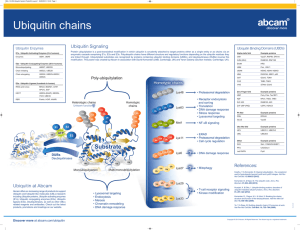
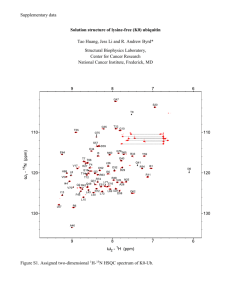
DEUBIQUITYLASES FROM GENES TO ORGANISM
advertisement

Physiol Rev 93: 1289 –1315, 2013 doi:10.1152/physrev.00002.2013 DEUBIQUITYLASES FROM GENES TO ORGANISM Michael J. Clague, Igor Barsukov, Judy M. Coulson, Han Liu, Daniel J. Rigden, and Sylvie Urbé Cellular and Molecular Physiology, Institute of Translational Medicine, and Institute of Integrative Biology, University of Liverpool, Liverpool, United Kingdom Clague MJ, Barsukov I, Coulson JM, Liu H, Rigden DJ, Urbé S. Deubiquitylases From Genes to Organism. Physiol Rev 93: 1289 –1315, 2013; doi:10.1152/ physrev.00002.2013.—Ubiquitylation is a major posttranslational modification that controls most complex aspects of cell physiology. It is reversed through the action of a large family of deubiquitylating enzymes (DUBs) that are emerging as attractive therapeutic targets for a number of disease conditions. Here, we provide a comprehensive analysis of the complement of human DUBs, indicating structural motifs, typical cellular copy numbers, and tissue expression profiles. We discuss the means by which specificity is achieved and how DUB activity may be regulated. Generically DUB catalytic activity may be used to 1) maintain free ubiquitin levels, 2) rescue proteins from ubiquitin-mediated degradation, and 3) control the dynamics of ubiquitin-mediated signaling events. Functional roles of individual DUBs from each of five subfamilies in specific cellular processes are highlighted with an emphasis on those linked to pathological conditions where the association is supported by whole organism models. We then specifically consider the role of DUBs associated with protein degradative machineries and the influence of specific DUBs upon expression of receptors and channels at the plasma membrane. L I. II. III. IV. V. VI. VII. VIII. IX. INTRODUCTION CATALYTIC MECHANISM DUBOMICS CLEAVAGE SPECIFICITY REGULATION OF DUB ACTIVITY THE FIVE DUB FAMILIES: ROLES IN... DUBs AS INTEGRAL COMPONENTS OF... INFLUENCE ON CELL PHYSIOLOGY... CONCLUSIONS 1289 1290 1291 1291 1293 1293 1305 1307 1308 I. INTRODUCTION The ubiquitin-proteasome system (UPS) is firmly established as an all-pervasive regulator of cellular function in eukaryotes (73). The tagging of proteins with the 76-amino acid polypeptide ubiquitin not only provides a signal for protein degradation, but also a reversible posttranslational modification used to modulate enzymatic activity and protein interactions. The deubiquitylases (DUBs) are a family of ⬃90 enzymes that control the cellular flux of ubiquitin by its removal from substrate proteins (13, 120, 187, 209, 210). In this review, we shall detail the five subfamilies of mammalian DUBs. We provide an updated analysis of their sequence and structural features. We discuss their cellular and organismal expression patterns and highlight associations with disease. Finally, we turn to their control of the cellular response to its environment, through influences upon the trafficking and activity of receptors and channels. The seven internal lysine residues intrinsic to ubiquitin (Lys 6, 11, 27, 29, 33, 48 and 63) all provide sites for the gen- eration of an isopeptide bond with the COOH terminus of another ubiquitin (FIGURE 1A). Linear ubiquitin chains, linked by peptide bonds between the NH2 and COOH termini, can be generated de novo as well as being directly translated from two of the four pro-ubiquitin genes (269). Types of polyubiquitin chains display varying topologies, illustrated by the dispositions of the proximal (free COOH terminus) ubiquitin compared with the distal ubiquitin in the overlaid crystal structures for five varieties of di-ubiquitin molecules so far determined (FIGURE 1B) (75, 133). Structures of linear ubiquitin and Lys63linked di-ubiquitin suggest open chain configurations that have been likened to “beads on a string.” Others are more compacted, providing restricted access to some surfaces. This is illustrated in FIGURE 1C for a hydrophobic patch centered around Ile44, which is often critical for protein interactions. In solution, a number of conformations likely exist in dynamic equilibrium for a given chain type, which can be selected for and stabilized by proteinprotein interactions (26, 263, 288). Polyubiquitin chains may be homogeneous assemblies of a single linkage type or heterogeneous linkages, which can include more than one linkage type to a common proximal ubiquitin (branched chains). In principle, DUBs can hydrolyze ubiquitin chains from the ends (exo-activity) or from within the polymer (endo-activity). Throughout this review the standard protease nomenclature of Schechter and Berger is adopted; interaction sites associated with the substrate are denoted as P1, P2 etc NH2 terminal to the scissile bond and P1=, P2= etc towards the COOH terminus, 0031-9333/13 Copyright © 2013 the American Physiological Society 1289 CLAGUE ET AL. A B K63 C M1 lin K48 K6 K29 K63 K33 K27 K11 K48 lin K63 K6 ub K11 C-term K11 K6 K48 FIGURE 1. Structures of ubiquitin chains. A: structure of ubiquitin (PDB ID 1ubq) in cartoon representation showing the side-chain positions of all seven Lys residues and Met1 (orange). B: superposition of the proximal ubiquitin molecules of di-ubiqutin structures on the mono-ubiquitin oriented as in A. Chain types are represented by different colors: mono-ubiquitin, green; linear chain (PDB ID 2w9n), cyan; K6 (PDB ID 2xk5), magenta; K11 (PDB ID 2xew), yellow; K48 (PDB ID 1aar), bronze; and K63 (PDB ID 2jf5), gray. C: surface representation of di-ubiquitin and mono-ubiquitin structures in the same orientation as in B. Hydrophobic I44 patch is highlighted in blue, proximal ubiquitin is colored in green in each molecule and distal ubiquitin colored as in B. with the corresponding sites on the enzyme denoted S1, S2 and S1=, S2= etc (226). The ubiquitin conjugated to substrate protein defines the proximal ubiquitin of a chain. Thus, when the proximal ubiquitin is being cleaved, the P1=, P2= sites correspond to DUB binding sites on the ubiquitylated protein itself. The complexity of ubiquitin chain types bears analogy to protein glycosylation, but its significance is still under active consideration (122, 133). Proteomic studies reveal that each of the isopeptide linkage types is represented to a significant extent in both yeast and mammalian cells (54, 117, 196, 282, 298). The attachment of ubiquitin chains with any isopeptide linkage excepting Lys63 appears to target substrates to the proteasome in vivo (54, 172). Lys63 linkages may be particularly important in targeting endosomal proteins to the lysosome (38, 142), whilst linear ubiquitin chains play a critical role in the NFB signaling pathway (269). One prevailing notion is that these different linkage types may provide some specificity for interaction with proteins containing different ubiquitin binding domains, particularly when such domains are organized in tandem (182, 204). 1290 II. CATALYTIC MECHANISM The DUBs can be subdivided into five families based on the architecture of their catalytic domains: ubiquitin specific proteases (USPs), ubiquitin COOH-terminal hydrolases (UCHs), ovarian tumor proteases (OTUs), Josephins, and the JAB1/MPN/MOV34 family (JAMMs). The first four families are cysteine proteases, which rely on a catalytic triad of conserved amino acids, in common with classical cysteine proteases such as papain (245). A nearby histidine lowers the pKa of the catalytic cysteine residue facilitating a nucleophilic attack, whilst a third residue (Asp or Asn) is normally required for alignment and polarization of this His residue. One feature of this mechanism is the formation of an acyl intermediate by the covalent linkage between the cysteine and the carboxyl group, which will be generated upon scission (FIGURE 2A). Whilst the cysteine protease DUBs have divergent catalytic domain structure, once bound to the ubiquitin COOH terminus, the catalytic residues superpose with little deviation (119). In distinction, JAMMs are Zn2⫹ metalloproteases, in which invariant His, Asp, and Ser residues coordinate the catalytic zinc. The reaction mechanism is predicted to be similar to other met- Physiol Rev • VOL 93 • JULY 2013 • www.prv.org DUBs FROM GENES TO ORGANISM alloproteases such as thermolysin (FIGURE 2B). The catalytic zinc ion is coordinated by two His, an Asp, and a water molecule. A neighboring Glu accepts a proton from the water molecule leaving a hydroxyl ion, which attacks the substrate bond at the carbonyl carbon. The transient tetrahedral intermediate, which is formed, collapses resulting in scission, with the hydroxyl group from the water replacing the leaving group at the COOH terminus of the distal ubiquitin (FIGURE 2B). III. DUBOMICS Aside from the catalytic domain, DUBs often contain other domains and shorter structural motifs, which may regulate activity and govern interactions. This is most prominent for the USP family. We provide an updated set of annotations across the DUB superfamily arranged according to sequence homology of their catalytic domains (FIGURE 3). This includes several newly recognized structures, such as PH domains in USPs 26, 29, and 37, specific insertions within the catalytic domain (289) and intrinsically disordered regions that are statistically more likely to provide sites for posttranslational modifications and proteinprotein interactions. TABLE 1 provides an overview of the counterparts to human DUBs that are found in other species routinely used in experimental biology, revealing a core set of 16 DUB family members common to humans, Danio rerio (zebrafish), Drosophila melanogaster, Caenorhabditis elegans, and at least one or other commonly used strain of yeast (additional accession information is provided in Supplementary Table 1. The online version of this article contains supplementary material.). We provide a comprehensive analysis of the evolutionary relationships between family members in Supplementary Figure 1. A systematic study of the 41 Drosophila DUBs has revealed key roles in development, adult motility, and longevity (260), while a morpholino-based functional screen has identified 57 DUBs that play key roles in early development of zebrafish (259). Quantitative proteomic analysis reveals that protein copy numbers of individual DUBs span several orders of magnitude in model cell lines such as Swiss 3T3 cells (79, 231) (FIGURE 4A). Highly expressed DUBs (⬎300,000 copies/cell) include components of the proteasome and COP9 signalosome, UCHL1 and UCHL3, which suppress the formation of ubiquitin adducts, and OTUB1, which is the most abundant active DUB represented in 3T3 cells. With the exception of UCHL1, these proteins tend to be highly expressed across all cell lines that have been scrutinized at the proteomic level (79, 231). While some expressed DUBs may be below the detection limit by current mass spectrometry, copy numbers in the low hundreds have been determined. Examples include the mitochondrial DUB, USP30, and the sumoylation target USP25 (173, 181, 231). Three systematic studies have mapped the subcellular distributions of human DUBs in cultured cells and the comple- ment of DUBs found in Saccharomyces pombe (128, 244, 261). FIGURE 4B illustrates prominent subcellular sites of accumulation for individual mammalian DUBs, derived from these studies and from more specific investigations. At the organismal level, based on transcriptional profiling, many DUBs are relatively overexpressed in the brain, hematopoietic system, and reproductive organs. FIGURE 5 provides a preliminary map of tissue “hot spots” for particular DUBs. Examples of transcriptional control of DUBs include vasopressin and aldosterone induction of USP10 and USP245, respectively (24, 68). IV. CLEAVAGE SPECIFICITY A. Chain Linkage Specificity One aspect of the appendage of particular polyubiquitin chain types to substrate proteins is that it can then restrict their processing to chain-specific deubiquitylase activities (TABLE 2). Early studies indicated a stringent preference of the JAMM family member, AMSH, for Lys63-linked chains over Lys48 chains (170, 171), which has now been extended to exclude activity towards other chain types (27, 123). Several other JAMM family members such as AMSH-LP and BRCC36 have similar selectivity for Lys63linkages (45, 70, 225). A corollary of this stringency is that these DUBs may be unable to efficiently remove the proximal ubiquitin moiety that is directly attached to substrate proteins. However, other JAMMs such as MYSM1 and POH1 are capable of making this cleavage (284, 297). The availability of substrates bearing all eight simple ubiquitin chain linkage types has prompted more extensive surveys of DUB specificity in vitro (27, 67, 123, 154, 268) (TABLE 2). In general, while the USP family of proteins displays variations in kcat and Km over orders of magnitude, they exhibit little specificity for particular isopeptide chain linkages (67, 123). Notable exceptions are provided by USPL1 which is a SUMO-specific protease (230), USP18 which may be specific for the ubiquitin-like modifier, ISG15 (164) and CYLD which shows a marked preference for the open conformation presented by Lys63 and linear ubiquitin chains (27, 123). The ubiquitin binding site of USP family enzymes such as USP21 contact Lys6, which precludes endoactivity for Lys6-linked chains. USPs can only interact with the distal end of this particular ubiquitin chain type and process it sequentially. In contrast, the OTU family protein OTUD3 can cleave such chains at any position (95). Remarkably, the OTU family catalytic domains display a wide diversity in chain preferences, despite a high degree of structural conservation. For example, OTUB1 and OTUB2 show opposite proclivities for Lys63- and Lys48-linked chains, respectively, while Cezanne/OTU7B and TRABID are most active towards Lys11- and Lys29-linked chains, respectively (27, 60, 154, 268, 270). One study has suggested that the Josephin protein Ataxin-3 shows a Physiol Rev • VOL 93 • JULY 2013 • www.prv.org 1291 CLAGUE ET AL. A a Proximal Lys Ubiquitin H O N C Distal Gly Isopeptide Ubiquitin bond Catalytic triad O S1' Asp S1 S– O– Cys NH+ HN His DUB (USP) d c b Attack Lys Ubiquitin H O N C Tetrahedral intermediate Gly Lys Ubiquitin O Ubiquitin S– – O Asp H O– N C O O Cys NH+ HN Oxyanion hole Asp NH3+ Lys O Ubiquitin C Gly Ubiquitin S O Release – S O– Cys NH+ HN His Asp HN His DUB (USP) Gly Ubiquitin Cys N His DUB (USP) DUB (USP) Acyl enzyme intermediate e f g O Oxyanion hole Deacylation (water attack) O O C H Gly Ubiquitin S Tetrahedral intermediate O– Asp HO Cys N Asp HN His a Ubiquitin O N Isopeptide bond Release S– – O NH Cys + Asp Cys NH+ HN His DUB (USP) DUB (USP) Distal H Proximal Lys63 Catalytic triad Gly Ubiquitin S His DUB (USP) B C O Gly Ubiquitin O– HN C O– O H HO C Gly O Ubiquitin Active site S1' Glu O– H O O H 2+ S1 HO Zn His Ser His Asp AMSH-LP b c d NH3+ Lys63 H N Lys63 Ubiquitin Glu – O O His O H H 2+ 1292 Gly O Ubiquitin HO Zn His Asp AMSH-LP Ubiquitin H C Ser Lys63 Ubiquitin Glu N C Gly HO O– Ubiquitin OH O His 2+ – HO Zn Ser Release Glu His Asp AMSH-LP Physiol Rev • VOL 93 • JULY 2013 • www.prv.org O C Gly O Ubiquitin Release O– O His 2+ HO Zn His Asp AMSH-LP Ser DUBs FROM GENES TO ORGANISM preference for Lys63- over Lys48-linked chains, but that branched chains containing both linkages provide a preferred substrate, from which Lys63 linkages are preferentially processed (279). B. Discrimination Between Ubiquitin and Ubiquitin-like Modifications Several ubiquitin-like proteins including ISG15, Nedd8, and SUMO modify proteins using similar mechanisms to ubiquitin itself (94). Whilst these all present ubiquitin-like folds, DUBs are nevertheless able to discriminate between these molecules. In some cases (SUMO, Atg12, FAT10), this is determined by divergent amino acid sequences adjacent to the COOH-terminal Gly-Gly residues, a region which corresponds to 71LRLRGG76 in ubiquitin. However, a high degree of similarity to ubiquitin in this region for Nedd8 (contains Ala72 for Arg72) and identity in the case of ISG15 allow for cross-reactivity (5). USP21 shows activity towards ubiquitin and ISG15 but not Nedd8. The structure of USP21 in complex with a noncleavable form of linear di-ubiquitin aldehyde provides an explanation of this specificity that can be generalized to the entire USP family. The Arg72 residue of ubiquitin interacts with an invariant Glu residue (Glu304 in USP21) to enhance affinity but furthermore, modeling of Nedd8 into the ubiquitin S1 site also results in steric clashes