Document 14226796
advertisement

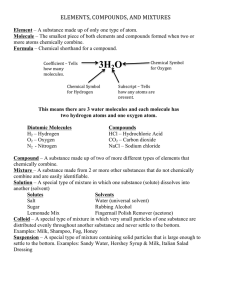
MATTER the stuff of the universe Ma4er • Ma#er: ______________________________ _____________________________________ • Mass: the amount of ____________________ • Weight: ______________________________ – on Earth, this is the same as mass • Law of Conserva4on of Ma#er: “__________ ________________________________”…but it can be rearranged (via chemical or physical reacDons) 1 Atoms Ma4er is composed of _________ • Atoms: ___________________________ _________________________________ _________________________________ • can not be further divided and sDll have _________ ___________ – Are composed of _________, ___________, and ___________ Pure Substance • A substance with – Ex:___________________ _______________________ • __________ and ______________are both considered to be pure substances 2 States of Ma4er Solid Liquid Gas Condensed States: Solids • Solids – Have a ___________________ __________________________ – Molecules are • Tightly ________ • Moving _________________________ ____________________ • ________________a4racted to each other • Not _________________ 3 Condensed States: Liquids • Liquids ¨ Have NO set__________but definite __________ – Molecules are • ____________than in a solid at the same temperature • _________________________ • _________ a4racted to each other • Not _______________ Expanded States: Gas • Gas (vapor) – Have no ________________ • Depends on the container – Have ________________ • Varies depending on _______________ and ___________ – Molecules are • ______________________a4racted to each other • ________________than those in a liquid at the same temperature • As a result are – _________________as far as possible – __________________past each other – _____________________ 4 Expanded States: Plasma • Plasmas – Occur at extremely ____________________ – Are ________________ • May be some atoms or molecules but mostly – ________________ floaDng around not a4ached – Usually are __________________________ – Most ________________ form of ma4er in the universe • ______________ – Here on Earth: _______________________________ ___________________________________________ 5 What is a non-­‐Newtonian fluid? the water to squirt out. To make the You have to pull the trigger on a water pistol to get water to come out faster, you have to pull the trigger harder. Fluids resist flow. The resistance to flow arises because of the friction between these layers. This phenomenon is known as viscosity. Newton devised a simple model for fluid flow that could be used to relate how hard you have to pull the trigger to how fast the liquid will squirt out of the pistol. Picture a flowing liquid as a series of layers of liquid sliding past each other. The slower one layer slides over another, the less resistance there is, so that if there was no difference between the speeds the layers were moving, there would be no resistance. Fluids like water and gasoline behave according to Newton's model, and are called Newtonian fluids. But ketchup, blood, yogurt, gravy, pie fillings, mud, and cornstarch paste DON'T follow the model. They're non-Newtonian fluids because doubling the speed that the layers slide past each other does not double the resisting force. It may less than double (like ketchup), or it may more than double (as in the case of quicksand and gravy). That's why stirring gravy thickens it, and why struggling in quicksand will make it even harder to escape. For some fluids (like mud, or snow) you can push and get no flow at all- until you push hard enough, and the substance begins to flow like a normal liquid. This is what causes mudslides and avalanches. Physical and Chemical ProperDes and Changes 6 Physical Property • CharacterisDc of a • Examples: substance that can – ______________ be observed – ______________ ________________ – ______________ ________________ – ______________ ________________ – ______________ ________________ – ______________ ________________ Chemical Property • Ability of a substance to _____________ _________________________________ • You must change the idenDty _________ _________________________________ _________________________________ • Examples: – ______________ – ______________ with acids and bases 7 Intrinsic v. Extrinsic ProperDes • Intrinsic (intensive) Property: A property that _______________ __________________________ of substance changes – _____________ – _____________ – _____________ – _____________ Intrinsic v. Extrinsic ProperDes • Extrinsic (extensive) property: A property that ___________________ ______________________________ ______________________________ – _____________ – _____________ – _____________ – _____________ 8 Density…Intrinsic or Extrinsic? ¤ Density data for a sample of gold. ¤ Density= mass/ volume ¤ Units are g/mL or g/cm3 Mass (g) Volume (mL) 46.71 2.42 297.80 15.43 662.76 34.34 147.07 7.62 431.162 22.34 85.69 4.44 Density Physical Change • Change in the _________________substance, not in its chemical nature – No chemical bonds ___________________________ • The chemical formula is the same before and aher the change – ________________________________are made or broken, not the molecules themselves – Examples: • __________________ • __________________ • __________________ • __________________ 9 Chemical Change (___________) • Change_____________________________ _____________________________ ¨ Atoms are __________________ – Bonds are ______________________ in new ways • Examples: – __________________ – __________________ – _______________ – __________________ Signs of Chemical Change • Change in: – ___________________ • Forming a _______________ (solid made from liquids) • ___________________________ – __________ – ________________ • _________ (exothermic) • _________ (endothermic) – _____________ • giving off ___________ 10 What is an element? • Element: – A substance made up of only one type of atom is an _____________________ How do we represent elements? – Represented by symbols on the periodic table • Usually comes from the ___________, a _____________, or a _____________ • One