorac Cardiovasc Surg 102: 297–308, 1991. 213. KO W, LANG D, HAWES AS, ZELANO JA, ISOM OW, AND KRIEGER KH. Platelet activating factor antagonism attenuates platelet and neutrophil activation and reduces myocardial injury during coronary reperfusion. J Surg Res 55: 504 –515, 1993. 214. KOBAYASHI I, KIM D, HOBSON RW II, AND DURAN WN. Platelet-activating factor modulates microvascular transport by stimulation of protein kinase C. Am J Physiol Heart Circ Physiol 266: H1214 – H1220, 1994. 215. KOBAYASHI F, SAGAWA N, IHARA Y, KITAGAWA K, YANO J, AND MORI T. Platelet activating factor-acetylhydrolase activity in maternal and umbilical venous plasma obtained from normotensive and hypertensive pregnancies. Obstet Gynecol 84: 360 –364, 1994. 216. KOCH AE, POLVERINI PJ, KUNKEL SL, HARLOW LA, DI PIETRO LA, ELNER VM, ELNER SG, AND STRIETER RM. Interleukin-8 as a macrophagederived mediator of angiogenesis. Science 258: 1798 –1801, 1992. 217. KOCHANEK PM, NEMOTO EM, MELICK JA, EVANS RW, AND BURKE DF. Cerebrovascular and cerebrometabolic effects of intracarotid infused platelet activating factor in rats. J Cereb Blood Flow Metab 8: 546 –551, 1988. 218. KOLTAY M, HOSFORD D, GUINOT P, ESANU A, AND BRAQUET P. Platelet activating factor (PAF). A review of its effects, antagonist and possible future clinical implication (part 1). Drugs 42: 9 –29, 1991. 219. KOLTAY M, LEPRAN I, SZEKERES L, VIOSSAT I, CHABRIER E, AND BRAQUET P. Effect of BN 52021, a specific PAF-acether antagonist, on cardiac anaphylaxis in Langendorff hearts isolated from passively sensitized guinea-pigs. Eur J Pharmacol 130: 133–136, 1986. 220. KOLTAY M, TOSAKI A, GUILLON JM, HOSFORD D, AND BRAQUET P. PAF antagonists as potential therapeutic agents in cardiac anaphylaxis and myocardial ischemia. In: Cardiovascular Drug Review 7. New York: Raven, 1989, p. 177–189. 221. KOLTAY M, TOSAKI A, HOSFORD D, AND BRAQUET P. BN 52021, a PAF receptor antagonist, protects isolated working rat heart against arrhytmias induced by ischemia but not reperfusion. Eur J Pharmacol 164: 293–302, 1989. 222. KOLTAY M, TOSAKI A, HOSFORD D, ESANU A, AND BRAQUET P. Effect of BN 50739, a new platelet activating factor antagonist, on ischemia induced ventricular arrhythmias in isolated working rat hearts. Cardiol Res 25: 391–397, 1991. 223. KORTH R, HIRAFUJI M, LALAU-KERALY C, DELAUTIER I, BIDAULT J, AND BENVENISTE J. Interaction of antagonist WEB 2086 and its hetrazepine analogue with human platelets and endothelial cells. Biochem Pharmacol 44: 223–229, 1989. 224. KRAVCHENKO VV, ZHIXING P, HAN J, HERBERT JM, ULEVITCH RJ, AND YE RD. Platelet activating factor induces NF-kB activation through a G protein-coupled pathway. J Biol Chem 270: 14928 –14934, 1995. 225. KRULL M, DOLD C, HIPPENSTIEL S, ROSSEAU S, LOHMEYER J, AND SUTTORP N. Escherichia coli hemolysin and Staphylococcus aureus alpha-toxin potently induce neutrophil adhesion to cultured human endothelial cells. J Immunol 157: 4133– 4140, 1996. 226. KUBES P, IBBOTSON G, RUSSEL J, WALLACE JL, AND GRANGER DN. Role of platelet-activating factor in ischemia/reperfusion-induced leuko- 227. 228. 229. 230. 231. 232. 233. 234. 235. 236. 237. 238. 239. 240. 241. 242. 243. 244. 245. 1693 cyte adherence. Am J Physiol Gastrointest Liver Physiol 259: G300 –G305, 1990. KUBES P, SUZUKI M, AND GRANGER DN. Platelet-activating factorinduced microvascular dysfunction: role of adherent leukocytes. Am J Physiol Gastrointest Liver Physiol 258: G158 –G163, 1990. KUIJPERS TW, HAKKERT BC, HART MHL, AND ROOS D. Neutrophil migration across monolayers of cytokine-prestimulated endothelial cells: a role for platelet activating factor and IL-8. J Cell Biol 117: 565–572, 1992. KUIJPERS TW, HAKKERT BC, HOOGERWERF M, LEEWENBERG JFM, AND ROOS D. Role of endothelial leukocyte adhesion molecule-1 and platelet activating factor in neutrophil adherence to IL-1 prestimulated endothelial cells. Endothelial leukocyte adhesion molecule1-mediated CD18 activation. J Immunol 147: 1369 –1376, 1991. KUIPERS B, VAN DER POLL T, AND LEVI M. Platelet-activating factor antagonist TCV-309 attenuates the induction of the cytokine network in experimental endotoxemia in chimpanzee. J Immunol 152: 2438 –2446, 1994. KUMAR A, THOTA V, DEE L, OLSON J, URETZ E, AND PARRILLO JE. Tumor necrosis factor alpha and interleukin 1 beta are responsive for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med 183: 949 –958, 1996. KUNZ D, GERARD NP, AND GERARD C. The human leukocyte plateletactivating factor receptor. J Biol Chem 267: 9101–9106, 1992. LAI FM, SHEPHERD CA, CERVONI P, AND WISSNER A. Hypotensive and vasodilatatory activity of (⫹)-1-O-octadecyl-2-acetyl-glyceryl-3phosphorylcholine in the normotensive rat. Life Sci 32: 1159 –1166, 1983. LANIYONU AA, COSTON AF, AND KLABUNDE RE. Endotoxin-induced microvascular leakage is prevented by a PAF antagonist and NO synthase inhibitor. Shock 7: 49 –54, 1997. LAURINDO FRM, EZRA D, CZAJA JF, FEUERSTEIN G, AND GOLDSTEIN RE. Acute right ventricular failure due to platelet-activating factor on thromboxane A2 analog. Clin Res 33: 203, 1985. LAURINDO FRM, GOLDSTEIN RE, DAVENPORT NJ, EZRA D, AND FEUERSTEIN GZ. Mechanisms of hypotension produced by platelet-activating factor. J Appl Physiol 66: 2681–2690, 1989. LAZAROUS DF, SCHEINOWITZ M, SHOU M, HODGE E, RAJANAYAGAM MAS, HUNSBERGER S, ROBISON G, STIBER JA, CORREA R, EPSTEIN SE, AND UNGER EF. Effects of chronic systemic administration of basic fibroblast growth factor on collateral development in canine heart. Circulation 91: 145–153, 1995. LEAVER HA, SMITH S, HOWEI A, ROSS WB, AND YAP PL. Endotoxin releases platelet activating factor from human monocytes in vitro. Immunopharmacology 20: 105–113, 1990. LEE TC, BLANK ML, FITZGERALD V, AND SNYDER F. Substrate specificity in the biocleavage of the O-alkyl-bond: 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine (a hypotensive and platelet activating lipid) and its metabolites. Arch Biochem Biophys 280: 353–357, 1981. LEE TC, LENIHAN DJ, MALONE B, RODDY LL, AND WASSERMAN SI. Increased biosynthesis of platelet activating factor in activated human eosinophils. J Biol Chem 259: 5526 –5530, 1984. LEE TC, MALONE B, BLANK M, AND SNYDER F. 1-Alkyl-2-acetyl-snglyceryl-3-phosphocholine (platelet activating factor) stimulates calcium influx in rabbit platelets. Biochem Biophys Res Commun 102: 1262–1268, 1981. LEE TC, MALONE B, WASSERMAN SI, FITZGERALD V, AND SNYDER F. Activities of enzyme that metabolize platelet activating factor (1alkyl-2-acetyl-sn-glycero-3-phosphocholine) in neutrophils and eosinophils from humans and the effect of a calcium ionophore. Biochem Biophys Res Commun 105: 1303–1308, 1982. LEE TC, MALONE B, AND SNYDER A. Formation of 1-alkyl-2-acetyl-snglycerols via de novo biosynthetic pathway for platelet activating factor. Characterization of 1-alkyl-2-acetyl-sn-glycero-3-phosphohydrolase in rat spleen. J Biol Chem 263: 1755–1760, 1988. LEE TC, MALONE B, AND SNYDER A. A new de novo pathway for the formation of 1-alkyl-2-acetyl-sn-glycerols, precursors of platelet activating factor. Biochemical characterization of 1-alkyl-2-lyso-snglycero-3-acetyl-CoA acetyltransferase in rat spleen. J Biol Chem 261: 5373–5377, 1986. LEFER AM, MULLER HF, AND SMITH BH. Pathophysiological mechanism of sudden death induced by platelet activating factor. Br J Pharmacol 83: 125–130, 1984. 