and charge repulsion (287). V. REGULATION OF DUB ACTIVITY Like most cellular enzymes, the activity of DUBs can be controlled through multiple mechanisms. Several DUBs require assembly into large multimolecular complexes for full activation, exemplified by the proteasomal DUBs which are discussed in detail below. Another example is provided by the allosteric activation of USP22 by multiple components of the SAGA coactivator complex (118, 138, 223). Simpler instances of allosteric regulation are found with the increase in kcat following interaction of USP1, USP12, and USP46 with UAF1 (WDR48) (40, 41). However, the interaction between USP1 and UAF1 is itself regulated by CDK1-mediated serine phosphorylation of USP1 (267). The COOHterminal region of USP7 contains 5 Ubiquitin-like (Ubl) domains organized into 2-1-2 Ubl units, the last pair of which (HUBL-45) activate USP7 by 100-fold (66, 71). The metabolic enzyme GMPS binds to the first three Ubl domains and hyperactivates USP7 by stabilization of the HUBL-45 interaction with the catalytic domain (66). Other examples of intramolecular domains influencing activity include an increase in Km of USP4 mediated by a Ubl domain inserted within the catalytic domain itself (160) and an increase in kcat of USP16 attributable to its ZnF-UBP domain (67). Interaction of DUBs with proteins bearing ubiquitin binding domains, exemplified by the interaction between the endosomal DUBs AMSH and USP8 with components of the ESCRT-0 complex, can enhance activity by providing more effective capture of substrate and reducing the apparent Km (171, 218). Cross-talk between phosphorylation and ubiquitin modification is a significant aspect of intracellular signaling networks (101, 174). In a most direct case, the enzymatic activity of OTUD5 (DUBA) is entirely contingent on phosphorylation at a single serine residue, which interacts directly with the COOH-terminal tail of ubiquitin (97). Differential phosphorylation of USP8 at S680 and dephosphorylation of USP37 during M-phase of the cell cycle correspond with enhanced and reduced activity, respectively (100, 176, 190). USP8 also undergoes translocation to endosomes following acute EGFR stimulation (219), whilst USP4 translocates from nucleus to cytoplasm following phosphorylation by Akt (290). These examples illustrate a further mechanism of regulation, by dynamically changing the palette of associates and substrates to colocalizing proteins. Other posttranslational modifications are emerging as modifiers of activity, including ubiquitylation itself. Ubiquitylation of Ataxin-3 in the vicinity of the catalytic site enhances its activity (255), whilst sumoylation of USP25 inhibits activity (173). Interestingly, the catalytic cysteine of the cysteine protease DUBs is widely subject to reversible inactivation by modification with reactive oxygen species (ROS), similarly to protein tyrosine phosphatases (48, 62, 132, 144, 217). VI. THE FIVE DUB FAMILIES: ROLES IN HEALTH AND DISEASE In the following section we discuss the major characteristics of each subfamily of DUBs, highlighting examples FIGURE 2. Reaction mechanisms. Schematic overview of USP (A) and JAMM (B) action upon di-ubiquitin substrates. Covalent, isopeptide, and noncovalent bonds are shown by black solid, red solid, and orange dashed lines, respectively. A, a: conserved residues form a catalytic triad. Nearby S1= and S1 sites accommodate the proximal and distal ubiquitin units, respectively. b: The deprotonated thiol group carries out a nucleophilic attack on the carbonyl carbon forming a negatively charged tetrahedral intermediate (c), with the negatively charged O⫺ occupying the oxyanion hole where it is stabilized by hydrogen bonding (not shown). d: Collapse of the tetrahedral intermediate results in scission and release of the distal ubiquitin leaving an acyl-enzyme intermediate. e: A water molecule attacks the acyl-enzyme intermediate, resulting in another negatively charged intermediate shown in f. g: Proximal ubiquitin is released, and the original configuration of the catalytic triad is recovered. B: metalloprotease activity of JAMM family members exemplified by AMSH-LP. a: Conserved residues coordinate zinc and water. b: AMSH-LP specifically binds to lysine-63-linked ubiquitin chain, through S1 and S1= sites allowing the hydroxyl group of active site water molecule to carry out nucleophilic attack on the carbonyl carbon between two ubiquitin molecules, forming a tetrahedral intermediate shown in c. d: Collapse of intermediate leads to scission and product release. Physiol Rev • VOL 93 • JULY 2013 • www.prv.org 1293 CLAGUE ET AL. of particular physiological significance or where linkage to disease is well established. Generically DUB catalytic activity may be used to 1) maintain free ubiquitin levels, 2) rescue proteins from any of the ubiquitin-mediated 1294 degradation pathways (proteasomal, endosomal, and autophagosomal), and 3) control the dynamics of ubiquitin-mediated signaling events (120). FIGURE 6 and Supplementary Table 2 provide a summary of DUBs linked to Physiol Rev • VOL 93 • JULY 2013 • www.prv.org DUBs FROM GENES TO ORGANISM FIGURE 3.—Continued disease through mutational or expression analysis. We also highlight examples where mouse models have provided significant physiological insight and further collate this information in TABLE 3. A. USP Family The USPs are the largest family of DUBs and contain an assortment of accessory domains (FIGURE 3). Several of FIGURE 3. Schematic representation of domain architectures of human DUBs. Domains, drawn approximately to scale, were assigned by HHsearch (240) analysis against the CDD (166), Pfam (202), and SMART (149) domain databases and against structures in the PDB (216). Regions indicated in pink are those of greater than 20 residues predicted as intrinsically disordered by IUPRED (59). Since short UIMs were not reliably predicted with HHsearch, consensus predictions for these were made considering Pfam search results and local searches of PROSITE (238) with ScanProsite (78). Some homologs of zinc fingers and EF-hand structures are predicted to lack metal binding capacity due to their lack of key ligating residues. Domains unique to PRPF8 are numbered as follows: 1) PRO8NT, 2) PROCN, 3) RRM_4, 4) U5_2-snRNA_bdg, 5) U6-snRNP_bdg, 6) PRP8_IV, 7) PROCT. For some sequences, certain stretches of amino acid (e.g., ⫹100) are not shown. Names used correspond to NCBI-approved gene symbols except for those listed below. The expanded USP17 gene family is represented by one member, DUB3, officially known as USP17-like protein 2. POH1 (PSMD14), AMSH (STAMBP), AMSH-LP (STAMBPL), YOD1 (OTU1), A20 (TNFAIP3), TRABID (ZRANB1), Cezanne (OTUD7B), Cezanne 2 (OTUD7A). Enzymes indicated by (*) are predicted to be inactive based on sequence or structural (PRPF8) analysis. Note that EIF3F has been proposed to deubiquitylate Notch (178) and USPL1 is a SUMO-specific protease (230). **HIN1L is annotated as a hypothetical protein. DUBs are ordered according to bootstrapped neighbor-joining phylogenetic analysis of their catalytic domains [using MEGA (249): see legend to Supplementary Figure 1 for detailed methods] shown as a tree to the left. Nodes supported by bootstrap values of ⬎50% are indicated with a dot. Physiol Rev • VOL 93 • JULY 2013 • www.prv.org 1295 CLAGUE ET AL. Table 1. Proposed orthologs of human DUBs assigned in silico as bidirectional best hits (9, 280) against the species shown by reciprocal BLAST analysis (10) USP1 USP2 USP3 USP4 USP5 USP6 USP7 USP8 USP9Y USP9X USP10 USP11 USP12 USP13 USP14 USP15 USP16 USP17L2 USP18 USP19 USP20 USP21 USP22 USP24 USP25 USP26 USP27X USP28 USP29 USP30 USP31 USP32 USP33 USP34 USP35 USP36 USP37 USP38 USP39 USP40 USP41 USP42 USP43 USP44 USP45 USP46 USP47 USP48 USP49 USP50 H. sapiens D. rerio C. elegans S. pombe S. cerevisiae O94782 O75604 Q9Y6I4 Q13107 P45974 P35125 Q93009 P40818 O00507 Q93008 Q14694 P51784 O75317 Q92995 P54578 Q9Y4E8 Q9Y5T5 Q6R6M4 Q9UMW8 O94966 Q9Y2K6 Q9UK80 Q9UPT9 Q9UPU5 Q9UHP3 Q9BXU7 A6NNY8 Q96RU2 Q9HBJ7 Q70CQ3 Q70CQ4 Q8NFA0 Q8TEY7 Q70CQ2 Q9P2H5 Q9P275 Q86T82 Q8NB14 Q53GS9 Q9NVE5 Q3LFD5 Q9H9J4 Q70EL4 Q9H0E7 Q70EL2 P62068 Q96K76 Q86UV5 Q70CQ1 Q70EL3 F1QNF4 E7FEC1 E7FC36 Q8I077 Q9VR54 Q1MT86 Q9VZU7 P91502 Q11119 P38237 I3ISS5 I3IT16 Q9VYQ8 Q9VDD8 Q7JKC3 G5EBW2 Q9UTT1 P50101 P32571 Q4FE55 E7F2D1 F1QPF4 A4FUN7 F1QFS9 F1R4B7 E7EZD6 A8HAL1 P55824 Q9W0L7 O94269 Q01477 E7EYZ1 F1RCS0 A5PN09 E9QFQ5 A6H8I0 F1Q6K1 E7FFB3 A2BGT0 E7F6P1 E7FCR5 A5PMR2 A8WFZ5 E7F4C0 E7F6T8 F1RBA1 A3KQ59 F1Q7D2 F1QSB4 F1Q8C6 Q7ZUM8 E9QG68 A5WWB0 H9GYG8 E7EY58 D. melanogaster I2HA92 Q9P7V9 Q9VKZ8 Q17361 Q92353 O60079 Q7JW61 P43593 P39538 P53874 Q9VVR1 Q09738 P50102 Q9P3U0 Q01476 G5EG81 Q09931 Q9W462 Q9BKQ6 Q9VW49 Q9VC56 Q7JQI1 Q9VRP5 Q9VWP1 O74442 O44787 Q9USR2 P43589 Q9W117 Q9W4C3 Q9VCT9 Q24574 Q9P7S5 P34547 Q22240 G5ECC7 P39967 Continued 1296 Physiol Rev • VOL 93 • JULY 2013 • www.prv.org DUBs FROM GENES TO ORGANISM Table 1.—Continued H. sapiens USP51 USP52 USP53 USP54 CYLD USPL1 UCHL1 UCHL3 UCHL5 BAP1 ATXN3 ATXN3L JOSD1 JOSD2 OTUB1 OTUB2 OTUD1 OTUD3 OTUD4 HIN1L OTUD5 OTUD6A OTUD6B YOD1 A20 Cezanne 2 Cezanne TRABID VCPIP1 BRCC3 COPS5 COPS6 POH1 PSMD7 AMSH AMSH-LP MPND MYSM1 PRPF8 EIF3F EIF3H Q70EK9 Q504Q3 Q70EK8 Q70EL1 Q9NQC7 Q5W0Q7 P09936 P15374 Q9Y5K5 Q92560 P54252 Q9H3M9 Q15040 Q8TAC2 Q96FW1 Q96DC9 Q5VV17 Q5T2D3 G3V0I6 Q7RTX8 Q96G74 Q7L8S5 Q8N6M0 Q5VVQ6 P21580 Q8TE49 Q6GQQ9 Q9UGI0 Q96JH7 P46736 Q92905 Q7L5N1 O00487 P51665 O95630 Q96FJ0 Q8N594 Q5VVJ2 Q6P2Q9 O00303 O15372 D. rerio E7FG33 E7FGV9 E7F383 E7FEV5 Q6DRC5 Q6YI49 Q504C0 Q6NWL6 A1L2G3 Q7ZU73* Q08C38 F1QCJ5 Q6DGP0 D. melanogaster C. elegans S. pombe S. cerevisiae A1Z7K9 P53015 Q09798 P53010 Q1W9Q0 Q8IPC5 Q7JMS4 Q10171 Q9UUB6 P35127 P35122 Q9XZ61 Q7K5N4 Q9UAV3 Q09444 O17850 Q9W422 Q9VL00 Q8IAA2 Q9XVR6 Q7JLI8 F1R341 F1QHE7 Q08BW0 Q9VTK7 O44438 Q7ZV00 Q567B1 E7F165 F1QRG1 Q9VUN9 Q9VRJ9 Q19681 A0JMQ9 F1QCM0 F1R395 Q6PC30 Q567F8* F1QHE5 Q7ZYX7 Q6TH47 E7F3M4 Q08CH3 Q5RGA4 F1R7K1 E7F3G6 Q6AXJ2 Q9VH90 Q9UUK3 O13974 P38747 P43558 O94454 Q12468 P41878 O74440 P43588 Q08723 Q9N4T5 Q9XZ58 Q9VCY3 Q9V3H2 P26270 P91001 Q95PZ0 O76577 O61792 Q9VA71 Q9VKJ1 A1Z8U0 Q9VN50 Q9U9Q4 Q9P371 P34369 Q18967 O01974 O14187 O43060 Q9UT48 P33334 Species are represented by reference proteomes retrieved from UniProt. In a few italicized cases, the reciprocal BLAST criterion was not satisfied for the human accession shown but for a different product of the same gene. Emboldened accessions in other species are not those retrieved by reciprocal BLAST but instead products of the same gene that are preferred for their greater length and/or reviewed status in UniProt. Asterisks mark two similar cases where the preferred accession shown is not the UniProt reference genome for D. rerio. these are drawn from inserts within the catalytic domain (289), which have been variously shown to influence activity (160) or localization (121, 254). Multiple USPs carry a ZnF-UBP binding domain, of which a subset (USP3, USP5, USP13, USP16, USP44, USP45, USP49), have been shown to (or by homology are predicted to) specifically recognize the free COOH terminus Gly-Gly motif of ubiquitin (21, 183, 193, 207). This interaction underpins the central function of the abundant USP5 (isopeptidase T) in processing newly synthesized linear polyubiquitin chains. It provides Physiol Rev • VOL 93 • JULY 2013 • www.prv.org 1297 CLAGUE ET AL. A PSMD7 OTUB1 EIF3F POH1 EIF3H CSN6 UCHL3 UCHL5 USP5 CSN5 PRPF8 USP14 USP9X OTUD6B USP7 USP47 USP15 BRCC3 OTUD4 AMSH USP4 USP10 USP19 VCPIP1 USP3 CYLD USP12 USP24 USP8 USP9Y USP36 USP34 USP48 USP32 USP25 USP40 USP30 102 103 104 105 106 Copy number/cell B USP1 USP36 USP6 pm USP3 USP39 USP19 USP7 er USP11 USP22 nucleus USP8 USP26 USP33 USP29 USP42 USP21 USP44 USP33 USP49 USPL1 USP21 mt ee mvb 1298 AMSH AMSH-LP golgi mito CYLD USP33v3 BAP1 MYSM1 USP2a USP30 Physiol Rev • VOL 93 • JULY 2013 • www.prv.org USP32 DUBs FROM GENES TO ORGANISM Trachea A20 Spinal cord AMSH Haemopoietic system EIF3H, AMSHLP, PRPF8, OTUD5, A20 CYLD, USP3, USP4, USP7, USP8, USP9Y, USP15, USP20, USP25, USP28, USP33, USP34, USP36, USP39, USPL1, PARP11 Bone MYSM1, POH1, USP9Y, USP16, USP36 Skin VCPIP1, USP15, USP53, USP54 Brain MYSM1, ATXN3L, OTUB1, Cezanne2, TRABID, UCHL1, USP6, USP9X, USP11, USP14, USP21, USP22, USP26, USP29, USP32, USP33, USP35, USP42, USP46, USP51, USP54 Lung PRPF8, AMSH, OTUD1, OTUD6A, A20, UCHL3, USP3 Heart OTUB1, OTUD6A, USP13 Reproductive system COPS5, COPS6, EIF3H, PRPF8, JOSD1, OTUD1, OTUD5, Cezanne, Cezanne2, TRABID, BAP1,UCHL3, USP1, USP4, USP7, USP9X, USP10, USP13, USP18, USP21, USP24, USP25, USP28, USP30 USP31, USP32, USP36, USP37, USP42, USP44, USP45, USP47, USP48, USP51, USPL1 Skeletal muscle COPS5, EIF3F, OTUB2, OTUD1, USP2, USP13, USP19, USP38, USP39, USP49, USP54 Liver & biliary system MPND, OTUB2, Cezanne, USP18, USP26, USP29, USP30, USP31, USP35, USP40, USP43, PARP11 Renal system OTUD3, USP2, USP21, USP45 Pancreas UCHL3, USP3, USP53 Digestive system BRCC3, MPND, AMSHLP, YOD1, USP12 FIGURE 5. Differential DUB expression in human tissues. DUB transcript expression data were collated from the EMBL gene atlas (112) and are shown for selected tissues. Inclusion criteria are that each DUB was significantly overexpressed, relative to their mean expression in other tissues, in at least two studies, and greater than 67% of all studies. Each DUB is shown for up to three tissues in which its expression was most prevalent. selectivity for unanchored ubiquitin and is necessary for optimal catalytic activity (207), whilst in combination with other ubiquitin binding domains it contributes to a high avidity for tetra-ubiquitin (208). However, it is possible that the ZnF-UBP domain also presents a protein interaction module for the 72 other human proteins which possess COOH-terminal di-Gly motifs. One interesting member of this list is histone H4, which could serve to recruit both USP3 and USP16 to histone complexes, where they have been proposed to deubiquitylate histone H2A (87, 110, 183). USP7/HAUSP has garnered a great deal of attention because of the prominence of some well-characterized substrates that are associated with tumor suppression (p53/MDM2, FOXO4, PTEN, INK4a) (50, 51, 151, 152, 163, 242, 262). USP7 ⫺/⫺ mice suffer embryonic lethality, in part due to FIGURE 4. A: estimated cellular copy number for individual DUBs in Swiss 3T3 cells determined by quantitative mass spectrometry. Data collated from Schwanhausser et al. (231) which provides estimates for copy number of 5,000 distinct proteins. Note that some further DUBs may be present, but just not detected by this mass spectrometry experiment. B: subcellular localization of human DUBs. Only those DUBs which have been localized to specific cytoplasmic organelles, the plasma membrane, or which exclusively localize to the nucleus are shown for simplification. Source data are derived from Urbé et al. (261) supplemented by additional data for USP2A (159), USP3 (77), USP22 (244), USP32 (4), USP33 (150, 254). pm, Plasma membrane; mito, mitochondria; mvb, multivesicular body; ee, early endosome; er, endoplasmic reticulum; mt, microtubules. *Nucleolus. Physiol Rev • VOL 93 • JULY 2013 • www.prv.org 1299 CLAGUE ET AL. Table 2. Selectivity of DUBs between polyubiquitin chain types USPs CYLD Ataxin 3 A20 OTUB1 OTUB2 OTUD3 OTUD5 Cezanne Trabid AMSH AMSH-LP Brcc36 Lys63 Lys48 Lys33 Lys29 Lys27 Lys11 Lys6 Linear Reference Nos. ⫹⫹⫹ ⫹⫹⫹ ⫹⫹⫹ ⫺ ⫺ ⫹⫹⫹ ND ⫹⫹⫹ ⫹ ⫹ ⫹⫹⫹ ⫹⫹⫹ ⫺ ⫹⫹ ⫹⫹⫹ ⫹⫹⫹ ⫹ ⫹ ⫺ ND ⫺ ⫺ ⫹⫹ ND ND ND ND ND ND ND ND ⫹ ⫺ ⫹⫹ ⫺ ND ⫺ ⫺ ND ND ND ⫺ ⫹⫹⫹ ⫺ ⫹ ND ND ND ND ND ND ND ND ⫺ ⫺ ⫹⫹⫹ ⫹ ⫹ ⫺ ⫺ ND ND ND ⫹⫹⫹ ⫺ ⫺ ⫹⫹⫹ ⫺ ND ⫺ ⫺ ND ⫹⫹⫹ ND ⫺ ⫺ ⫺ Variable ⫹⫹⫹ ND ⫺ ND ND ND ND ND ⫺ ⫺ 27, 67 27, 123, 268 27, 184, 279 27, 123, 268 60, 268, 270 60 95 114 27, 268 154, 268 27, 45, 123, 171, 225 Collation of data from published studies that inform on the discrimination of full-length or DUB catalytic domains between polyubiquitin chain types. Most USPs are reported as relatively nonselective, with the exception of CYLD and show a variable ability to hydrolyze linear chains. A (⫺) indicates where no activity was determined, and number of (⫹) signs gives an indication of preference for that chain over others for a specific DUB and is not an absolute indicator of activity. ND, not determined. p53 activation, as embryonic development is extended in USP7/p53 double-knockout embryos (124, 125). Several USPs are associated with DNA repair pathways, most prominently USP1. Its deubiquitylating activity regulates Fanconi anemia, complementation group D2 (FANCD2) and proliferating cell nuclear antigen (PCNA), which are both components of the cross-link repair pathway (41, 98, 145, 186). Recently, USP1 has also been shown to control the stability of ID (inhibitors of DNA binding) proteins, which inhibit differentiation and maintain stem cell characteristics in osteosarcoma. USP1 overexpression impairs osteoblastic differentiation of mesenchymal precursors, while depletion of USP1 induced such differentiation in osteosarcoma cells (277). Accordingly, USP1 ⫺/⫺ mice show defects in skeletal development including ossification of the cranial and long bones, further to a genomic instability and Fanconi anemia phenotype previously reported (116, 277). B. UCH Family Structural studies indicate that UCHL1 and UCHL3 substrates are limited by a requirement for the leaving group to pass through a loop region, which sits directly over the active site (55, 108, 109, 175). Accordingly, UCHL1 and UCHL3 both show negligible in vitro activity against Lys48, Lys63, and linear ubiquitin chains (123). However, the ability to hydrolyze Lys48 and Lys63 linked chains can be conferred upon UCHL3 through expansion of this loop by the insertion of 5 or 10 glycine residues (201). Two classes of physiological substrate have been proposed, based on in vitro enzymatic analysis (140). 