• Physical Change Chemical vs. Physical Changes –No new compounds are formed
advertisement

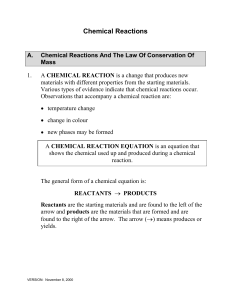
Chemical vs. Physical Changes • Physical Change –No new compounds are formed –Ex. Cutting, ripping, dissolving, phase changes… Chemical vs. Physical Changes • Chemical Change –Bonds are broken and the atoms in compounds are rearranged –New Compounds are created –Are the result of chemical reactions Chemical Reactions • Evidence – Bubbling, Cloudy solution, Temperature Change, Color Change, Smoke, Light, Heat • Reactants: what you start with • Products: what you end with • Can be written as a sentence or an equation: Methane reacts with oxygen to form carbon dioxide and water. or CH4 + O2 CO2 + H2O (REACTANTS) (PRODUCTS) Types of Reactions • Addition/Synthesis: two or more elements combine to form a new compound A + B AB Shortcut – only one product Types of Reactions • Decomposition: one compound breaks apart to form two or more products AB A + B Shortcut – only one reactant Types of Reactions • Single-Replacement (Displacement): an element replaces another element in a compound A +BX AX + B Shortcut one element & one compound on both sides Types of Reactions • Double-Replacement (Displacement): two elements in different compounds switch places AX + BY BX + AY Shortcut Two compounds with elements switching places in each Combustion: a compound containing carbon and hydrogen combines with oxygen to form carbon dioxide and water AB + O2 CO2 + H2O Types of Reactions • Combustion: a compound containing carbon and hydrogen combines with oxygen to form carbon dioxide and water AB + O2 CO2 + H2O Types of Reactions • Exothermic: reactions that give energy off to their surroundings. • See a flame or feel heat (hand warmers, MREs) • Endothermic: reactions that absorb energy from their surroundings. • Feel cold (instant ice packs) Law of Conservation of Mass • During a chemical reaction, atoms CANNOT be created nor destroyed, only rearranged! • In other words, the number of atoms of each element in the reactants must equal the number of atoms in the products. • CHEMICAL EQUATIONS MUST BE BALANCED!!! How to Balance Equations 1. Count the number of atoms on each side of the reaction. 2. Put a coefficient in front of one molecule that has too few atoms of an element. 3. Count the number of atoms again. 4. If all are equal—YOU ARE DONE! If not—REPEAT steps 2-3 until they are! CH4 + O2 CO2 + H2O Count the number of atoms: • Reactants: C–1 H–4 O–2 NOT BALANCED!!!!!!! • Products: C–1 H–2 O–2+1=3 CH4 + 2O2 CO2 + 2H2O Put in coefficients: • Reactants: C–1 H–4 O–2*2=4 BALANCED!!!!!!!!!! • Products: C–1 H–2*2=4 O–2+2=4