1694 MONTRUCCHIO, ALLOATTI, AND CAMUSSI 246. LEHR HA, SEEMULLER J, HUBNER C, MENGER MD, AND MESSMER K. Oxidized LDL-induced leukocyte/endothelium interaction in vivo involves the receptor for platelet activating factor. Arteriosclerosis Thrombosis 13: 1013–1018, 1993. 247. LEONG LLL AND TAYLOR R. The lyso-precursor of platelet-activating factor (lyso-PAF) in ischaemic myocardium. J Lipid Mediat 4: 277–287, 1991. 248. LEONG LLL, STEPHENS CJ, STURM MJ, AND TAYLOR RR. Effect of WEB 2086 on myocardyal infarct size and regional size and regional blood flow in the dog. Cardiol Res 26: 126 –132, 1992. 249. LEONG LLL, STURM MJ, PAPADIMITRIOU JM, STEPHENS CJ, AND TAYLOR RR. The effects of a PAF antagonist on ischemic myocardial damage and arrhytmia in the dog. J Mol Cell Cardiol 24: 641– 649, 1992. 250. LEPRAN I AND LEFER AM. Ischemia aggravating effects of plateletactivating factor in acute myocardial ischemia. Basic Res Cardiol 80: 135–141, 1985. 251. LEVI R, BURKE JA, GUO ZG, HATTORI Y, HOPPENS CM, MCMANUS LM, HANAHAN DDJ, AND PINKARD RN. Acetyl glyceryl ether phosphorylcholine (AGEPC), a putative mediator of cardiac anaphylaxis in the guinea pig. Circ Res 54: 117–124, 1984. 252. LEVI R, GENOVESE A, AND PINCKARD RN. Alkyl chain homologs of platelet-activating factor and their effects on the mammalian heart. Biochem Biophys Res Commun 161: 1341–1347, 1989. 253. LEVI R AND MULLANE KM. Isolated coronary-perfused mammalian heart: assessment of eicosanoid and platelet-activating factor release and effects. Methods Enzymol 187: 610 – 621, 1990. 254. LEVY JV. Calmodulin antagonist inhibits aggregation of human, guinea-pig, and rabbit platelets induced with platelet-activating factor. FEBS Lett 154: 262–264, 1983. 255. LEWIS MS, WHATLEY RE, CAIN P, MCINTYRE TM, PRESCOTT TM, AND ZIMMERMAN GA. Hydrogen peroxide stimulates the synthesis of platelet activating factor by endothelium and induces endothelial cell-dependent neutrophil adhesion. J Clin Invest 82: 2045–2055, 1988. 256. LIBBY P. Molecular bases of the acute coronary syndromes. Circulation 91: 2844 –2850, 1995. 257. LIU H, CHAO W, AND OLSON MS. Regulation of the surface expression of the platelet activating factor receptor in IC-21 peritoneal macrophages. Effect of lipopolysaccharide. J Biol Chem 267: 20811– 20819, 1992. 258. LOPEZ DIEZ F, NIETO ML, FERNANDEZ-GALLARDO S, GIJON MA, AND SANCHEZ CRESPO M. Occupancy of platelet receptor for platelet activating factor in patients with septicemia. J Clin Invest 83: 1733–1740, 1989. 259. LORANT DE, TOPHAN MK, WHATLEY RE, MCEVER RE, MCINTYRE RP, PRESCOTT SM, AND ZIMMERMAN GA. Inflammatory roles of P-selectin. J Clin Invest 92: 559 –570, 1993. 260. LOTNER GZ, LYNCH JM, BETZ SJ, AND HENSON PM. Human neutrophilderived platelet activating factor. J Immunol 124: 676 – 684, 1980. 261. LUCCHESI BR. Modulation of leukocyte-mediated myocardial reperfusion injury. Annu Rev Physiol 52: 561–576, 1990. 262. LUDWIG JC AND PINCKARD RN. Diversity in the chemical structures of neutrophil-derived platelet activating factors. In: New Horizons in Platelet Activating Factor Research, edited by Winslow CM and Lee JL. New York: Wiley, 1987, p. 57–71. 263. MA XL, WEYRICH AS, KRANTZ S, AND LEFER AM. Mechanisms of the cardioprotective actions of WEB 2170, bepafant, a platelet activating factor antagonist, in myocardial ischemia and reperfusion. J Pharmacol Exp Ther 260: 1229 –1236, 1992. 264. MAGNI F, ROSSONI G, AND BERTI F. BN 52021 protects guinea-pig from heart anaphylaxis. Pharmacol Res Commun 20 Suppl 5: 75–78, 1988. 265. MAN RY AND KINNAIRD AA. Similar coronary vascular effects in the rat perfused heart of platelet activating factor structural analogues with agonist and antagonist properties. Br J Pharmacol 116: 2359 – 2364, 1995. 266. MARCHESELLI VL, ROSSOWSKA MJ, DOMINGO MT, BRAQUET P, AND BAZAN NG. Distinct platelet activating factor binding sites in synaptic endings and in intracellular membranes of rat cerebral cortex. J Biol Chem 265: 9140 –9145, 1990. 267. MARUYAMA M, FARBER NE, VERCELLOTTI GM, JACOB HS, AND GROSS GJ. Inhibition of platelet-activating factor does not alter myocardial stunning. J Mol Cell Cardiol 21 Suppl 2: S115, 1989. Volume 80 268. MARUYAMA M, FARBER NE, VERCELLOTTI GM, JACOB HS, AND GROSS GJ. Evidence for a role of platelet activating factor in the pathogenesis of irreversible but not reversible myocardial injury after reperfusion in dogs. Am Heart J 120: 510 –520, 1990. 269. MARUYAMA M, VERCELLOTTI GM, JACOB HS, AND GROSS GJ. Platelet activating factor as a mediator for myocardial infarction. Circulation 2 Suppl: 233, 1989. 270. MARUYAMA M, VERCELLOTTI GM, JACOB G, GROSS G, AND CHRISTENSEN C. Inhibition of platelet-activating factor reduces myocardial infarct size. J Mol Cell Cardiol 21 Suppl 2: S114, 1989. 271. MARZI I, BAUER M, REISDORF E, AND WALCHER F. Involvement of platelet activating factor in pathologic leukocyte-endothelium interactions in the liver after hemorrhagic shock. Zentralbl Chir 119: 814 – 821, 1994. 272. MASSEY CV, KOHOUT TA, GAA ST, LEDERER WJ, AND ROGERS TB. Molecular and cellular actions of platelet-activating factor in rat heart cells. J Clin Invest 88: 2106 –2116, 1991. 273. MASUGI F, OGIHARA T, OTSUKA A, SAEKI S, AND KUMAHARA Y. Potent hypotensive activity of 1-O-hexadecyl-2-O-acetyl-sn-glycero-3-phosphocholine in spontaneously hypertensive rat. Biochem Biophys Res Commun 104: 280 –284, 1982. 274. MATSUMOTO K, TAKI F, KONDOH Y, TANIGUCHI H, AND TAKAGI K. Platelet activating factor in bronchoalveolar lavage fluid of patients with adult respiratory distress syndrome. Clin Exp Pharmacol Physiol 19: 509 –515, 1992. 275. MCGOWAN HM, ANDONGEN RV, KELLY LD, AND HILL KJ. Increased levels of platelet-activating factor (1-O-alkyl-2-acetylglycero-phosphocholine) in blood after reversal of renal clip hypertension in the rat. Clin Sci 74: 393–396, 1988. 276. MCINTYRE T, ZIMMERMAN GA, AND PRESCOTT SM. Leukotrienes C4 and D4 stimulate human endothelial cells to synthesize platelet activating factor and bind neutrophils. Proc Natl Acad Sci USA 83: 2204 – 2208, 1986. 277. MCINTYRE TM, REINHOLD SL, PRESCOTT SM, AND ZIMMERMAN GA. Protein kinase C activity appears to be required for the synthesis of platelet-activating factor and leukotriene B4 by human neutrophils. J Biol Chem 262: 15370 –15376, 1987. 278. MCINTYRE TM, ZIMMERMAN GA, SATOH K, AND PRESCOTT SM. Cultured endothelial cells synthesize both platelet activating factor and prostacyclin in response to histamine, bradykinin, and adenosine triphosphate. J Clin Invest 76: 271–280, 1985. 279. MCMANUS LM AND PINCKARD RN. Kinetics of acetyl glyceryl ether phosphorylcholine (AGEPC)-induced acute lung alterations in the rabbit. Am J Pathol 121: 55– 68, 1985. 280. MCMANUS LM, WOODARD DS, DEAVERS SI, AND PINCKARD RL. Biology of disease. PAF molecular heterogeneity: pathobiological implications. Lab Invest 69: 639 – 650, 1993. 