1) The proubiquitin genes in most organisms contain head to tail repeats of the ubiquitin sequence with an additional amino acid or short peptide capping the COOH terminus, which is highly variable between species. 2) All of the intermediates in the enzymatic activation of the ubiquitin COOH terminus are thioesters, which can form adventitious adducts by thiol or amine modification that may be recycled by UCHL1/3 action. This function is congruent with the high copy numbers observed for these enzymes in proteomics experiments and their lack of protein-protein interaction domains (FIGURES 3 AND 4) (79, 231). A back of the envelope calculation suggests that without countervailing measures, all free ubiquitin would be converted to glutathione thiol ester or otherwise conjugated with intracellular polyamines within a matter of minutes (199). The other two UCH family members, the proteasome associated UCHL5/UCH37 and the tumor suppressor BAP1, have more extended cross-over loops, permissive for cleavage of ubiquitin chains (136, 296). It is likely that these FIGURE 6. Association of DUBs with disease. Cancer expression data were accessed for each DUB from Oncomine (211), criteria used for differential expression were P ⬍0.0001, 2-fold change and within top 10% of genes, for at least three studies. Cancer mutation data were taken from COSMIC (74), with criteria that the DUB was mutated in at least 2% of all tumors; examples of tumor types are given, where two or more samples were tested and greater than 10% showed mutations. Key to cancers: Ba, brain; Bl, bladder; Br, breast; Ce, cervical; Co, colorectal; Es, esophagus; He, hemopoietic; Ki, Kidney; Le, leukemia; Li, liver; Lu, lung (NSCLC); LuS, lung (SCLC); Ly, lymphoma; LI, large intestine; Me, melanoma; Ms, mesothelioma; Ov, ovary; UM, uveal melanoma; Pa, pancreatic; Pr, prostate; Sa, sarcoma; Se, seminoma; Sk, skin; St, stomach; UA, upper aerodigestive tract; UT, urinary tract. Further information and full references for other expression, mutation, and disease association data are given in Supplementary TABLE 2. 1300 Physiol Rev • VOL 93 • JULY 2013 • www.prv.org DUBs FROM GENES TO ORGANISM longer loops can accommodate di-ubiquitin substrates as a consequence of greater flexibility rather than providing an opening through which the leaving group must pass. For Overexpressed in cancer BRCC3 COPS5 COPS6 EIF3H MPND MYSM1 PRPF8 POH1 AMSH AMSH-LP ATXN3 ATXN3L JOSD1 OTUB1 OTUB2 OTUD1 OTUD3 OTUD4 OTUD6B Cezanne2 Cezanne A20 VCPIP1 BAP1 UCHL1 UCHL3 CYLD DUB3 USP1 USP2 USP3 USP4 USP5 USP6 USP7 USP8 USP9X USP9Y USP10 USP11 USP13 USP14 USP15 USP16 USP17 USP19 USP21 USP22 USP24 USP25 USP26 USP28 USP29 USP31 USP32 USP33 USP34 USP36 USP37 USP38 USP40 USP42 USP44 USP45 USP46 USP47 USP48 USP49 USP51 USP53 USP54 USPL1 PARP11 Downregulated in cancer Ly, Se Lu, Me both BAP1 and UCHL5, the in vitro activity against ubiquitin chains observed for the isolated catalytic domain is held in check by inhibitory domains in the full-length pro- Mutated in cancer Other disease association Le Moyamoya (cerebrovascular angiopathy ) Br Br, Pr Co Co LI, Sk Ly, LI, Sk, UA Retinitis pigmentosa many St Se Co, Ki MIC-CAP syndrome Machado Joseph disease LI, Sk Lu Bl Br, Co Ly, Co Ly, Co, Lu Se LI LI, UA Co Ba Co Ly many Lu, Co, Pa Co LI, Sk Li, UA Ly, UA LI, Sk, UT, UA UM, Ki, Ms Ba, Co, Ki Se many Le, se Co, Ki, Br Ba, Sa, St Pr, Ov Ba Lu, LuS, Bl, Pr LuS Ki Pr Lu Pa Me, Co Me, Le, Sa Le Br, ki, Ov Inflammatory conditions Cancer predisposition syndrome Neurodegenerative diseases Sk LI, Sk, UA Familial cylindromatosis LI, Sk, UT LI LI, Sk, UA, UT LI, Sk, UT LI, Sk, UA LI, Sk, UA LI, Sk, UA He, Ly, LI, Sk, UA, Pa LI, Sk, UA Heart failure Aneurysmal bone cyst Male infertility, prostate cancer Premature ovarian failure Male infertility Ba LI LI, Sk, UA Ov Br Ba, Br, Ov LI Chronic myeloid leukemia LI, Sk, UA LI copy number gain in cancer Lu, Co, Es, Ce Me, Co, Br Ly, Br Br Br Ly Ly Br Se Ki Co Ly Ly Se Br Le Br, Es, Ki Br, Se Me Br, Co, Lu, Ly Co amplification overexpression deletion downregulation Ly, Se translocation many LI, Li, Sk LI, Sk, UA, UT LI, Sk, UA LI, Sk, UA LI, Sk LI, Sk LI LI, Ov, Pr, Sk LI, Sk, UA Sk Li LI LI, Sk LI, Sk, UA LI, UA Parkinson's (late onset) Crohn's disease Male infertility Parkinson's (late onset) Depression LI, Sk, UA LI, Sk, UA, UT LI, Sk LI, Sk, UA LI, Sk LI LI, Sk LI expansion Cantu syndrome SNP association somatic mutation Physiol Rev • VOL 93 • JULY 2013 • www.prv.org germline mutation 1301 CLAGUE ET AL. Table 3. Phenotypes associated with genetically modified DUB mouse models DUB Family DUB Genotype JAMM/MPN⫹ COPS5 JAMM/MPN⫹ COP6S Reference Nos. Phenotype Molecular Mechanism Csn5Sfl/fl Lck-Cre Targeted to thymocytes. Decreased growth and development Defective S-phase progression and massive apoptosis, dysregulated turnover of P53, iB␣,ß-catenin Cop6S⫺/⫺ Embryonic lethal (E7.5) Cop6S⫹/⫺ Decreased growth, increased sensitivity to gamma-radiation and decreased radiation induced tumors Regulating DNA damageassociated apoptosis and tumorigenesis through MDM2-p53 pathway 294 Oxidative stress and genomic instability 188 194 294 JAMM/MPN⫹ MYSM1 Mysm1tmla/tmla Defects in bone marrow hematopoiesis, with lymphopenia, anemia, thrombocytosis JAMM/MPN⫹ PRPF8 Prpf8tm1.1Eap/⫹ Prpf8tm1.1Eap/⫹ tm1.1Eap Knock-in of disease-associated mutation. Retinal degeneration in heterozygotes, more severe in homozygotes 81 JAMM/MPN⫹ PSMD7 Mov34⫺/⫺ Embryonic lethal (develop to the blastocyst stage and die shortly after implantation) 243 JAMM/MPN⫹ STAMBP Amsh⫺/⫺ Postnatal growth retardation and lethality at P19-P23, Brain atrophy and loss of hippocampal/cerbral cortex neurons 105 Amsh⫺/⫺ Josephin ATXN3 Atxn3⫺/⫺ Atxn3 ⫺/⫺ Failure to degrade proteins including glutamate receptors, leading to accumulation of ubiquitylated protein aggregates 248 No overt phenotype, but heightened anxiety Increased levels of ubiquitinated proteins 229 Defective cellular stress response Decreased basal and stressinduced hsp70 transcription 205 OTU OTUD5 Otud5Gt(A021B07)Wrst/Y OTU TNFAIP3 Tnfaip3⫺/⫺ Multi-organ inflammation Increased NFB signaling 236 Tnfaip3fl/fl CD19-Cre Targeted to B cells. Autoantibodies and autoimmune disease similar to SLE Increased NFB signaling 236 Tnfaip3fl/fl CD11c-Cre Targeted to dendritic cells. Splenomegaly, lymphadenopathy and autoimmune disease, e.g., colitis Increased NFB signaling 236 UCH BAP1 Abnormal embryo turning and developmental patterning Bap1⫺/⫺ Embryonic lethal (E9.5) Bap1fl/fl ERT2⫹-Cre Ubiquitous deletion in adult tissues. Splenomegaly, myeloid transformation. 63 57 Destabilization of epigenetic regulators (HCF1 & OGT), interaction ASXL1/ASXL2 57 UCH UCHL1 Gad⫺/⫺ (lack Uchl1) Defective fertilization and preimplantation embryo development Gad⫺/⫺ (lack Uchl1) Defective spermatozoa Decreased apoptosis 134 UCH UCHL3 Uchl3⫺/⫺ Testicular atrophy and germ cell loss Increased apoptosis 135 Uchl3⫺/⫺ Defective adipogenesis and resistance to HFD-induced obesity Decreased IGF signaling, increased muscle AMPK activation 233, 247 Uch37⫺/⫺ Prenatal lethality, severely abnormal brain development UCH USP UCHL5 CYLD CYLD fl/fl ALFP-Cre 179 8 Hepatic dysfunction, apoptosis and subsequent cancer Activation of TAK1 (TGFbeta pathway) and JNK signalling 189 Cyld⫺/⫺ Lung fibrosis in response to injury Increased TGFbeta signalling and smad3 stability through loss of Akt deubiquitylation 155 Elevated perinatal lethality, male infertility. Fanconi anemia Impaired Fancd2 foci during DNA damage repair by homologous recombination 116 Osteopeonia Loss of ID proteins 277 USP USP1 Usp1⫺/⫺ USP USP2 Usp2⫺/⫺ Defective fertilization and sperm motility USP USP4 Usp4⫺/⫺ Viable and developmentally normal. Enhanced apoptosis in thymus and spleen in response to ionizing radiation USP USP5 Usp5⫺/⫺ Embryonic lethal (E7) 17 Hyperactive DNA damage checkpoints and upregulated levels and activity of p53 292 63 Continued 1302 Physiol Rev • VOL 93 • JULY 2013 • www.prv.org DUBs FROM GENES TO ORGANISM Table 3.—Continued DUB Family USP USP DUB USP7 USP8 Genotype Phenotype Molecular Mechanism Reference Nos. Usp7⫺/⫺ Embryonic lethal (E6.5-E7.5) Dysregulated Mdm2/p53 pathway 124 Hauspfl/fl nes-Cre Brain targeted knockout. Neonatal lethality, deficiencies in brain development Increased p53 levels, transcriptional activity and p53-mediated apoptosis 125 Ubpy⫺/⫺ Grossly disorganized embryogenesis and complete embryonic lethality (E9.5) Ubpyfl/fl Mx1-Cre Conditional knockout in adults. Liver failure Reduced levels of RTKs and abnormal trafficking 185 No protein aggregates, instead depletion of synaptic ubiquitin pool, increased GABA(A) receptor turnover 14, 18, 32, 33, 49, 139, 278 USP USP14 Usp14ax⫺J/ax⫺J Mutation in intron leads to reduced USP14 levels. Tremors, abnormal brain morphology, altered synaptic transmission and increased apoptosis USP USP9x Usp9xGt(XK141)Byg Abnormal embrogenesis and embryo size, open neural tube, failure to form heart USP USP16 Usp16⫺/⫺ Compete embryonic lethality (E6) USP USP18 Usp18⫺/⫺ Tremors, seizures, abnormal nervous system, death Usp18⫹/⫺ Increased susceptibility to bacterial infection Usp18⫺/⫺ 185 63 63 ISG15 214 Chemically induced mutation. Increased inflammatory response to Salmonella Reduced IFN-gamma, increased IL-6 212 USP USP22 Usp22⫺/⫺ Embryonic lethal (E9.5-E10.5) Sirt1 instability, increased p53 transcriptional activity, apoptosis 157 USP USP25 USP25⫺/⫺ Enhanced inflammatory response to IL-17 Loss of USP25 leads to deubiquitylation of TRAF5 & TRAF6, increased Il17dependent TRAF5 interaction with splicing factor SF2, and stabilization of proinflammatory transcripts CXCL1, IL-6 and TNF 295 USP USP44 USP44⫺/⫺ Spontaneous tumor formation, particularly in lung Regulates mitotic checkpoint, interacts with centrin to control centriole positioning 293 USP USP46 Usp46CS/CS (mutation) Low immobility in tail suspension and forced swim tests GABAergic transmission 256 USP USP47 Usp47⫺/⫺ Usp46⫺/⫺ 103 Increased sensitivity to ultraviolet radiation teins. Binding to the appropriate physiological partners relieves this inhibition, providing a control mechanism for the appropriate expression of activity (228, 286). UCHL1 is one of the most abundant brain proteins, estimated at 1–2% total protein (58, 106, 275). Several lines of evidence link UCHL1 to neurodegenerative conditions. A homozygous missense mutation, identified in three siblings of a Turkish family, has reduced affinity and catalytic activity towards ubiquitin and is proposed to lead to childhood-onset multisystem neurodegenerative syndrome (19). A separate mutation showing reduced activity has been linked to increased risk of Parkinson’s disease (PD), but mouse models suggest that this could represent gain, rather than loss, of function (148, 234). Conversely, a common polymorphism, S18Y, may reduce PD’s susceptibility in certain populations (165). A proteomic analysis has revealed that UCHL1 is a major target of oxidative damage in Alz- 63 heimer’s disease (AD) and idiopathic PD brains (35). Transduction of UCHL1, coupled to the HIV-transactivator protein, into mouse hippocampal brain slices alleviates defects induced by treatment with oligomeric Aß protein and in the mouse APP/PSI model of AD. Furthermore, intraperitoneal injections of the UCHL1 fusion protein improve the contextual memory of APP/PSI mice (80). Interestingly, the induced expression of a neuron-specific UCH enzyme has been associated with long-term facilitation in Aplysia (91). Aberrant expression of UCHL1 is observed in a variety of cancer types, including lung, colon, and pancreas (93, 253, 283) and has been functionally associated with the determination of cellular invasive properties and determination of chemosensitivity (28, 115). Mice lacking UCHL1 due to an intragenic deletion exhibit defective spermatogenesis and gracile axonal dystrophy (Gad), which is thought to reflect defective axonal transport Physiol Rev • VOL 93 • JULY 2013 • www.prv.org 1303 CLAGUE ET AL. (221). Focal degeneration in the gracile fasciculus, observed in Gad mice, resembles the symptoms associated with chronic deprivation of the antioxidant vitamin E, but cannot be alleviated by vitamin E administration. However, it is interesting to note that upregulation of UCHL1 gene transcription is prominent in skeletal muscles of ␣-tocopherol (one form of vitamin E)-deficient mice (264). Gad mice show reduced levels of unconjugated ubiquitin in neurons, while expression of UCHL1 in cultured cells and mice enhances the free ubiquitin pool (191). tin chains on substrate proteins. It can also antagonize interactions between various other E3 enzymes with the E2 enzymes UBC13 and UbcH5c (237). Furthermore, ubiquitin chain binding by intrinsic A20 ZnF domains influence ubiquitin dynamics on the NFB pathway (23, 239). In vitro data indicate DUB specificity for Lys48over Lys63-linked chains, but cellular models suggest a critical role in cleaving activating Lys63 chains en bloc from mediators of NFB signaling, such as receptor interacting protein 1 (RIP1) (123, 156, 271). BAP1 may be the most commonly mutated DUB in cancer. Somatic inactivating mutations have been found at high incidence in uveal melanomas, clear cell renal carcinoma, and pleural malignant mesotheliomas (1, 82, 86). Germline mutations have been linked to a tumor predisposition syndrome for melanocytic tumors and mesothelioma (252, 274). BAP1 gene deletion in mice is embryonically lethal, but conditional knockout mice develop a myeloid disorder resembling chronic myelomonocytic leukemia (CMML) (57). BAP1 protein is largely confined to the nucleus, where it interacts with several transcriptional regulators including host cell factor-1 (HCF-1), the polycomb group proteins additional sex-combs like 1 and 2 (ASXL1 and ASXL2), and the DNA binding protein FOXK1, which are likely to form a modular complex (57, 162). Deubiquitylating activity of BAP1 maintains protein levels of the pleiotropic transcriptional regulator HCF-1 and its interacting partner Olinked N-acetylglucosamine transferase (OGT), which itself positively regulates HCF-1 activity by glycosylation (57). This complex plays a critical role in glucose sensing. Levels of the promoter of gluconeogenesis, peroxisome proliferator-activated receptor-␥ coactivator (PGC)-1␣, are increased in euglycemic conditions. It is proposed that N-acetylglucosylation of PGC-1␣ by HCF-1/OGT promotes its stability, through the recruitment of BAP1 deubiquitylating activity (220). Germ-line single-nucleotide polymorphisms of A20 in humans have been linked with susceptibility to a number of inflammatory conditions, including systemic lupus erythematosus, rheumatoid arthritis, and Crohn’s disease (161). The widespread inflammation and perinatal mortality of A20-deficient mice has spurred the generation of lineage-specific, conditional knockout models that allow functional analysis in specific cell types. A20-deficient B cells show hypersensitivity to stimuli of the NFB pathway and increased survival of germinal center B cells. This provides a framework for understanding the role of A20 in suppressing B-cell lymphomas, which is suggested by human genetic studies (36, 96, 161). Other studies have found critical functions for A20 in dendritic cells (83, 126), macrophages (167), and intestinal epithelial cells (265). C. OTU Family Linkage of OTU proteins to ubiquitin chain processing was first