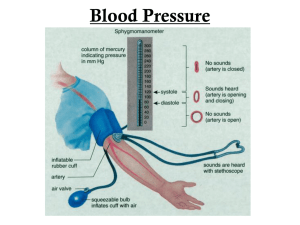
Regulation of Coronary Blood Flow During Exercise
advertisement

Physiol Rev 88: 1009 –1086, 2008; doi:10.1152/physrev.00045.2006. Regulation of Coronary Blood Flow During Exercise DIRK J. DUNCKER AND ROBERT J. BACHE Division of Experimental Cardiology, Department of Cardiology, Thoraxcenter, Cardiovascular Research Institute COEUR, Erasmus University Medical Center, Rotterdam, The Netherlands; and Division of Cardiology, Department of Medicine, Minnesota Medical School, Minneapolis, Minnesota I. Introduction II. Coronary Blood Flow in the Normal Heart During Exercise A. Myocardial oxygen demand B. Myocardial oxygen supply C. Determinants of coronary blood flow D. Transmural distribution of left ventricular myocardial blood flow E. Coronary blood flow to the right ventricle and the atria F. Control of coronary vascular resistance G. Coronary blood flow in the exercise-trained heart III. Coronary Blood Flow Distal to a Coronary Artery Obstruction During Exercise A. Regulation of coronary blood flow distal to a coronary artery stenosis B. Regulation of coronary blood flow to collateral-dependent myocardium during exercise C. Exercise training and the coronary collateral circulation 1010 1010 1010 1012 1016 1019 1021 1024 1040 1047 1047 1060 1068 Duncker DJ, Bache RJ. Regulation of Coronary Blood Flow During Exercise. Physiol Rev 88: 1009 –1086, 2008; doi:10.1152/physrev.00045.2006.—Exercise is the most important physiological stimulus for increased myocardial oxygen demand. The requirement of exercising muscle for increased blood flow necessitates an increase in cardiac output that results in increases in the three main determinants of myocardial oxygen demand: heart rate, myocardial contractility, and ventricular work. The approximately sixfold increase in oxygen demands of the left ventricle during heavy exercise is met principally by augmenting coronary blood flow (⬃5-fold), as hemoglobin concentration and oxygen extraction (which is already 70 – 80% at rest) increase only modestly in most species. In contrast, in the right ventricle, oxygen extraction is lower at rest and increases substantially during exercise, similar to skeletal muscle, suggesting fundamental differences in blood flow regulation between these two cardiac chambers. The increase in heart rate also increases the relative time spent in systole, thereby increasing the net extravascular compressive forces acting on the microvasculature within the wall of the left ventricle, in particular in its subendocardial layers. Hence, appropriate adjustment of coronary vascular resistance is critical for the cardiac response to exercise. Coronary resistance vessel tone results from the culmination of myriad vasodilator and vasoconstrictors influences, including neurohormones and endothelial and myocardial factors. Unraveling of the integrative mechanisms controlling coronary vasodilation in response to exercise has been difficult, in part due to the redundancies in coronary vasomotor control and differences between animal species. Exercise training is associated with adaptations in the coronary microvasculature including increased arteriolar densities and/or diameters, which provide a morphometric basis for the observed increase in peak coronary blood flow rates in exercise-trained animals. In larger animals trained by treadmill exercise, the formation of new capillaries maintains capillary density at a level commensurate with the degree of exercise-induced physiological myocardial hypertrophy. Nevertheless, training alters the distribution of coronary vascular resistance so that more capillaries are recruited, resulting in an increase in the permeabilitysurface area product without a change in capillary numerical density. Maintenance of ␣- and ß-adrenergic tone in the presence of lower circulating catecholamine levels appears to be due to increased receptor responsiveness to adrenergic stimulation. Exercise training also alters local control of coronary resistance vessels. Thus arterioles exhibit increased myogenic tone, likely due to a calcium-dependent protein kinase C signalingmediated alteration in voltage-gated calcium channel activity in response to stretch. Conversely, training augments endothelium-dependent vasodilation throughout the coronary microcirculation. This enhanced responsiveness appears to result principally from an increased expression of nitric oxide (NO) synthase. Finally, physical conditioning decreases extravascular compressive forces at rest and at comparable levels of exercise, www.prv.org 0031-9333/08 $18.00 Copyright © 2008 the American Physiological Society 1009 1010 DIRK J. DUNCKER AND ROBERT J. BACHE mainly because of a decrease in heart rate. Impedance to coronary inflow due to an epicardial coronary artery stenosis results in marked redistribution of myocardial blood flow during exercise away from the subendocardium towards the subepicardium. However, in contrast to the traditional view that myocardial ischemia causes maximal microvascular dilation, more recent studies have shown that the coronary microvessels retain some degree of vasodilator reserve during exercise-induced ischemia and remain responsive to vasoconstrictor stimuli. These observations have required reassessment of the principal sites of resistance to blood flow in the microcirculation. A significant fraction of resistance is located in small arteries that are outside the metabolic control of the myocardium but are sensitive to shear and nitrovasodilators. The coronary collateral system embodies a dynamic network of interarterial vessels that can undergo both long- and short-term adjustments that can modulate blood flow to the dependent myocardium. Long-term adjustments including recruitment and growth of collateral vessels in response to arterial occlusion are time dependent and determine the maximum blood flow rates available to the collateral-dependent vascular bed during exercise. Rapid short-term adjustments result from active vasomotor activity of the collateral vessels. Mature coronary collateral vessels are responsive to vasodilators such as nitroglycerin and atrial natriuretic peptide, and to vasoconstrictors such as vasopressin, angiotensin II, and the platelet products serotonin and thromboxane A2. During exercise, ßadrenergic activity and endothelium-derived NO and prostanoids exert vasodilator influences on coronary collateral vessels. Importantly, alterations in collateral vasomotor tone, e.g., by exogenous vasopressin, inhibition of endogenous NO or prostanoid production, or increasing local adenosine production can modify collateral conductance, thereby influencing the blood supply to the dependent myocardium. In addition, vasomotor activity in the resistance vessels of the collateral perfused vascular bed can influence the volume and distribution of blood flow within the collateral zone. Finally, there is evidence that vasomotor control of resistance vessels in the normally perfused regions of collateralized hearts is altered, indicating that the vascular adaptations in hearts with a flow-limiting coronary obstruction occur at a global as well as a regional level. Exercise training does not stimulate growth of coronary collateral vessels in the normal heart. However, if exercise produces ischemia, which would be absent or minimal under resting conditions, there is evidence that collateral growth can be enhanced. In addition to ischemia, the pressure gradient between vascular beds, which is a determinant of the flow rate and therefore the shear stress on the collateral vessel endothelium, may also be important in stimulating growth of collateral vessels. I. INTRODUCTION Energy production in the normally functioning heart is primarily dependent on oxidative phosphorylation, with ⬍5% of ATP production resulting from glycolytic metabolism (436). Because of this dependence on oxidative energy production, increases of cardiac activity are dependent on almost instantaneous parallel increases of oxygen availability. In contrast to skeletal muscle, which is quiescent with very low metabolic requirements during resting conditions, the heart continues to pump at a rate of 60 –70 beats/min in humans even at rest. Consequently, at rest, oxygen consumption normalized per gram of myocardium is 20-fold higher than that of skeletal muscle. As an adaptation to the high oxygen demands, the heart maintains a very high level of oxygen extraction so that 70 – 80% of the arterially delivered oxygen is extracted, compared with 30 – 40% in skeletal muscle (181, 346). This high level of oxygen extraction is facilitated by a high capillary density of 3,000 – 4,000/mm2 (353), which is substantially higher than the 500 –2,000 capillaries/mm2 found in skeletal muscle (226). Because of the high level of oxygen extraction by the myocardium during resting conditions, increases in oxygen demand produced by exercise (increasing up to 6-fold during maximal exercise) are mediated principally by an increase in coronary blood flow. Physiol Rev • VOL John Hunter stated in 1794 that “blood goes where it is needed” (485). How the blood “knows” where it is needed, i.e., the mechanism(s) that enable coronary flow to respond to the oxygen demands of the myocardium, most notably during exercise, has been the subject of cardiovascular research for over a century (485). Significant advances in our understanding of coronary blood flow regulation have been made over the past 40 years and are summarized in several previous reviews (53, 138, 181, 346). The aim of this review is to provide a comprehensive update of the acute and chronic adaptations that regulate coronary blood flow in response to dynamic exercise under physiological conditions in the healthy heart (sect. II) and in the heart with impaired coronary arterial inflow (sect. III). II. CORONARY BLOOD FLOW IN THE NORMAL HEART DURING EXERCISE A. Myocardial Oxygen Demand Oxygen consumption by the heart is principally required for contraction, with requirements for maintaining basal metabolism comprising only 10 –20% of total oxygen consumption (58, 617). The oxygen required for contraction is related to heart rate (62, 302, 582), ventricular wall tension (217), muscle shortening (78, 217), and contrac- 88 • JULY 2008 • www.prv.org CORONARY BLOOD FLOW tility (43, 162, 217, 417). Precise determination of the relative contributions of these individual variables in vivo has been difficult, as pharmacological or electrical modulation of one of these variables often results in alterations of one or more of the other variables. An integrated model of these mechanical determinants of oxygen consumption has been proposed by Suga and Sagawa (544, 545). These authors demonstrated that the oxygen consumption per heart beat is determined by ventricular work (represented by the ventricular pressure-volume area which integrates wall tension and shortening) and contractility (Emax, represented by the slope of the end-systolic pressure volume relation) (Fig. 1, top panels). Exercise is the most important physiological stimulus for increasing myocardial oxygen demands. The requirement of exercising skeletal muscle for increased blood flow is met by vasodilation of resistance vessels in the skeletal muscle, which requires an increase in cardiac output, and is facilitated by an increase in arterial pressure. The hemodynamic adjustments that result in the increased cardiac output and arterial pressure during exercise cause increases in each of the major determinants of myocardial oxygen demands: heart rate, contractility, and ventricular work (Fig. 1, bottom panels, and Fig. 2). 1011 1. Heart rate Early studies comparing myocardial oxygen consumption during heavy exercise with similar increases in heart rate produced by cardiac pacing suggested that 30 – 40% of the increase in coronary blood flow during exercise can be attributed to the increase in heart rate (302, 582). However, the contribution of the increase in heart rate to the increase in myocardial oxygen consumption was likely underestimated, as pacing decreases enddiastolic volume and stroke volume, thereby reducing ventricular work (72, 531). Indeed, other studies (214, 242, 258, 396, 588) suggest that the increase in heart rate accounts for 50 –70% of the increase in myocardial oxygen consumption during exercise (Fig. 2). 