281. MCNAMARA MJC, SCHMITT JD, WYKLE RL, AND DANIEL LW. 1-Hexadecyl-2-acetyl-sn-glycerol stimulates differentiation of HL-60 human promyelocytic leukemia cells to macrophage-like cells. Biochem Biophys Res Commun 122: 824 – 830, 1984. 282. METHA J, WARGOVICH T, AND NICHOLS W. Biphasic effects of plateletactivating factor on coronary blood flow in anesthetized dog. Prostaglandins Leukotrienes Med 21: 87–95, 1986. 283. MICKELSON JK, SIMPSON PJ, AND LUCCHESI BR. Myocardial dysfunction and coronary vasoconstriction induced by platelet-activating factor in the post-infarcted rabbit isolated heart. J Mol Cell Cardiol 20: 547–561, 1988. 284. MILHOAN KA, LANE TA, AND BLOOR CM. Hypoxia induces endothelial cells to increase their adherence for neutrophils: role of PAF. Am J Physiol Heart Circ Physiol 263: H956 –H962, 1992. 285. MIYAURA S, EGUCHI H, AND JOHNSTON M. Effect of a cigarette smoke extract on the metabolism of the proinflammatory autacoid platelet activating factor. Circ Res 70: 341–347, 1992. 286. MIZOGUCHI Y, ICHIKAWA Y, KICKA K, KAWADA N, KOBAYASHI K, AND YAMAMOTO S. Effects of arachidonic acid metabolites and interleukin-1 on platelet activating factor production by hepatic sinusoidal endothelial cells from mice. J Gastroenterol Hepatol 6: 283–291, 1991. 287. MONTRUCCHIO G, ALLOATTI G, MARIANO F, COMINO A, CACACE G, POLLONI R, DE FILIPPI PG, EMANUELLI G, AND CAMUSSI G. Role of plateletactivating factor in polymorphonuclear neutrophil recruitment in reperfused ischemic rabbit heart. Am J Pathol 142: 471– 480, 1993. October 2000 PAF AND THE CARDIOVASCULAR SYSTEM 288. MONTRUCCHIO G, ALLOATTI G, MARIANO F, DE PAULIS R, COMINO A, EMANUELLI G, AND CAMUSSI G. Role of platelet-activating factor in the reperfusion injury of rabbit ischemic heart. Am J Phatol 137: 71– 83, 1990. 289. MONTRUCCHIO G, ALLOATTI G, MARIANO F, LUPIA E, GRECO-LUCCHINA P, MUSSO E, EMANUELLI G, AND CAMUSSI G. Role of platelet-activating factor in hypotension and platelet activation induced by infusion of thrombolitic agents in rabbits. Circ Res 72: 658 – 670, 1993. 290. MONTRUCCHIO G, ALLOATTI G, MARIANO F, MEDA E, TETTA C, EMANUELLI G, AND CAMUSSI G. The pattern of cardiovascular alterations induced by infusion of platelet activating factor in rabbit is modified by pretreatment with H1-H2 receptor antagonists but not by cyclooxygenase inhibition. Agents Actions 21: 72–78, 1987. 291. MONTRUCCHIO G, ALLOATTI G, TETTA C, DE LUCA R, SAUNDERS RN, EMANUELLI G, AND CAMUSSI G. Release of platelet-activating factor from ischemic-reperfused rabbit heart. Am J Physiol Heart Circ Physiol 256: H1236 –H1246, 1989. 292. MONTRUCCHIO G, ALLOATTI G, TETTA C, ROFFINELLO C, EMANUELLI G, AND CAMUSSI G. In vitro contractile effect of platelet activating factor on guinea-pig myometrium. Prostaglandins 32: 539 –554, 1986. 293. MONTRUCCHIO G, BERGERONE S, BUSSOLINO F, ALLOATTI G, SILVESTRO L, LUPIA E, CRAVETTO A, DI LEO M, EMANUELLI G, AND CAMUSSI G. Streptokinase induces intravascular release of platelet activating factor in patients with acute myocardial infarction and stimulates its synthesis by cultured human endothelial cells. Circulation 88: 1476 –1483, 1993. 294. MONTRUCCHIO G, CAMUSSI G, TETTA C, EMANUELLI G, ORZAN F, LIBERO L, AND BRUSCA A. Intravascular release of platelet-activating factor during atrial pacing. Lancet 2: 293, 1986. 295. MONTRUCCHIO G, LUPIA E, BATTAGLIA E, BUSSOLINO F, EMANUELLI G, AND CAMUSSI G. Tumor necrosis factor ␣-induced angiogenesis depends on “in situ” platelet activating factor biosynthesis. J Exp Med 180: 377–382, 1994. 296. MONTRUCCHIO G, LUPIA E, BATTAGLIA E, DEL SORBO L, BOCCELLINO M, BIANCONE L, EMANUELLI G, AND CAMUSSI G. Platelet-activating factor enhances VEGF-induced endothelial cell motility and neo-angiogenesis in a murine matrigel model. Arterioscler Thomb Vasc Biol 20: 80 – 88, 2000. 297. MONTRUCCHIO G, LUPIA E, DE MARTINO A, BATTAGLIA E, ARESE M, TIZZANI A, BUSSOLINO F, AND CAMUSSI G. Nitric oxide mediates angiogenesis induced in vivo by platelet-activating factor and tumor necrosis factor-alpha. Am J Pathol 151: 557–563, 1997. 298. MONTRUCCHIO G, LUPIA E, DE MARTINO A, SILVESTRO L, RIZEA SAVU S, CACACE G, DE FILIPPI PG, EMANUELLI G, AND CAMUSSI G. Plasmin promotes an endothelium-dependent adhesion of neutrophils. Involvement of platelet activating factor and P-Selectin. Circulation 93: 2152–2160, 1996. 299. MORITOKI H, HISAYAMA T, TAKEUCHI S, MIYAMO H, AND KONDOH W. Involvement of nitric oxide pathway in the PAF-induced relaxation of rat thoracic aorta. Br J Pharmacol 107: 196 –201, 1992. 300. MORLEY J, PAGE CP, AND PAUL W. Inflammatory actions of platelet activating factor (PAF-acether) in guinea-pig skin. Br J Pharmacol 80: 503, 1982. 301. MUELLER HW, O’FLAHERTY JT, AND WYKLE RL. The molecular species distribution of platelet activating factor synthesized by rabbit and human neutrophils. J Biol Chem 259: 14554 –14559, 1984. 302. MUELLER HW, HAUGHT CA, MCNATT JM, CUI K, GASKELL SJ, JOHNSTON DA, AND WILLERSON JT. Measurement of platelet activating factor in a canine model of coronary thrombosis and in endarterectomy samples from patients with advanced coronary artery disease. Circ Res 77: 54 – 63, 1995. 303. MUIRHEAD EE, BYERS LW, DESIDERIO DM, BROOKS B, AND BROSIUS WM. Antihypertensive lipids from the kidney: alkyl ether analogs of phosphatidylcholine. Federation Proc 40: 2285–2290, 1981. 304. MUIRHEAD EE, BYERS LW, DESIDERIO DM, PITCOCK JA, BROOKS B, BROWN PS, AND BROSIUS LW. Derivation of antihypertensive neutral renomedullary lipid from renal venous effluent. J Lab Clin Med 99: 64 –75, 1982. 305. MUIRHEAD EE, PITCOCK JA, AND BROOKS B. Renal medullary system of blood pressure control. J Hypertens 4 Suppl 4: S27–S32, 1986. 306. MULDER MF, VAN LAMBALGEN AA, VAN KRAATS AA, SCHEFFER PG, BOUMAN AA, VAN DEN BOS GC, AND THIJS LG. Systemic and regional 307. 308. 309. 310. 311. 312. 313. 314. 315. 316. 317. 318. 319. 320. 321. 322. 323. 324. 325. 326. 1695 haemodynamic changes during endotoxin or platelet activating factor (PAF)-induced shock in rats. Circ Shock 41: 221–229, 1993. MUROHARA T, BUERKE M, AND LEFER AM. Polymorphonuclear leukocyte-induced vasocontraction and endothelial dysfunction. Role of selectins. Arteriosclerosis Thrombosis 14: 1509 –1519, 1994. MUROHARA T, HOROWITZ JR, SILVER M, TSURUMI Y, CHEN D, SULLIVAN A, AND ISNER JM. Vascular endothelial growth factor/vascular permeability factor enhances vascular permeability via nitric oxide and prostacyclin. Circulation 97: 99 –107, 1998. NAKAJIMA T, SUGIMOTO T, AND KURACHI Y. Platelet activating factor activates cardiac via arachidonic acid metabolites. FEBS Lett 289: 239 –243, 1991. NAKAMURA M, HONDA Z, IZUMI T, SAKANAKA C, MUTOH H, MINAMI M, BITO H, SEYAMA Y, MATSUMOTO T, NOMA M, AND SHIMIZU T. Molecular cloning and expression of platelet-activating factor receptor from human leukocyte. J Biol Chem 266: 20400 –20405, 1991. NAKASHIMA S, SUGANUMA A, SATO M, TOHMATSU T, AND NOZAWA Y. Mechanism of arachidonic acid liberation in platelet activating factor-stimulated human