suggested by their binding to active site ubiquitin probes and their structural similarity to cysteine proteases (15, 22, 64). Despite structural conservation within the catalytic domain, the family shows diverse specificity for ubiquitin chain linkages (TABLE 2). The strongest vein of biological data for this family, which has been reviewed extensively elsewhere (34), links the OTU protein A20 (also known as TNFAIP3) to regulation of the proinflammatory NFB pathway (88, 161, 236). The A20 gene can be induced by NFB signaling and operates within a negative-feedback loop to restrict the duration or intensity of signaling. A20 function is particularly complex as it has been proposed to possess both intrinsic DUB and E3-ligase activities, which may coordinate both the assembly and disassembly of ubiqui- 1304 OTUB1 is one of the most highly expressed of all DUBs (FIGURE 4B; Refs. 79, 231). This may reflect a particular feature of this protein that is independent of its DUB activity. OTUB1 is a potent suppressor of Lys63-linked polyubiquitylation at DNA double-strand breaks, independent of its catalytic activity (111). This is accomplished by binding to and inhibiting transfer from the ubiquitin-charged E2 ubiquitin conjugating enzyme UBC13 (111). Furthermore, binding of OTUB1 to multiple E2 enzymes of the UBE2D and UBE2E class has been reported in a proteomic study (244). These include UbcH5, for which inhibition by OTUB1 is proposed to lead to p53 stabilization (246). Elegant structural and biochemical studies show that OTUB1 can also bind free ubiquitin. This binding induces conformational changes in the catalytic domain, which allow simultaneous binding to the E2-ubiquitin, such that both ubiquitins together mimic the configuration of a cleaved Lys48 di-ubiquitin. Thus OTUB1 is proposed to utilize a mechanism akin to product inhibition to inhibit the activity of associated E2 enzymes (111, 224, 273). D. Josephin Family Machado Joseph Disease (MJD) is the most common form of spinocerebellar ataxia worldwide. This progressive condition is characterized by polyQ expansion in the ataxin 3 (ATXN3) gene (168). The NH2-terminal Josephin domain possesses deubiquitylating activity (30, 227), while the COOH terminus of the most abundant isoform contains Physiol Rev • VOL 93 • JULY 2013 • www.prv.org DUBs FROM GENES TO ORGANISM two UIM domains followed by the polyQ sequence and then a third UIM (89). The etiology of MJD is linked to the formation of cellular aggregates once a threshold of polyQ extension has been reached, in common with other polyQ diseases such as Huntington’s. It is currently contentious if any specific attributes of MJD relate to associated depletion of enzymatic activity. However, these structures fail to clearly explain the Lys63 specificity of other JAMMs. The biological function of ATXN3 remains poorly characterized. ATXN3 knockout in mice produces no obvious physiological changes, possibly because of redundancy with other DUBs. However, there is a general elevation in the amount of ubiquitylated protein (229). ATXN3 regulates transcription of multiple genes (65, 215), a property which may allow for a coordinated response to proteotoxic stresses, which have been shown to promote nuclear accummulation of ATXN3 (206). In C. elegans, ATXN3 has been linked to the control of longevity by the IGF-I signaling axis (131). In the following section, we discuss DUBs associated with two of the major protein degradation pathways, the proteasomal and lysosomal routes, for which some common principles related to ubiquitin homeostasis and protein rescue apply. DUBs associated with the ubiquitin-dependent degradative pathway of autophagy largely remain to be elucidated, although USP10 and USP13 have been implicated in the control of the stability of the autophagy gene product BECLIN1 (158). E. JAMM Family The 26S proteasome is a ⬃2.5 MDa assembly of proteins, which is responsible for the degradation of most cytosolic proteins. It is comprised of two large subcomplexes corresponding to the 20S catalytic core (CP; core particle) and two copies of the 19S regulatory particle (RP; regulatory particle) that can be subdivided into base and lid components. The base contains a ring of 6 homologous ATPases that promote substrate unfolding and translocation into the 20S catalytic chamber through a narrow (⬃13 Å) gated channel. The RP includes two ubiquitin receptors and three distinct DUB activities amongst its constituent proteins. One of these, POH1/PSMD14/Rpn11 (hereafter referred to as POH1), is constitutively incorporated in stoichiometric quantities and required for RP assembly. The other two, USP14 (Ubp6 in yeast) and UCH37/UCHL5 (not present in yeast), are reversibly associated (146). The JAMM/MPN⫹ branch constitutes a subset of proteins from the broader MPN (Mpr1-Pad1-N-terminal) family which is conserved in bacteria and archaea. JAMMs contain a signature ‘H-x-H-P-x[6]-S-x[2]-D’ motif within the MPN domain that, through its invariant His and Asp residues, coordinates a zinc atom, which is required for activity (12, 47, 56, 169, 170, 225, 284). JAMMs are generally incorporated into large multimeric complexes such as the proteasome lid complex (POH1/Rpn11), COP9 signalosome (CSN5), and the endocytic ESCRT machinery (AMSH) (39, 46, 72). BRCC36 is associated with two complexes involved in DNA repair, BRISC and BRCA1RAP180 (45, 235), whilst MYSM1 expresses its histone H2A deubiquitylating activity from within a complex containing the histone acetyltransferase (HAT) p300/CBPassociated factor (p/CAF) (297). Orchestration of histone modifications by MYSM1 has recently been shown to function as part of an epigenetic switch controlling B-cell development (107). Accordingly, mice deficient in MYSM1 show defects in lymphocyte and erythroid development (188). Several members of this family show a strong preference for Lys63 polyubiquitin linkages including AMSH, AMSH-LP, BRCC36, and POH1 (45, 170, 171, 225). However, both MYSM1 and POH1 apparently cleave ubiquitin proximal to a protein substrate. In the case of MYSM1, this can be the monoubiquitin attached to histone H2A (297), while POH1 can cleave polyubiquitin chains en bloc, from unfolded proteasomal substrates (284). The structure of the catalytic domain of AMSH-LP in complex with Lys63 diubiquitin and later of the AMSH catalytic domain illustrated specific interactions of ubiquitin with the catalytic core, but also with two AMSH-specific insertions that interact with the proximal and distal ubiquitins, respectively (56, 225). VII. DUBs AS INTEGRAL COMPONENTS OF PROTEIN MACHINERIES ASSOCIATED WITH DEGRADATION A. Proteasomal DUBs Proteasomal DUBs have been suggested to be involved in the recycling of ubiquitin, even directly coupling this to protein degradation, or in “proof-reading” at the proteasome whereby certain proteins may be reprieved from degradation (72). Deubiquitylation is required for release of substrate from ubiquitin receptor proteins. If ubiquitin chain trimming outpaces substrate unfolding/translocation, it can result in dissociation from the proteasome and rescue of the protein. Conversely, retarded deubiquitylation leads to occlusion of substrate binding sites and clogs up the proteasome (291). Only siRNA knockdown of POH1 interferes with proteasome assembly, while depletion of USP14 or UCH37 alone both enhance protein degradation rates, but their combined depletion inhibits proteasomal activity (127). Yeast cells respond to ubiquitin depletion by upregulating the USP14 ortholog Ubp6, which restores ubiquitin levels (85). The ataxia (axj) mouse exhibits severe tremors at 2–3 wk of age, reflecting defective synaptic transmission, which results from an intronic mutation, leading to loss of Physiol Rev • VOL 93 • JULY 2013 • www.prv.org 1305 CLAGUE ET AL. full-length USP14 expression (14, 278). The observed phenotypes probably reflect depletion of the synaptic ubiquitin pool observed in axj mice, as they can be rescued by either neuron specific expression of Usp14 or ubiquitin itself (32, 33, 49). Although crystal structures of proteasomal complexes are unavailable, sub-nanometer resolution structures derived from electron microscopy single-particle analyses provide information on the organization of constituent proteins (16, 53, 137, 141). POH1 is adjacent to the ubiquitin receptor Adrm1/Rpn13 and positioned directly above the AAA-ATPase N-ring (16). The activity of POH1 is enigmatic. Based on in vitro enzymatic and structural studies of AMSH, BRCC3, and POH1, it has been proposed that the JAMM family proteins may collectively possess a stringent specificity for Lys63 ubiquitin chain linkages (44, 45, 123, 171, 225, 235). However, elegant preceding work suggested that POH1 activity on proteasomal substrates 1) indirectly requires ATPase activity presumably for unfolding of the substrate, 2) is coupled to proteasomal degradation, and 3) completely removes ubiquitin by cleavage at the base of the ubiquitin chain (266, 284). In fact, the catalytic activities of all three proteasomal DUBs are dependent on incorporation or association with the 19S particle. Proteasomal binding activates Ubp6/USP14 by several hundredfold (143, 147). Binding of the 19S component Adrm1 (Rpn13) to the COOH-terminal tail of UCH37 is proposed to remove an autoinhibitory barrier, leading to acceleration of ubiquitin-AMC hydrolysis (but see Ref. 29 for a note of caution on this). Full incorporation into the 19S complex is required for efficient processing of polyubiquitin chains by UCH37, which occurs from the distal end (203, 286). Not all proteasomal DUB functions require catalytic activity. Binding of ubiquitin chains to USP14/Ubp6 or UCH37 opens the gate of the 20S channel, and in combination with an unfolded substrate domain stimulates proteasomal ATPase activity. Although this offers the possibility of coupling ubiquitin recycling with degradation, gate opening or ATPase stimulation does not require catalytic activity (197, 198). Expression of catalytically inactive USP14/ Ubp6 has been shown to have either positive or negative effects on proteasomal degradative activity, and these observations remain to be fully reconciled (84, 143, 197). Deletion of 31 amino acids from the COOH terminus of yeast Rpn11 leads to cell cycle defects and altered mitochondrial morphology. These morphological changes can be suppressed by expression of the Rpn11 COOH-terminal fragment alone, without any indication that this interacts directly with the proteasome (213). POH1 and UCH37 also present further moonlighting functions independent of proteasome assembly or degradation. 1306 UCH37 associates with the Ino180 chromatin remodeling complex, where it is held in an inactive state. Inhibition is relieved by transient interaction with the proteasome, leading to the suggestion of cooperation between these two complexes in either transcription or DNA repair, both processes to which each complex has been linked (285). POH1 DUB activity has also recently been proposed to contribute to the choreography of the double-strand break DNA repair response (31). Finally, UCH37 also associates with Mothers against Decapentaplegic proteins (SMAD) proteins, in particular SMAD7, and inhibits type I transforming growth factor (TGF)- receptor degradation (272). Owing to the success in the clinic of the proteasomal inhibitor Bortezomib in treating multiple myeloma, there is great interest in developing further modes of proteasomal inhibition. Small molecule inhibitors of POH1 represent one such possibility. The small molecule b-AP15 was first identified as a candidate proteasome inhibitor on the basis of a gene expression signature shared with other known proteasome inhibitors. Its mechanism of action proved to be through dual inhibition of both cysteine protease deubiquitylating activities associated with the proteasome, whilst total cellular DUB activity and the activity of several recombinant USP proteins is unaffected (52). One may presume that the mode of inhibition may be indirect, via drug-induced conformational changes within the 19S particle. Nevertheless, some promising effects of b-AP15 on the progression of tumors in mouse models were reported (52). A selective inhibitor of USP14, IU1, was identified using a small molecule screening approach. Application of the drug to cells leads to enhanced degradation rates for a variety of overexpressed proteins, by opposing ubiquitin chain trimming (143). These observations point to potential benefits in the treatment of certain neurological conditions, where proteasome activity may be limiting for the suppression of aggregate formation of misfolded proteins. B. ESCRT DUBs Trafficking to the lysosome provides the major degradative pathway for the majority of plasma membrane channels, pumps, and receptors (38, 200). In many cases this is achieved by the capture of ubiquitylated proteins, which have entered the sorting endosome, by the endosomal sorting complex required for transport (ESCRT) machinery (92, 102, 276). This machinery is comprised of four subcomplexes, ESCRT-0, I, II and III, which were originally proposed to act in sequential fashion. This view has become more nuanced with time, and a higher degree of integration between these components seems likely. Under the influence of this machinery, the sorting endosome matures into multivesicular bodies (MVBs) with the accrual of luminal vesicles, laden with cargo molecules that bud from the limiting membrane. MVBs then deliver cargo to lysosomes by direct fusion (76). Physiol Rev • VOL 93 • JULY 2013 • www.prv.org DUBs FROM GENES TO ORGANISM Two DUBs, AMSH and USP8/UBPY, form a network of interactions with various components of ESCRT-0 and ESCRT-III (39). Both contain MIT domains that promote endosomal association, through distinct but overlapping sets of interactions with the CHMP protein constituents of the ESCRT-III subcomplex (3, 171, 218, 241, 258). Furthermore, they share a binding site on the SH3 domain of the ESCRT-0 component STAM (113, 250). It is striking that both proteasomal and ESCRT complexes carry a JAMM family DUB (POH1 or AMSH, respectively) specific for Lys63-linked chains as well as a more promiscuous enzyme (USP14 or USP8). Accordingly, this parallel can be extended to consideration of function, in that ESCRT DUBs may couple ubiquitin recycling with commitment to degradation, i.e., inclusion into luminal vesicles of the MVB. Equally they may perform proofreading functions as detailed above for proteasomal DUBs (39). This function was first proposed for AMSH based on the observation that its depletion leads to enhanced rates of EGFR degradation (25, 39, 170, 195). By deubiquitylating receptor, AMSH inhibits inclusion into luminal vesicles and receptors recycle to the plasma membrane. The body of data around USP8 is more complex. Its depletion has more severe effects on the organization of the endocytic pathway that combine to inhibit EGFR downregulation (25, 177, 218, 219). One contributing factor to such defective EGFR trafficking is that USP8 controls the stability of ESCRT-0 components, Hrs and STAM in cells and in conditional knockout mice (185, 219). However, for Frizzled receptor (Fz) and Smoothened (Smo), key components of Wingless (Wnt) and Hedgehog signaling pathways, respectively, USP8 plays a negative regulatory role with regard to receptor degradation more akin to that originally proposed for AMSH (153, 180, 281). VIII. INFLUENCE ON CELL PHYSIOLOGY THROUGH CONTROL OF RECEPTORS AND CHANNELS The influence of DUBs on membrane trafficking has a profound effect on cell physiology through the regulation of receptor and channel densities at the plasma membrane. Here we highlight some examples in addition to effects on EGFR, Smo, and Fz receptors (described above), where the physiological consequences of this regulation are supported by whole organismal models. The further influence of DUBs on specific effector pathways, downstream of ligands such as Wnt and TGF-, has recently been reviewed elsewhere (7, 37, 251). Surface levels of glutamate neurotransmitter receptors can be regulated by both direct and indirect ubiquitylation (43, 130). Usp-46 was identified in a C. elegans RNAi screen for DUBs regulating the abundance of the glutamate receptor GLR-1 at synapses in the ventral chord. Follow-up studies indicated that usp-46 mutant worms show defects in glutamate-dependent behaviors and that the effect on GLR-1 is accomplished by direct deubiquitylation of the receptor at the level of the sorting endosome (129). USP46 has been further linked to GABAergic transmission