2. Contractility The exercise-induced increase in contractility is due to -adrenergic activation as well as the direct positive inotropic effect of heart rate (treppe) (320). Studies in which the inotropic effect produced by the -adrenergic nervous system during exercise was blocked with propranolol while heart rate was maintained constant by atrial pacing were interpreted to suggest that up to 30% of the increase in myocardial oxygen demands during exercise could be attributed to the adrenergically mediated FIG. 1. Schematic illustrations of ventricular pressure-volume area [PVA, total mechanical energy, consisting of external work (EW) and potential work (PW)] and maximum elastance (Emax, i.e., contractility) in a pressure-volume diagram (top left panel; ESPVR, end-systolic pressure volume relation), and the relation to myocardial oxygen consumption (top right panel). The PVA-independent MV̇O2 constitutes basal metabolism and the cost of contractility (i.e., electromechanical activation). In the bottom panels are shown representative alterations produced by exercise in dogs with an increase in heart rate from ⬃110 to ⬃250 beats/min. An increase in PVA occurs due to a small increase in end-diastolic volume and a marked increase in systolic arterial pressure, which together with the increase in Emax (bottom left panel), cause an estimated 80% increase in MV̇O2 per beat (bottom right panel). Together with the 120% increase of heart rate, a quadrupling of MV̇O2 per min (121, 568) is explained. [Illustrations are based on Suga (545).] Physiol Rev • VOL 88 • JULY 2008 • www.prv.org 1012 DIRK J. DUNCKER AND ROBERT J. BACHE FIG. 2. Schematic overview of the effect of exercise on myocardial oxygen balance. Shown are the contributions of various variables to the exercise-induced increase in myocardial oxygen demand (⌬O2 demand) and supply (⌬O2 supply). For example, the increase in oxygen demand is produced exclusively (100%) by an increase in contractile activity, which in turn is principally (for 60%) the result of an increase in heart rate. Similarly, the increase in oxygen supply is primarily met (for 80%) by an increase in blood flow, which is predominantly (for 90%) achieved by a decrease in coronary vascular resistance (Rcoronary). PPERFUSION, perfusion pressure; PTISSUE, intramyocardial tissue pressure; [O2]ART, arterial oxygen content; [O2]CV, coronary venous oxygen content; [Hb], hemoglobin content; O2 sat, oxygen saturation. augmentation of contractility (43, 162, 303, 417). The effect of increased contractility on myocardial oxygen consumption in these studies was likely overestimated, as ß-blockade blunted not only the exercise-induced increase in contractility, but also left ventricular systolic pressure and stroke volume and consequently left ventricular work per beat (43, 162, 303, 417). Therefore, it is estimated that contractility contributes 15–25% of the increase in oxygen consumption during exercise (Figs. 1 and 2). flow (Fig. 2). In some species such as the dog (313, 583), horse (167, 448), and sheep (415), oxygen delivery is facilitated by a prominent increase in hemoglobin (by up to 30 –50%), but in swine (148, 247, 345) and human (484, 486), hemoglobin concentrations increase much less. Although myocardial oxygen extraction also increases during exercise (377, 391, 402, 426, 587, 588), the high level of basal oxygen extraction (typically 70 – 80% during resting conditions) limits further increases. In chronically instrumented dogs, von Restorff et al. (587) found that heavy treadmill exercise caused an increase of myocardial oxygen consumption from 0.09 ⫾ 0.01 at rest to 0.57 ⫾ 0.05 ml䡠min⫺1 䡠g⫺1 during exercise that was provided by a 434% increase of coronary blood flow, an increase of arterial oxygen content from 20 ⫾ 1 to 23 ⫾ 1 ml/dl, and an increase of myocardial oxygen extraction from 79 ⫾ 2 to 93 ⫾ 1% (Fig. 3). Thus the principal mechanism for augmenting myocardial oxygen delivery is by increasing coronary blood flow and, as a result, coronary flow is strongly correlated with myocardial oxygen consumption. The increase in myocardial blood flow results from a combination of coronary vasodilation, with a decrease of coronary vascular resistance during heavy exercise to 20 –30% of the resting level, and a 20 – 40% increase in mean arterial pressure (37, 275, 313, 377, 426, 448, 469, 587, 588). 1. Coronary blood flow Left ventricular myocardial blood flow during resting conditions in chronically instrumented large animals in 3. Ventricular work Left ventricular work increases during exercise in proportion to the increased systolic arterial pressure and secondary to a modest increase of left ventricular enddiastolic volume (279, 456, 581). Stroke volume, and hence external work, is augmented as the increased contractility during exercise causes the ventricle to eject to a smaller end-systolic volume (279, 456, 581) so that total ventricular work increases, accounting for an estimated 15–25% of the increase in oxygen consumption (Figs. 1 and 2). B. Myocardial Oxygen Supply Increased myocardial oxygen demands during exercise are met principally by augmenting coronary blood Physiol Rev • VOL FIG. 3. Myocardial oxygen balance in awake dogs at rest and during four incremental levels of treadmill exercise. The increase in myocardial oxygen consumption was principally accounted for by an increase in coronary blood flow with only modest contributions of increase in hematocrit and oxygen extraction. MV̇O2, myocardial oxygen consumption; Hct, hematocrit; ArtSO2, arterial oxygen saturation; CVSO2, coronary venous oxygen saturation. Data are means ⫾ SE. *P ⬍ 0.05 vs rest. [Data from Restorff et al. (588).] 88 • JULY 2008 • www.prv.org CORONARY BLOOD FLOW the awake state and in normal human subjects is generally reported in the range of 0.5–1.5 ml䡠min⫺1 䡠g myocardium⫺1 (17, 24 –26, 33, 34, 37, 41, 44, 132, 137, 139 –141, 144, 146, 147, 174, 212, 214, 236, 289, 316, 327, 356, 379, 420, 427, 440, 444, 448, 473, 499, 500, 524, 539, 540, 547, 567–569, 588, 593, 603, 614, 615, 624). The wide range of resting values of left ventricular blood flow in awake animals appears to be related to the state of alertness. Animals conditioned to rest quietly in the laboratory have the lowest reported values, whereas animals standing on a treadmill ready to run have higher heart rates and higher coronary flow rates. Dynamic exercise increases coronary blood flow in proportion to the heart rate, with peak values during maximal exercise typically three to five times the resting level (378, 448, 498 –500, 574, 588, 603). The strong correlation between coronary flow and heart rate occurs because heart rate is a common multiplier for the other determinants of myocardial oxygen demand (contractility, cardiac work), which are computed per beat. Regression analysis of published left ventricular blood flow data against heart rate demonstrates remarkably similar relationships between human, canine, equine, and porcine data during dynamic exercise (Fig. 4) (17, 24 –26, 33, 34, 37, 41, 44, 76, 77, 132, 137, 139 –141, 144, 146, 147, 162, 174, 212, 214, 236, 250, 258, 275, 289, 302, 303, 316, 327, 345, 356, 378, 379, 420, 426, 427, 430, 431, 440, 444, 448, 469, 473, 499, 500, 524, 539, 540, 547, 567–569, 588, 593, 603, 614, 615, 624). The values reported by Sanders et al. (498) are for total heart flow and are therefore lower than from other studies in which blood flow from the left ventricle was reported. In some laboratories using fluorescent microspheres, absolute blood flow measurements of 7.5– 8.5 ml䡠 min⫺1 䡠g myocardium⫺1 have 1013 been reported in swine exercising at heart rates of ⬃260 beats/min (106, 327); these values are much higher than reported by most other groups in which myocardial blood flow was measured with radioactive microspheres (Fig. 4). These studies have not been included in Figure 4. A) UNIQUE RESPONSES TO EXERCISE IN RODENTS. An inexpensive small animal model that would reproduce the effects of exercise seen in larger animals and in humans would be extremely useful, especially in light of the availability of genetically modified mouse strains that could be used to assess the contribution of a gene product to the acute and chronic coronary blood flow responses to exercise (55). However, the technical difficulty of measuring coronary blood flow in small animals is formidable. Consequently, no studies of coronary or myocardial blood flow responses to exercise in mice are currently available. Flaim et al. (185) studied myocardial blood flow, measured with radioactive microspheres injected into the left ventricle of rats exercised by swimming or running on a treadmill. The increase in oxygen consumption produced by swimming was due solely to an increased oxygen extraction, so that compared with resting conditions swimming caused no increases of heart rate or cardiac output and no increase of myocardial blood flow. Treadmill exercise at 70 ft./min for 5 min or until exhaustion increased heart rates from ⬃380 to 480 beats/min and increased cardiac output, while arterial blood pressure was unchanged. Left ventricular myocardial blood flow increased far less during exercise than in large animals, being 5.9 ml䡠min⫺1 䡠g⫺1 at rest and 7.8 ml䡠min⫺1 䡠g⫺1 during exercise, an ⬃30% increase. The very high left ventricular myocardial blood flow rates at rest (⬃5-fold greater than in large animals or humans) appeared to result from the high basal heart FIG. 4. Relations between heart rate (HR) and left ventricular myocardial blood flow (LVMBF) at rest and during treadmill exercise in dogs (24 –26, 33, 34, 37, 41, 44, 132, 137, 140, 141, 144, 174, 212, 214, 289, 356, 427, 440, 444, 473, 524, 567–569, 588, 624), swine (76, 77, 139, 146, 147, 236, 345, 430, 431, 499, 500, 539, 540, 547, 603), and horses (17, 378, 379, 448). Data from humans were obtained principally from young healthy male subjects performing upright bicycle exercise (162, 258, 275, 302, 303, 316, 420, 426, 469, 593, 614, 615). Data from rats (185) have been added (solid circles representing rest and exercise data) to illustrate that the high LVMBF values in this species are the result of the high heart rates, so that the rat data fall right on the regression line for the human data. Physiol Rev • VOL 88 • JULY 2008 • www.prv.org 1014 DIRK J. DUNCKER AND ROBERT J. BACHE rates in rats, and the modest relative increase in myocardial blood flow in response to exercise was proportionate to the modest further increase in heart rate (Fig. 4). Thus myocardial blood flow in the rat is much higher than in larger animals during resting conditions, but the increase in blood flow during treadmill exercise is much less than in larger animals. The lack of hemodynamic response to swimming indicates that this stress is not likely to be useful for examining coronary responses to exercise (35, 185). Furthermore, the response to treadmill exercise in rats is so dissimilar to that of larger animals (including humans) that even treadmill exercise is likely to be of limited value in understanding regulation of coronary blood flow responses to exercise in larger animal species or in humans. 