polymorphonuclear neutrophils. J Immunol 143: 1295–1302, 1989. NAKAYAMA R AND SAITO K. Presence of 1-O-alk-1-enyl-2-O-acetylglycerophosphocholine (vinyl form of PAF) in perfused rat and guinea pig hearts. J Biochem 105: 494 – 496, 1989. NAMM DH, TADEPALLI AS, AND HIGH JA. Species specificity of the platelet responses to 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine. Thromb Res 25: 341–350, 1982. NATHAN N, CORNU E, DENIZOT Y, FEISS P, AND ARNOUX B. Serum platelet activating factor acetylhydrolase during coronary artery bypass surgery. J Cardiothorac Vasc Anesth 8: 254 –255, 1994. NICK JA, AVDI NJ, YOUNG SK, KNALL C, GERWINS P, JOHNSON GL, AND WORTHEN GS. Common and distinct intracellular signaling pathways in human neutrophils utilized by platelet-activating factor and FMLP. J Clin Invest 99: 975–986, 1997. NIDORF S, STURM M, STROPHAIR J, KENDREW PJ, AND TAYLOR R. Whole blood aggregation, thromboxane release and the lyso-derivative of platelet activating factor in myocardial infarction and unstable angina. Cardiovasc Res 23: 273–278, 1989. NIETO ML, VENABLE ME, BAULDRY SA, GREENE DG, KENNEDY M, BASS DA, AND WYKLE RL. Evidence the hydrolysis of ethanolamine plasmalogens triggers synthesis of platelet activating factor via a transacylase reaction. J Biol Chem 266: 18699 –18706, 1991. NIJSSEN JG, ROSENBOOM CF, AND VAN DEN BOSCH H. Identification of a calcium-independent phospholipase A2 in rat lung cytosol and differentiation from acetylhydrolase for 1-alkyl-2-acetyl-sn-glycero3-phosphocholine (PAF-acether). Biochim Biophys Acta 876: 611– 618, 1986. NINIO E, MENCIA-HUERTA JM, HEYMANS F, AND BENVENISTE J. Biosynthesis of platelet activating factor. 1. Evidence for a acetyl-transferase activity in murine macrophages. Biochim Biophys Acta 710: 23–31, 1982. O’FLAHERTY JT AND NISHIHIRA J. Arachidonate metabolites, platelet activating factor, and the mobilization of protein kinase C in human polymorphonuclear neutrophils. J Immunol 138: 1889 –1895, 1987. O’FLAHERTY JT, SURLES JR, REDMAN J, JACOBSON D, PIANTADOSI C, AND WYKLE RL. Binding and metabolism of platelet activating factor by human neutrophils. J Clin Invest 78: 381–388, 1986. O’FLAHERTY JT, WYKLE RL, MILLER CH, LEWIS JC, WAITE M, BASS DA, MCCALL CE, AND DECHATELET LR. 1-O-alkyl-sn-glyceryl-3-phosphorylcholine: a novel class of neutrophil stimulants. Am J Pathol 103: 70, 1981. OHAR JA, WALLER KS, DEMELLO D, AND LAGUNOFF D. Administration of chronic intravenous platelet activating factor induces pulmonary arterial atrophy and hypertension in rabbits. Lab Invest 65: 451– 458, 1991. OKANO S, TAGAWA M, AND URAKAWA N. Effect of TCV-309, a novel platelet activating factor antagonist, on hemodynamics in dogs with endotoxin-induced shock. J Vet Med Sci 57: 385–387, 1995. OLSON NC, JOYCE PB, AND FLEISHER LN. Role of platelet-activating factor and eicosanoids during endotoxin-induced lung injury in pigs. Am J Physiol Cell Physiol 258: C283–C288, 1992. OTSUKA A, MASUGI F, OGIHARA T, SAEKI S, KOYAMA Y, AND KUMAHARA Y. Adrenergic blocking action of 1-O-hexadecyl-2-O-acetyl-sn-glyc- 1696 327. 328. 329. 330. 331. 332. 333. 334. 335. 336. 337. 338. 339. 340. 341. 342. 343. 344. 345. 346. 347. MONTRUCCHIO, ALLOATTI, AND CAMUSSI ero-3-phosphocholine in rats. Clin Exp Hypertens A Theory Practice 5: 625– 635, 1983. OTSUKA A, MASUGI F, OGIHARA T, SAEKI S, NAGANO M, KOYAMA Y, TABUCHI Y, AND KUMAHARA Y. Hypotensive mechanism of acetyl glyceryl ether phosphorylcholine (AGEPC) in dogs. Effects of haemodynamic and humoral factors. Prostaglandins Leukotrienes Med 19: 25–35, 1985. OU MC, KAMBAYASHI J, KAWASAKI T, UEMURA Y, SHINOZAKI K, SHIBA E, SAKON M, YUKAWA M, AND MORI T. Potential etiologic role of PAF in two major septic complications: disseminated intravascular coagulation and multiple organ failure. Thromb Res 73: 227–238, 1994. PAN Z, KRAVCHENKO VV, AND YE RD. Platelet activating factor stimulates transcription of the heparin-binding epidermal growth factor-like growth factor in monocytes. J Biol Chem 270: 7787–7790, 1995. PERICO N, REMUZZI A, DADAN J, BATTAGLIA C, AND REMUZZI G. Plateletactivating factor alters glomerular barrier size selectivity for macromolecules in rats. Am J Physiol Renal Fluid Electrolyte Physiol 261: F85–F90, 1991. PIETSCH P, HUNGER T, BRAUN M, ROEDIGER A, BAUMANN G, AND FELIX SB. Effect of platelet-activating factor on intracellular Ca2⫹ concentration and contractility in isolated cardiomyocytes. J Cardiovasc Pharmacol 31: 758 –763, 1998. PINCKARD RM, FARR RS, AND HANAHAN DJ. Physicochemical and functional identity of rabbit platelet activating factor (PAF) release in vivo during IgE anaphylaxis with PAF released in vitro from IgE sensitized basophils. J Immunol 123: 1847–1857, 1979. PINCKARD RN, JACKSON EM, HOPPENS C, WEINTRAUB ST, LUDWIG JC, MCMANUS LM, AND MOTT GE. Molecular heterogeneity of platelet activating factor produced by stimulated human polymorphonuclear leukocytes. Biochem Biophys Res Commun 122: 325–332, 1984. PIPER PJ AND STEWART AG. Coronary vasoconstriction in the rat isolated perfused heart induced by platelet activating factor is mediated by leukotriene C4. Br J Pharmacol 88: 595– 605, 1986. PIPER PJ AND STEWART AJ. Antagonism of vasoconstriction induced by platelet-activating factor in guinea-pig perfused hearts by selective platelet-activating factor antagonists. Br J Pharmacol 90: 771– 783, 1987. PRESCOTT SM, ZIMMERMAN GA, AND MCINTYRE TM. Human endothelial cells in culture produce platelet-activating factor when stimulated with thrombin. Proc Natl Acad Sci USA 81: 3534 –3538, 1984. PRESCOTT SM, ZIMMERMAN GA, AND MCINTYRE TM. Platelet-activating factor. J Biol Chem 265: 17381–17384, 1990. PREWITT PL, LEACH BE, BYERS LW, BROOKS B, LANDS WEM, AND MUIRHEAD EE. Antihypertensive polar renomedullary lipids, a semisynthetic vasodilator. Hypertension 1: 299 –308, 1979. PUGSLEY MK, SALARI H, BEATCH GN, AND WALKER MJ. The effects of platelet activating factor on isolated rat hearts. Proc West Pharmacol Soc 33: 129 –132, 1990. PUGSLEY MK, SALARI H, AND WALKER MJ. Actions of platelet-activating factor on isolated rat heart. Circ Shock 35: 207–214, 1991. QUIAN C, LEE TC, AND SNYDER F. Metabolism of platelet activating factor (PAF) and related ether lipids by neonatal rat myocytes. J Lipid Mediators 1: 113–123, 1989. QUYYUMI AA, DIODATI JG, LAKATOS E, BONOW RO, AND EPSTEIN SE. Angiogenic effects of low molecular weight heparin in patients with stable coronary artery disease: a pilot study. J Am Coll Cardiol 22: 635– 641, 1993. RABINOVICI R, YUE TL, FARHAT M, SMITH EF, ESSER KM, SLIVJAK M, AND FEUERSTEIN G. Platelet activating factor (PAF) and tumor necrosis factor-alpha (TNF-alpha) interactions in endotoxemic shock: studies with BN 50739, a novel PAF antagonist. J Pharmacol Exp Ther 255: 256 –264, 1990. RABINOVICI R, YUE TL, AND FEUERSTEIN G. Platelet activating factor in cardiovascular stress situations. Lipids 26: 1257–1263, 1991. RAINGER GE, FISHER AC, AND NASH GB. Endothelial-borne plateletactivating factor and interleukin-8 rapidly immobilize rolling neutrophils. Am J Physiol Heart Circ Physiol 272: H114 –H122, 1997. RAMOS-FRANCO J, LO CF, AND BREITWIESER GE. Platelet activating factor receptor-dependent activation of the muscarinic K current in bullfrog atrial myocytes. Circ Res 72: 786 –794, 1993. RANDOLPH AE, MERTZ TE, AND KAPLAN HR. Blood pressure and heart 348. 