in mouse models. Quantitative trait locus analysis of CS mice, which exhibit depressive behavior mapped to a 3-bp in-frame deletion of USP46, which reduces catalytic activity (256). Depressive behavioral effects have since been recapitulated in USP46 knockout mice, which can be alleviated by Nitazepam, which enhances GABA binding to receptors (103). Four DUBs have been implicated in the regulation of TGF- receptor stability, UCH37 and the highly related USP4, USP11, and USP15. The individual effects of these may be more or less pronounced depending on cellular context (2, 6, 61, 272, 290). USP15 has also been associated with many other cellular signaling events including the MAP kinase pathway (90), -catenin stability (99), and the NFB pathway (232). It is recruited to TGF- receptors in complex with the E3-ligase SMURF2 and SMAD7, which acts as a scaffold. Its deubiquitylating activity promotes receptor expression through stabilization of the receptor (61), and it appears that USP15 can enhance the role of TGF- signaling in glioblastoma multiforme (GBM). However, it has also been shown that USP15 empowers transcriptional activation of R-SMADs, the ultimate effectors of the TGF- pathway, by removing inactivating monoubiquitin (104); so fully discriminating the physiological importance of each of these effects may prove challenging. One consideration is that USP15 also promotes bone morphogenic protein (BMP) signaling, which utilizes overlapping SMAD family members with the TGF- pathway, as effectors of an entirely distinct receptor type (104). USP11 was also identified as the major SMAD7 interacting DUB by proteomics and is proposed to be recruited to TGF- receptor, which it deubiquitylates, in a similar manner to USP15 (6). USP4 is closely related to USP11 and USP15 (FIGURE 3) and was identified as a top hit in a genome-wide gain-of-function screen for enhancers of TGF- signaling (other hits included USP11, USP15, USP19 but were not substantively followed up). Depletion of USP4 inhibits TGF- but not BMP signaling, and in the zebrafish embryo leads to early morphogenetic defects. It also impedes cell migration in vitro and metastasis in a zebrafish xenograft model (292). In common with USP15, USP4 can deubiquitylate the activated TGF- receptor but in contrast binds directly to it, independent of SMAD7. USP10 is localized to sorting endosomes in human airway epithelial cells where it is proposed to directly deubiquitylate the cystic fibrosis transmembrane conductance regulator (CFTR) (20). An alternative mode of action of vasopressin-induced USP10 has been posited for its control of the epithelial sodium channel ENaC in renal cells. In this case, the relevant substrate is suggested to be Sorting Nexin 3, a positive regulator of recycling, that is stabilized by USP10 Physiol Rev • VOL 93 • JULY 2013 • www.prv.org 1307 CLAGUE ET AL. expression (24), recalling the stabilization of ESCRT-0 sorting factors by USP8 (see above). In some instances, ubiquitylation may provide a direct sorting signal for internalization of receptors from the plasma membrane in addition to sorting into MVBs described above (38). For example, it may be at this point that Cezanne exerts its negative regulation on EGFR downregulation (195). DUBs may affect this step by direct deubiquitylation of receptors or through influencing components of the vesicular entry routes. The ubiquitin-dependent interaction of cargo molecules with clathrin-coated vesicle (CCV) adaptor proteins, such as epsin, promotes endocytosis (257). The Drosophila DUB Fat Facets (Faf) (USP9X in humans) interacts directly with the epsin homolog Liquid facets (Laf). Deubiquitylation of Laf by Faf is proposed to facilitate Notch signaling by promoting the internalization of the Notch ligand Delta during fly development (192). IX. CONCLUSIONS 2. Aggarwal K, Massague J. Ubiquitin removal in the TGF-beta pathway. Nat Cell Biol 14: 656 – 657, 2012. 3. Agromayor M, Martin-Serrano J. Interaction of AMSH with ESCRT-III and deubiquitination of endosomal cargo. J Biol Chem 281: 1374 –1387, 2006. 4. Akhavantabasi S, Akman HB, Sapmaz A, Keller J, Petty EM, Erson AE. USP32 is an active, membrane-bound ubiquitin protease overexpressed in breast cancers. Mammalian Genome 21: 388 –397, 2010. 5. Akutsu M, Ye Y, Virdee S, Chin JW, Komander D. Molecular basis for ubiquitin and ISG15 cross-reactivity in viral ovarian tumor domains. Proc Natl Acad Sci USA 108: 2228 –2233, 2011. 6. Al-Salihi MA, Herhaus L, Macartney T, Sapkota GP. USP11 augments TGFbeta signaling by deubiquitylating ALK5. Open Biol 2: 120063, 2012. 7. Al-Salihi MA, Herhaus L, Sapkota GP. Regulation of the transforming growth factor beta pathway by reversible ubiquitylation. Open Biol 2: 120082, 2012. 8. Al-Shami A, Jhaver KG, Vogel P, Wilkins C, Humphries J, Davis JJ, Xu N, Potter DG, Gerhardt B, Mullinax R, Shirley CR, Anderson SJ, Oravecz T. Regulators of the proteasome pathway, Uch37 and Rpn13, play distinct roles in mouse development. PLoS ONE 5: e13654, 2010. 9. Altenhoff AM, Dessimoz C. Phylogenetic and functional assessment of orthologs inference projects and methods. PLoS Computat Biol 5: e1000262, 2009. Interest in the DUB family of enzymes is burgeoning as the range of biological processes shown to be under their control expands. In the last few years, rapid strides have been made in cataloguing their varying specificities for different types of polyubiquitin chains, but in most cases this is not fully understood at the structural level. Screening strategies have identified DUB’s associated with important regulatory pathways in cellular systems, which are now increasingly being supported by animal models that show corresponding developmental defects or disease states. One must bear in mind that a requirement for catalytic activity has not been shown in all cases, and some assays may reflect other aspects of a particular DUB’s function. Nevertheless, several DUBs are emerging as attractive drug targets, for which first generation tool compounds have been developed (11, 42). The stage is now set for the development of clinically useful therapies that build upon this body of knowledge. 10. Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389 –3402, 1997. 11. Altun M, Kramer HB, Willems LI, McDermott JL, Leach CA, Goldenberg SJ, Kumar KG, Konietzny R, Fischer R, Kogan E, Mackeen MM, McGouran J, Khoronenkova SV, Parsons JL, Dianov GL, Nicholson B, Kessler BM. Activity-based chemical proteomics accelerates inhibitor development for deubiquitylating enzymes. Chem Biol 18: 1401– 1412, 2011. 12. Ambroggio XI, Rees DC, Deshaies RJ. JAMM: a metalloprotease-like zinc site in the proteasome and signalosome. PLOS Biol 2: 0001– 0007, 2003. 13. Amerik AY, Hochstrasser M. Mechanism and function of deubiquitinating enzymes. Biochim Biophys Acta 1695: 189 –207, 2004. 14. Anderson C, Crimmins S, Wilson JA, Korbel GA, Ploegh HL, Wilson SM. Loss of Usp14 results in reduced levels of ubiquitin in ataxia mice. J Neurochem 95: 724 –731, 2005. 15. Balakirev MY, Tcherniuk SO, Jaquinod M, Chroboczek J. Otubains: a new family of cysteine proteases in the ubiquitin pathway. EMBO Rep 4: 517–522, 2003. 16. Beck F, Unverdorben P, Bohn S, Schweitzer A, Pfeifer G, Sakata E, Nickell S, Plitzko JM, Villa E, Baumeister W, Forster F. Near-atomic resolution structural model of the yeast 26S proteasome. Proc Natl Acad Sci USA 109: 14870 –14875, 2012. ACKNOWLEDGMENTS Address for reprint requests and other correspondence: M. J. Clague, Cellular and Molecular Physiology, Institute of Translational Medicine, Univ. of Liverpool, Liverpool, UK (e-mail: clague@liv.ac.uk). DISCLOSURES No conflicts of interest, financial or otherwise, are declared by the authors. 17. Bedard N, Yang Y, Gregory M, Cyr DG, Suzuki J, Yu X, Chian RC, Hermo L, O’Flaherty C, Smith CE, Clarke HJ, Wing SS. Mice lacking the USP2 deubiquitinating enzyme have severe male subfertility associated with defects in fertilization and sperm motility. Biol Reprod 85: 594 – 604, 2011. 18. Bhattacharyya BJ, Wilson SM, Jung H, Miller RJ. Altered neurotransmitter release machinery in mice deficient for the deubiquitinating enzyme Usp14. Am J Physiol Cell Physiol 302: C698 –C708, 2012. 19. Bilguvar K, Tyagi NK, Ozkara C, Tuysuz B, Bakircioglu M, Choi M, Delil S, Caglayan AO, Baranoski JF, Erturk O, Yalcinkaya C, Karacorlu M, Dincer A, Johnson MH, Mane S, Chandra SS, Louvi A, Boggon TJ, Lifton RP, Horwich AL, Gunel M. Recessive loss of function of the neuronal ubiquitin hydrolase UCHL1 leads to early-onset progressive neurodegeneration. Proc Natl Acad Sci USA 110: 3489 –3494, 2013. 20. Bomberger JM, Barnaby RL, Stanton BA. The deubiquitinating enzyme USP10 regulates the post-endocytic sorting of cystic fibrosis transmembrane conductance regulator in airway epithelial cells. J Biol Chem 284: 18778 –18789, 2009. REFERENCES 1. Abdel-Rahman MH, Pilarski R, Cebulla CM, Massengill JB, Christopher BN, Boru G, Hovland P, Davidorf FH. Germline BAP1 mutation predisposes to uveal melanoma, 1308 lung adenocarcinoma, meningioma, and other cancers. J Med Genet 48: 856 – 859, 2011. 21. Bonnet J, Romier C, Tora L, Devys D. Zinc-finger UBPs: regulators of deubiquitylation. Trends Biochem Sci 33: 369 –375, 2008. Physiol Rev • VOL 93 • JULY 2013 • www.prv.org DUBs FROM GENES TO ORGANISM 22. Borodovsky A, Kessler BM, Casagrande R, Overkleeft HS, Wilkinson KD, Ploegh HL. A novel active site-directed probe specific for deubiquitylating enzymes reveals proteasome association of USP14. EMBO J 20: 5187–5196, 2001. 23. Bosanac I, Wertz IE, Pan B, Yu C, Kusam S, Lam C, Phu L, Phung Q, Maurer B, Arnott D, Kirkpatrick DS, Dixit VM, Hymowitz SG. Ubiquitin binding to A20 ZnF4 is required for modulation of NF-kappaB signaling. Mol Cell 40: 548 –557, 2010. 24. Boulkroun S, Ruffieux-Daidie D, Vitagliano JJ, Poirot O, Charles RP, Lagnaz D, Firsov D, Kellenberger S, Staub O. Vasopressin-inducible ubiquitin-specific protease 10 increases ENaC cell surface expression by deubiquitylating and stabilizing sorting nexin 3. Am J Physiol Renal Physiol 295: F889 –F900, 2008. 25. Bowers K, Piper SC, Edeling MA, Gray SR, Owen DJ, Lehner PJ, Luzio JP. Degradation of endocytosed epidermal growth factor and virally ubiquitinated major histocompatibility complex class I is independent of mammalian ESCRTII. J Biol Chem 281: 5094 – 5105, 2006. 42. Colland F. The therapeutic potential of deubiquitinating enzyme inhibitors. Biochem Soc Trans 38: 137–143, 2010. 43. Colledge M, Snyder EM, Crozier RA, Soderling JA, Jin Y, Langeberg LK, Lu H, Bear MF, Scott JD. Ubiquitination regulates PSD-95 degradation and AMPA receptor surface expression. Neuron 40: 595– 607, 2003. 44. Cooper EM, Boeke JD, Cohen RE. Specificity of the BRISC deubiquitinating enzyme is not due to selective binding to Lys63-linked polyubiquitin. J Biol Chem 285: 10344 – 10352, 2010. 45. Cooper EM, Cutcliffe C, Kristiansen TZ, Pandey A, Pickart CM, Cohen RE. K63specific deubiquitination by two JAMM/MPN⫹ complexes: BRISC-associated Brcc36 and proteasomal Poh1. EMBO J 2009. 46. Cope GA, Deshaies RJ. COP9 signalosome: a multifunctional regulator of SCF and other cullin-based ubiquitin ligases. Cell 114: 663– 671, 2003. 26. Bozza WP, Liang Q, Gong P, Zhuang Z. Transient kinetic analysis of USP2-catalyzed deubiquitination reveals a conformational rearrangement in the K48-linked diubiquitin substrate. Biochemistry 51: 10075–10086, 2012. 47. Cope GA, Suh GS, Aravind L, Schwarz SE, Zipursky SL, Koonin EV, Deshaies RJ. Role of predicted metalloprotease motif of Jab1/Csn5 in cleavage of Nedd8 from Cul1. Science 298: 608 – 611, 2002. 27. Bremm A, Freund SM, Komander D. Lys11-linked ubiquitin chains adopt compact conformations and are preferentially hydrolyzed by the deubiquitinase Cezanne. Nat Struct Mol Biol 17: 939 –947, 2010. 48. Cotto-Rios XM, Bekes M, Chapman J, Ueberheide B, Huang TT. Deubiquitinases as a signaling target of oxidative stress. Cell Reports 2012. 49. Crimmins S, Jin Y, Wheeler C, Huffman AK, Chapman C, Dobrunz LE, Levey A, Roth KA, Wilson JA, Wilson SM. Transgenic rescue of ataxia mice with neuronal-specific expression of ubiquitin-specific protease 14. J Neurosci 26: 11423–11431, 2006. 28. Brinkmann K, Zigrino P, Witt A, Schell M, Ackermann L, Broxtermann P, Schull S, Andree M, Coutelle O, Yazdanpanah B, Seeger JM, Klubertz D, Drebber U, Hacker UT, Kronke M, Mauch C, Hoppe T, Kashkar H. Ubiquitin C-terminal hydrolase-L1 potentiates cancer chemosensitivity by stabilizing NOXA. Cell reports 3: 881– 891, 2013. 50. Cummins JM, Rago C, Kohli M, Kinzler KW, Lengauer C, Vogelstein B. Tumour suppression: disruption of HAUSP gene stabilizes p53. Nature 428: 486, 2004. 29. Burgie SE, Bingman CA, Soni AB, Phillips GN Jr. Structural characterization of human Uch37. Proteins 2011. 51. Cummins JM, Vogelstein B. HAUSP is required for p53 destabilization. Cell Cycle 3: 689 – 692, 2004. 30. Burnett B, Li F, Pittman RN. The polyglutamine neurodegenerative protein ataxin-3 binds polyubiquitylated proteins and has ubiquitin protease activity. Hum Mol Genet 12: 3195–3205, 2003. 52. D’Arcy P, Brnjic S, Olofsson MH, Fryknas M, Lindsten K, De Cesare M, Perego P, Sadeghi B, Hassan M, Larsson R, Linder S. Inhibition of proteasome deubiquitinating activity as a new cancer therapy. Nat Med 17: 1636 –1640, 2011. 31. Butler LR, Densham RM, Jia J, Garvin AJ, Stone HR, Shah V, Weekes D, Festy F, Beesley J, Morris JR. The proteasomal de-ubiquitinating enzyme POH1 promotes the double-strand DNA break response. EMBO J 31: 3918 –3934, 2012. 53. Da Fonseca PC, He J, Morris EP. Molecular model of the human 26S proteasome. Mol Cell 46: 54 – 66, 2012. 32. Chen PC, Bhattacharyya BJ, Hanna J, Minkel H, Wilson JA, Finley D, Miller RJ, Wilson SM. Ubiquitin homeostasis is critical for synaptic development and function. J Neurosci 31: 17505–17513, 2011. 54. Dammer EB, Na CH, Xu P, Seyfried NT, Duong DM, Cheng D, Gearing M, Rees H, Lah JJ, Levey AI, Rush J, Peng J. Polyubiquitin linkage profiles in three models of proteolytic stress suggest the etiology of Alzheimer disease. J Biol Chem 286: 10457– 10465, 2011. 33. Chen PC, Qin LN, Li XM, Walters BJ, Wilson JA, Mei L, Wilson SM. The proteasomeassociated deubiquitinating enzyme Usp14 is essential for the maintenance of synaptic ubiquitin levels and the development of neuromuscular junctions. J Neurosci 29: 10909 –10919, 2009. 55. Das C, Hoang QQ, Kreinbring CA, Luchansky SJ, Meray RK, Ray SS, Lansbury PT, Ringe D, Petsko GA. Structural basis for conformational plasticity of the Parkinson’s disease-associated ubiquitin hydrolase UCH-L1. Proc Natl Acad Sci USA 103: 4675– 4680, 2006. 34. Chiu YH, Zhao M, Chen ZJ. Ubiquitin in NF-kappaB signaling. Chem Rev 109: 1549 – 1560, 2009. 56. Davies CW, Paul LN, Kim MI, Das C. Structural and thermodynamic comparison of the catalytic domain of AMSH and AMSH-LP: nearly identical fold but different stability. J Mol Biol 413: 416 – 429, 2011. 35. Choi J, Levey AI, Weintraub ST, Rees HD, Gearing M, Chin LS, Li L. Oxidative modifications and down-regulation of ubiquitin carboxyl-terminal hydrolase L1 associated with idiopathic Parkinson’s and Alzheimer’s diseases. J Biol Chem 279: 13256 – 13264, 2004. 36. Chu Y, Vahl JC, Kumar D, Heger K, Bertossi A, Wojtowicz E, Soberon V, Schenten D, Mack B, Reutelshofer M, Beyaert R, Amann K, van Loo G, Schmidt-Supprian M. B cells lacking the tumor suppressor TNFAIP3/A20 display impaired differentiation and hyperactivation and cause inflammation and autoimmunity in aged mice. Blood 117: 2227–2236, 2011. 37. Clague MJ, Coulson JM, Urbe S. Cellular functions of the DUBs. J Cell Sci 125: 277– 286, 2012. 38. Clague MJ, Liu H, Urbe S. Governance of endocytic trafficking and signaling by reversible ubiquitylation. Dev Cell 23: 457– 467, 2012. 39. Clague MJ, Urbe S. Endocytosis: the DUB version. Trends Cell Biol 16: 551–559, 2006. 40. Cohn MA, Kee Y, Haas W, Gygi SP, D’Andrea AD. UAF1 is a subunit of multiple deubiquitinating enzyme complexes. J Biol Chem 2008. 41. Cohn MA, Kowal P, Yang K, Haas W, Huang TT, Gygi SP, D’Andrea AD. A UAF1containing multisubunit protein complex regulates the Fanconi anemia pathway. Mol Cell 28: 786 –797, 2007. 57. Dey A, Seshasayee D, Noubade R, French DM, Liu J, Chaurushiya MS, Kirkpatrick DS, Pham VC, Lill JR, Bakalarski CE, Wu J, Phu L, Katavolos P, LaFave LM, Abdel-Wahab O, Modrusan Z, Seshagiri S, Dong K, Lin Z, Balazs M, Suriben R, Newton K, Hymowitz S, Garcia-Manero G, Martin F, Levine RL, Dixit VM. Loss of the tumor suppressor BAP1 causes myeloid transformation. Science 337: 1541–1546, 2012. 