Name: __________________________________ Final Review: Integrated Science How to Study for the Final:
advertisement

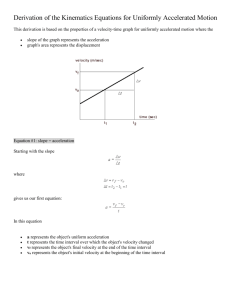
Name: __________________________________ Final Review: Integrated Science How to Study for the Final: First go through this study guide to get an idea of what will be on the final. Answer any questions, fill out any definitions, and do any problems that are listed. We have taken example problems from your quizzes, tests, and labs to give you SOME practice. However, you will likely need more practice in some areas. If you need more practice you should refer to the hard copy of your tests/quizzes, as well as you labs and notes. If you do not have these, almost everything that we have done in class can be accessed electronically from my website. PLEASE MAKE TIME TO COME AFTER SCHOOL IF YOU NEED HELP!!! I WILL BE AFTER EVERY DAY BETWEEN NOW AND THE FINAL! TOPIC A: Scientific Method 1) What are the 5 steps of the scientific method? 2) Define Control and Variable True/False _______1. _______2. _______3. _______4. _______5. The first step of the scientific method is to identify the problem. An experiment should test many variables at the same time. A hypothesis is your prediction for how an experiment will turn out. The metric unit for measuring mass is grams. The metric unit for measuring length is inches. PRACTICE: SCIENTIFIC METHOD Patty Power Mr. Krabbs wants to make Bikini Bottoms a nicer place to live. He has created a new sauce that he thinks will reduce the production of body gas associated with eating crabby patties from the Krusty Krab. He recruits 100 customers with a history of gas problems. He has 50 of them (Group A) eat crabby patties with the new sauce. The other 50 (Group B) eat crabby patties with sauce that looks just like new sauce but is really just mixture of mayonnaise and food coloring. Both groups were told that they were getting the sauce that would reduce gas production. Two hours after eating the crabby patties, 30 customers in group A reported having fewer gas problems and 8 customers in group B reported having fewer gas problems. Which people are in the control group? Which people are the treatment group? What should Mr. Krabs’ conclusion be? Why do you think 8 people in group B reported feeling better? Slimotosis Sponge Bob notices that his pal Gary is suffering from slimotosis, which occurs when the shell develops a nasty slime and gives off a horrible odor. His friend Patrick tells him that rubbing seaweed on the shell is the perfect cure, while Sandy says that drinking Dr. Kelp will be a better cure. Sponge Bob decides to test this cure by rubbing Gary with seaweed for 1 week and having him drink Dr. Kelp. After a week of treatment, the slime is gone and Gary’s shell smells better. What was the initial observation? What is the control group? Experimental Group? What is the Independent Variable, Dependent Variable? What should Sponge Bob’s conclusion be? Make sure you can design an experiment using the scientific method to CORRECTLY test a single variable. Write a testable hypothesis that clearly states the Controls, Ind. Variable, and Dependent Variable Make sure you can evaluate the effectiveness of an example experiment (are their steps missing, does the test correctly test a single variable, is there a hypothesis?) Below write out 2 different experiments of your choosing…you can use one from your classmates projects, but you can’t use one you have done already. No MENTOS! Make sure to include the following… o Problem, o Hypothesis, o Experimental Design (How is it set up, Independent Variable/Dependent Variabls, Controls, Control Group, Experimental Group) DATA You should be able to set up a simple scatter plot given a set of data. You should be able to set up a line graph given a simple set of data. You should be able to set up a series of bar graphs given a set of data. Each graph must include all elements needed (title, axes, labels, key, etc.) TOPIC B: Metric and Measurement With each jump between prefixes in the metric system, the amount changes by an order of ___________________. (How much do you multiply or divide by in one step) For the following things answer with the correct measurements (meter, liter or gram) mass of a penny length of a football field amount of air in a basketball density of a marble use a graduated cylinder to measure this use a triple beam balance to measure this METRIC SYSTEM List all the base units from the metric system (length, mass, volume) List all the metric prefixes… Remember your pneumonic device!!! With each jump between prefixes in the metric system, the amount changes by an order of ___________________. (How much do you multiply or divide by in one step) 456 m = _____________________ mm 675.69 km = ___________________ mm 345 kl = ________________________ml 6) 957.95 mg = _____________________ kg You should be able to use a ruler, scale, and graduated cylinder to find length, mass, and volume respectively. Calculate density, volume or mass given the other 2 variables in a word problem. ~A block of lead has dimensions of L=5.12 cm by W=3.45 cm by H=? The block weighs 1201 g. The density of the block is 1.1303 g/cm3. From this information, calculate the height of the block. ~The mass of a beaker is 45.5g. When a marble is placed in the beaker, its combined weight of the beaker and marble is 64.5g. Also, when a graduated cylinder is filled with water to 10ml of water, and the marble is dropped in, the water level rises to 25.5ml. What is the density of the marble? Calculate density of irregular objects using tools available in class TOPIC C: INTRO TO MATTER 1. What is Matter? 