349. 350. 351. 352. 353. 354. 355. 356. 357. 358. 359. 360. 361. 362. 363. 364. 365. 366. 367. Volume 80 rate effects of alkyl ether phospholipids in conscious renal, spontaneously hypertensive, and normotensive rats. Clin Exp Hypertens A Theory Pract 5: 741–758, 1983. RAYNER TE, CHEN BN, MCLOUGHLIN JW, MENADUE MF, NORMAN RJ, AND OLIVER JR. Prostaglandin F2alpha mediates platelet activating factor stimulated atrial natriuretic factor release from the isolated rat heart. Endocrinology 133: 1108 –1115, 1993. RAYNER TE, MENADUE MF, AND OLIVER JR. Platelet-activating factor stimulates the release of atrial natriuretic factor from the rat heart. J Endocrinol 130: 281–288, 1991. RENOOIJ W AND SNYDER F. Biosynthesis of 1-alkyl-2-acetyl-sn-glycero-phosphocholine (platelet activating factor and a hypotensive lipid) by cholinephosphotransferase in various rat tissues. Biochim Biophys Acta 663: 545–556, 1981. RIEDEL A AND MEST HJ. The effect of PAF (platelet-activating factor) on experimental cardiac arrhythmias and its inhibition by substances influencing arachidonic acid metabolites. Adv Prostaglandins Leukotrienes Med 28: 103–109, 1987. ROBERTSON DA, GENOVESE A, AND LEVI R. Negative inotropic effect on platelet-activating factor on human myocardium: a pharmacological study. J Pharmacol Exp Ther 243: 834 – 839, 1987. ROBERTSON DA, WANG DY, LEE CO, AND LEVI R. Negative inotropic effect of platelet-activating factor: association with a decrease in intracellular sodium activity. J Pharmacol Exp Ther 245: 124 –128, 1988. ROBINSON M, BLANK ML, FITZGERALD V, AND SNYDER F. Activation of lysophospholipids by rabbit alveolar macrophages: specificity of CoA-dependent, and CoA-independent reactions. J Biol Chem 260: 7889 –7895, 1985. ROSS R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362: 801– 809, 1993. ROUIS M, NIGON F, AND CHAPMAN MJ. Platelet activating factor is a potent stimulant of the production of active oxygen species by human monocyte-derived macrophages. Biochem Biophys Res Commun 56: 1293–1298, 1988. SAEKI S, MASUGI F, OGIHARA T, OTSUKA A, KUMAHARA Y, WATANABE K, TUMURA K, AKASHI A, AND KUMAGAI A. Effects of 1-O-alkyl-2-acetylsn-glycero-3-phosphocholine (platelet-activating factor) on cardiac function in perfused guinea-pig heart. Life Sci 37: 325–329, 1985. SAGACH VF, DMITRIEVA AV, AND BRAQUET P. Influence of BN 52021 on cardio- and hemodynamic changes during the development of postischemic shock reaction. In: Ginkgolides—Chemistry, Biology, Pharmacology and Clinical Perspective, edited by Braquet P. Barcelona, Spain: Prous Science, 1989, vol. 2, p. 341–392. SAKAGUCHI K, MORIMOTO S, MASUGI F, SAEKI S, OGIHARA T, YAMADA K, AND YAMATSU I. Studies on the role of platelet activating factor in blood pressure regulation. Lipids 26: 1264 –1268, 1991. SANCHEZ-CRESPO M, ALONSO F, INARREA P, ALAVERZ V, AND EGIDO J. Vascular actions of synthetic PAF acether (a synthetic plateletactivating factor) in the rat: evidence for a platelet independent mechanism. Immunopharmacology 4: 173–185, 1982. SANCHEZ-CRESPO M, FERNANDEZ-GALLARDO S, NIETO ML, BARANIS J, AND BRAQUET P. Inhibition of the vascular actions of immunoglobulin G aggregates by BN 52021, a highly specific antagonist of PAF-acether. Immunopharmacology 10: 69 –75, 1985. SAUNDERS RN AND HANDLEY DA. Platelet activating factor antagonists. Annu Rev Pharmacol Toxicol 27: 237–255, 1987. SCHERF H, NIES AS, SCHWERTSCHLAG U, HUGHES M, AND GERBER JG. Hemodynamic effects of platelet activating factor in the dog kidney in vivo. Hypertension 8: 737–741, 1986. SCHLONDORFF D AND NEUWIRTH R. Platelet activating factor and the kidney. Am J Physiol Renal Fluid Electrolyte Physiol 251: F1–F11, 1986. SCHLONDORFF D, SINGHAL P, HASSID A, SATRIANO JA, AND DECANDIDO S. Relationship of GTP-binding proteins, phospholipase C and PGE2 synthesis in rat glomerular mesangial cells. Am J Physiol Renal Fluid Electrolyte Physiol 256: F171–F178, 1989. SCHRODER E, POULEUR H, VAN MECHELEN H, KEYEUX A, RAIGOSO J, AND CHARLIER A. Alterations in endocardial vascular resistance after reperfusion in a flow, high demand model of ischemia: effects of dipyridamole and WEB 2086, a platelet activating factor antagonist. J Am Coll Cardiol 16: 1750 –1759, 1990. SCHULAM PG, KURUVILLA A, PUTCHA G, MANGUS L, FRANKILIN-JOHNSON October 2000 368. 369. 370. 371. 372. 373. 374. 375. 376. 377. 378. 379. 380. 381. 382. 383. 384. 385. 386. 387. PAF AND THE CARDIOVASCULAR SYSTEM J, AND SHEARER WT. Platelet activating factor induces phospholipid turnover, calcium flux, arachidonic acid liberation, eicosanoid generation, and oncogene expression in a human B cell line. J Immunol 146: 1642–1648, 1991. SCHWERTSCHLAG U, SCHERF H, GERBER JG, MATHIAS M, AND NIES AS. Platelet activating factor induced changes on renal vascular resistance, vascular reactivity, and renin release in the isolated perfused rat kidney. Circ Res 60: 534 –539, 1987. SCHWERTSCHLAG US AND WHORTON AR. Platelet activating factorinduced homologous and heterologous desensitization in cultured vascular smooth muscle cells. J Biol Chem 263: 13791–13796, 1988. SESSLER CN, GLAUSER FL, DAVIS D, AND FLOWER AA. Effects of platelet-activating factor antagonist SRI-63441 on endotoxemia in sheep. J Appl Physiol 65: 2624 –2631, 1988. SHALEY Y, ZANDER G, AND PORT S. Elevated blood levels of platelet activating factor in patients with unstable angina (Abstract). J Am Coll Cardiol 15: 129A, 1990. SHAW JO AND HENSON PM. The binding of rabbit basophil derived platelet activating factor to rabbit platelets. Am J Pathol 98: 791– 810, 1980. SHAW JO, PINCKARD RN, FERRIGNI KS, MCMANUS L, AND HANAHAN J. Activation of human neutrophils with 1-O-hexadecyl/octadecyl-2acetyl-sn-glyceryl-3-phosphorylcoline (platelet activating factor). J Immunol 127: 1250 –1255, 1981. SHIMIZU T, HONDA Z, NAKAMURA M, BITO H, AND IZUMI T. Plateletactivating factor receptor and signal transduction. Biochem Pharmacol 44: 1001–1008, 1992. SHIMIZU T, MORI M, BITO H, SAKANAKA C, TABUCHI S, AIHARA M, AND KUME K. Platelet-activating factor and somatostatin activate mitogen-activated protein kinase (MAP kinase) and arachidonate release. J Lipid Mediat Cell Signal 14: 103–108, 1996. SILVESTRO L, RUIKUN C, SOMMER F, DUC TM, BIANCONE L, MONTRUCCHIO G, AND CAMUSSI G. Platelet activating factor-induced endothelial cells expression of adhesion molecules and modulation of surface glycocalyx, evaluated by electron spectroscopy chemical analysis. Semin Thromb Haemostasis 20: 214 –222, 1994. SIMINIAK T, FLORES NA, AND SHERIDAN DJ. Neutrophil interactions with endothelium and platelets: possible role in the development of cardiovascular injury. Eur Heart J 16: 160 –170, 1995. SIMINIAK T, O’GORMAN DJ, SHAHI M, HACKETT D, AND SHERIDAN DJ. Plasma mediated neutrophil stimulation during coronary angioplasty: autocrine effect of platelet activating factor. Br Heart J 74: 625– 630, 1995. SIRAGANIAN RP AND OSLER AG. Destruction of rabbit platelets in the allergic response of sensitized leukocytes. 