58. Doran JF, Jackson P, Kynoch PA, Thompson RJ. Isolation of PGP 9.5, a new human neurone-specific protein detected by high-resolution two-dimensional electrophoresis. J Neurochem 40: 1542–1547, 1983. 59. Dosztanyi Z, Csizmok V, Tompa P, Simon I. The pairwise energy content estimated from amino acid composition discriminates between folded and intrinsically unstructured proteins. J Mol Biol 347: 827– 839, 2005. 60. Edelmann MJ, Iphofer A, Akutsu M, Altun M, di Gleria K, Kramer HB, Fiebiger E, Dhe-Paganon S, Kessler BM. Structural basis and specificity of human otubain 1-mediated deubiquitination. Biochem J 418: 379 –390, 2009. 61. Eichhorn PJ, Rodon L, Gonzalez-Junca A, Dirac A, Gili M, Martinez-Saez E, Aura C, Barba I, Peg V, Prat A, Cuartas I, Jimenez J, Garcia-Dorado D, Sahuquillo J, Bernards R, Baselga J, Seoane J. USP15 stabilizes TGF-beta receptor I and promotes oncogenesis through the activation of TGF-beta signaling in glioblastoma. Nat Med 18: 429 – 435, 2012. Physiol Rev • VOL 93 • JULY 2013 • www.prv.org 1309 CLAGUE ET AL. 62. Enesa K, Ito K, Luong le A, Thorbjornsen I, Phua C, To Y, Dean J, Haskard DO, Boyle J, Adcock I, Evans PC. Hydrogen peroxide prolongs nuclear localization of NF-kappaB in activated cells by suppressing negative regulatory mechanisms. J Biol Chem 283: 18582–18590, 2008. 63. Eppig JT, Blake JA, Bult CJ, Kadin JA, Richardson JE. The Mouse Genome Database (MGD): comprehensive resource for genetics and genomics of the laboratory mouse. Nucleic Acids Res 40: D881– 886, 2012. 64. Evans PC, Smith TS, Lai MJ, Williams MG, Burke DF, Heyninck K, Kreike MM, Beyaert R, Blundell TL, Kilshaw PJ. A novel type of deubiquitinating enzyme. J Biol Chem 278: 23180 –23186, 2003. 65. Evert BO, Vogt IR, Vieira-Saecker AM, Ozimek L, de Vos RA, Brunt ER, Klockgether T, Wullner U. Gene expression profiling in ataxin-3 expressing cell lines reveals distinct effects of normal and mutant ataxin-3. J Neuropathol Exp Neurol 62: 1006 –1018, 2003. 66. Faesen AC, Dirac AM, Shanmugham A, Ovaa H, Perrakis A, Sixma TK. Mechanism of USP7/HAUSP activation by its C-terminal ubiquitin-like domain and allosteric regulation by GMP-synthetase. Mol Cell 44: 147–159, 2011. 67. Faesen AC, Luna-Vargas MPA, Geurink PP, Clerici M, Merkx R, van Dijk WJ, Hameed DS, El Oualid F, Ovaa H, Sixma TK. The differential modulation of USP activity by internal regulatory domains, interactors and eight ubiquitin chain types. Chem Biol 18: 1550 –1561, 2011. 68. Fakitsas P, Adam G, Daidie D, van Bemmelen MX, Fouladkou F, Patrignani A, Wagner U, Warth R, Camargo SM, Staub O, Verrey F. Early aldosterone-induced gene product regulates the epithelial sodium channel by deubiquitylation. J Am Soc Nephrol 18: 1084 –1092, 2007. 69. Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 39: 783–791, 1985. 70. Feng L, Wang J, Chen J. The Lys63-specific deubiquitinating enzyme BRCC36 is regulated by two scaffold proteins localizing in different subcellular compartments. J Biol Chem 285: 30982–30988, 2010. 71. Fernandez-Montalvan A, Bouwmeester T, Joberty G, Mader R, Mahnke M, Pierrat B, Schlaeppi JM, Worpenberg S, Gerhartz B. Biochemical characterization of USP7 reveals post-translational modification sites and structural requirements for substrate processing and subcellular localization. FEBS J 274: 4256 – 4270, 2007. 72. Finley D. Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu Rev Biochem 78: 477–513, 2009. 73. Finley D, Ciechanover A, Varshavsky A. Ubiquitin as a central cellular regulator. Cell 116: S29 –32, 2004. 74. Forbes SA, Tang G, Bindal N, Bamford S, Dawson E, Cole C, Kok CY, Jia M, Ewing R, Menzies A, Teague JW, Stratton MR, Futreal PA. COSMIC (the Catalogue of Somatic Mutations in Cancer): a resource to investigate acquired mutations in human cancer. Nucleic Acids Res 38: D652– 657, 2010. 75. Fushman D, Wilkinson KD. Structure and recognition of polyubiquitin chains of different lengths and linkage. Biol Reports 3: 26, 2011. 76. Futter CE, Pearse A, Hewlett LJ, Hopkins CR. Multivesicular endosomes containing internalized EGF-EGF receptor complexes mature and then fuse directly with lysosomes. J Cell Biol 132: 1011–1023, 1996. 77. Garcia-Santisteban I, Banuelos S, Rodriguez JA. A global survey of CRM1-dependent nuclear export sequences in the human deubiquitinase family. Biochem J 441: 209 – 217, 2012. RNA splicing factor RP show late-onset RPE and retinal degeneration. Invest Ophthalmol Vis Sci 52: 190 –198, 2011. 82. Guo G, Gui Y, Gao S, Tang A, Hu X, Huang Y, Jia W, Li Z, He M, Sun L, Song P, Sun X, Zhao X, Yang S, Liang C, Wan S, Zhou F, Chen C, Zhu J, Li X, Jian M, Zhou L, Ye R, Huang P, Chen J, Jiang T, Liu X, Wang Y, Zou J, Jiang Z, Wu R, Wu S, Fan F, Zhang Z, Liu L, Yang R, Liu X, Wu H, Yin W, Zhao X, Liu Y, Peng H, Jiang B, Feng Q, Li C, Xie J, Lu J, Kristiansen K, Li Y, Zhang X, Li S, Wang J, Yang H, Cai Z, Wang J. Frequent mutations of genes encoding ubiquitin-mediated proteolysis pathway components in clear cell renal cell carcinoma. Nat Genet 44: 17–19, 2012. 83. Hammer GE, Turer EE, Taylor KE, Fang CJ, Advincula R, Oshima S, Barrera J, Huang EJ, Hou B, Malynn BA, Reizis B, DeFranco A, Criswell LA, Nakamura MC, Ma A. Expression of A20 by dendritic cells preserves immune homeostasis and prevents colitis and spondyloarthritis. Nat Immunol 12: 1184 –1193, 2011. 84. Hanna J, Hathaway NA, Tone Y, Crosas B, Elsasser S, Kirkpatrick DS, Leggett DS, Gygi SP, King RW, Finley D. Deubiquitinating enzyme Ubp6 functions noncatalytically to delay proteasomal degradation. Cell 127: 99 –111, 2006. 85. Hanna J, Meides A, Zhang DP, Finley D. A ubiquitin stress response induces altered proteasome composition. Cell 129: 747–759, 2007. 86. Harbour JW, Onken MD, Roberson ED, Duan S, Cao L, Worley LA, Council ML, Matatall KA, Helms C, Bowcock AM. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 330: 1410 –1413, 2010. 87. Hard RL, Liu J, Shen J, Zhou P, Pei D. HDAC6 and Ubp-M BUZ domains recognize specific C-terminal sequences of proteins. Biochemistry 49: 10737–10746, 2010. 88. Harhaj EW, Dixit VM. Deubiquitinases in the regulation of NF-kB signaling. Cell Res 21. 89. Harris GM, Dodelzon K, Gong L, Gonzalez-Alegre P, Paulson HL. Splice isoforms of the polyglutamine disease protein ataxin-3 exhibit similar enzymatic yet different aggregation properties. PLoS ONE 5: e13695, 2010. 90. Hayes SD, Liu H, Macdonald E, Sanderson CM, Coulson JM, Clague MJ, Urbe S. Direct and indirect control of mitogen-activated protein kinase pathway-associated components, BRAP/IMP E3 ubiquitin ligase and CRAF/RAF1 kinase, by the deubiquitylating enzyme USP15. J Biol Chem 287: 43007– 43018, 2012. 91. Hegde AN, Inokuchi K, Pei W, Casadio A, Ghirardi M, Chain DG, Martin KC, Kandel ER, Schwartz JH. Ubiquitin C-terminal hydrolase is an immediate-early gene essential for long-term facilitation in Aplysia. Cell 89: 115–126, 1997. 92. Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell 21: 77–91, 2011. 93. Hibi K, Westra WH, Borges M, Goodman S, Sidransky D, Jen J. PGP9.5as a candidate tumor marker for non-small-cell lung cancer. Am J Pathol 155: 711–715, 1999. 94. Hochstrasser M. Origin and function of ubiquitin-like proteins. Nature 458: 422– 429, 2009. 95. Hospenthal MK, Freund SM, Komander D. Assembly, analysis and architecture of atypical ubiquitin chains. Nat Struct Mol Biol 2013. 96. Hovelmeyer N, Reissig S, Xuan NT, Adams-Quack P, Lukas D, Nikolaev A, Schluter D, Waisman A. A20 deficiency in B cells enhances B-cell proliferation and results in the development of autoantibodies. Eur J Immunol 41: 595– 601, 2011. 97. Huang OW, Ma X, Yin J, Flinders J, Maurer T, Kayagaki N, Phung Q, Bosanac I, Arnott D, Dixit VM, Hymowitz SG, Starovasnik MA, Cochran AG. Phosphorylation-dependent activity of the deubiquitinase DUBA. Nat Struct Mol Biol 19: 171–175, 2012. 78. Gattiker A, Gasteiger E, Bairoch A. ScanProsite: a reference implementation of a PROSITE scanning tool. Applied Bioinformatics 1: 107–108, 2002. 98. Huang TT, Nijman SM, Mirchandani KD, Galardy PJ, Cohn MA, Haas W, Gygi SP, Ploegh HL, Bernards R, D’Andrea AD. Regulation of monoubiquitinated PCNA by DUB autocleavage. Nat Cell Biol 8: 339 –347, 2006. 79. Geiger T, Wehner A, Schaab C, Cox J, Mann M. Comparative proteomic analysis of eleven common cell lines reveals ubiquitous but varying expression of most proteins. Mol Cell Proteomics 11: M111 014050, 2012. 99. Huang X, Langelotz C, Hetfeld-Pechoc BK, Schwenk W, Dubiel W. The COP9 signalosome mediates beta-catenin degradation by deneddylation and blocks adenomatous polyposis coli destruction via USP15. J Mol Biol 391: 691–702, 2009. 80. Gong B, Cao Z, Zheng P, Vitolo OV, Liu S, Staniszewski A, Moolman D, Zhang H, Shelanski M, Arancio O. Ubiquitin hydrolase Uch-L1 rescues beta-amyloid-induced decreases in synaptic function and contextual memory. Cell 126: 775–788, 2006. 100. Huang X, Summers MK, Pham V, Lill JR, Liu J, Lee G, Kirkpatrick DS, Jackson PK, Fang G, Dixit VM. Deubiquitinase USP37 is activated by CDK2 to antagonize APC(CDH1) and promote S phase entry. Mol Cell 42: 511–523, 2011. 81. Graziotto JJ, Farkas MH, Bujakowska K, Deramaudt BM, Zhang Q, Nandrot EF, Inglehearn CF, Bhattacharya SS, Pierce EA. Three gene-targeted mouse models of 101. Hunter T. The age of crosstalk: phosphorylation, ubiquitination, and beyond. Mol Cell 28: 730 –738, 2007. 1310 Physiol Rev • VOL 93 • JULY 2013 • www.prv.org DUBs FROM GENES TO ORGANISM 102. Hurley JH, Stenmark H. Molecular mechanisms of ubiquitin-dependent membrane traffic. Annu Rev Biophys 40: 119 –142, 2011. 123. Komander D, Reyes-Turcu F, JDFL, Odenwaelder P, Wilkinson KD, Barford D. Molecular discrimination of structurally equivalent Lys63-linked and linear polyubiquitin chains. EMBO Rep 10: 466 – 473, 2009. 103. Imai S, Mamiya T, Tsukada A, Sakai Y, Mouri A, Nabeshima T, Ebihara S. Ubiquitinspecific peptidase 46 (Usp46) regulates mouse immobile behavior in the tail suspension test through the GABAergic system. PLoS ONE 7: e39084, 2012. 124. Kon N, Kobayashi Y, Li M, Brooks CL, Ludwig T, Gu W. Inactivation of HAUSP in vivo modulates p53 function. Oncogene 29: 1270 –1279, 2010. 104. Inui M, Manfrin A, Mamidi A, Martello G, Morsut L, Soligo S, Enzo E, Moro S, Polo S, Dupont S, Cordenonsi M, Piccolo S. USP15 is a deubiquitylating enzyme for receptoractivated SMADs. Nat Cell Biol 13: 1368 –1375, 2011. 125. Kon N, Zhong J, Kobayashi Y, Li M, Szabolcs M, Ludwig T, Canoll PD, Gu W. Roles of HAUSP-mediated p53 regulation in central nervous system development. Cell Death Differ 18: 1366 –1375, 2011. 105. Ishii N, Owada Y, Yamada M, Miura S, Murata K, Asao H, Kondo H, Sugamura K. Loss of neurons in the hippocampus and cerebral cortex of AMSH-deficient mice. Mol Cell Biol 21: 8626 – 8637, 2001. 126. Kool M, van Loo G, Waelput W, De Prijck S, Muskens F, Sze M, van Praet J, BrancoMadeira F, Janssens S, Reizis B, Elewaut D, Beyaert R, Hammad H, Lambrecht BN. The ubiquitin-editing protein A20 prevents dendritic cell activation, recognition of apoptotic cells, and systemic autoimmunity. Immunity 35: 82–96, 2011. 106. Jackson P, Thompson RJ. The demonstration of new human brain-specific proteins by high-resolution two-dimensional polyacrylamide gel electrophoresis. J Neurol Sci 49: 429 – 438, 1981. 107. Jiang XX, Nguyen Q, Chou Y, Wang T, Nandakumar V, Yates P, Jones L, Wang L, Won H, Lee HR, Jung JU, Muschen M, Huang XF, Chen SY. Control of B cell development by the histone H2A deubiquitinase MYSM1. Immunity 35: 883– 896, 2011. 108. Johnston SC, Larsen CN, Cook WJ, Wilkinson KD, Hill CP. Crystal structure of a deubiquitinating enzyme (human UCH-L3) at 1.8 A resolution. EMBO J 16: 3787– 3796, 1997. 109. Johnston SC, Riddle SM, Cohen RE, Hill CP. Structural basis for the specificity of ubiquitin C-terminal hydrolases. EMBO J 18: 3877–3887, 1999. 110. Joo HY, Zhai L, Yang C, Nie S, Erdjument-Bromage H, Tempst P, Chang C, Wang H. Regulation of cell cycle progression and gene expression by H2A deubiquitination. Nature 449: 1068 –1072, 2007. 111. Juang YC, Landry MC, Sanches M, Vittal V, Leung CC, Ceccarelli DF, Mateo AR, Pruneda JN, Mao DY, Szilard RK, Orlicky S, Munro M, Brzovic PS, Klevit RE, Sicheri F, Durocher D. OTUB1 co-opts Lys48-linked ubiquitin recognition to suppress E2 enzyme function. Mol Cell 45: 384 –397, 2012. 112. Kapushesky M, Emam I, Holloway E, Kurnosov P, Zorin A, Malone J, Rustici G, Williams E, Parkinson H, Brazma A. Gene expression atlas at the European bioinformatics institute. Nucleic Acids Res 38: D690 – 698, 2010. 113. Kato M, Miyazawa K, Kitamura N. A de-ubiquitinating enzyme UBPY interacts with the SH3 domain of Hrs binding protein via a novel binding motif Px(V/I)(D/N)RxxKP. J Biol Chem 275: 37481–37487, 2000. 114. Kayagaki N, Phung Q, Chan S, Chaudhari R, Quan C, O’Rourke KM, Eby M, Pietras E, Cheng G, Bazan JF, Zhang Z, Arnott D, Dixit VM. DUBA: a deubiquitinase that regulates type I interferon production. Science 318: 1628 –1632, 2007. 115. Kim HJ, Kim YM, Lim S, Nam YK, Jeong J, Lee KJ. Ubiquitin C-terminal hydrolaseL1 is a key regulator of tumor cell invasion and metastasis. Oncogene 28: 117–127, 2009. 116. Kim JM, Parmar K, Huang M, Weinstock DM, Ruit CA, Kutok JL, D’Andrea AD. Inactivation of murine Usp1 results in genomic instability and a Fanconi anemia phenotype. Dev Cell 16: 314 –320, 2009. 117. Kim W, Bennett EJ, Huttlin EL, Guo A, Li J, Possemato A, Sowa ME, Rad R, Rush J, Comb MJ, Harper JW, Gygi SP. Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol Cell 44: 325–340, 2011. 118. Kohler A, Zimmerman E, Schneider M, Hurt E, Zheng N. Structural basis for assembly and activation of the heterotetrameric SAGA histone H2B deubiquitinase module. Cell 141: 606 – 617, 2010. 119. Komander D, Barford D. Structure of the A20 OTU domain and mechanistic insights into deubiquitination. Biochem J 409: 77– 85, 2008. 120. Komander D, Clague MJ, Urbe S. Breaking the chains: structure and function of the deubiquitinases. Nat Rev Mol Cell Biol 10: 550 –563, 2009. 121. Komander D, Lord CJ, Scheel H, Swift S, Hofmann K, Ashworth A, Barford D. The structure of the CYLD USP domain explains its specificity for Lys63-linked polyubiquitin and reveals a B box module. Mol Cell 29: 451– 464, 2008. 122. Komander D, Rape M. The ubiquitin code. Annu Rev Biochem 81: 203–229, 2012. 127. Koulich E, Li X, Demartino GN. Relative structural and functional roles of multiple deubiquitylating proteins associated with mammalian 26S proteasome. Mol Biol Cell 19: 1072–1082, 2008. 128. Kouranti I, McLean JR, Feoktistova A, Liang P, Johnson AE, Roberts-Galbraith RH, Gould KL. A global census of fission yeast deubiquitinating enzyme localization and interaction networks reveals distinct compartmentalization profiles and overlapping functions in endocytosis and polarity. PLoS Biol 8: 2010. 129. Kowalski JR, Dahlberg CL, Juo P. The deubiquitinating enzyme USP-46 negatively regulates the degradation of glutamate receptors to control their abundance in the ventral nerve cord of Caenorhabditis elegans. J Neurosci 31: 1341–1354, 2011. 130. Kowalski JR, Juo P. The role of deubiquitinating enzymes in synaptic function and nervous system diseases. Neural Plasticity 2012. 131. Kuhlbrodt K, Janiesch PC, Kevei E, Segref A, Barikbin R, Hoppe T. The MachadoJoseph disease deubiquitylase ATX-3 couples longevity and proteostasis. Nat Cell Biol 13: 273–281, 2011. 132. Kulathu Y, Garcia FJ, Mevissen TE, Busch M, Arnaudo N, Carroll KS, Barford D, Komander D. Regulation of A20 and other OTU deubiquitinases by reversible oxidation. Nat Commun 4: 1569, 2013. 133. Kulathu Y, Komander D. Atypical ubiquitylation: the unexplored world of polyubiquitin beyond Lys48 and Lys63 linkages. Nat Rev Mol Cell Biol 13: 508 –523, 2012. 134. Kwon J, Sekiguchi S, Wang YL, Setsuie R, Yoshikawa Y, Wada K. The region-specific functions of two ubiquitin C-terminal hydrolase isozymes along the epididymis. Exp Anim 55: 35– 43, 2006. 135. Kwon J, Wang YL, Setsuie R, Sekiguchi S, Sakurai M, Sato Y, Lee WW, Ishii Y, Kyuwa S, Noda M, Wada K, Yoshikawa Y. Developmental regulation of ubiquitin C-terminal hydrolase isozyme expression during spermatogenesis in mice. Biol Reprod 71: 515– 521, 2004. 136. Lam YA, DeMartino GN, Pickart CM, Cohen RE. Specificity of the ubiquitin isopeptidase in the PA700 regulatory complex of 26 S proteasomes. J Biol Chem 272: 28438 – 28446, 1997. 137. Lander GC, Estrin E, Matyskiela ME, Bashore C, Nogales E, Martin A. Complete subunit architecture of the proteasome regulatory particle. Nature 482: 186 –191, 2012. 138. Lang G, Bonnet J, Umlauf D, Karmodiya K, Koffler J, Stierle M, Devys D, Tora L. The tightly controlled deubiquitination activity of the human SAGA complex differentially modifies distinct gene regulatory elements. Mol Cell Biol 2011. 