2. What is the difference between a pure substance and a mixture? 3. Know the difference between an atom, molecule, compounds, mixture and examples of each. 4. What are the 3 phases of matter? Sketch and explain how they differ. 5. What is the difference between a physical and a chemical property? Give 3 examples of each. 6. What are intensive vs. extensive physical properties and examples CHEMICAL AND PHYSICAL CHANGES 1. What is a chemical change? Give 2 examples from labs we have done or the webquest. 2. What is a physical change? Give 2 examples from labs we have done or the webquest. 3. What are 4 signs that indicate a chemical change has occurred? SOLUTION, COLLOID AND SUSPENSION 1. What is a true solution? 2. How do particle sizes differ between solutions, colloids and suspensions? 3. What are 2 tests that you can perform to see if you have a solution, colloid or suspension? 4. What is the Tyndall effect? Of the 3 types of mixtures in the lab, which will always show the tyndall? 7. Of the 3 types of mixtures, which will always separate upon standing? PRACTICE: 1) For the following things give the proper state of matter (solid, liquid, gas) CO2 H20 Sugar metal steam Fixed Shape and Fixed Volume No Fixed shape No fixed shape and no fixed volume 2) What is matter? 3) Identify the following as intensive or extensive properties: Color Mass Volume Malleability Conductivity Length Reactivity 3) ID the following as Mixture, Element or Compound: water sand in water salt water oil in water milk carbon carbon dioxide hydrogen 1. What is the Tyndall effect? What type of solutions show tyndall? 2. What is a period on the periodic table? Give one example 3. What is a group on the periodic table? Give one example 4. How many valence electrons does Carbon have? Boron? Neon? Out of the 3, which is the most stable and why? 5. How many outer shells does Hydrogen have? Helium? Silicon? 6. Metals are likely to (give up or take) electrons while non-metals are more likely to (give up or take) electrons. 7. What are 4 properties of metals? ALCHEMY 1. What is Alchemy? 2. Why was Alchemy important to science? 3. What part of alchemy were you trying to re-create with the penny lab? 4. What results did you use to determine that the substance you created was not actually gold? 5. What is an alloy? 6. Define all the parts of an atom: protons, neutrons, electrons, nucleus, electron cloud. TOPIC D: PERIODIC TABLE How is the periodic table arranged that makes it very convenient? Make sure to be able to differentiate between Metals, Nonmetals, and Metalloids. How can you test a substance to determine whether you have Metals, Nonmetals, and Metalloids. List all of the element groups of the periodic table below. Make sure to describe MANY of the common characteristics of each group, and know where each group is located. Draw the Bohr Diagram of each of these atoms: a) Calcium b) Oxygen c) Fluorine d) Magnesium RADIOACTIVTY What is radioactivity? What is radioactive decay? What are the 3 types of radioactive decay? TOPIC E: Gas Laws List all of the Gas Laws equations you need to know. Go to your gas laws worksheets/quizzes and practice some gas laws problems. Gases have all of the following properties except: a. They are compressible b. Will fill an entire container c. Particles have a large volume d. They change direction upon collision As pressure goes up, volume: a) Goes down b) Goes Up c) Stays the same d) Explodes As temperature goes up, volume: a. Goes down b. Goes up c. Stays the same d. Explodes The unit for temperature in gas laws equations is: a. Farenheit b. Celcius c. Kelvin TOPIC F: Energy and Energy Transformations What is Kinetic Energy? What is Potential Energy? How are they calculated? (What are the equations?) What variables are used, how are they determined, what units to you use for each? Be able to do a series of word problems (like on your practice sheets and quiz sheet) What is weight, and how is it different from mass? What are the units for weight? What are the units for energy? TOPIC F: Kinematics What is kinematics? What are the 5 variables we use in kinematics? Given a particular word problem, you need to determine what variables are present, and figure out how to solve for the other variables not present using your formula sheet. Know how to pick the appropriate equation from your equation sheet given a problem Directions: Use the information below and your kinematics equations sheet to find the 2 missing variables for each interval (A, B, C…). Remember D= Displacement, NOT DISTANCE. Show your work below each description to find the 2 missing variables and fill your answers in the chart below. Scenario: Mr. Bell thinks he is King Shiz on the streets, and is a master rollerblader (not the kind where you do tricks, but the kind where you have a headband, tights, and a walkman). This is the story of his unfortunate rollerblading incident. Mr. Bell was getting ready to cruise down the boardwalk in his sweet rollerblades, wearing his favorite neon green singlet, matching headband, and his tape player, stocked with smooth jazz by Kenny G. Interval Information: A: He starts from rest and begins to accelerate at 2.5m/s2 for 8 seconds. B: Then Mr. Bell sees that he is over the rollerblading speed limit and slows down to 14 m/s in 2 seconds. (Hint: you might need to use the final velocity of your previous situation as your initial in this situation and subsequent situations!) C: Then he cruises at a constant velocity(this tells you a relationship about initial and final velocity). He then maintains