1. Demonstration of a fluid phase intermediate. J Immunol 106: 1244 –1251, 1971. SIREN AL AND FEUERSTEIN GZ. Effect of platelet-activating factor and its antagonist BN 52021 on cardiac function and regional blood flow in the conscious rat. Am J Physiol Heart Circ Physiol 257: H25–H32, 1989. SIROIS MG AND EDELMAN ER. VEGF effect on vascular permeability is mediated by synthesis of platelet-activating factor. Am J Physiol Heart Circ Physiol 272: H2746 –H2756, 1997. SMILEY PL, STREMLER KE, PRESCOTT SM, ZIMMERMAN GA, AND MCINTYRE TM. Oxidatively fragmented phosphatidylcholines activate human neutrophils through the receptor for platelet-activating factor. J Biol Chem 266: 11104 –11110, 1991. SMITH KA, PREWITT RL, BYERS LW, AND MUIRHEAD EE. Analogs of phosphatidylcholine: adrenergic antagonists from the renal medulla. Hypertension 3: 460 – 470, 1981. SNYDER F. Enzymatic pathways for platelet activating factor, related alkyl glycerolipids and their precursors. In: Platelet-Activating Factor and Related Lipid Mediators, edited by Snyder F. New York: Plenum, 1987. SNYDER F. Platelet-activating factor and related acetylated lipids as potent biologically active cellular mediators. Am J Physiol Cell Physiol 259: C697–C708, 1990. SNYDER F, LEE TC, AND BLANK ML. The role of transacylase in the metabolism of arachidonate and platelet-activating factor. Prog Lipid Res 31: 65– 86, 1992. SOLDI R, SANAVIO F, AGLIETTA M, PRIMO L, DEFILIPPI P, MARCHISIO PC, AND BUSSOLINO F. Platelet-activating factor (PAF) induces the early 388. 389. 390. 391. 392. 393. 394. 395. 396. 397. 398. 399. 400. 401. 402. 403. 404. 405. 406. 407. 408. 1697 tyrosine phosphorylation of focal adhesion kinase (p125FAK) in human endothelial cells. Oncogene 13: 515–525, 1996. SOLOVIEV AE AND BRAQUET P. The role of PAF-acether in the mechanism of isolated coronary artery spasm under hypoxia and its inhibition by BN 52021. In: Ginkgolides—Chemistry, Biology, Pharmacology and Clinical Perspectives, edited by Braquet P. Barcelona, Spain: Prous Science, 1990, vol. 2, p. 353–367. SORENSEN J, KALD B, TAGESSON C, AND LINDAHL M. Platelet activating factor and phospholipase A2 in patients with septic shock and trauma. Intensive Care Med 20: 555–561, 1994. SPRINGER TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 76: 301–314, 1994. STAFFORINI DM, MCINTYRE TM, CARTER ME, AND PRESCOT SM. Human plasma platelet-activating factor acetylhydrolase: association with lipoprotein particles in the degradation of platelet-activating factor. J Biol Chem 262: 4125– 4222, 1987. STAFFORINI DM, PRESCOTT SM, ZIMMERMAN GA, AND MCINTYRE TM. Platelet-activating factor acetylhydrolase in human tissue and blood cells. Lipids 26: 979 –985, 1991. STAHL GL, BITTERMAN H, TERASHITA Z, AND LEFER AM. Beneficial effect of platelet activating factor receptor antagonist in traumatic shock (Abstract). Circ Shock 24: 214, 1988. STAHL GL, BITTERMAN H, TERASHITA ZL, AND LEFER AM. Salutary consequences of platelet activating factor in hemorrhagic shock. Eur J Pharmacol 149: 233–240, 1988. STAHL GL, CRAFT DV, LENTO PH, AND LEFER AM. Detection of platelet activating factor during traumatic shock. Circ Shock 26: 237–244, 1988. STAHL GL AND LEFER AM. Heterogeneity of vascular smooth muscle responsiveness to lipid vasoactive mediators. Blood Vessels 24: 24 –30, 1987. STAHL GL AND LEFER AM. Mechanism of platelet-activating factorinduced in cardiac depression in the isolated perfused rat heart. Circ Shock 23: 165–177, 1987. STAHL GL, LEFER DJ, AND LEFER AM. PAF-acether induced cardiac dysfunction in the isolated perfused guinea pig-heart. NaunynSchmiedeberg’s Arch Pharmacol 336: 459 – 463, 1987. STAHL GL, TERASHITA Z, AND LEFER AM. Role of platelet-activating factor in propagation of cardiac damage during myocardial ischemia. J Pharmacol Exp Ther 244: 898 –904, 1988. STEPHENS CJ, GRAHAM RM, STURM MJ, RICHARDSON M, AND TAYLOR RR. Variation in plasma platelet activating factor degradation and serum lipids after acute myocardial infarction. Coronary Artery Dis 4: 187–193, 1993. STEWART AG AND PIPER PJ. Platelet-activating factor and the cardiovascular system: involvement in cardiac anaphylaxis. Prog Biochem Pharmacol 22: 132–140, 1988. SUBBANAGOUNDER G, LEITINGER N, SHIH PT, FAULL KF, AND BERLINER JA. Evidence that phospholipid oxidation and/or platelet activating factor play an important role in early atherogenesis. In vitro and in vivo inhibition by WEB 2086. Circ Res 85: 311–318, 1999. SUGIMOTO T, TSUCHINOCHI H, MCGREGOR CG, MUTOH H, SHIMIZU T, AND KURACHI DY. Molecular cloning and characterization of the platelet activating factor receptor gene expressed in the human heart. Biochem Biophys Res Commun 189: 617– 624, 1992. SYBERTZ EJ, SABIN C, BAUM T, EYNON E, NELSON S, AND MORAN R. Studies on the interactions of acetyl glycerol ether phosphoryl choline with the sympathetic nervous system in rats. J Pharmacol Exp Ther 223: 594 –598, 1982. SYBERTZ EJ, WATKINS RW, BAUM T, PULA K, AND RIVELLI M. Cardiac, coronary and peripheral vascular effects of acetyl glyceryl ether phosphorylcholine in the anesthetized dog. J Pharmacol Exp Ther 232: 156 –162, 1985. SZABO C, WU CC, MITCHELL JA, GROSS SS, THIEMERMANN C, AND VANE JR. Platelet-activating factor contributes to the induction of nitric oxide synthase by bacterial lipopolysaccharide. Circ Res 73: 991– 999, 1993. TAMARGO J, TEJERINA T, DELGADO C, AND BARRIGON S. Electrophysiological effects of platelet-activating factor (PAF-acether) in guinea pig-papillary muscles. Eur J Pharmacol 109: 219 –227, 1985. TAMURA K, KIMURA Y, TAMURA T, KITASHIRO S, TZUOKA T, TSUJI H, TWASAKA T, AND INADA M. The effect of platelet activating factor 1698 409. 410. 411. 412. 413. 414. 415. 416. 417. 418. 419. 420. 421. 422. 423. 424. 425. 426. 427. 428. 429. MONTRUCCHIO, ALLOATTI, AND CAMUSSI antagonist TCV-309 on arrhythmias and functional recovery during myocardial reperfusion. Coronary Artery Dis 5: 267–273, 1994. TANIGUCHI H, IWASAKA T, TAKAYAMA Y, SUGIURA T, AND INADA M. Role of platelet activating factor in pulmonary edema after coronary ligation in dogs. Chest 102: 1245–1250, 1992. TANNIERE M AND ROCHETTE L. Direct effects of platelet-activating factor (PAF) on cardiac function in isolated guinea pig heart. Drug Dev Res 11: 177–186, 1987. TAO W, MOLSKI FP, AND SHA’AFI RI. Arachidonic acid release in rabbit neutrophils. Biochem J 257: 633– 637, 1989. TERASHITA ZI, IMURA Y, NISHIKAWA S, AND SUMIDA S. Is platelet activating factor a mediator of endotoxic shock? Eur J Pharmacol 109: 257–261, 1985. TERASHITA ZI, STAHL GL, AND LEFER AM. Protective effect of platelet activating factor (PAF) antagonist and its combined treatment with prostaglandin (PGE1) in traumatic shock. J Cardiovasc Pharmacol 12: 505–511, 1988. TETTA C, MONTRUCCHIO G, ALLOATTI G, ROFFINELLO C, EMANUELLI G, BENEDETTO C, CAMUSSI G, AND MASSOBRIO M. Platelet activating factor contracts human myometrium in vitro. Proc Soc Exp