or two le4ers for those with official names – ___________________________________ – ___________________________________ • Unofficially named elements have three le4ers, starDng with a capital ____ 11 Elements in Nature • ____90 naturally occurring – Of the __________on the Periodic Table, only ___ and ____ are not naturally occurring • Some elements are _________________ __________________, but commonly are found in compounds – ex: ______________is virtually non-­‐existent in pure form, but is found in common_______, a compound we call table salt. Elements 12 When elements were discovered Allotropes • Allotropes are ___________________ __________________________ • Shown here are allotropes of carbon • ______________, ______________, and ______________ • Difference is how the atoms are __________________________ 13 Molecules • Two (2) or more _______________ _____________________ – O2 – H2 – H2O – C6H12O6 Compounds • Two (2) or more ___________________ ________________________________ _________________________________ – Has own, unique: • ________ (law of__________________) • properDes – ___________________________________ – Need a ___________________to separate them into the elements it is made from • 2 H2O -­‐> 2H2 and O2 14 Law of Definite ProporDons • In samples of any chemical compound, the ________________________________ _________________________________ • ________________________________ – H2O is always H2O, and never H2O2 – Once the formula is different, the materials and the properDes are different 15 What is the difference between compounds and molecules? Molecules: two or more atoms bonded Compounds: two or more atoms of _______ ________ bonded • The term molecule is more _________, compound is more _________. • Molecules can be _______________ like ___________ • Compounds by definiDon ________ _______________ SeparaDon of compounds into their elements • Compounds can be broken into their elements by _______________, such as • ______________. This is the decomposiDon of a substance (here, water) into it’s elements by an ______________. 16 Mixture • A blend of 2 or more____________ • Have __________ composiDons – No _____________________ • Examples: Air: A Mixture 17 Types of Mixtures • Homogeneous – ________________ – __________________ – cannot see ________________ – Examples • ___________ • ___________ • ___________ • ___________ Types of mixture, con’t • Heterogeneous – ______________ – not __________________ – can see ______________ – Examples: • _________________ • _________________ • _________________ • _________________ 18 Subtypes of mixtures... • Solu4ons: one thing dissolved in another; they do not ___________________or when filtered (parDcles are molecules) – Two parts: • Solvent-­‐______________________________ • Solute-­‐ _______________________________ Ex: Salt water – water is the ________, salt the _______ – Can be any state of ma4er • Alloys are _____________________(a solid soluDon) Subtypes of mixtures... • Suspensions: – Heterogeneous mixture of two or more substances – may look like a soluDon but can ____________________________ ____________________________ – parDcles are not dissolved • parDcles are larger than molecules; someDmes they can be seen by the naked eye 19 Subtypes of mixtures... • Colloid-­‐ microscopically mixed homogeneous soluDons that will _______________ (____________) – They will ________________out upon standing – Emulsion-­‐ a type of colloid made from of two liquids that normally will not mix (are_________________________) 20 Tyndall Effect • Twenty-­‐four-­‐karat gold is an _____________ • Eighteen-­‐karat gold is an ________. • Fourteen-­‐karat gold is an ___________. – (Alloys are homogeneous mixtures-­‐ specifically soluDons-­‐ of metals) – What is the solvent in 18K and 14K gold? – What is/ are the solute(s) in 18K and 14K gold? 21 ClassificaDon of Ma4er 1_15 Matter (materials) Physical processes Substances Mixtures Chemical Elements reactions Compounds Homogeneous mixtures (solutions) Heterogeneous mixtures SeparaDng Mixtures • Mixtures can be separated by _________________________ – No chemical reacDons or changes needed – 5 main methods are based on physical properDes • _________________ • _________________ • _________________ • _________________ • _________________ 22 SeparaDng Mixtures • Evapora4on: ____________________ ________________________________ • Crystalliza4on: ___________________ ____________________________________ ______________________ • • • • – Usually paired _____________ _____________ _____________ _____________ Chromatography: “color wriDng” • Using how ______________________to separate them – Use specific raDos of movement through a medium (paper, water, column) based on how some molecules move through the medium – Phases: • StaDonary phase – _____________________(paper, the column) – ____________________of the mixture _________________ • Mobile phase – moves over the staDonary phase – _________________________of the mixture – Rf values constant for component of a mixture • RaDo of ________________________________________ _______________________________________________ • Ex: running colors in ink when wet 23 chromatograms Gas and Column Chromotography 24 FiltraDon • Use a funnel and ________ _____________ • Separates a _____________ _____________ • Based on _____________ • Separates _______ ________________ DisDllaDon • One liquid has to have a lower _____ ________________ – Heat both; the one with the lower BP is boiled out first – Can use _______________ to collect materials as they boil out • Used with crude oil (______________) to isolate separate products 25 DisDllaDon of Crude Oil: Cracking Other methods… • Use ____________ (____________) – _____________________ • Use _____________ – For solids: Mixture of pieces is placed in a soluDon • Some float and can be skimmed off • Some sink • Used in recycling plants to separate plasDcs – For liquids that are immiscible (will not mix, like oil and water): use a separatory funnel 26 Separatory Funnel Used for liquids that are ___________ (will not mix, like oil and water) End of Chapter 27