2. Oxygen-carrying capacity of arterial blood A) HEMOGLOBIN. In the dog, horse, and sheep, oxygen delivery to the myocardium is facilitated by prominent increases in hemoglobin concentration during exercise and the resultant increase in oxygen carrying capacity of arterial blood. The hemoglobin concentration increases because exercise elicits splenic contraction that expresses erythrocyte-rich blood into the general circulation. Thus dogs performing near-maximal treadmill exercise have been reported to sustain a 12–21% increase in hematocrit (313, 588). Vatner et al. (583) reported a 23% increase in hematocrit during heavy exercise in free running dogs which was abolished by splenectomy. Manohar (378) reported that in ponies hemoglobin increased from 11.4 g/dl at rest to 16.9 g/dl at maximal exercise, a 48% increase. This resulted principally from splenic contraction, as in splenectomized ponies hemoglobin content increased from 12.3 g/dl at rest to 13.9 g/dl at maximal exercise, an increase of only 13%. Augmentation of the arterial oxygen content is an important response to exercise, since splenectomized ponies required higher myocardial blood flow rates at similar work loads (378). Furthermore, normal ponies had residual coronary vasodilator reserve in response to adenosine infusion even during maximal exercise, whereas in splenectomized animals, vasodilator reserve was exhausted in the subendocardium during heavy exercise (378). In sheep, hemoglobin concentration increased from 9.1 ⫾ 0.4 g/dl at rest to 13.1 ⫾ 0.8 g/dl during maximal exercise (415). Pretreatment with the nonselective ␣-adrenergic receptor blocker phenoxybenzamine blunted the increase from 9.0 ⫾ 1.1 to 11.3 ⫾ 0.9 g/dl, indicating that contraction of the spleen is mediated by ␣-adrenergic receptor activation during exercise. In swine, hemoglobin increases by 10 –20% (16, 148, 247, 345, 395, 539, 540, 547); this increase was abolished by pretreatment with the mixed ␣1/␣2-adrenergic receptor antagonist phentolamine, supporting a role for ␣-adrenergic-mediated splenic contraction during exer- Physiol Rev • VOL cise (148). The ␣-adrenergic receptor involved in the exercise-induced splenic contraction is likely of the ␣1-subtype, as the exercise-induced increase in hemoglobin can be mimicked with the ␣1-adrenergic receptor agonist phenylephrine (502). Blockade of the increase in hemoglobin in both swine or dogs requires a greater increase in coronary blood flow at each level of myocardial oxygen consumption (148, 502), indicating that the increase in hemoglobin is physiologically important in these species, although less than in horses. The hemoglobin concentration in humans increases by no more than 15% in response to upright exercise (97); this increase is not the result of splenic contraction, but rather is due principally to a decrease in plasma volume resulting from extravasation of fluid from the capillaries (244, 483, 484). B) ARTERIAL OXYGEN SATURATION OF HEMOGLOBIN. Arterial oxygen tension and saturation are generally unchanged during submaximal and maximal exercise in normal humans (97). However, in endurance athletes with extremely high values of maximum total body oxygen consumption, decreases in arterial oxygen tension have been reported (127, 296, 551). Arterial oxygen tensions were not changed during maximal exercise in chronically instrumented dogs (247, 587), whereas a 12- to 20-mmHg decrease in arterial oxygen tension has been reported in chronically instrumented ponies during heavy exercise (301, 377), and a 5- to 10-mmHg decrease in exercising swine (16, 145, 247, 394), although this is not a uniform finding in either ponies (378, 448) or swine (149, 345). It is important to note that small changes in arterial oxygen tension contribute little to the arterial oxygen content, since the arterial oxygen-hemoglobin dissociation curve operates at its upper plateau even during resting conditions. Consequently, even in the studies that reported a 12- to 20-mmHg decrease in oxygen tension, arterial hemoglobin oxygen saturation was maintained (301, 377). 3. Myocardial oxygen extraction A) RELATION TO MYOCARDIAL OXYGEN CONSUMPTION. In many species, the increase in oxygen delivery to the heart during exercise does not fully match the increased oxygen demand, necessitating an increase of myocardial oxygen extraction, with widening of the arteriovenous oxygen difference and a decrease in coronary venous oxygen content (24, 121, 258, 275, 291, 313, 316, 377, 541, 588). In normal male human subjects who performed bicycle exercise to achieve heart rates of 171 beats/min (⬃85% of predicted maximum heart rate), coronary sinus oxygen content decreased from 8.5 ⫾ 1.6 ml/dl at rest to 6.6 ⫾ 0.7 ml/dl at peak exercise (22% decrease) (316). Similar findings have been reported by other investigators in human subjects during heavy exercise (258, 275), but lesser exercise loads (⬍70% of maximum heart rate) did not result in increased myocardial oxygen extraction or decreased coronary ve- 88 • JULY 2008 • www.prv.org CORONARY BLOOD FLOW nous oxygen content compared with resting conditions (57, 211, 372, 402, 469, 471). It is likely that the relatively light levels of exercise used in the latter studies can account for the lesser increases of oxygen extraction. Von Restorff et al. (588) reported that heavy treadmill exercise in dogs, which increased heart rate to 284 beats/min, increased myocardial oxygen extraction from 75 ⫾ 2% at rest to 93 ⫾ 1% during exercise, with a decrease in coronary venous oxygen saturation from 24 ⫾ 1 to 9 ⫾ 1%. Bache and Dai (27) observed a decrease in coronary sinus oxygen tension from 21 ⫾ 2 to 13 ⫾ 1 mmHg over a similar exercise range. Similarly, Manohar (377) reported that in horses coronary venous oxygen tension decreased from 22 ⫾ 1 mmHg at rest to 15 ⫾ 1 mmHg during heavy exercise. In contrast, in swine, myocardial oxygen extraction and coronary venous oxygen tension remain unaltered during treadmill exercise, even at levels of up to 80 –90% of maximum heart rate (145, 147–149, 393, 395, 396). Interestingly, Heiss et al. (258) observed in young healthy male volunteers that the coronary venous oxygen content decreased by 8% with no change in coronary venous oxygen tension (Fig. 5). Similarly, in young healthy male volunteers, Grubbstrom et al. (224) observed a marked decrease in coronary venous oxygen saturation from 33 ⫾ 2% at rest to 24 ⫾ 2% during heavy exercise at ⬃90% of maximum heart rate, whereas coro- FIG. 5. Relation between myocardial oxygen consumption (MV̇O2) and coronary venous oxygen tension (left panel) and oxygen saturation (right panel) at rest and during treadmill exercise in swine (148), humans (258), horses (377, 448), and dogs (24, 588). Note that exercise does not alter coronary venous oxygen tension in swine, whereas it is already reduced at low levels of exercise in dogs. Humans and horses demonstrate an intermediate oxygen tension response. During exercise, oxygen saturation decreases in horses and humans (similar to dogs), whereas in swine saturation is not affected, suggesting a rightward shift of the hemoglobin-oxygen dissociation curve during exercise in humans and horses. Also note that despite similar resting coronary venous oxygen tensions, horses and humans have higher hemoglobin oxygen saturations compared with swine and dogs, consistent with the higher P50 values reported in the latter species (286, 474). Data are means ⫾ SE. *P ⬍ 0.05 vs. corresponding rest. See text for further explanation. Physiol Rev • VOL 1015 nary venous oxygen tension was minimally affected (21 ⫾ 1 mmHg at rest versus 18 ⫾ 2 mmHg during exercise). These observations suggest that a rightward shift of the hemoglobin oxygen dissociation curve acts to facilitate oxygen delivery to the myocardium during heavy exercise in humans. The decrease in blood pH resulting from lactate production by working skeletal muscle has been reported to cause a rightward shift of the hemoglobin oxygen dissociation curve in humans (224, 258) and horses (377). However, in dogs, the coronary venous blood pH did not change even during maximal exercise (247) and therefore cannot contribute to this rightward shift. This may explain why in dogs coronary venous oxygen tension is decreased at relatively low levels (⬍70% of maximum) of exercise, whereas in horses and humans coronary venous oxygen tensions decrease less despite similar decrements in oxygen saturation (Fig. 5). The observation that coronary venous oxygen saturation levels are maintained in exercising swine is consistent with the unchanged arterial carbon dioxide tension and pH at submaximal levels of exercise (80 –90% of maximum) (149). It is possible that during exhaustive exercise lactate production by skeletal muscle (430, 431) and the associated decrease in blood pH (247) also facilitate a decrease in coronary venous oxygen saturation in swine. B) RELATION TO VASOMOTOR CONTROL IN CORONARY RESISTANCE VESSELS. Adrenergic vasoconstrictor mechanisms restrain the increase in coronary blood flow during exercise, thereby contributing to the increased oxygen extraction. Thus nonselective ␣-adrenergic blockade with phentolamine or selective ␣1-adrenergic blockade with prazosin increased myocardial blood flow during exercise, which was accompanied by decreased myocardial oxygen extraction and increased coronary venous oxygen tension (27, 31, 122, 214, 266). However, ␣-adrenergic blockade did not completely eliminate the increase in myocardial oxygen extraction that occurred during exercise (27, 31), which could have resulted from incomplete blockade or from other unidentified factors which restrain coronary vasodilation during exercise. Alternatively, a decrease in coronary venous oxygen tension has been proposed to represent a metabolic error signal, needed for negativefeedback metabolic control of myocardial oxygen delivery and hence coronary blood flow. However, the observation that oxygen tension is minimally affected in exercising swine (148), and during submaximal exercise in humans (258, 471), indicates that a decrease in oxygen tension is not obligatory for the exercise-induced increase in coronary blood flow. C) RELATION TO VASODILATOR RESERVE, OXIDATIVE METABOLISM, AND CONTRACTILE FUNCTION. The increased oxygen extraction indicates that the increase of myocardial blood flow during exercise does not fully compensate for the increased oxygen demands. Failure of blood flow to fully meet the increase in myocardial oxygen consumption is not the 88 • JULY 2008 • www.prv.org 1016 DIRK J. DUNCKER AND ROBERT J. BACHE result of exhaustion of coronary vasodilator reserve during exercise, since a further increase in coronary blood flow can be elicited with a pharmacological or ischemic vasodilator stimulus. For example, during maximal treadmill exercise in dogs and swine, a brief total coronary occlusion has been shown to result in reactive hyperemia with a further increase in blood flow (588, 603). Furthermore, intravenous administration of adenosine to swine resulted in a 15–26% increase in myocardial blood flow during maximum exercise, despite a significant drop in arterial pressure (77, 500, 603). When the results were expressed in terms of coronary resistance, adenosine caused a 20 ⫾ 1% further decrease in coronary vascular resistance in swine during maximal exercise (603). Similarly, dipyridamole administered intravenously or into the left atrium of dogs or swine during near-maximal or maximal treadmill exercise caused a 20 – 46% further increase in myocardial blood flow (41, 342). An even greater adenosine-recruitable flow reserve was reported in ponies during near-maximal exercise (378, 448), causing an increase in myocardial blood flow from 5.34 ⫾ 0.31 to 9.34 ⫾ 0.66 ml䡠min⫺1 䡠g⫺1 (448). Taken together, these studies demonstrate that in the normal heart substantial coronary vasodilator reserve exists even during maximal exercise. Although the increase in myocardial oxygen extraction indicates that coronary blood flow does not keep pace with the increased myocardial oxygen demands during exercise, there is no evidence to suggest that this disparity results in ischemia in the normal heart even during heavy exercise. Studies examining myocardial lactate metabolism for evidence of anaerobic glycolysis demonstrated continued lactate consumption even during heavy exercise (258, 275, 291, 402). Nevertheless, Gwirtz and co-workers (230, 232) found that pharmacologically induced increases of coronary blood flow during exercise can enhance contractile function, which is known as the Gregg effect (see Refs. 181, 599 for in depth reviews). These investigators observed that intracoronary administration of the selective ␣1-adrenergic blocker prazosin during treadmill exercise in dogs caused a 21 ⫾ 3% increase in coronary artery blood flow that was associated with a 21 ⫾ 4% increase in the maximal rate of regional myocardial segment shortening (total systolic shortening was unchanged) and a 26% increase in myocardial oxygen consumption. The increased velocity of shortening was not mediated by enhanced -adrenergic activation, since a similar response to prazosin was observed after -adrenergic blockade with propranolol (232). Similarly, intracoronary administration of adenosine during exercise caused a 25–30% increase in coronary blood flow (230, 291), and this was associated with a 27% increase in the rate of systolic segment shortening (230) and a 16% increase of myocardial oxygen consumption (291). The effects on contractile function produced by both intracoronary adenosine and prazosin occurred with no change in Physiol Rev • VOL heart rate, left ventricular systolic pressure, or myocardial end-diastolic segment length (230, 232). The observed increases in the velocity of segment shortening and