Biol Med 183: 376 –381, 1986. TEW DG, SOUTHAN C, RICE SQ, LAWRENCE MP, LI H, BOYD HF, MOORES K, GLOGER IS, AND MACPHEE CH. Purification, properties, sequencing, and cloning of a lipoprotein-associated, serine-dependent phospholipase involved in the oxidative modification of low-density lipoproteins. Arterioscler Thromb Vasc Biol 16: 591–599, 1996. THIERRY AT, DOLLY M, BRAQUET P, CLUZEL J, AND MEYNIEL G. Presence of specific platelet activating factor binding sites in the rat retina. Eur J Pharmacol 163: 97–101, 1989. TIPPINS JR, ANTONIW JW, ALISON MR, GARVEY B, AND MASERI A. WEB 2086 inhibits neutrophil dependent increases in coronary resistance in blood perfused rabbit heart. Cardiovasc Res 26: 162–169, 1992. TJOELKER LW, WILDER C, EBERHARDT C, STAFFORINI DM, DIETSCH G, SCHIMPF B, HOOPER S, LE TRONG H, COUSENS LS, ZIMMERMAN GA, YAMADA Y, MCINTYRE TM, PRESCOTT SM, AND GRAY PW. Anti-inflammatory properties of a platelet-activating factor acetylhydrolase. Nature 374: 549 –553, 1995. TOKUMURA A, TOUJIMA M, YOSHIOKA Y, AND FUKUZAWA K. Lipid peroxidation in low density lipoproteins from human plasma and egg yolk promotes accumulation of 1-acyl analogues of platelet-activating factor-like lipids. Lipids 31: 1251–1258, 1996. TOLINS JP, VERCELLOTTI GM, WILKOWSKE M, HA B, JACOB HS, AND RAIJ L. Role of platelet activating factor in endotoxemic acute renal failure in the male rat. J Lab Clin Med 113: 316 –324, 1989. TORR SR, HASKEL EJ, VON VOIGTLANDER PF, BERGMANN SR, AND RABENDSCHEIN D. Inhibition of cyclic flow variation and reocclusion after thrombolysis in dogs by a novel antagonist of platelet activating factor. J Am Coll Cardiol 18: 1804 –1810, 1991. TOYOFUKU T, KUBO K, KOBAYASHI T, AND KUSAMA S. Effects of ONO6240 a platelet activating factor antagonist on endotoxin shock in unanesthetized sheep. Prostaglandins 31: 271–281, 1986. TRIGGIANI M AND CHILTON FH. Metabolism of platelet-activating factor in the guinea-pig heart. J Moll Cell Cardiol 24: 1101–1111, 1992. TRIGGIANI M, D’SOUZA DM, AND CHILTON FH. Metabolism of 1-acyl-2acetyl-sn-glycero-3-phosphocholine in the human neutrophils. J Biol Chem 266: 6928 – 6935, 1991. TRIGGIANI M, FONTEH AN, AND CHILTON FH. Factors that influence the proportions of platelet-activating factor and 1-acyl-2-acetyl-sn-glycero-3-phosphocholine synthesized by the mast cell. Biochem J 286: 497–503, 1992. TRIGGIANI M, GOLDMAN DW, AND CHILTON FH. Biological effects of 1-acyl-2-acetyl-sn-glycero-3-phosphocholine in the human neutrophil. Biochim Biophys Acta Lipids Lipid Metab 1084: 41– 47, 1991. TRIGGIANI M, HUBBARD WC, AND CHILTON FH. Synthesis of 1-O-acyl2-acetyl-sn-glycero-3-phosphocholine by an enriched preparation of the human lung mast cell. J Immunol 144: 4773– 4780, 1990. TRIGGIANI M, SCHLEIMER RP, WARNER JA, AND CHILTON FH. Differential synthesis of 1-acyl-2-acetyl-sn-glycero-3-phosphocholine and platelet-activating factor by human inflammatory cells. J Immunol 146: 660 – 666, 1991. TSELEPIS AD, DENTAN C, KARABINA SAP, CHAPMAN MJ, AND NINIO E. PAF-degrading acetylhydrolase is preferentially associated with 430. 431. 432. 433. 434. 435. 436. 437. 438. 439. 440. 441. 442. 443. 444. 445. 446. 447. 448. Volume 80 dense LDL and VHDL-1 in human plasma. Catalytic characteristics and relation to the monocyte-derived enzyme. Arterioscler Thromb Vasc Biol 15: 1764 –1773, 1995. TSELEPIS AD, EVANGELOU A, TSOUKATOS D, DEMOPOULTOS CA, AND KAPOULAS VM. Electrocardiographic alterations induced by AGEPC in Wistar rats in relation to its hypotensive and hematologic effects. Comp Biochem Physiol 87: 41– 46, 1987. TSELEPIS AD, TSOUKATOS D, DROUDES C, DONAS A, AND EVANGELOU A. Platelet response to the aggregatory effect of platelet activating factor (PAF) ex vivo in patients with acute myocardial infarction. Eur J Clin Invest 21: 490 – 496, 1991. TUFANO MA, BIANCONE L, ROSSANO F, CAPASSO C, BARONI A, DE MARTINO A, IORIO EL, SILVESTRO L, AND CAMUSSI G. Outer-membrane porins from gram-negative bacteria stimulate platelet activating factor biosynthesis by cultured human endothelial cells. Eur J Biochem 214: 685– 693, 1993. UCHIDA Y, YANAGISAWA-MIWA A, NAKAMURA F, YAMADA K, TOMARU T, KIMARA K, AND MORITA T. Angiogenic therapy of acute myocardial infarction by intrapericardial injection of basic fibroblast growth factor and heparin sulfate: an experimental study. Am Heart J 130: 1182–1188, 1995. UEMURA Y, LEE Y, AND SNYDER F. A coenzyme A-independent transacylase is linked to the formation of platelet-activating factor (PAF) by generating the lyso-PAF intermediate in the remodeling pathway. J Biol Chem 266: 8268 – 8272, 1991. UKENA D, DENT G, BIRKE FW, ROBAULT C, SYBRECHT GW, AND BARNES PJ. Radioligand binding of antagonists of platelet activating factor to intact human platelets. FEBS Lett 228: 285–289, 1988. UKENA D, KROGEL C, DENT G, YUKAWA T, SYBRECHT G, AND BARNES PJ. PAF-receptors on eosinophils: identification with a novel ligand, 3 H-WEB 2086. Biochem Pharmacol 38: 1702–1705, 1989. VALLARI S, RECORD M, AND SNYDER F. Conversion of alkylacetylglycerol to platelet activating factor in HL-60 cells and subcellular localization of the mediator. Arch Biochem Biophys 276: 538 –545, 1990. VALONE FH. Identification of platelet activating factor receptors in P388D murine macrophages. J Immunol 140: 2389 –2394, 1988. VALONE FH, COLES E, REINHOLD VR, AND GOETZL EJ. Specific binding of phospholipid platelet activating factor by human platelets. J Immunol 129: 1639 –1641, 1982. VALONE FH AND EPSTEIN LB. Biphasic platelet activating factor synthesis by human monocytes stimulated with IL-1beta, tumor necrosis factor or IFN-gamma. J Immunol 141: 3945–3950, 1988. VALONE FH AND GOETZL EJ. Specific binding by human polymorphonuclear leucocytes of the immunological mediator 1-O-hexadecyl/ octadecyl-2-acetyl-sn-glycero-3 phosphorylcholine. Immunology 48: 141–149, 1983. VARGAFTIG BB, PRETOLANI M, COEFFIER E, AND CHIGNARD M. Platelet activating factor: biology receptors and antagonists. Handb Inflammation 6: 113–146, 1989. VARON D, JACKSON DE, SHENKMAN B, DARDIK R, TAMARIN I, SAVION N, AND NEWMAN P. Platelet/endothelial cell adhesion molecule-1 serves as a costimulatory agonist receptor that modulates integrin-dependent adhesion and aggregation of human platelets. Blood 91: 500 – 507, 1998. VEMULAPALLI S, CHIU PJS, AND BARNETT A. Cardiovascular and renal action of platelet activating factor in anesthetized dogs. Hypertension 6: 489 – 493, 1984. VENABLE ME, NIETO ML, SCHMITT JD, AND WYKLE RL. Conversion of 1-O-3H-alkyl-2-arachidonoyl-sn-glycero-3-phoshocholine to lysoplatelet-activating factor by the CoA-independent transacylase in membrane fraction of human neutrophils. J Biol Chem 266: 18691– 18698, 1991. VENABLE ME, ZIMMERMAN GA, MCINTYRE TM, AND PRESCOTT SM. Platelet-activating factor: a phospholipid autacoid with diverse actions. J Lipid Res 34: 691–702, 1993. VIOSSAT I, CHABRIER PE, CHAPELAT M, AND BRAQUET P. The coronary, inotropic, chronotropic and arrhythmogenic effects of PAF-acether on isolated guinea-pig heart their selective inhibition by BN 52021. In: M. Paubert—Lipid Mediators in the Immunology of Shock, edited by Braquet P. New York: Plenum, 1987, p. 513–517. VIOSSAT I, CHAPELAT M, CHABRIER PE, AND BRAQUET P. Effects of platelet-activating factor (PAF) and its receptor antagonist BN October 2000 449. 