his velocity for 10 seconds. D: He then slows down for 5 seconds to avoid hitting Snooki who is “trying to find the beach”, but instantly comes to a complete stop when he runs into the formidable Snooki as she drops him cold with a closeline. PRACTICE: KINEMATIC EQUATION WORD PROBLEMS Know how to find initial, average, and final velocity. Given a graph of either time vs distance, time vs velocity, or time vs acceleration, be able to get information about the other variables from these graphs. o What does the slope on a T vs D graph tell us? o What does the slope on a T vs V graph tell us? o What does the slope on a T vs A graph tell us? o What does the concavity on a T v D graph tell us? o What does the concavity on a T v V graph tell us? Make sure you understand what is happening in a free fall situation. Write what you know about freefall below. Make sure you can set up a problem where a ball is tossed in the air and falls back down. Diagram that situation below with all the variables you know PRACTICE: INTERPRETING GRAPHS Scenario: The graph above describes an unfortunate situation. Ryan was very excited because he got a brand new Schwin bicycle for his 17th birthday, complete with a horn and a basket. He named his bike Flash. Ryan was riding Flash down the street a cruising speed until he came to a red light. He stopped at first, but then decided to gun it, at which point he was hit by an 18 wheeler and was thrown off his bike backwards flipping through the air, until he came to rest when he hit the ground. Ryan got up and the only thing that was hurt was his pride, and his prized possession, Flash. Don’t run redlights Ryan. 15 D d (m) C 10 E B 5 A 5 10 15 20 t (sec) Directions: Based on question, answers require a specific interval (Letter), distance, velocity, or time. 1) _____During which interval did Ryan have positive acceleration? 2) _____How long was Ryan at rest during the interval? 3) _____What interval did Ryan have negative velocity? 4) _____At what time did Ryan get hit by the truck? 5) _____How far did Ryan fly when he got hit by the truck? 6) _____During what interval did Ryan have positive velocity, and zero acceleration? 7) _____How long was Ryan moving forward throughout the entire scenario? 8) _____At what time did Ryan hit the ground? 9) _____How fast was Ryan cruising during interval A? 10) Draw a line on the graph that you could use to approximate Ryan’s velocity at 9 seconds. 11) Put a star on the graph where Ryan had the highest velocity. 12) Put a title on the graph. Make sure to include the necessary components. Velocity v. Time of a Mr. Bell Driving His Whip 15 30 C D E V 10 (m/s) 20 B 20 10 A 13) 10 15 20 14) t (sec) 13) _____During what interval does the car have positive acceleration? 14) _____During what interval(s) does the car have positive velocity? a. B only b. B, C, and E only c. All intervals d. None of the intervals 15) _____ During what interval did Mr. Bell slam on his breaks to avoid getting a ticket? 16) _____ During what interval(s) was the acceleration equal to zero? a. B only b. C, and E only c. All intervals d. None of the intervals e. C, D, and E 17) _____ The speed limit was 20 m/s, how long was Mr. Bell speeding? a. 0 seconds b. 9.5 seconds c. 10 seconds d. 10.5 seconds 18) How far did Mr. Bell travel between the interval 5 and 10 seconds? 5 Answer: ___________ 19) Estimate Mr. Bell’s Instantaneous Acceleration at 4 seconds using the interval method we have done in 𝚫𝒚 class (2 points). Hint: Slope is equal to 𝚫𝒙. You need to look at points on the graph to get your info to answer this!!! Answer: ___________ ______1. When you look at the speedometer in your car, you can see the car’s: A. Velocity B. Average speed C. Instantaneous speed ______2. Sam rides his bicycle 20 km. If it takes him 1 hour, what is his average speed? A. 20 km/hour B. 5 km/hour C. 1 km/hour ______3. Suppose you took a trip that covers 40 km. If it takes you 2 hours, what is your average speed? A, 5 km/hour B . 8 km/hour C. 20 km/hour ______4. Shelley runs 36 meters in 9 seconds. Her average speed is A. 9 m/sec B. 10 m/sec C. 4 m/sec ______ 5. Which of the following is a unit of speed? A. meters B. meters/sec C. seconds _______6. Velocity is different from speed in that A. velocity is a change in speed B. velocity involves the force of gravity C. velocity is speed plus direction _______7. A car’s velocity changes if it: A. speeds up or slows down B. turns a corner C. Both A and B _____ 8. Acceleration is defined as: A. distance traveled divided by time B. the rate of change in velocity of an object C. a change in direction _____9. Your car accelerates if it A. changes speed B. changes direction C. Both A and B Distance Time _____ 10. The graph above shows A. An object that is not moving B. An object that is moving at a constant velocity C. An object that is accelerating _____ 11. Friction is a force that A. opposes an object’s motion B. decreases with large mass C. is less when a surface if rough ______12. A force A. is expressed in Newtons (N) B. is a push or a pull C. Both A and B ______ 13. The upward force that a table exerts on a book is called A. frictional force B. gravitational force C. normal force _______ 14. When forces on an object are balanced, the object A. moves B. does not move C. accelerates _______ 15. Gravity is A. a force of attraction between objects B. the same on all planets C. largest in small objects TOPIC F: Newton’s Laws Know all three (be able to state them each way possible) of Newton’s Laws Be able to describe what Newton’s Laws are present in a given scenario Refer to your project sheet.