myocardial oxygen utilization in response to pharmacological coronary vasodilation during exercise suggest that coronary flow may modulate the increase of myocardial contractility that occurs during exercise. C. Determinants of Coronary Blood Flow 1. Effective perfusion pressure: extravascular compressive forces The effective perfusion pressure of the coronary bed is determined by the pressure drop across the coronary vascular bed, with the entrance pressure being aortic pressure. However, because extravascular forces are exerted on the compressible intramural coronary vasculature by the surrounding myocardium, the effective back pressure that acts to impede coronary blood flow cannot simply be equated to right atrial pressure. The interaction between the intravascular distending pressure and the extravascular compressive forces can be described by the “vascular waterfall” and is especially important during systole (134, 262) but also, albeit to a lesser extent, during diastole (52, 571, 585, 592). Thus, during systole, the contracting myocardium generates a high level of intramyocardial pressure that compresses the coronary microvasculature, thereby impeding blood flow (Figs. 6 and 7; Refs. 257, 309, 313, 500, 599). Conversely, during diastole, intraventricular pressures transmitted into the left ventricular wall exert a small compressive force on the intramural vasculature (20, 154, 163, 274), creating waterfalls at the level of the arterioles and the venules, and possibly the epicardial veins (571, 585, 599). For an in depth review of the interaction of the myocardium and coronary vasculature, the reader is referred to a recent article in this journal (599). Isolating the impeding effects of cardiac contraction on coronary blood flow requires elimination of active vasomotor influences by producing maximal vasodilation of the coronary vascular bed. An early study reported that “minimum” coronary vascular resistance (calculated as aortic pressure/mean coronary blood flow during intravenous administration of adenosine) was lower during treadmill exercise than at rest (448). However, computation of vascular resistance from single measurements of pressure and flow does not fully characterize the effects of changes in extravascular forces. Comprehensive analysis of mechanical effects of cardiac contraction on blood flow is provided by the pressure-flow relationship, obtained from multiple measurements of coronary blood flow over a range of perfusion pressures. During maximum vasodilation, the pressure-flow relationship is determined by the maximum vascular conductance, repre- 88 • JULY 2008 • www.prv.org 1017 CORONARY BLOOD FLOW FIG. 6. Hemodynamic responses to treadmill exercise in dogs. L.CIRC., left circumflex coronary artery; COR, coronary; SYST, systolic; DIAST, diastolic. See text for further explanation. [Modified from Khouri et al. (313).] sented by the slope of the relationship, and the x-intercept or pressure at which flow ceases (zero-flow pressure; Pzf); in the maximally dilated circulation, changes in Pzf are determined principally by changes of the extravascular compressive forces (15, 318, 503, 621). Duncker et al. (151) used this technique to study the effect of exercise on coronary blood flow in dogs. Maximum coronary vasodilation was maintained by intra-arterial infusion of adenosine (50 g䡠 kg⫺1 䡠min⫺1); this dose was determined to cause maximum vasodilation, since larger doses caused no further increase in blood flow. As heart rate increased from 118 beats/min at rest to 213 beats/min during treadmill exercise, blood flow in the maximally vasodilated coronary circulation decreased from 5.66 ⫾ 0.41 ml䡠min⫺1 䡠g⫺1 during resting conditions to 4.62 ⫾ 0.43 ml䡠min⫺1 䡠g myocardium⫺1 despite a significant increase in coronary perfusion pressure (aortic pressure). The decrease of coronary blood flow resulted from an increase of Pzf from 13 ⫾ 1 mmHg at rest to 23 ⫾ 2 mmHg during exercise, as well as a decrease in the slope of the pressure-flow relationship from 12.3 ⫾ 0.9 to 10.9 ⫾ 0.9 (ml 䡠min⫺1 䡠g⫺1)/mmHg during exercise (Fig. 8). Several factors may contribute to this alteration of the coronary pressure-flow relationship during exercise. First, the increase in heart rate decreases maximum coronary blood flow rates by increasing the total time spent in systole (23). Second, the increased contractility increases systolic compression of the intramural coronary vessels (325, 385, 533, 563). However, the increased contractility simulPhysiol Rev • VOL taneously augments myocardial relaxation which increases the diastolic perfusion time (142, 465, 625). Finally, an increase of left ventricular diastolic filling pressure decreases maximum coronary blood flow (20, 154, 163). Analysis of the individual contributions of each of these variables to the exercise-induced changes in the coronary pressure-flow relationship demonstrated that heart rate and left ventricular diastolic pressure contributed to the increases in the Pzf, whereas the increase in contractility did not have a significant effect (137, 151), likely because the impeding effect of the increased force of contraction was offset by enhanced relaxation which increased the diastolic perfusion time. The increase in extravascular compressive forces during exercise is unlikely to be of physiological significance in the normal coronary circulation, because coronary vasodilator reserve capacity persists even during maximal exercise (41, 77, 342, 378, 448, 500, 603). However, when the oxygen-carrying capacity of the blood is reduced by anemia or hypoxia, or when atherosclerotic coronary artery disease reduces vascular caliber (see sect. III), then the increased extravascular forces produced by exercise could produce a functionally significant limitation of coronary blood flow rates during exercise. 2. Coronary vascular resistance During exercise, the increase in aortic pressure only slightly exceeds the increase in effective back pressure so 88 • JULY 2008 • www.prv.org 1018 DIRK J. DUNCKER AND ROBERT J. BACHE FIG. 7. Hemodynamic responses to treadmill exercise in swine. HR, heart rate; AoBP, aortic blood pressure; LAP, left atrial pressure; CO, cardiac output; df/dt, cardiac output differentiated over time; CBF, coronary blood flow; SS, stroke systolic; SD, stroke diastolic. See text for further explanation. [Modified from Sanders et al. (500).] that the effective perfusion pressure increases by no more than 20 –30% (151, 377). Consequently, the exercise-induced four- to sixfold increase in coronary blood flow is mediated principally by a decrease in coronary vascular resistance. Indeed, maximal exercise is associated with decreases in calculated coronary vascular resistance to 20 –30% of basal resting values in dogs, swine, and horses (77, 378, 448, 587). Total coronary resistance is the sum of both passive (structural) and active (smooth muscle tone) components (346, 411). In the completely vasodilated bed, flow to the different regions of the heart is determined by the crosssectional area of the vessels, the length of the vasculature, and the number of parallel vessels that supply a defined perfusion territory. Measurements of intravascular presPhysiol Rev • VOL sure during basal conditions have shown that ⬃90% of resistance resides in the small arteries and arterioles, hence the term resistance vessels (101, 105). The total length of the vessels supplying the subendocardium is longer than those supplying the subepicardium. In addition, cardiac contraction compresses the intramural vasculature during systole, impeding blood flow especially to the subendocardium (see sect. IID). To facilitate augmented flow during diastole to compensate for systolic underperfusion, the subendocardium has a 10% higher arteriolar and capillary density (53) so that during maximal pharmacological vasodilation under resting conditions, flow to the subendocardium is similar to flow to the subepicardium (140, 470). In addition, there is evidence that the subendocardial resistance vessels are more sen- 88 • JULY 2008 • www.prv.org CORONARY BLOOD FLOW FIG. 8. Coronary pressure-flow relation in the dog heart under conditions of maximal coronary vasodilation with intracoronary adenosine (50 g 䡠 kg⫺1 䡠 min⫺1). Shown are the relations at rest and during three incremental levels of treadmill exercise. Note the rightward shift of the pressure-flow relation with an increase in the zero-flow pressure intercept. See text for further explanation. Data are means ⫾ SE. *P ⬍ 0.05 vs. corresponding rest. [Data from Duncker et al. (151).] sitive to mediators of vasodilation including adenosine (470) and endothelium-dependent dilators (449). These structural and functional adaptations aid in maintaining blood flow to the subendocardial layers. D. Transmural Distribution of Left Ventricular Myocardial Blood Flow 1. Systolic compression of intramyocardial vessels Cardiac contraction impedes myocardial blood flow during systole so that during basal conditions arterial inflow occurs predominantly during diastole. Measurements of proximal coronary artery flow in chronically instrumented dogs (313) and swine (500) demonstrate that during resting conditions only 15–20% of left ventricular flow occurs during systole (Figs. 6 and 7). However, the high heart rates associated with exercise result in progressive encroachment of systole on the diastolic interval, while absolute blood flow rates during systole increase. As a result, during heavy exercise 40 –50% of total coronary artery blood flow occurs during systole (313, 500). The increase in the fraction of coronary flow during systole has implications for the transmural distribution of left ventricular myocardial blood flow, as the throttling effect of cardiac contraction on the intramural coronary vessels is expressed nonuniformly across the left ventricular wall (Fig. 9). Myocardial compressive force increases from intrathoracic pressure at the epicardial surface to equal or to exceed intraventricular presPhysiol Rev • VOL 1019 sure at the endocardial surface (15, 71). Interaction of this gradient of effective tissue pressure with the intravascular distending pressure acts to create an array of vascular waterfalls across the wall of the left ventricle that selectively impedes subendocardial blood flow during systole (134, 140, 262, 305). Furthermore, blood from vessels within the innermost ventricular layers is squeezed retrograde into more superficial subepicardial arterial vessels during each systole so that subendocardial vessels have to be refilled in diastole (analogous to the emptying and filling of a capacitor; Refs. 274, 305, 599). Consequently, systolic flow is directed toward the subepicardium, while antegrade subendocardial blood flow occurs exclusively during diastole. Moreover, with shortening of diastole when the heart rate increases, a relatively greater part of diastole is required to refill the subendocardial vessels, thereby delaying net forward flow in the subendocardial microcirculation. The effects of the increased force of cardiac contraction and increased heart rate during exercise can be studied by maximally vasodilating the coronary circulation with an intracoronary infusion of adenosine (infused regionally to avoid systemic hemodynamic effects), allowing selective study of the impeding effects of cardiac contraction without the confounding influence of metabolic vasoregulation of coronary resistance vessel tone. Using this approach, we observed that exercise caused a redistribution of blood flow away from the subendocardium toward the subepicardium (Fig. 10). The increase in subepicardial blood flow during exercise is consistent with the presence of an intramyocardial pump (274) but was aided, at least in part, by the exercise-induced increase in coronary perfusion pressure (140). Despite the mechanical effects of cardiac contraction that act to increase impedance to blood flow to the deeper myocardial layers during exercise, in the normal heart with intact coronary tone a modest net transmural gradient of blood flow favoring the subendocardium exists, reflecting the higher systolic tension development and oxygen requirements of this layer (596). Maintenance of this normal pattern of transmural perfusion requires augmentation of subendocardial flow during diastole in proportion to the degree of systolic underperfusion. This diastolic gradient of blood flow favoring the subendocardium is dependent on a transmural gradient of vasomotor tone, with vascular resistance during diastole being lowest in the subendocardium (23). 