450. 451. 452. 453. 454. 455. 456. 457. 458. 459. 460. 461. 462. 463. 464. 465. PAF AND THE CARDIOVASCULAR SYSTEM 52021 on isolated perfused guinea-pig heart. Prostaglandins Leukotrienes Essent Fatty Acids 38: 189 –194, 1989. VOELKEL NF, WORTHEN S, REEVES JT, HENSON PM, AND MURPHY RC. Nonimmunological production of leukotrienes induced by plateletactivating factor. Science 218: 286 –288, 1982. WAHLER GM, COYLE DE, AND SPERELAKIS N. Effects of platelet-activating factor on single potassium channel currents in guinea pig ventricular myocytes. Mol Cell Biochem 93: 69 –76, 1990. WANG J AND DUNN MJ. Platelet activating factor mediates endotoxininduced acute renal insufficiency in rats. Am J Physiol Renal Fluid Electrolyte Physiol 253: F1283–F1289, 1987. WEINTRAUB ST, LEAR CL, AND PINCKARD RN. Analysis of platelet activating factor by GC/MS after direct derivatization with penthafluorobenzoyl chloride and heptafluorobutyric anhydride. J Lipid Res 31: 719 –725, 1990. WEINTRAUB ST, LUDWIG JC, MOTT GE, MCMANUS LM, AND PINCKARD RN. Fast atom bombardment-mass spectrometric identification of molecular species of platelet activating factor produced by stimulated human polymorphonuclear leukocytes. Biochem Biophys Res Commun 129: 868 – 876, 1985. WERR J, XIE X, HEDQVIST P, RUOSLAHTI E, AND LINDBOM L. 1 integrins are critically involved in neutrophil locomotion in extravascular tissue in vivo. J Exp Med 2091: 2096, 1998. WEYRICH AS, ELSTAD MR, MCEVER RP, MCINTYRE TM, MOORE KL, MORRISSEY JH, PRESCOTT SM, AND ZIMMERMAN GA. Activated platelets signal chemokine synthesis by human monocytes. J Clin Invest 97: 1525–1534, 1996. WEYRICH AS, MCINTYRE TM, MCEVER RP, PRESCOTT SM, AND ZIMMERMAN GA. Monocytes tethering by p-selectin regulates monocyte chemotactic protein-1 and tumor necrosis factor-alpha secretion: signal integration and NF-kB translocation. J Clin Invest 95: 2297– 2303, 1995. WHATLEY RE, CLAY KL, CHILTON FH, TRIGGIANI M, ZIMMERMAN GA, MCINTYRE TM, AND PRESCOTT SM. Relative amounts of 1-O-alkyl- and 1-acyl-2-acetyl-sn-glycero-3-phosphocholine in stimulated endothelial cells. Prostaglandins 43: 21–29, 1992. WHATLEY RE, ZIMMERMAN G, MCINTYRE TM, TAYLOR BS, AND PRESCOTT SM. Production of platelet activating factor by endothelial cells. Semin Thromb Haemostasis 13: 445– 453, 1987. WILLIAMS FM, COLLINS PD, TANNIERE-ZELLER M, AND WILLIAMS TJ. The relationship between neutrophils and increased microvascular permeability in a model of myocardial ischaemia and reperfusion in the rabbit. Br J Pharmacol 100: 729 –734, 1990. WINKLER JD AND CHILTON FH. The role of CoA-independent transacylase in the production of platelet-activating factor and the metabolism of arachidonate. Drug News Perspect 6: 133–138, 1993. WINKLER JD, SUNG CM, HUBBARD WC, AND CHILTON FH. Influence of arachidonic acid on indices of phospholipase A2 activity in the human neutrophil. Biochem J 291: 825– 831, 1993. WOOLLEY DS, PUGLISI RN, QUINN JV, AND SLOTMAN GJ. Platelet activating factor mediates cardiopulmonary dysfunction during graded bacteremic shock. J Trauma 41: 291–296, 1996. WRIGTH SD, RAMOS RA, TOBIAS PS, ULEVITCH RJ, AND MATHISON JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249: 1431–1433, 1990. WYKLE RL, MALONE B, AND SNYDER F. Enzymatic synthesis of 1-Oalkyl-2-acetyl-sn-glycero-3-phosphocholine, a hypotensive and platelet-aggregation lipid. J Biol Chem 255: 10256 –10260, 1980. XIE J, KO J, BAGBY GJ, GILES TD, AND GREENBERG SS. Dissociation of TNF-alpha from endotoxin-induced nitric oxide and acute-phase 466. 467. 468. 469. 470. 471. 472. 473. 474. 475. 476. 477. 478. 479. 480. 1699 hypotension. Am J Physiol Heart Circ Physiol 273: H164 –H174, 1997. YAMAMOTO N, ST CLAIRE DA, HOMMA S, AND NGWENYA BZ. Activation of mouse macrophages by alkylglycerols, inflammation products of cancerous tissues. Cancer Res 48: 6044 – 6049, 1988. YAMANAKA S, IWAO H, YUKIMURA T, KIM S, AND MIURA K. Effect of the platelet activating factor antagonist, TCV-309, and the cyclo-oxygenase inhibitor, ibuprofen, on the haemodynamic changes in canine experimental endotoxic shock. Br J Pharmacol 110: 1501– 1507, 1993. YAMANAKA S, MIURA K, YUKIMURA T, OKUMURA M, AND YAMAMOTO K. Putative mechanism of hypotensive action of platelet activating factor in dogs. Circ Res 70: 893–901, 1992. YE RD, KRAVCHENKO VV, PAN Z, AND FENG L. Stimulation of NF-kappa B activation and gene expression by platelet activating factor. Adv Exp Med Biol 416: 143–151, 1996. YE RD, PROSSITZ ER, ZUO A, AND COCHRANE CG. Characterization of a human cDNA that encodes a functional receptor for platelet activating factor. Biochem Biophys Res Commun 180: 105–111, 1991. YOSHIDA N, GRANGER DN, ANDERSON DC, ROTHLEIN R, LANE C, AND KVIETYS PR. Anoxia/reoxygenation induced neutrophil adherence to cultured endothelial cells. Am J Physiol Heart Circ Physiol 262: H1891–H1898, 1992. YOUSUFZAI SYK AND ABDEL-LATIF AA. Effect of platelet activating factor on the release of arachidonic acid and prostaglandins by rabbit iris smooth muscle. Inhibition by calcium-channel antagonists. Biochem J 228: 697–706, 1985. YUE TL, FARHAT M, RABINOVICI R, PERERA PY, VOGEL SN, AND FEUERSTEIN G. Protective effect of BN 50739, a new PAF antagonist, on endotoxin treated rabbits. J Pharmacol Exp Ther 254: 976 –981, 1990. ZAVOICO GB, HRBOLICH JK, GIMBRONE MA, AND SCHAFER IA. Enhancement of thrombin and ionomycin-stimulated prostacyclin and platelet activating factor production in cultured endothelial cells by a tumor-promoting phorbol ester. J Cell Physiol 143: 596 – 605, 1990. ZHIXING P, KRAVCHENKO VV, AND YE RD. Platelet activating factor stimulates transcription of the heparin-binding epidermal growth factor-like growth factor in monocytes. J Biol Chem 270: 7787– 7790, 1995. ZIMMERMAN BJ, HOLT JW, PAULSON JC, ANDERSON DC, MIYASAKA M, TAMATANI T, TODD RF, RUSCHE JR, AND GRANGER DN. Molecular determinants of lipid mediator-induced leukocyte adherence and emigration in rat mesenteric venules. Am J Physiol Heart Circ Physiol 266: H847–H853, 1994. ZIMMERMAN GA, MCINTYRE TM, MEHRA M, AND PRESCOTT SM. Endothelial cell-associated platelet activating factor: a novel mechanism for signaling intercellular adhesion. J Cell Biol 110: 529 –540, 1990. ZIMMERMAN GA, MCINTYRE TM, AND PRESCOTT SM. Production of platelet activating factor by human vascular endothelial cells: evidence for a requirement for a specific agonist and modulation by prostacyclin. Circulation 72: 718 –727, 1985. ZIMMERMAN GA, PRESCOTT SM, AND MCINTYRE TM. Platelet activating factor: a fluid-phase and cell-mediator of inflammation. In: Inflammation: Basic Principles and Clinical Correlates, edited by Gallin JI and Goldstein IM., New York: Raven, 1992, p. 1–28. ZIMMERMAN GA, PRESCOTT SM, AND MCINTYRE TM. Endothelial cell interactions with granulocytes: tethering and signaling molecules. Immunol Today 13: 93–100, 1992.