139. Lappe-Siefke C, Loebrich S, Hevers W, Waidmann OB, Schweizer M, Fehr S, Fritschy JM, Dikic I, Eilers J, Wilson SM, Kneussel M. The ataxia (axJ) mutation causes abnormal GABAA receptor turnover in mice. PLoS Genet 5: e1000631, 2009. 140. Larsen CN, Krantz BA, Wilkinson KD. Substrate specificity of deubiquitinating enzymes: ubiquitin C-terminal hydrolases. Biochemistry 37: 3358 –3368, 1998. 141. Lasker K, Forster F, Bohn S, Walzthoeni T, Villa E, Unverdorben P, Beck F, Aebersold R, Sali A, Baumeister W. Molecular architecture of the 26S proteasome holocomplex determined by an integrative approach. Proc Natl Acad Sci USA 109: 1380 –1387, 2012. 142. Lauwers E, Erpapazoglou Z, Haguenauer-Tsapis R, Andre B. The ubiquitin code of yeast permease trafficking. Trends Cell Biol 20: 196 –204, 2010. Physiol Rev • VOL 93 • JULY 2013 • www.prv.org 1311 CLAGUE ET AL. 143. Lee BH, Lee MJ, Park S, Oh DC, Elsasser S, Chen PC, Gartner C, Dimova N, Hanna J, Gygi SP, Wilson SM, King RW, Finley D. Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature 467: 179 –184, 2010. 163. Maertens GN, El Messaoudi-Aubert S, Elderkin S, Hiom K, Peters G. Ubiquitinspecific proteases 7 and 11 modulate Polycomb regulation of the INK4a tumour suppressor. EMBO J 29: 2553–2565, 2010. 144. Lee JG, Baek K, Soetandyo N, Ye Y. Reversible inactivation of deubiquitinases by reactive oxygen species in vitro and in cells. Nat Commun 4: 1568, 2013. 164. Malakhov MP, Malakhova OA, Kim KI, Ritchie KJ, Zhang DE. UBP43 (USP18) specifically removes ISG15 from conjugated proteins. J Biol Chem 277: 9976 –9981, 2002. 145. Lee KY, Yang K, Cohn MA, Sikdar N, D’Andrea AD, Myung K. Human ELG1 regulates the level of ubiquitinated proliferating cell nuclear antigen (PCNA) through its interactions with PCNA and USP1. J Biol Chem 285: 10362–10369, 2010. 165. Maraganore DM, Lesnick TG, Elbaz A, Chartier-Harlin MC, Gasser T, Kruger R, Hattori N, Mellick GD, Quattrone A, Satoh J, Toda T, Wang J, Ioannidis JP, de Andrade M, Rocca WA. UCHL1 is a Parkinson’s disease susceptibility gene. Ann Neurol 55: 512–521, 2004. 146. Lee MJ, Lee BH, Hanna J, King RW, Finley D. Trimming of ubiquitin chains by proteasome-associated deubiquitinating enzymes. Mol Cell Proteomics 10: R110 – 003871, 2011. 147. Leggett DS, Hanna J, Borodovsky A, Crosas B, Schmidt M, Baker RT, Walz T, Ploegh H, Finley D. Multiple associated proteins regulate proteasome structure and function. Mol Cell 10: 495–507, 2002. 148. Leroy E, Boyer R, Auburger G, Leube B, Ulm G, Mezey E, Harta G, Brownstein MJ, Jonnalagada S, Chernova T, Dehejia A, Lavedan C, Gasser T, Steinbach PJ, Wilkinson KD, Polymeropoulos MH. The ubiquitin pathway in Parkinson’s disease. Nature 395: 451– 452, 1998. 149. Letunic I, Doerks T, Bork P. SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Res 40: D302–305, 2012. 150. Li J, D’Angiolella V, Seeley ES, Kim S, Kobayashi T, Fu W, Campos EI, Pagano M, Dynlacht BD. USP33 regulates centrosome biogenesis via deubiquitination of the centriolar protein CP110. Nature 495: 255–259, 2013. 151. Li M, Brooks CL, Kon N, Gu W. A dynamic role of HAUSP in the p53-Mdm2 pathway. Mol Cell 13: 879 – 886, 2004. 152. Li M, Chen D, Shiloh A, Luo J, Nikolaev AY, Qin J, Gu W. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature 416: 648 – 653, 2002. 153. Li S, Chen Y, Shi Q, Yue T, Wang B, Jiang J. Hedgehog-regulated ubiquitination controls smoothened trafficking and cell surface expression in Drosophila. PLoS Biol 10: e1001239, 2012. 154. Licchesi JD, Mieszczanek J, Mevissen TE, Rutherford TJ, Akutsu M, Virdee S, El Oualid F, Chin JW, Ovaa H, Bienz M, Komander D. An ankyrin-repeat ubiquitin-binding domain determines TRABID’s specificity for atypical ubiquitin chains. Nat Struct Mol Biol 19: 62–71, 2011. 155. Lim JH, Jono H, Komatsu K, Woo CH, Lee J, Miyata M, Matsuno T, Xu X, Huang Y, Zhang W, Park SH, Kim YI, Choi YD, Shen H, Heo KS, Xu H, Bourne P, Koga T, Yan C, Wang B, Chen LF, Feng XH, Li JD. CYLD negatively regulates transforming growth factor-beta-signaling via deubiquitinating Akt. Nat Commun 3: 771, 2012. 156. Lin SC, Chung JY, Lamothe B, Rajashankar K, Lu M, Lo YC, Lam AY, Darnay BG, Wu H. Molecular basis for the unique deubiquitinating activity of the NF-kappaB inhibitor A20. J Mol Biol 376: 526 –540, 2008. 157. Lin Z, Yang H, Kong Q, Li J, Lee SM, Gao B, Dong H, Wei J, Song J, Zhang DD, Fang D. USP22 antagonizes p53 transcriptional activation by deubiquitinating Sirt1 to suppress cell apoptosis and is required for mouse embryonic development. Mol Cell 46: 484 – 494, 2012. 166. Marchler-Bauer A, Zheng C, Chitsaz F, Derbyshire MK, Geer LY, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Lanczycki CJ, Lu F, Lu S, Marchler GH, Song JS, Thanki N, Yamashita RA, Zhang D, Bryant SH. CDD: conserved domains and protein threedimensional structure. Nucleic Acids Res 41: D348 –352, 2013. 167. Matmati M, Jacques P, Maelfait J, Verheugen E, Kool M, Sze M, Geboes L, Louagie E, Mc Guire C, Vereecke L, Chu Y, Boon L, Staelens S, Matthys P, Lambrecht BN, Schmidt-Supprian M, Pasparakis M, Elewaut D, Beyaert R, van Loo G. A20 (TNFAIP3) deficiency in myeloid cells triggers erosive polyarthritis resembling rheumatoid arthritis. Nat Genet 43: 908 –912, 2011. 168. Matos CA, de Macedo-Ribeiro S, Carvalho AL. Polyglutamine diseases: the special case of ataxin-3 and Machado-Joseph disease. Prog Neurobiol 95: 26 – 48, 2011. 169. Maytal-Kivity V, Reis N, Hofmann K, Glickman MH. MPN⫹, a putative catalytic motif found in a subset of MPN domain proteins from eukaryotes and prokaryotes, is critical for Rpn11 function. BMC Biochem 3: 28, 2002. 170. McCullough J, Clague MJ, Urbe S. AMSH is an endosome-associated ubiquitin isopeptidase. J Cell Biol 166: 487– 492, 2004. 171. McCullough J, Row PE, Lorenzo O, Doherty M, Beynon R, Clague MJ, Urbe S. Activation of the endosome-associated ubiquitin isopeptidase AMSH by STAM, a component of the multivesicular body-sorting machinery. Curr Biol 16: 160 –165, 2006. 172. Meierhofer D, Wang X, Huang L, Kaiser P. Quantitative analysis of global ubiquitination in HeLa cells by mass spectrometry. J Proteome Res 7: 4566 – 4576, 2008. 173. Meulmeester E, Kunze M, Hsiao HH, Urlaub H, Melchior F. Mechanism and consequences for paralog-specific sumoylation of ubiquitin-specific protease 25. Mol Cell 30: 610 – 619, 2008. 174. Minguez P, Parca L, Diella F, Mende DR, Kumar R, Helmer-Citterich M, Gavin AC, van Noort V, Bork P. Deciphering a global network of functionally associated post-translational modifications. Mol Syst Biol 8: 599, 2012. 175. Misaghi S, Galardy PJ, Meester WJ, Ovaa H, Ploegh HL, Gaudet R. Structure of the ubiquitin hydrolase UCH-L3 complexed with a suicide substrate. J Biol Chem 280: 1512–1520, 2005. 176. Mizuno E, Kitamura N, Komada M. 14 –3-3-dependent inhibition of the deubiquitinating activity of UBPY and its cancellation in the M phase. Exp Cell Res 313: 3624 – 3634, 2007. 177. Mizuno E, Kobayashi K, Yamamoto A, Kitamura N, Komada M. A deubiquitinating enzyme UBPY regulates the level of protein ubiquitination on endosomes. Traffic 7: 1017–10131, 2006. 158. Liu J, Xia H, Kim M, Xu L, Li Y, Zhang L, Cai Y, Norberg HV, Zhang T, Furuya T, Jin M, Zhu Z, Wang H, Yu J, Hao Y, Choi A, Ke H, Ma D, Yuan J. Beclin1 controls the levels of p53 by regulating the deubiquitination activity of USP10 and USP13. Cell 147: 223–234, 2011. 178. Moretti J, Chastagner P, Gastaldello S, Heuss SF, Dirac AM, Bernards R, Masucci MG, Israel A, Brou C. The translation initiation factor 3f (eIF3f) exhibits a deubiquitinase activity regulating Notch activation. PLoS Biol 8: e1000545, 2010. 159. Liu Z, Zanata SM, Kim J, Peterson MA, Di Vizio D, Chirieac LR, Pyne S, Agostini M, Freeman MR, Loda M. The ubiquitin-specific protease USP2a prevents endocytosismediated EGFR degradation. Oncogene 2012. 179. Mtango NR, Sutovsky M, Susor A, Zhong Z, Latham KE, Sutovsky P. Essential role of maternal UCHL1 and UCHL3 in fertilization and preimplantation embryo development. J Cell Physiol 227: 1592–1603, 2012. 160. Luna-Vargas MP, Faesen AC, van Dijk WJ, Rape M, Fish A, Sixma TK. Ubiquitinspecific protease 4 is inhibited by its ubiquitin-like domain. EMBO Rep 12: 365–372, 2011. 180. Mukai A, Yamamoto-Hino M, Awano W, Watanabe W, Komada M, Goto S. Balanced ubiquitylation and deubiquitylation of Frizzled regulate cellular responsiveness to Wg/Wnt. EMBO J 29: 2114 –2125, 2010. 161. Ma A, Malynn BA. A20: linking a complex regulator of ubiquitylation to immunity and human disease. Nat Rev Immunol 12: 774 –785, 2012. 181. Nakamura N, Hirose S. Regulation of mitochondrial morphology by USP30, a deubiquitinating enzyme present in the mitochondrial outer membrane. Mol Biol Cell 19: 1903–1911, 2008. 162. Machida YJ, Machida Y, Vashisht AA, Wohlschlegel JA, Dutta A. The deubiquitinating enzyme BAP1 regulates cell growth via interaction with HCF-1. J Biol Chem 284: 34179 –34188, 2009. 1312 182. Nathan JA, Kim HT, Ting L, Gygi SP, Goldberg AL. Why do cellular proteins linked to K63-polyubiquitin chains not associate with proteasomes? EMBO J 32: 552–565, 2013. Physiol Rev • VOL 93 • JULY 2013 • www.prv.org DUBs FROM GENES TO ORGANISM 183. Nicassio F, Corrado N, Vissers JH, Areces LB, Bergink S, Marteijn JA, Geverts B, Houtsmuller AB, Vermeulen W, Di Fiore PP, Citterio E. Human USP3 is a chromatin modifier required for S phase progression and genome stability. Curr Biol 2007. 203. Qiu XB, Ouyang SY, Li CJ, Miao S, Wang L, Goldberg AL. hRpn13/ADRM1/GP110 is a novel proteasome subunit that binds the deubiquitinating enzyme, UCH37. EMBO J 25: 5742–5753, 2006. 184. Nicastro G, Todi SV, Karaca E, Bonvin AM, Paulson HL, Pastore A. Understanding the role of the Josephin domain in the PolyUb binding and cleavage properties of ataxin-3. PLoS ONE 5: e12430, 2010. 204. Rahighi S, Dikic I. Selectivity of the ubiquitin-binding modules. FEBS Lett 586: 2705– 2710, 2012. 205. Reina CP, Nabet BY, Young PD, Pittman RN. Basal and stress-induced Hsp70 are modulated by ataxin-3. Cell Stress Chaperones 2012. 185. Niendorf S, Oksche A, Kisser A, Lohler J, Prinz M, Schorle H, Feller S, Lewitzky M, Horak I, Knobeloch KP. Essential role of ubiquitin-specific protease 8 for receptor tyrosine kinase stability and endocytic trafficking in vivo. Mol Cell Biol 27: 5029 –5039, 2007. 206. Reina CP, Zhong X, Pittman RN. Proteotoxic stress increases nuclear localization of ataxin-3. Hum Mol Genet 19: 235–249, 2010. 186. Nijman SM, Huang TT, Dirac AM, Brummelkamp TR, Kerkhoven RM, D’Andrea AD, Bernards R. The deubiquitinating enzyme USP1 regulates the Fanconi anemia pathway. Mol Cell 17: 331–339, 2005. 207. Reyes-Turcu FE, Horton JR, Mullally JE, Heroux A, Cheng X, Wilkinson KD. The ubiquitin binding domain ZnF UBP recognizes the C-terminal diglycine motif of unanchored ubiquitin. Cell 124: 1197–1208, 2006. 187. Nijman SM, Luna-Vargas MP, Velds A, Brummelkamp TR, Dirac AM, Sixma TK, Bernards R. A genomic and functional inventory of deubiquitinating enzymes. Cell 123: 773–786, 2005. 208. Reyes-Turcu FE, Shanks JR, Komander D, Wilkinson KD. Recognition of polyubiquitin isoforms by the multiple ubiquitin binding modules of isopeptidase T. J Biol Chem 283: 19581–19592, 2008. 188. Nijnik A, Clare S, Hale C, Raisen C, McIntyre RE, Yusa K, Everitt AR, Mottram L, Podrini C, Lucas M, Estabel J, Goulding D, Adams N, Ramirez-Solis R, White JK, Adams DJ, Hancock RE, Dougan G. The critical role of histone H2A-deubiquitinase Mysm1 in hematopoiesis and lymphocyte differentiation. Blood 119: 1370 –1379, 2012. 209. Reyes-Turcu FE, Ventii KH, Wilkinson KD. Regulation and cellular roles of ubiquitinspecific deubiquitinating enzymes. Annu Rev Biochem 78: 363–397, 2009. 189. Nikolaou K, Tsagaratou A, Eftychi C, Kollias G, Mosialos G, Talianidis I. Inactivation of the deubiquitinase CYLD in hepatocytes causes apoptosis, inflammation, fibrosis, and cancer. Cancer Cell 21: 738 –750, 2012. 211. Rhodes DR, Kalyana-Sundaram S, Mahavisno V, Varambally R, Yu J, Briggs BB, Barrette TR, Anstet MJ, Kincead-Beal C, Kulkarni P, Varambally S, Ghosh D, Chinnaiyan O. Oncomine 3 AM genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia 9: 166 –180, 2007. 190. Olsen JV, Vermeulen M, Santamaria A, Kumar C, Miller ML, Jensen LJ, Gnad F, Cox J, Jensen TS, Nigg EA, Brunak S, Mann M. Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci Signal 3: ra3, 2010. 191. Osaka H, Wang YL, Takada K, Takizawa S, Setsuie R, Li H, Sato Y, Nishikawa K, Sun YJ, Sakurai M, Harada T, Hara Y, Kimura I, Chiba S, Namikawa K, Kiyama H, Noda M, Aoki S, Wada K. Ubiquitin carboxy-terminal hydrolase L1 binds to and stabilizes monoubiquitin in neuron. Hum Mol Genet 12: 1945–1958, 2003. 192. Overstreet E, Fitch E, Fischer JA. Fat facets and Liquid facets promote Delta endocytosis and Delta signaling in the signaling cells. Development 131: 5355–5366, 2004. 193. Pai MT, Tzeng SR, Kovacs JJ, Keaton MA, Li SS, Yao TP, Zhou P. Solution structure of the Ubp-M BUZ domain, a highly specific protein module that recognizes the C-terminal tail of free ubiquitin. J Mol Biol 370: 290 –302, 2007. 194. Panattoni M, Sanvito F, Basso V, Doglioni C, Casorati G, Montini E, Bender JR, Mondino A, Pardi R. Targeted inactivation of the COP9 signalosome impairs multiple stages of T cell development. J Exp Med 205: 465– 477, 2008. 195. Pareja F, Ferraro DA, Rubin C, Cohen-Dvashi H, Zhang F, Aulmann S, Ben-Chetrit N, Pines G, Navon R, Crosetto N, Kostler W, Carvalho S, Lavi S, Schmitt F, Dikic I, Yakhini Z, Sinn P, Mills GB, Yarden Y. Deubiquitination of EGFR by Cezanne-1 contributes to cancer progression. Oncogene 2012. 196. Peng J, Schwartz D, Elias JE, Thoreen CC, Cheng D, Marsischky G, Roelofs J, Finley D, Gygi SP. A proteomics approach to understanding protein ubiquitination. Nat Biotechnol 21: 921–926, 2003. 197. Peth A, Besche HC, Goldberg AL. Ubiquitinated proteins activate the proteasome by binding to Usp14/Ubp6, which causes 20S gate opening. Mol Cell 36: 794 – 804, 2009. 198. Peth A, Kukushkin N, Bosse M, Goldberg AL. Ubiquitinated proteins activate the proteasomal ATPases by binding to usp14 or uch37 homologs. J Biol Chem 288: 7781–7790, 2013. 199. Pickart CM, Rose IA. Ubiquitin carboxyl-terminal hydrolase acts on ubiquitin carboxyl-terminal amides. J Biol Chem 260: 7903–7910, 1985. 200. Poole B, Ohkuma S, Warburton M. Protein degradation in cells in culture. Ciba Found Symp 189 –203, 1979. 210. Reyes-Turcu FE, Wilkinson KD. Polyubiquitin binding and disassembly by deubiquitinating enzymes. Chem Rev 2009. 212. Richer E, Prendergast C, Zhang DE, Qureshi ST, Vidal SM, Malo D. N-ethyl-Nnitrosourea-induced mutation in ubiquitin-specific peptidase 18 causes hyperactivation of IFN-alphass signaling and suppresses STAT4-induced IFN-gamma production, resulting in increased susceptibility to Salmonella typhimurium. J Immunol 185: 3593– 3601, 2010. 213. Rinaldi T, Hofmann L, Gambadoro A, Cossard R, Livnat-Levanon N, Glickman MH, Frontali L, Delahodde A. Dissection of the carboxyl-terminal domain of the proteasomal subunit Rpn11 in maintenance of mitochondrial structure and function. Mol Biol Cell 19: 1022–1031, 2008. 214. Ritchie KJ, Malakhov MP, Hetherington CJ, Zhou L, Little MT, Malakhova OA, Sipe JC, Orkin SH, Zhang DE. Dysregulation of protein modification by ISG15 results in brain cell injury. Genes Dev 16: 2207–2212, 2002. 215. Rodrigues AJ, Coppola G, Santos C, Costa Mdo C, Ailion M, Sequeiros J, Geschwind DH, Maciel P. Functional genomics and biochemical characterization of the C. elegans orthologue of the Machado-Joseph disease protein ataxin-3. FASEB J 21: 1126 –1136, 2007. 216. Rose PW, Bi C, Bluhm WF, Christie CH, Dimitropoulos D, Dutta S, Green RK, Goodsell DS, Prlic A, Quesada M, Quinn GB, Ramos AG, Westbrook JD, Young J, Zardecki C, Berman HM, Bourne PE. The RCSB Protein Data Bank: new resources for research and education. Nucleic Acids Res 41: D475–D482, 2013. 217. Ross SH, Lindsay Y, Safrany ST, Lorenzo O, Villa F, Toth R, Clague MJ, Downes CP, Leslie NR. Differential redox regulation within the PTP superfamily. Cell Signal 19: 1521–1530, 2007. 218. Row PE, Liu H, Hayes S, Welchman R, Charalabous P, Hofmann K, Clague MJ, Sanderson CM, Urbe S. The MIT domain of UBPY constitutes a CHMP binding and endosomal localization signal required for efficient epidermal growth factor receptor degradation. J Biol Chem 282: 30929 –30937, 2007. 219. Row PE, Prior IA, McCullough J, Clague MJ, Urbe S. The ubiquitin isopeptidase UBPY regulates endosomal ubiquitin dynamics and is essential for receptor down-regulation. J Biol Chem 281: 12618 –12624, 2006. 