2. Subendocardial/subepicardial blood flow ratio The transmural distribution of myocardial blood flow during exercise has been measured with radioactive microspheres. In most cases, the left ventricular wall has been divided into three or four layers, and the transmural distribution of perfusion was expressed as ratio of blood flow to the innermost layer (subendocar- 88 • JULY 2008 • www.prv.org 1020 DIRK J. DUNCKER AND ROBERT J. BACHE FIG. 9. Graph showing a schematic drawing of the intramyocardial microvasculature (top panel) and the extravascular forces acting on the coronary microvasculature during diastole (bottom left panel) and systole (bottom right panel). PIM, intramyocardial pressure; PLUMEN, pressure in left ventricular lumen; PPERI, pressure in pericardial space. See text for further explanation. dium) divided by blood flow to the outermost layer (subepicardium) (ENDO/EPI ratio). In chronically instrumented awake dogs and swine, ENDO/EPI blood flow ratios at rest have been reported between 1.09 and 1.49 (24, 26, 34, 37, 41, 76, 77, 132, 140, 146, 147, 236, 327, 342, 427, 440, 499, 500, 588, 603). ENDO/EPI ratios are somewhat dependent on the size microsphere used. In early studies in which 7- to 10-m-diameter microspheres were used, ENDO/EPI ratios decreased during exercise, with values near 1.0 during heavy exercise (37, 41, 603). In contrast, when 15-m-diameter microspheres were used, higher ENDO/EPI ratios have generally been reported with values of 1.10 –1.31 during heavy exercise (Fig. 11) (24, 26, 327, 430, 431, 440, 499, 500), although several studies in swine reported values near 1.00 (76, 77, 147, 236, 342). Conversely, Symons Physiol Rev • VOL and Stebbins (547) reported higher ENDO/EPI values in awake swine at rest (1.68) and during moderate exercise (1.53) than any other laboratories; an explanation for their findings is not readily found. The reason for the disparity in the transmural distribution of microspheres during exercise based on size is uncertain, but could be the result of streaming of the larger microspheres into the penetrating arteries that deliver blood to the subendocardium, or to arteriovenous shunting of a fraction of the 7- to 10-m-diameter microspheres from the subendocardial microvessels. Size-dependent characteristics of microsphere measurements in the myocardium have been previously reviewed (573). Ponies and standard bred horses appear to have a more prominent decrease in ENDO/EPI ratio during exercise than either dogs or swine (Fig. 11). Using 15-m-diam- 88 • JULY 2008 • www.prv.org CORONARY BLOOD FLOW 1021 EPI ratio during heavy exercise in dogs increased from 1.03 during control conditions to 1.15 after the administration of dipyridamole. Intravenous adenosine had no effect on the ENDO/EPI ratio during maximal exercise in ponies (378, 448). Furthermore, Breisch et al. (77) reported that during heavy exercise in swine, the ENDO/EPI ratio was maintained near unity during vasodilation with adenosine. The reason for this disparity is unclear but does not appear to be due to adenosine-induced alterations in blood pressure (Table 1). Of greatest importance, however, is the finding that absolute subendocardial blood flow rates increased in response to exogenous adenosine or dipyridamole during heavy exercise (41, 77, 140, 342, 378, 448, 500, 603), indicating that vasodilator reserve had not been exhausted (Table 1). E. Coronary Blood Flow to the Right Ventricle and the Atria FIG. 10. Distribution of left ventricular myocardial blood flow in the dog at rest (open symbols) and during exercise (solid symbols) in the presence of intact vasomotor tone (circles) and during maximum vasodilation with intracoronary adenosine (squares). Data are means ⫾ SE. *P ⬍ 0.05 vs corresponding rest. Dot inside symbol denotes significant (P ⬍ 0.05) increase in flow produced by adenosine. [Data from Duncker et al. (140).] 1. Right ventricular blood flow During quiet resting conditions, right ventricular blood flow in the dog and horse expressed per gram of eter microspheres, Manohar and associates (378, 448) reported that ENDO/EPI ratios in ponies decreased from 1.18 –1.27 at rest to 0.97– 0.99 during heavy treadmill exercise. Similarly, Armstrong et al. (17) reported a decrease in ENDO/EPI ratio from 1.24 at rest to 1.05 during exercise in standard bred horses. This may be in part the result of the marked increase in left ventricular end-diastolic pressure that occurred in horses from 11 ⫾ 2 mmHg at rest to 36 ⫾ 4 mmHg during heavy exercise, which contrasts with increases in left ventricular end-diastolic or left atrial pressure from 2–5 mmHg at rest to only 5–15 mmHg during heavy exercise in dogs (7, 24, 26, 37, 153, 279, 440) and swine (147, 500, 603). 3. Influence of vasomotor tone on the transmural distribution of myocardial blood flow Several studies suggest that active coronary vasomotor tone is important for maintaining subendocardial blood flow during exercise. Thus studies in swine and dogs have demonstrated that coronary vasodilation with adenosine or dipyridamole during exercise caused the ENDO/EPI ratio to fall significantly below 1.0 (Fig. 10; Table 1; Refs. 140, 342, 500, 603). These findings could be interpreted to suggest that at high heart rates during exercise, active vasomotion is required to maintain a gradient of vascular resistance favoring perfusion of the deeper myocardial layers during diastole. In contrast to these reports, Barnard et al. (41) reported that the ENDO/ Physiol Rev • VOL FIG. 11. Relation between heart rate (HR) and left ventricular subendocardial to subepicardial blood flow ratio (LV endo/epi) at rest and during treadmill exercise in dogs (24, 26, 34, 132, 140, 427, 440), swine (76, 77, 147, 236, 327, 342, 430, 431, 499, 500), and horses (17, 378, 448), measured with microspheres 15 m in diameter. No data on transmural distribution of left ventricular blood flow are available in humans. Note that exercise results in a modest decrease in endo/epi ratio, but this typically does not decrease below 1.0 even during heavy exercise. Also note that endo/epi ratios are lower and decrease more during exercise in horses (LV E/E ⫽ ⫺1.37HR ⫹ 1.30) than in either swine (LV E/E ⫽ ⫺1.17HR ⫹ 1.38) or dogs (LV E/E ⫽ ⫺1.06HR ⫹ 1.41). 88 • JULY 2008 • www.prv.org 1. Dog Barnard et al. (41) Sanders et al. (500) Physiol Rev • VOL 88 • JULY 2008 • Swine Swine Breisch et al. (77) Laughlin et al. (342) Parks and Manohar (448) Manohar (378) www.prv.org Reactive hyperemia to “brief” occlusion Dipyridamole, 0.75 mg/kg ia Adenosine, 1.5 mg 䡠 kg⫺1 䡠 min⫺1 iv Adenosine, 0.8 mg 䡠 kg⫺1 䡠 min⫺1 iv Reactive hyperemia to 10-s occlusion Adenosine, 0.8 mg 䡠 kg⫺1 䡠 min⫺1 iv Dipyridamole, 1-2 mg/kg, iv Adenosine, 4 mol 䡠 kg⫺1 䡠 min⫺1 iv Adenosine, 3 mol 䡠 kg⫺1 䡠 min⫺1 iv Method of Vasodilation 283 ⫾ 5 ? 293 ⫾ 9* 227 ⫾ 6 224 ⫾ 5 281 ⫾ 6 238 ⫾ 15 225 ⫾ 7 224 ⫾ 6 239 ⫾ 3 240 ⫾ 3 283 ⫾ 5 260 ⫾ 9 271 ⫾ 8 282 ⫾ 6 ? 284 ⫾ 6 283 ⫾ 5 Exercise ⫹ Vasodilation Exercise 155 ⫾ 8 160 ⫾ 4 164 ⫾ 10 146 ⫾ 7 138 ⫾ 5 138 ⫾ 5 161 ⫾ 5 ? 125 ⫾ 5 Exercise 138 ⫾ 6* 153 ⫾ 9 133 ⫾ 7* 71 ⫾ 3* 136 ⫾ 5 128 ⫾ 6* 155 ⫾ 6 ? ? Exercise ⫹ Vasodilation Mean Arterial Blood Pressure, mmHg 5.30 ⫾ 0.32 5.34 ⫾ 0.31 5.26 ⫾ 0.47 4.05 ⫾ 0.20 3.82 ⫾ 0.24 3.82 ⫾ 0.24 2.46 ⫾ 0.24 4.24 ⫾ 0.30 2.56 ⫾ 0.10 Exercise 9.40 ⫾ 0.71* 9.34 ⫾ 0.66* 6.17 ⫾ 0.71* 5.10 ⫾ 0.30* 4.99 ⫾ 0.35* 4.41 ⫾ 0.29* 3.14 ⫾ 0.24* 6.20 ⫾ 0.35* 3.84* Exercise ⫹ Vasodilation Mean CBF, ml 䡠 min⫺1 䡠 g⫺1 0.97 ⫾ 0.01 0.99 ⫾ 0.02 1.02 ⫾ 0.05 1.00 ⫾ 0.04 1.04 ⫾ 0.07 1.04 ⫾ 0.07 1.05 ⫾ 0.07 1.03 ⫾ 0.05 ? Exercise 1.02 ⫾ 0.11 1.11 ⫾ 0.15 0.87 ⫾ 0.03* 0.98 ⫾ 0.03 1.41 ⫾ 0.07* 0.89 ⫾ 0.08* 0.87 ⫾ 0.13* 1.15 ⫾ 0.08 ? Exercise ⫹ Vasodilation Endo/Epi 5.4 ⫾ 0.3 5.1 ⫾ 0.4 5.21 ⫾ 0.44 4.05 ⫾ 0.20 3.89 3.89 2.48 ⫾ 0.19 4.31 ? Exercise 9.5 ⫾ 1.1* 8.7 ⫾ 0.8* 5.62 ⫾ 0.51 5.09 ⫾ 0.32* 5.84a 4.15a 2.82 ⫾ 0.19* 6.64a ? Exercise ⫹ Vasodilation Subendocardial Blood Flow, ml 䡠 min⫺1 䡠 g⫺1 Data are means ⫾ SE. HR, heart rate; CBF, coronary blood flow; Endo/Epi, subendocardial-to-subepicardial blood flow ratio; “?”, not reported; ia, intra-atrial (infusion into the left a atrium). Computed from mean myocardial flow and Endo/Epi ratio. *P ⬍ 0.05, effect of vasodilation during vs. before vasodilation. Pony Pony Swine White et al. (603) Swine Dog von Restorff et al. (588) Species HR, beats/min Coronary vasodilator reserve during severe treadmill exercise Investigators TABLE 1022 DIRK J. DUNCKER AND ROBERT J. BACHE CORONARY BLOOD FLOW myocardium is typically 50 – 60% of left ventricular blood flow, while the transmural distribution of perfusion is uniform or slightly favors the subendocardium (34, 37, 44, 378, 379, 440). In resting swine, right ventricular blood flow per gram of myocardium is ⬃70 –90% of left ventricular blood flow with an ENDO/EPI ratio of 1.50 (147, 342, 499). The lower resting flows in the right ventricle are the result of the lower oxygen consumption (245, 626), consistent with the markedly lower right ventricular, compared with left ventricular, systolic pressure. Interestingly, the lower levels of oxygen consumption are associated with significantly lower levels of myocardial oxygen extraction (46 ⫾ 3%) by the right ventricle (245, 620). During graded treadmill exercise in dogs, swine, and horses, right ventricular blood flow increases as a direct function of heart rate (Fig. 12). Right ventricular blood flow expressed per gram of myocardium is ⬃75–90% of flow to the left ventricle during the heaviest levels of exercise (34, 37, 44, 342, 378, 379), while in one study of swine exercising at 85–90% of maximum heart rate, right ventricular blood flow slightly exceeded left ventricular flow (147). The relatively greater exercise-induced increase in right ventricular blood flow during heavy exercise (3- to 6-fold) compared with left ventricular blood 1023 flow (2.5- to 4-fold), likely reflects the greater increase in right ventricular myocardial oxygen consumption (245) secondary to the marked increase in pulmonary artery pressure during exercise (147, 627). The relative increase in right ventricular blood flow during heavy exercise appears to be greatest in ponies (8- to 10-fold; Refs. 378, 379) compared with dogs (5- to 6-fold; Refs. 34, 37), and swine (3- to 4-fold; Refs. 147, 342). This is likely the result of the pronounced pulmonary hypertension that occurs during exercise in horses, with mean pulmonary pressure increasing from 19 –30 mmHg at rest to 66 – 89 mmHg during maximal exercise (378, 379). In the left ventricle, the high resting level of oxygen extraction (70 – 80%) necessitates an increase in coronary blood flow even at low levels of exercise. Right ventricular oxygen extraction during resting conditions is much lower, so that ⬃85% of the increment in oxygen consumption produced by mild exercise (60% of maximal heart rate) was accommodated by an increase in oxygen extraction from 46 ⫾ 3% at rest to 68 ⫾ 2%, with extraction further increasing to 82 ⫾ 1% during exercise at 80% of maximal heart rate (Fig. 13) (245). The difference in regulation of oxygen extraction between the right and left ventricles is incompletely understood, but the blunted response of right ventricular blood flow to exercise with FIG. 12. Relation between heart rate and right ventricular blood flow (top panels) and left and right atrial blood flow (bottom panels) in dogs (34, 44), swine (342), and horses (378, 379) at rest and during treadmill exercise. Exercise produced significant (P ⬍ 0.05) increases in flow to all four cardiac chambers at each level of exercise studied. Note that the absolute increases in right ventricular and atrial flows are similar to the increases in left ventricular flow, but that as a result of the lower resting right ventricular and atrial flows, the relative increases are greater in these cardiac chambers compared with the left ventricle. Data are means ⫾ SE. See text for further explanation. Physiol Rev • VOL 88 • JULY 2008 • www.prv.org 1024 DIRK J. DUNCKER AND ROBERT J. BACHE is likely the result of the lower systolic compressive forces acting on the intramyocardial vasculature in the right compared with the left ventricle (53). 