Study Guide for Chemistry EOC Assessment
advertisement

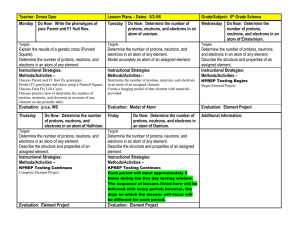
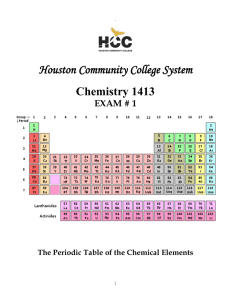
Study Guide for Chemistry EOC Assessment Your test will consist of multiple-choice questions over the following topics. 1. Metric units for mass, length, volume 2. Metric to metric conversions (K H Da m/l/g D C M) 3. Know how to put numbers in scientific notation and take them out. 4. Significant Digit rules 5. Difference between chemical and physical properties and changes. There will be multiple questions on this. 6. Ways to tell if a chemical change (chemical reaction) is taking place. 7. Pure substances and mixtures a. heterogeneous mixture b. homogeneous mixture 8. Density & how it relates to floating and sinking 9. Use the equation for density Density = Mass/volume (g/ml) (g) (ml) 10. Know the phases of matter and the characteristics of each phase. 11. Law of Conservation of Matter 12. What is a mole in chemistry? 13. Convert grams to moles, and moles to grams 14. Define an atom 15. Structure of an atom and where each subatomic particle is located. a. Nucleus (contains protons and neutrons) b. Protons (positive) c. Neutrons (neutral) d. Electrons (negative) 16. Isotopes 17. Elements 18. If given atomic # and mass, find the number of protons and neutrons in an atom. 19. Find the number of valence electrons, and tell what it says about reactivity. 20. Periodic Table a. Know about the families b. Group vs. period 21. Know about the properties of metals and nonmetals. a. Ductile, malleable, conductors 22. Ionic & Covalent Compounds How do they form? (Transfer or gain electrons) Characteristics of Each 23. Be able to name ionic compounds 24. Predict oxidation numbers 25. Chemical Equations: Know the parts of an equation 5 main types of reactions-know about them and be able to identify them (Synthesis, Decomposition, Single Displacement, Double Displacement, Combustion) 26. Acids & BasesMacro and Microscopic properties of each What ions do they form, how do they affect litmus, what is their pH range? Identify examples of each- Mono, di, and polyprotic acids Tell if they are strong or weak Acid-base indicator