201. Popp MW, Artavanis-Tsakonas K, Ploegh HL. Substrate filtering by the active site crossover loop in UCHL3 revealed by sortagging and gain-of-function mutations. J Biol Chem 284: 3593–3602, 2009. 220. Ruan HB, Han X, Li MD, Singh JP, Qian K, Azarhoush S, Zhao L, Bennett AM, Samuel VT, Wu J, Yates JR, 3rd, Yang X. O-GlcNAc transferase/host cell factor C1 complex regulates gluconeogenesis by modulating PGC-1alpha stability. Cell Metab 16: 226 – 237, 2012. 202. Punta M, Coggill PC, Eberhardt RY, Mistry J, Tate J, Boursnell C, Pang N, Forslund K, Ceric G, Clements J, Heger A, Holm L, Sonnhammer EL, Eddy SR, Bateman A, Finn RD. The Pfam protein families database. Nucleic Acids Res 40: D290 –301, 2012. 221. Saigoh K, Wang YL, Suh JG, Yamanishi T, Sakai Y, Kiyosawa H, Harada T, Ichihara N, Wakana S, Kikuchi T, Wada K. Intragenic deletion in the gene encoding ubiquitin carboxy-terminal hydrolase in gad mice. Nat Genet 23: 47–51, 1999. Physiol Rev • VOL 93 • JULY 2013 • www.prv.org 1313 CLAGUE ET AL. 222. Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4: 406 – 425, 1987. 223. Samara NL, Datta AB, Berndsen CE, Zhang X, Yao T, Cohen RE, Wolberger C. Structural insights into the assembly and function of the SAGA deubiquitinating module. Science 328: 1025–1029, 2010. 224. Sato Y, Yamagata A, Goto-Ito S, Kubota K, Miyamoto R, Nakada S, Fukai S. Molecular basis of Lys-63-linked polyubiquitination inhibition by the interaction between human deubiquitinating enzyme OTUB1 and ubiquitin-conjugating enzyme UBC13. J Biol Chem 287: 25860 –25868, 2012. 225. Sato Y, Yoshikawa A, Yamagata A, Mimura H, Yamashita M, Ookata K, Nureki O, Iwai K, Komada M, Fukai S. Structural basis for specific cleavage of Lys 63-linked polyubiquitin chains. Nature 455: 358 –362, 2008. 226. Schechter I, Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun 27: 157–162, 1967. 227. Scheel H, Tomiuk S, Hofmann K. Elucidation of ataxin-3 and ataxin-7 function by integrative bioinformatics. Hum Mol Genet 12: 2845–2852, 2003. 228. Scheuermann JC, de Ayala Alonso AG, Oktaba K, Ly-Hartig N, McGinty RK, Fraterman S, Wilm M, Muir TW, Muller J. Histone H2A deubiquitinase activity of the Polycomb repressive complex PR-DUB. Nature 2010. 229. Schmitt I, Linden M, Khazneh H, Evert BO, Breuer P, Klockgether T, Wuellner U. Inactivation of the mouse Atxn3 (ataxin-3) gene increases protein ubiquitination. Biochem Biophys Res Commun 362: 734 –739, 2007. 230. Schulz S, Chachami G, Kozaczkiewicz L, Winter U, Stankovic-Valentin N, Haas P, Hofmann K, Urlaub H, Ovaa H, Wittbrodt J, Meulmeester E, Melchior F. Ubiquitinspecific protease-like 1 (USPL1) is a SUMO isopeptidase with essential, non-catalytic functions. EMBO Rep 13: 930 –938, 2012. 231. Schwanhausser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M. Global quantification of mammalian gene expression control. Nature 473: 337– 342, 2011. 232. Schweitzer K, Bozko PM, Dubiel W, Naumann M. CSN controls NF-kappaB by deubiquitinylation of IkappaBalpha. EMBO J 26: 1532–1541, 2007. 233. Setsuie R, Suzuki M, Kabuta T, Fujita H, Miura S, Ichihara N, Yamada D, Wang YL, Ezaki O, Suzuki Y, Wada K. Ubiquitin C-terminal hydrolase-L3-knockout mice are resistant to diet-induced obesity and show increased activation of AMP-activated protein kinase in skeletal muscle. FASEB J 23: 4148 – 4157, 2009. 234. Setsuie R, Wang YL, Mochizuki H, Osaka H, Hayakawa H, Ichihara N, Li H, Furuta A, Sano Y, Sun YJ, Kwon J, Kabuta T, Yoshimi K, Aoki S, Mizuno Y, Noda M, Wada K. Dopaminergic neuronal loss in transgenic mice expressing the Parkinson’s diseaseassociated UCH-L1 I93M mutant. Neurochem Int 50: 119 –129, 2007. 235. Shao G, Lilli DR, Patterson-Fortin J, Coleman KA, Morrissey DE, Greenberg RA. The Rap80-BRCC36 de-ubiquitinating enzyme complex antagonizes RNF8-Ubc13-dependent ubiquitination events at DNA double strand breaks. Proc Natl Acad Sci USA 106: 3166 –3171, 2009. 236. Shembade N, Harhaj EW. Regulation of NF-kappaB signaling by the A20 deubiquitinase. Cell Mol Immunol 9: 123–130, 2012. 237. Shembade N, Ma A, Harhaj EW. Inhibition of NF-kappaB signaling by A20 through disruption of ubiquitin enzyme complexes. Science 327: 1135–1139, 2010. 238. Sigrist CJ, de Castro E, Cerutti L, Cuche BA, Hulo N, Bridge A, Bougueleret L, Xenarios I. New and continuing developments at PROSITE. Nucleic Acids Res 41: D344 –347, 2013. 239. Skaug B, Chen J, Du F, He J, Ma A, Chen ZJ. Direct, noncatalytic mechanism of IKK inhibition by A20. Mol Cell 44: 559 –571, 2011. 240. Soding J. Protein homology detection by HMM-HMM comparison. Bioinformatics 21: 951–960, 2005. 241. Solomons J, Sabin C, Poudevigne E, Usami Y, Hulsik DL, Macheboeuf P, Hartlieb B, Gottlinger H, Weissenhorn W. Structural basis for ESCRT-III CHMP3 recruitment of AMSH. Structure 19: 1149 –1159, 2011. 242. Song MS, Salmena L, Carracedo A, Egia A, Lo-Coco F, Teruya-Feldstein J, Pandolfi PP. The deubiquitinylation and localization of PTEN are regulated by a HAUSP-PML network. Nature 455: 813– 817, 2008. 1314 243. Soriano P, Gridley T, Jaenisch R. Retroviruses and insertional mutagenesis in mice: proviral integration at the Mov 34 locus leads to early embryonic death. Genes Dev 1: 366 –375, 1987. 244. Sowa ME, Bennett EJ, Gygi SP, Harper JW. Defining the human deubiquitinating enzyme interaction landscape. Cell 138: 389 – 403, 2009. 245. Storer AC, Menard R. Catalytic mechanism in papain family of cysteine peptidases. Methods Enzymol 244: 486 –500, 1994. 246. Sun XX, Challagundla KB, Dai MS. Positive regulation of p53 stability and activity by the deubiquitinating enzyme Otubain 1. EMBO J 31: 576 –592, 2012. 247. Suzuki M, Setsuie R, Wada K. Ubiquitin carboxyl-terminal hydrolase l3 promotes insulin signaling and adipogenesis. Endocrinology 150: 5230 –5239, 2009. 248. Suzuki S, Tamai K, Watanabe M, Kyuuma M, Ono M, Sugamura K, Tanaka N. AMSH is required to degrade ubiquitinated proteins in the central nervous system. Biochem Biophys Res Commun 408: 582–588, 2011. 249. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 2731–2739, 2011. 250. Tanaka N, Kaneko K, Asao H, Kasai H, Endo Y, Fujita T, Takeshita T, Sugamura K. Possible involvement of a novel STAM-associated molecule “AMSH” in intracellular signal transduction mediated by cytokines. J Biol Chem 274: 19129 –19135, 1999. 251. Tauriello DV, Maurice MM. The various roles of ubiquitin in Wnt pathway regulation. Cell Cycle 9: 3700 –3709, 2010. 252. Testa JR, Cheung M, Pei J, Below JE, Tan Y, Sementino E, Cox NJ, Dogan AU, Pass HI, Trusa S, Hesdorffer M, Nasu M, Powers A, Rivera Z, Comertpay S, Tanji M, Gaudino G, Yang H, Carbone M. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet 43: 1022–1025, 2011. 253. Tezel E, Hibi K, Nagasaka T, Nakao A. PGP9.5as a prognostic factor in pancreatic cancer. Clin Cancer Res 6: 4764 – 4767, 2000. 254. Thorne C, Eccles RL, Coulson JM, Urbe S, Clague MJ. Isoform-specific localization of the deubiquitinase USP33 to the Golgi apparatus. Traffic 2011. 255. Todi SV, Scaglione KM, Blount JR, Basrur V, Conlon KP, Pastore A, Elenitoba-Johnson K, Paulson HL. Activity and cellular functions of the deubiquitinating enzyme and polyglutamine disease protein ataxin-3 are regulated by ubiquitination at lysine 117. J Biol Chem 285: 39303–39313, 2010. 256. Tomida S, Mamiya T, Sakamaki H, Miura M, Aosaki T, Masuda M, Niwa M, Kameyama T, Kobayashi J, Iwaki Y, Imai S, Ishikawa A, Abe K, Yoshimura T, Nabeshima T, Ebihara S. Usp46 is a quantitative trait gene regulating mouse immobile behavior in the tail suspension and forced swimming tests. Nat Genet 41: 688 – 695, 2009. 257. Traub LM, Lukacs GL. Decoding ubiquitin sorting signals for clathrin-dependent endocytosis by CLASPs. J Cell Sci 120: 543–553, 2007. 258. Tsang HT, Connell JW, Brown SE, Thompson A, Reid E, Sanderson CM. A systematic analysis of human CHMP protein interactions: additional MIT domain-containing proteins bind to multiple components of the human ESCRT III complex. Genomics 2006. 259. Tse WKF, Eisenhaber B, Ho SHK, Ng Q, Eisenhaber F, Jiang YJ. Genome-wide lossof-function analysis of deubiquitylating enzymes for zebrafish development. BMC Genomics 10: 637, 2009. 260. Tsou WL, Sheedlo MJ, Morrow ME, Blount JR, McGregor KM, Das C, Todi SV. Systematic analysis of the physiological importance of deubiquitinating enzymes. PLoS ONE 7: e43112, 2012. 261. Urbe S, Liu H, Hayes SD, Heride C, Rigden DJ, Clague MJ. Systematic survey of deubiquitinase localisation identifies USP21 as a regulator of centrosome and microtubule associated functions. Mol Biol Cell 23: 1095–1103, 2012. 262. van der Horst A, de Vries-Smits AM, Brenkman AB, van Triest MH, van den Broek N, Colland F, Maurice MM, Burgering BM. FOXO4 transcriptional activity is regulated by monoubiquitination and USP7/HAUSP. Nat Cell Biol 8: 1064 –1073, 2006. 263. Varadan R, Walker O, Pickart C, Fushman D. Structural properties of polyubiquitin chains in solution. J Mol Biol 324: 637– 647, 2002. Physiol Rev • VOL 93 • JULY 2013 • www.prv.org DUBs FROM GENES TO ORGANISM 264. Vasu VT, Ott S, Hobson B, Rashidi V, Oommen S, Cross CE, Gohil K. Sarcolipin and ubiquitin carboxy-terminal hydrolase 1 mRNAs are over-expressed in skeletal muscles of alpha-tocopherol deficient mice. Free Radic Res 43: 106 –116, 2009. 283. Yamazaki T, Hibi K, Takase T, Tezel E, Nakayama H, Kasai Y, Ito K, Akiyama S, Nagasaka T, Nakao A. PGP9.5as a marker for invasive colorectal cancer. Clin Cancer Res 8: 192–195, 2002. 265. Vereecke L, Sze M, Mc Guire C, Rogiers B, Chu Y, Schmidt-Supprian M, Pasparakis M, Beyaert R, van Loo G. Enterocyte-specific A20 deficiency sensitizes to tumor necrosis factor-induced toxicity and experimental colitis. J Exp Med 207: 1513–1523, 2010. 284. Yao T, Cohen RE. A cryptic protease couples deubiquitination and degradation by the proteasome. Nature 419: 403– 407, 2002. 266. Verma R, Aravind L, Oania R, McDonald WH, Yates JR 3rd, Koonin EV, Deshaies RJ. Role of Rpn11 metalloprotease in deubiquitination and degradation by the 26S proteasome. Science 298: 611– 615, 2002. 285. Yao T, Song L, Jin J, Cai Y, Takahashi H, Swanson SK, Washburn MP, Florens L, Conaway RC, Cohen RE, Conaway JW. Distinct modes of regulation of the Uch37 deubiquitinating enzyme in the proteasome and in the Ino80 chromatin-remodeling complex. Mol Cell 31: 909 –917, 2008. 267. Villamil MA, Liang Q, Chen J, Choi YS, Hou S, Lee KH, Zhuang Z. Serine phosphorylation is critical for the activation of ubiquitin-specific protease 1 and its interaction with WD40-repeat protein UAF1. Biochemistry 2012. 268. Virdee S, Ye Y, Nguyen DP, Komander D, Chin JW. Engineered diubiquitin synthesis reveals Lys-29-isopeptide specificity of an OTU deubiquitinase. Nature Chem Biol 6: 750 –756, 2010. 269. Walczak H, Iwai K, Dikic I. Generation and physiological roles of linear ubiquitin chains. BMC Biol 10: 23, 2012. 270. Wang T, Yin L, Cooper EM, Lai MY, Dickey S, Pickart CM, Fushman D, Wilkinson KD, Cohen RE, Wolberger C. Evidence for bidentate substrate binding as the basis for the K48 linkage specificity of otubain 1. J Mol Biol 2009. 271. Wertz IE, O’Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, Wu P, Wiesmann C, Baker R, Boone DL, Ma A, Koonin EV, Dixit VM. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signaling. Nature 430: 694 – 699, 2004. 272. Wicks SJ, Haros K, Maillard M, Song L, Cohen RE, Dijke PT, Chantry A. The deubiquitinating enzyme UCH37 interacts with Smads and regulates TGF-beta signaling. Oncogene 24: 8080 – 8084, 2005. 273. Wiener R, Zhang X, Wang T, Wolberger C. The mechanism of OTUB1-mediated inhibition of ubiquitination. Nature 483: 618 – 622, 2012. 274. Wiesner T, Obenauf AC, Murali R, Fried I, Griewank KG, Ulz P, Windpassinger C, Wackernagel W, Loy S, Wolf I, Viale A, Lash AE, Pirun M, Socci ND, Rutten A, Palmedo G, Abramson D, Offit K, Ott A, Becker JC, Cerroni L, Kutzner H, Bastian BC, Speicher MR. Germline mutations in BAP1 predispose to melanocytic tumors. Nat Genet 43: 1018 –1021, 2011. 286. Yao T, Song L, Xu W, DeMartino GN, Florens L, Swanson SK, Washburn MP, Conaway RC, Conaway JW, Cohen RE. Proteasome recruitment and activation of the Uch37 deubiquitinating enzyme by Adrm1. Nat Cell Biol 8: 994 –1002, 2006. 287. Ye Y, Akutsu M, Reyes-Turcu F, Enchev RI, Wilkinson KD, Komander D. Polyubiquitin binding and cross-reactivity in the USP domain deubiquitinase USP21. EMBO Rep 2011. 288. Ye Y, Blaser G, Horrocks MH, Ruedas-Rama MJ, Ibrahim S, Zhukov AA, Orte A, Klenerman D, Jackson SE, Komander D. Ubiquitin chain conformation regulates recognition and activity of interacting proteins. Nature 492: 266 –270, 2012. 289. Ye Y, Scheel H, Hofmann K, Komander D. Dissection of USP catalytic domains reveals five common insertion points. Mol Biosyst 5: 1797–1808, 2009. 290. Zhang L, Zhou F, Drabsch Y, Gao R, Snaar-Jagalska BE, Mickanin C, Huang H, Sheppard KA, Porter JA, Lu CX, Ten Dijke P. USP4 is regulated by AKT phosphorylation and directly deubiquitylates TGF-beta type I receptor. Nat Cell Biol 2012. 291. Zhang NY, Jacobson AD, Macfadden A, Liu CW. Ubiquitin chain trimming recycles the substrate binding sites of the 26S proteasome and promotes degradation of lysine 48-linked polyubiquitin conjugates. J Biol Chem 286: 25540 –25546, 2011. 292. Zhang X, Berger FG, Yang J, Lu X. USP4 inhibits p53 through deubiquitinating and stabilizing ARF-BP1. EMBO J 30: 2177–2189, 2011. 293. Zhang Y, Foreman O, Wigle DA, Kosari F, Vasmatzis G, Salisbury JL, van Deursen J, Galardy PJ. USP44 regulates centrosome positioning to prevent aneuploidy and suppress tumorigenesis. J Clin Invest 122: 4362– 4374, 2012. 276. Williams RL, Urbe S. The emerging shape of the ESCRT machinery. Nat Rev Mol Cell Biol 8: 355–368, 2007. 294. Zhao R, Yeung SC, Chen J, Iwakuma T, Su CH, Chen B, Qu C, Zhang F, Chen YT, Lin YL, Lee DF, Jin F, Zhu R, Shaikenov T, Sarbassov D, Sahin A, Wang H, Lai CC, Tsai FJ, Lozano G, Lee MH. Subunit 6 of the COP9 signalosome promotes tumorigenesis in mice through stabilization of MDM2 and is upregulated in human cancers. J Clin Invest 121: 851– 865, 2011. 277. Williams SA, Maecker HL, French DM, Liu J, Gregg A, Silverstein LB, Cao TC, Carano RA, Dixit VM. USP1 deubiquitinates ID proteins to preserve a mesenchymal stem cell program in osteosarcoma. Cell 146: 918 –930, 2011. 295. Zhong B, Liu X, Wang X, Chang SH, Liu X, Wang A, Reynolds JM, Dong C. Negative regulation of IL-17-mediated signaling and inflammation by the ubiquitin-specific protease USP25. Nat Immunol 13: 1110 –1117, 2012. 278. Wilson SM, Bhattacharyya B, Rachel RA, Coppola V, Tessarollo L, Householder DB, Fletcher CF, Miller RJ, Copeland NG, Jenkins NA. Synaptic defects in ataxia mice result from a mutation in Usp14, encoding a ubiquitin-specific protease. Nat Genet 32: 420 – 425, 2002. 296. Zhou ZR, Zhang YH, Liu S, Song AX, Hu HY. Length of the active-site crossover loop defines the substrate specificity of ubiquitin C-terminal hydrolases for ubiquitin chains. Biochem J 441: 143–149, 2012. 279. Winborn BJ, Travis SM, Todi SV, Scaglione KM, Xu P, Williams AJ, Cohen RE, Peng J, Paulson HL. The deubiquitinating enzyme ataxin-3, a polyglutamine disease protein, edits Lys63 linkages in mixed linkage ubiquitin chains. J Biol Chem 283: 26436 –26443, 2008. 297. Zhu P, Zhou W, Wang J, Puc J, Ohgi KA, Erdjument-Bromage H, Tempst P, Glass CK, Rosenfeld MG. A histone H2A deubiquitinase complex coordinating histone acetylation and H1 dissociation in transcriptional regulation. Mol Cell 27: 609 – 621, 2007. 275. Wilkinson KD, Deshpande S, Larsen CN. Comparisons of neuronal (PGP 9.5) and non-neuronal ubiquitin C-terminal hydrolases. Biochem Soc Trans 20: 631– 637, 1992. 280. Wolf YI, Koonin EV. A tight link between orthologs and bidirectional best hits in bacterial and archaeal genomes. Genome Biol Evol 4: 1286 –1294, 2012. 281. Xia R, Jia H, Fan J, Liu Y, Jia J. USP8 promotes smoothened signaling by preventing its ubiquitination and changing its subcellular localization. PLoS Biol 10: e1001238, 2012. 298. Ziv I, Matiuhin Y, Kirkpatrick DS, Erpapazoglou Z, Leon S, Pantazopoulou M, Kim W, Gygi SP, Haguenauer-Tsapis R, Reis N, Glickman MH, Kleifeld O. A perturbed ubiquitin landscape distinguishes between ubiquitin in trafficking and in proteolysis. Mol Cell Proteomics 10: M111 009753, 2011. 282. Xu P, Duong DM, Seyfried NT, Cheng D, Xie Y, Robert J, Rush J, Hochstrasser M, Finley D, Peng J. Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell 137: 133–145, 2009. 299. Zuckerkandl E, Pauling L. Evolutionary divergence and convergence in proteins. In: Evolving Genes and Proteins, edited by Bryson V, Vogel HJ. New York: Academic, 1965, p. 97–166. Physiol Rev • VOL 93 • JULY 2013 • www.prv.org 1315