2. Atrial blood flow FIG. 13. Relation beween myocardial oxygen consumption and coronary venous oxygen tension (CVPO2) in the right ventricle (RV) and the left ventricle (LV) in dogs during treadmill exercise. Note the lower levels of oxygen consumption and higher coronary venous oxygen tensions in the RV compared with the LV. Data are means ⫾ SE. See text for further explanation. [Data from Hart et al. (245) for RV flows and from Gorman et al. (214) for LV flows.] increased oxygen extraction is not the result of exhaustion of vasodilator reserve. Thus Manohar (378) demonstrated that infusion of adenosine in ponies during maximal treadmill exercise caused right ventricular blood flow to increase from 4.80 ⫾ 0.31 ml䡠 min⫺1 䡠g⫺1 during maximal exercise to 7.54 ⫾ 0.30 ml䡠min⫺1 䡠g⫺1. Furthermore, Bauman et al. (45) reported similar vasodilator reserve in right and left ventricles of dogs at rest and during exercise. Zong et al. (626) demonstrated that the large increase in oxygen extraction during exercise could be in part explained by an exaggerated ␣-adrenergic vasoconstrictor influence on the right ventricular vasculature. Nevertheless, following ␣-adrenergic blockade, a significant increase in right ventricular oxygen extraction still occurred, indicating that other mechanisms must be involved as well. The transmural distribution of right ventricular blood flow in dogs and swine does not change from rest to exercise (34, 37, 342, 440, 499). Furthermore, during maximal exercise in ponies, vasodilator reserve existed in all transmural layers of the right ventricular wall; the right ventricular ENDO/EPI ratio during maximal exercise was 1.00 ⫾ 0.02 and actually increased to 1.32 ⫾ 0.10 when adenosine was infused while exercise continued (378). The finding that maximal pharmacological coronary vasodilation did not result in a relative redistribution of blood flow away from the subendocardium in the right ventricle Physiol Rev • VOL During quiet resting conditions, right and left atrial blood flows expressed per gram of myocardium are typically 20 – 40% of left ventricular blood flow in dogs (44), swine (342), and horses (379). During treadmill exercise, atrial flows can increase up to 15-fold reaching 50 – 60% of left ventricular flow levels during heavy exercise in dogs and swine, and up to 70% of left ventricular flows in ponies (Fig. 12). These increases in blood flow do not require maximal vasodilation of atrial vasculature, as the degree of vasodilator reserve during exercise was reported to be comparable to those in the ventricular chambers (45). Within the atria, blood flow was reported to be lowest in the appendage (0.15– 0.30 ml䡠min⫺1 䡠g⫺1) compared with the nonappendage regions (0.33– 0.37 ml䡠min⫺1 䡠g⫺1), whereas the exercise-induced increase in flow was greater in the appendages (6- to 11-fold in right and left atria, respectively) compared with the nonappendage regions (4- to 5-fold). These observations suggest that the appendages perform less work than the body of the atria under resting conditions, but become increasingly more active as atrial filling is enhanced during exercise (44). F. Control of Coronary Vascular Resistance Regulation of coronary vascular resistance is the result of a balance between a myriad of vasodilator and vasoconstrictor signals exerted by neurohormonal influences, the endothelium, and metabolic signals from the myocardium (Fig. 14). These vasomotor influences allow the myocardium to match the coronary blood supply to the requirement for oxygen and nutrients, while maintaining a consistently high level of oxygen extraction. The ability of coronary resistance vessels to dilate in response to increments in myocardial oxygen demand, as illustrated by the tight correlation between myocardial oxygen consumption and coronary blood flow, is critical for maintaining an adequate supply of oxygen to the myocardium. The matching of coronary vasomotor tone to myocardial metabolism is best studied by examining the relation between coronary venous oxygen tension and myocardial oxygen consumption (27, 143, 266, 267, 565, 566). For example, an increase in coronary resistance vessel tone will limit coronary blood flow and hence the oxygen supply, thereby forcing the myocardium to increase oxygen extraction, with a consequent reduction in coronary venous oxygen levels. Conversely, a decrease in coronary tone will increase blood flow and the oxygen supply to the heart; if oxygen consumption remains constant, oxygen 88 • JULY 2008 • www.prv.org CORONARY BLOOD FLOW 1025 FIG. 14. Schematic drawing of a coronary arteriole and the various influences that determine coronary vasomotor tone and diameter. PO2, oxygen tension; TxA2, thromboxane A2 (receptor); 5HT, serotonin or 5-hydroxytryptamine (receptor); P2X and P2Y, purinergic receptor subtypes 2X and 2Y that mediate ATP-induced vasoconstriction and vasodilation, respectively; ACh, acetylcholine; M, muscarinic receptor; H1 and H2, histamine receptors type 1 and 2; B2, bradykinin receptor subtype 2; ANG I and ANG II, angiotensin I and II; AT1, angiotensin II receptor subtype 1; ET, endothelin; ETA and ETB, endothelin receptor subtypes A and B; A2, adenosine receptor subtype 2; ß2, ß2-adrenergic receptor; ␣1 and ␣2, ␣-adrenergic receptors; NO, nitric oxide; eNOS, endothelial NO synthase; PGI2, prostacyclin; IP, prostacyclin receptor; COX-1, cyclooxygenase-1; EDHF, endotheliumderived hyperpolarizing factor; CYP450, cytochrome P450 2C9; KCa, calcium-sensitive K⫹ channel; KATP, ATP-sensitive K⫹ channel; KV, voltage-sensitive K⫹ channel; AA, arachidonic acid; L-Arg, L-arginine; O䡠-2, superoxide. Receptors, enzymes, and channels are indicated by an oval or rectangle, respectively. See text for further explanation. extraction will decrease and coronary venous oxygen levels will increase. Thus coronary venous oxygen tension is an index of tissue oxygenation that reflects the balance between the oxygen supply and demand of the heart and is ultimately determined by coronary resistance vessel tone (565). 1. Autonomic nervous system Autonomic influences have been studied by examining coronary blood flow at rest and during exercise after surgical or chemical denervation of the heart or in the presence of selective autonomic receptor antagonists. A) CARDIAC NEURAL ABLATION. The coronary vascular bed is richly innervated by both the sympathetic and parasympathetic divisions of the autonomic nervous system (46, 104, 181, 182, 598). To study their contributions to coronary blood flow regulation during exercise, techniques have been devised to produce surgical denervation of the Physiol Rev • VOL heart confirmed by depletion of myocardial norepinephrine stores and lack of an inotropic response to cardiac sympathetic nerve stimulation (133, 311). It should be noted that the results obtained using these preparations are complicated by supersensitivity to circulating catecholamines which develops after sympathetic neural ablation. This supersensitivity appears to be selective, in that the response of myocardial contractility to norepinephrine is augmented (102, 133), whereas ␣-adrenergic responses of the coronary vasculature do not appear to be enhanced (102, 129, 202). Cardiac neural ablation does not impair the ability to maintain steady-state levels of exercise, although the initial hemodynamic adjustment to exercise is delayed. For example, Gregg et al. (220) observed that in dogs with surgical cardiac denervation, the onset of heavy treadmill exercise resulted in a 25% decrease in arterial blood pressure that did not return to control levels until 36 –90 s 88 • JULY 2008 • www.prv.org 1026 DIRK J. DUNCKER AND ROBERT J. BACHE after beginning exercise, while in normal dogs arterial pressure decreased only slightly and recovered within 3– 6 s after beginning exercise. Similarly, the increase in coronary blood flow was delayed 10 –30 se after the onset of treadmill exercise in animals with cardiac denervation, in contrast to a 3- to 6-s delay observed in normal dogs. Gwirtz et al. (231) observed that cardiac sympathectomy decreased left ventricular peak systolic pressure and dP/ dtmax during exercise, suggesting that the reduction of oxygen consumption was due, at least in part, to decreased myocardial contractility. Myocardial oxygen consumption and coronary blood flow were substantially decreased in dogs following surgical denervation at rest and during treadmill exercise (220, 231). Oxygen extraction at rest was similar in control and denervated hearts, suggesting that autonomic control of coronary vasomotor tone is minimal under resting conditions. In contrast, the exercise-induced increase in myocardial oxygen extraction and decrease in coronary venous oxygen content were enhanced in denervated compared with innervated hearts (Fig. 15), indicating that intact innervation contributes in a feed-forward manner to exercise hyperemia. Further evidence for a role of neural control of coronary blood flow during exercise was provided by Schwartz and Stone (516) who reported that the increases of heart rate and contractility during exercise were attenuated by ablation of the right but not the left stellate ganglion in dogs, while the increase in coronary blood flow was slightly increased after left stellate ganglionectomy. DiCarlo et al. (130) also found that coronary flow during submaximal exercise was higher after left stellate ganglion ablation, and that nonselective ␣-adrenergic blockade with intracoronary phentolamine (1 mg) had no further effect, implying that sympathetic neural ablation FIG. 15. Effect of chronic cardiac neural ablation (left panel; Ref. 220) or selective sympathetic denervation (right panel; Ref. 231) on the relation between myocardial oxygen consumption (MV̇O2) and coronary venous oxygen content (CVO2 content) in the left ventricles of dogs during treadmill exercise. Data are means ⫾ SE. *P ⬍ 0.05 vs. control. In the study by Gregg et al. (220), statistical analysis was not presented. See text for further explanation. Physiol Rev • VOL abolishes ␣-adrenergic vasoconstriction during exercise. In contrast, Chilian et al. (102) reported that regional sympathetic denervation of the posterior left ventricular wall by epicardial application of phenol caused no difference in mean myocardial blood flow or the transmural distribution of perfusion either at rest or during treadmill exercise, thus failing to support neurogenic coronary vasoconstriction during exercise. After -adrenergic blockade with propranolol, nonselective ␣-adrenergic blockade with phentolamine decreased coronary vascular resistance in both the innervated (28%) and sympathectomized (23%) regions (likely in part to compensate for an 18% decrease in mean aortic pressure caused by the systemic ␣-adrenergic blockade). The authors concluded that ␣-adrenergic coronary vasoconstriction does occur during exercise, but is principally mediated by circulating cathecholamines rather than by direct neural connections. Finally, Furuya et al. (202) reported that coronary flow during exercise was slightly higher in denervated than in normal hearts at comparable levels of myocardial oxygen demand (reflected by the heart rate ⫻ mean aortic pressure product), but that ␣-adrenergic receptor blockade with phentolamine (2 mg/kg iv) caused a slight further increase in coronary flow. The authors interpreted these findings to suggest that adrenergic coronary vasoconstriction during exercise is mediated both by circulating catecholamines and by direct neural influences (202). Interpretation of these studies (102, 130, 202, 516) is complicated because myocardial oxygen consumption and coronary venous oxygen content or tension were not determined. In conclusion, cardiac autonomic denervation (220), or selective sympathetic denervation (130, 231, 516), limits exercise hyperemia in the heart, indicating that sympathetic activity contributes to exercise hyperemia in a feed-forward manner. Although there is evidence to suggest a role for circulating cathecholamines in the control of coronary blood flow during exercise as well (102), the weight of evidence is consistent with the concept that autonomic influences on the coronary circulation are principally neurally mediated. B) ␣-ADRENERGIC CONTROL. Blockade of ␣-adrenergic receptors can influence coronary blood flow through three separate mechanisms. First, blockade of prejunctional ␣2-adrenoceptors interrupts the negative-feedback control of norepinephrine release (268, 336). The resultant increase in norepinephrine levels augments cardiac ßadrenergic stimulation and increases coronary blood flow secondary to the increased myocardial oxygen consumption. Second, ␣-adrenergic blockade can increase coronary flow by interrupting vasoconstriction mediated by postjunctional ␣1- and ␣2-adrenoceptors located on the smooth muscle cells of small coronary arteries ⬎100 m (predominantly ␣1) and arterioles ⬍100 m (both ␣1 and ␣2) (98, 104, 410). Finally, ␣2-adrenergic receptors on 88 • JULY 2008 • www.prv.org CORONARY BLOOD FLOW coronary vascular endothelium stimulate release of nitric oxide (NO) (109), which can oppose the ␣-adrenergic vasoconstrictor effect (109, 287). Under basal resting conditions, cardiac sympathetic activity is minimal so that ␣-adrenergic blockade has a neligible effect on coronary flow in awake resting dogs (100) and humans (263, 272). In contrast, in dogs and swine, exercise produces a greater increase in coronary blood flow after systemic ␣-adrenergic blockade than during control conditions (27, 122, 148, 233, 266, 365). Systemic nonselective ␣-adrenergic antagonists exaggerate the -adrenoceptor-mediated increases of heart rate, left ventricular systolic pressure, and dP/dtmax during exercise as the result of blockade of prejunctional ␣2-adrenergic receptors which normally inhibit norepinephrine release (148, 268, 336). In this situation, -adrenergic blockade inhibits the marked increase in hemodynamic determinants of myocardial oxygen consumption produced by systemic nonselective ␣-adrenergic blockade. When nonselective ␣-adrenergic antagonists were administered to dogs by the intracoronary route to minimize systemic hemodynamic effects, coronary blood flow during exercise was still 10 –30% higher than during control exercise (123, 130, 232, 235, 282). Furthermore, at comparable levels of myocardial oxygen consumption, ␣-adrenergic blockade increased coronary venous oxygen tension (Fig. 16) and decreased coronary vascular resistance, indicating competition between ␣-adrenergic vasoconstriction and metabolic coronary vasodilation during exercise (27, 31, 122, 266). However, in swine, nonselective ␣-adrenergic blockade with phentolamine had no effect on the relation between myocardial oxygen consumption and coronary venous oxygen tension (Fig. 16), indicating FIG. 16. Effect of mixed ␣1-/␣2-adrenergic receptor blockade with phentolamine [2 mg/kg iv (27) or 1 mg/kg iv (148)] or selective ␣1adrenergic receptor blockade with prazosin (0.1 mg/kg iv) on the relation between myocardial oxygen consumption and coronary venous oxygen tension in the left ventricles of dogs (27; left panel) and swine (148; right panel) during treadmill exercise. Data are means ⫾ SE. *P ⬍ 0.05 prazosin and phentolamine vs. control. See text for further explanation. Physiol Rev • VOL 1027 negligible ␣-adrenergic control of coronary resistance vessel tone in this species (148, 514). To date, well-controlled studies of ␣-adrenergic control of coronary resistance vessel tone during exercise in humans are lacking (263). Both ␣1- and ␣2-adrenoceptors can mediate coronary vasoconstriction, but adrenergic coronary vasoconstriction during exercise in the normal dog heart appears to involve principally ␣1-adrenoceptors. Thus selective ␣1adrenergic blockade with intracoronary prazosin resulted in higher levels of coronary blood flow and lower coronary vascular resistance during graded treadmill exercise (123, 542), whereas intracoronary administration of the selective ␣2-adrenergic blockers yohimbine or idazoxan did not alter coronary blood flow or coronary resistance during exercise (123, 542). Since the effect of increased release of norepinephrine produced by ␣2-adrenoceptor blockade might be concealed by the ␣1-adrenergic vasoconstriction, which it can produce, studies were repeated with the addition of ␣1-adrenergic blockade. Combined intracoronary administration of ␣1- and ␣2-adrenergic blockers was not more effective in increasing coronary blood flow or coronary venous oxygen tension during exercise than was ␣1-adrenergic blockade alone (123). Although ␣2-adrenergic receptors on coronary vascular endothelium stimulate release of NO (109, 287), the lack of effect of blockade of ␣2-adrenoceptors was not due to simultaneous reduction in NO release, as blockade of ␣2-adrenoceptors failed to increase coronary flow during exercise in the presence of NO blockade (287). Thus the ␣-adrenergic vasoconstrictor tone that opposes metabolically mediated coronary vasodilation during exercise is mediated principally by postjunctional ␣1-adrenoceptor activity. Feigl and co-workers (183, 282) proposed that adrenergic coronary vasoconstriction can augment subendocardial blood flow during exercise. They observed that the ENDO/EPI blood flow ratio during exercise was slightly higher in regions with ␣-adrenoceptors intact than in regions where ␣-adrenoceptors were blocked with intracoronary phenoxybenzamine, although total blood flow was higher after adrenergic blockade (282). In contrast, ␣-adrenoceptor blockade caused a transmurally uniform increase of blood flow in myocardial regions perfused by a stenotic coronary artery (355), as well as in the pressure-overloaded hypertrophied left ventricular wall of dogs (155), indicating transmurally uniform ␣-adrenergic coronary vasoconstriction during exercise. Heusch and co-workers (46) addressed the issue of the uniformity of humoral and neuronal ␣-adrenergic vasoconstriction across the left ventricular wall in anesthetized dogs under conditions of intact vasomotor tone and during maximal coronary vasodilation with dipyridamole. During humoral adrenergic activation, produced by intravenous atropine/ norepinephrine, intracoronary phentolamine increased 88 • JULY 2008 • www.prv.org 1028 DIRK J. DUNCKER AND ROBERT J. BACHE blood flow uniformly in all myocardial layers, irrespective of whether coronary vasomotor tone was intact or abolished. During cardiac sympathetic nerve stimulation, phentolamine increased flow to all layers when coronary tone was intact, but during maximal coronary vasodilation, phentolamine increased flow to subepicardium with a tendency for subendocardial blood flow and the ENDO/ EPI ratio to decrease (both P ⫽ NS). It is possible that the differing results might be explained in part by the higher heart rates in the study of Huang and Feigl (⬃240 beats/min) compared with heart rates of ⬃215 beats/min (155, 355) and ⬍200 beats/min (46). Thus ␣-adrenergic stiffening of the intramural penetrating arteries that traverse the myocardium to the subendocardium, and the consequent reduction of systolic retrograde flow, could be of particular importance at high heart rates (183). To address this question, Morita et al. (412) mimicked exercise by combining intravenous norepinephrine infusion with cardiac pacing at 150, 200, and 250 beats/min in open-chest dogs with intact coronary vasomotor tone. At heart rates of 150 and 200 beats/min, intracoronary phenoxybenzamine produced a transmurally homogeneous increase in myocardial blood flow, whereas at 250 beats/min phenoxybenzamine produced a small decrease in blood flow to all myocardial layers. Interpretation of this transmurally homogeneous flow decrease is difficult because myocardial oxygen consumption was not measured. However, the lack of change in ENDO/EPI ratio fails to support the concept that the ␣-adrenergic vasoconstriction acts to protect the subendocardium from hypoperfusion during high work loads. Furthermore, even during maximal exercise, the coronary bed is not maximally vasodilated and retains residual vasomotor tone (see sect. IIB3C). These conditions favor a transmurally homogeneous ␣-adrenergic vasoconstrictor influence in the normal heart during exercise (46, 412). To definitively address the issue of whether ␣-adrenergic vasoconstriction serves to maintain subendocardial blood flow during heavy exercise, future studies investigating the effects of intracoronary ␣-blockade on transmural myocardial blood flow during exercise at maximal heart rates (⬃300 beats/min in dogs) are needed. In conclusion, study of ␣-adrenergic influences is complicated because pharmacological blockers often cause changes in contractile function that produce myocardial metabolic effects on the coronary circulation which exceed their direct vascular effects. For example, postjunctional ␣-adrenergic receptors mediate coronary vasoconstriction, but blockade of prejunctional ␣2-adrenoceptors interrupts the normal inhibition of norepinephrine release from sympathetic axons so that nonselective ␣-adrenergic blockers increase -adrenergic stimulation of contractility with a resultant increase of myocardial oxygen requirements and, therefore, coronary vasodilation. Consequently, although the primary vascular effect Physiol Rev • VOL of ␣-adrenergic activation is vasoconstriction, the overall response to nonselective ␣-adrenergic blockade is dominated by an increase in myocardial oxygen consumption which causes coronary vasodilation. ␣1-Adrenergic mechanisms act to blunt the increase in coronary flow that occurs during exercise, and this results in a decrease in coronary venous oxygen tension in some species as the dog, and possibly humans, but appears to be absent in swine. The weight of evidence indicates that ␣-adrenergic vasoconstriction limits myocardial blood flow in a transmurally homogeneous manner during moderate levels of exercise. Whether ␣-adrenergic tone acts to maintain subendocardial blood flow during (near) maximal levels of exercise remains to be established. C) -ADRENERGIC CONTROL. The direct influence of adrenergic control of coronary vascular resistance via -adrenoceptors [principally 2-receptors which are located on coronary arterioles ⬍100 m (104, 256, 416)], is difficult to separate from metabolic alterations in coronary vasomotion that result from -adrenergic inotropic and chronotropic effects on the myocardium. In awake resting dogs (43, 267) and swine (148), nonselective -adrenergic blockade with propranolol decreased myocardial oxygen consumption, and this resulted in a parallel reduction of coronary blood flow; myocardial oxygen extraction was unchanged, suggesting that -adrenergic control of the coronary circulation is minimal under resting conditions. However, during graded treadmill exercise, propranolol decreased coronary flow more than expected from the decrease in myocardial oxygen consumption, resulting in an increase in myocardial oxygen extraction and a decrease in coronary venous oxygen tension in both dogs and swine (Fig. 17) (43, 148, 214, 215, 266). Similarly, in normal human subjects or patients with angiographically normal coronary arteries (608), -adrenergic blockade with propranolol or sotalol decreased myocardial blood flow during bicycle exercise out of proportion to the reduction of myocardial oxygen consumption, necessitating an increase in myocardial oxygen extraction (162, 303). The findings imply that -adrenergic control of the coronary resistance vessels is minimal under resting conditions, but -adrenergic activation contributes to coronary vasodilation during exercise in a feed-forward manner (408, 409, 566). Intracoronary administration of the nonselective -adrenergic blocker propranolol to exercising dogs caused a slightly greater decrease of coronary blood flow than did the selective 1-adrenergic blocker atenolol, indicating that 2-adrenergic mechanisms can contribute to adrenergic coronary vasodilation. In support of this, intracoronary administration of the selective 2-adrenoceptor blocker ICI 118,551 during exercise caused no change in contractile function but produced an 11–14% decrease in coronary flow during moderate treadmill exercise in dogs (130, 386). These findings indicate that 2-adreno- 88 • JULY 2008 • www.prv.org CV CV CORONARY BLOOD FLOW FIG. 17. Top panels show the effect of mixed ß1-/ß2-adrenergic receptor blockade with propranolol [1 mg/kg iv (214) or 0.5 mg/kg iv (139)] on the relation between myocardial oxygen consumption and coronary venous oxygen tension in the left ventricles of dogs (214; left panel) and swine (139; right panel) during treadmill exercise. Dogs were studied under ␣-adrenergic blockade with phentolamine (Phento, 1 mg/kg iv) to avoid unopposed ␣-adrenergic vasoconstriction following ß-blockade. Bottom panels show the effect of combined ␣1/␣2- and ß1/ß2-adrenergic receptor blockade in swine (139) and dogs (214). Data are means ⫾ SE. *P ⬍ 0.05 vs. Phento alone or vs. control. See text for further explanation. ceptor activation during exercise causes a small but significant degree of coronary resistance vessel dilation independent of the myocardial effects of 1-adrenergic stimulation. In conclusion, -adrenergic blockade blunts the myocardial response to exercise, with a consequent reduction of oxygen consumption. However, -adrenergic blockade causes a greater reduction of coronary flow than of myocardial oxygen consumption, resulting in increased oxygen extraction by the heart and demonstrating a direct feed-forward -adrenergic vasodilator effect on the coronary vessels. In swine, -adrenergic activation exerts a feed-forward effect that is unopposed by ␣-adrenergic vasoconstriction so that coronary venous oxygen tension does not fall during exercise. D) PARASYMPATHETIC CONTROL. The coronary resistance vessels are richly innervated by the parasympathetic diPhysiol Rev • VOL 1029 vision of the autonomic nervous system (181). In dogs, pretreated with propranolol and paced to maintain a constant heart rate, stimulation of the vagosympathetic trunk produces coronary vasodilation independent of the cardiac effects of vagal stimulation (79). The coronary vasodilation produced by vagal stimulation was blocked by atropine and was mimicked by acetylcholine, which involves the release of endothelial NO in the dog (79, 525). To study the vasodilator influence of vagal activity on coronary blood flow during exercise, Gwirtz and Stone (234) administered the muscarinic receptor antagonist atropine into a coronary artery of dogs during submaximal exercise (heart rates of 190 –210 beats/min). Atropine had no effect on heart rate or coronary blood flow, indicating that parasympathetic effects on both the myocardium and coronary bed were negligible at this level of exercise. This is in accord with the finding that vagal tone to the myocardium is progressively withdrawn during increasing levels of exercise (434, 584). Importantly, the high vagal tone in the resting dog exerts a small vasodilator influence on the coronary circulation, the inhibition of which will limit rather than support the exercise-induced coronary vasodilation. In swine, the acetylcholine-induced NO-mediated vasodilation (which predominates in dogs) is outweighed by a direct vasoconstrictor effect on coronary smooth muscle, resulting in a net vasoconstrictor response to acetylcholine (119, 201, 248) or vagal nerve stimulation (201). Despite ample evidence that stimulation of the parasympathetic system can influence coronary vasomotor tone, the effects of vagal activity under basal resting conditions are generally considered to be negligible even during basal resting conditions when vagal activity is high (119). However, these studies have been conducted principally in anesthetized animal models that could have blunted vagal tone (580). In addition, coronary flow was not related to myocardial oxygen consumption which, in view of potential myocardial effects of vagal inhibition, makes interpretation of these studies difficult. Duncker et al. (148) investigated the effects of muscarinic receptor blockade with intravenous atropine in chronically instrumented swine at rest and during graded treadmill exercise. Atropine elicited a vasodilator response in the coronary resistance vessels under resting conditions that waned with increasing levels of exercise intensity. Vasodilation was likely the result of increased -adrenergic activity, since it was fully blocked when studies were repeated in the presence of propranolol. Thus the vasoconstrictor influence that was exerted by the parasympathetic nervous system was due to inhibition of ß-adrenergic vasodilator activity. These observations suggest that withdrawal of vagal tone may contribute to ß-adrenergic vasodilation at lower levels of exercise. Due to progressive withdrawal of vagal tone with increasing exercise intensity, parasympathetic influences are unlikely to be 88 • JULY 2008 • www.prv.org 1030 DIRK J. DUNCKER AND ROBERT J. BACHE physiologically significant during heavy exercise (139, 140, 234, 584). In conclusion, parasympathetic activation can exert weak species-dependent effects on the coronary vessels. In animals in which acetylcholine causes vasodilation, parasympathetic activity causes a weak dilator influence at rest when vagal tone is high, but vagal tone is withdrawn during exercise so that parasympathetic effects are negligible during exercise. In species in which acetylcholine causes coronary vasoconstriction, as in swine, withdrawal of vagal tone may augment the increase in coronary flow during exercise. E) SUMMARY AND INTEGRATION. Although coronary blood flow is strongly responsive to local myocardial metabolic requirements, the autonomic nervous system provides a modulating influence that can alter the coupling between coronary flow and myocardial metabolism. During resting conditions, cardiac sympathetic activity is minimal so that adrenergic blockade has a negligible effect on coronary flow. During exercise, adrenergic activation exerts paradoxical effects that both oppose (alpha) and reinforce (beta) the increase in coronary flow that occurs in response to the increase in cardiac work. The net effect of sympathetic stimulation is ßadrenergic feed-forward vasodilation (Fig. 17), which has been proposed to account for as much as 25% of the exercise hyperemia (215). However, cardiac denervation or pharmacological inhibition of autonomic control does not result in myocardial ischemia during exercise, implying that other vasodilator mechanisms act to compensate and mediate exercise hyperemia when autonomic control is blocked. These findings are consistent with the concept that autonomic control serves to optimize the matching of coronary blood flow to myocardial metabolic needs but is not essential for exercise hyperemia. 2. Angiotensin II Angiotensin II (ANG II) is a vasoactive octapeptide produced by cleavage of the decapeptide angiotensin I by angiotensin converting enzyme. ANG II exerts important cardiovascular effects, including positive inotropism and vasoconstriction, as well as causing norepinephrine and aldosterone release, all of which are mediated via the AT1 receptor (557). Although the AT2 receptor can mediate a vasodilator response, the (patho)physiological function of this receptor is less clear, as the vasomotor response to exogenous ANG II is principally vasoconstriction (557). In the coronary circulation, exogenous ANG II produces constriction of epicardial conductance arteries (419), as well as small arteries (298) and arterioles (623). Furthermore, intravenous as well as intracoronary administration of ANG II produces coronary vasoconstriction in a variety of species, including rat (374, 394, 513), dog (435, 624), Physiol Rev • VOL and swine (330), indicating that the AT1 receptor is present in the coronary microvasculature and is capable of influencing coronary vasomotor tone. Furthermore, during treadmill exercise, sympathetic activity, and hence activity of the renin-angiotensin system, increases. This suggests that the increased levels of ANG II during exercise could act to increase coronary vasomotor tone. Studies in awake swine examining the role of endogenous ANG II in the control of coronary resistance vessel tone have yielded ambiguous results, with AT1 receptor blockade having either no effect at rest (539, 547) or during exercise (106), or causing coronary vasodilation during exercise (539, 547). None of these studies corrected for alterations in coronary vasomotor tone resulting from changes in myocardial metabolic demands produced by AT1 receptor blockade, making interpretation of the data difficult. This is particularly important since systemic administration of the AT1 blocker (106, 539, 547) results in alterations of systemic hemodynamics. However, even when systemic hemodynamic effects are avoided by intracoronary administration of the AT1 antagonist (457, 475), myocardial oxygen demands can be reduced as a result of a decrease in contractility both directly, through blockade of AT1 receptors on the cardiomyocytes (125), and indirectly, through blockade of AT1 receptors on the sympathetic nerve endings with consequent blunting of norepinephrine release (310, 493, 550). Hence, the reported failure of coronary blood flow to increase in response to intracoronary losartan in humans with endothelial dysfunction (457) may have been due to a decrease in myocardial oxygen demands produced by presynaptic and cardiomyocyte AT1 blockade. To isolate the influence of AT1 receptors on coronary vasomotor tone, Merkus et al. (394) studied the effect of AT1 blockade on the relation between coronary blood flow and myocardial oxygen demand (143, 565). They found that AT1 receptor blockade caused coronary vasodilation at rest and during exercise of awake swine, indicating that endogenous ANG II exerts a tonic vasoconstrictor influence on the coronary resistance vessels (Fig. 18). AT1 receptor blockade can decrease norepinephrine levels by blockade of presynaptic AT1 receptors that act to facilitate norepinephrine release (310, 493, 550). Conversely, the decrease in blood pressure that often accompanies AT1 receptor blockade will activate the baroreceptor reflex-mediated release of norepinephrine. The baroreceptor-mediated increase in norepinephrine is likely the predominant effect, as circulating norepinephrine levels increased slightly following AT1 receptor blockade, while the transmyocardial norepinephrine gradient remained unchanged, consistent with reports that ANG II-induced presynaptic modulation of catecholamine release in the heart is minimal (310, 330). In swine, norepinephrine exerts its effects on coronary vasomotor tone principally 88 • JULY 2008 • www.prv.org CORONARY BLOOD FLOW 1031 3. Autacoids FIG. 18. Effect of AT1 receptor blockade with telmisartan (0.3 mg/kg iv) or irbesartan (1 mg/kg iv) on the relation between myocardial oxygen consumption and coronary venous oxygen tension in the left ventricles of dogs (624; left panel) and swine (394; right panel) during treadmill exercise. Data are means ⫾ SE. *P ⬍ 0.05 vs. control. See text for further explanation. through activation of -adrenoceptors, thereby causing vasodilation (148) so that the small increase in norepinephrine following AT1 receptor blockade may have contributed to the observed coronary vasodilation. Nevertheless, a vasodilator effect of AT1 receptor blockade remained after -receptor blockade, indicating that the coronary vasodilation produced by systemic AT1 receptor blockade was not simply the result of increased coronary -adrenergic stimulation, but also included a direct effect on the coronary resistance vessels. In contrast to observations in awake swine, studies in awake dogs have failed to reveal a significant role for ANG II in control of coronary resistance vessel tone either at rest or during exercise. Thus Zhang et al. (624) found that AT1 receptor blockade with telmisartan had no effect on the relation between myocardial oxygen consumption and coronary venous oxygen tension in awake normal dogs (Fig. 18), suggesting that ANG II does not contribute to the regulation of coronary resistance vessel tone in the dog. Similarly, a study in resting humans reported that intracoronary losartan had no effect on coronary blood flow or coronary vascular resistance (457). However, a role of ANG II in the regulation of coronary resistance vessel tone in exercising healthy humans, determining the relation between myocardial oxygen consumption and coronary venous oxygen tension has not been investigated to date. In summary, in swine, ANG II exerts a weak direct vasoconstrictor influence on the coronary circulation, particularly during exercise. In contrast, a role for ANG II in coronary vasomotor control during exercise in the canine and human heart has not been convincingly demonstrated. Physiol Rev • VOL Histamine and bradykinin are autacoids involved in inflammatory processes that can exert powerful effects on vasomotor tone. However, their role in the regulation of coronary vasomotor tone under normal conditions is still incompletely understood. A) HISTAMINE. Histamine, formed from histidine by histidine-decarboxylase and stored in mast cells and basophils, plays an important role in hypersensitivity and allergic reactions and can modulate vasomotor tone via stimulation of H1 and H2 receptors in the vascular wall. In the coronary circulation, H1 receptors located on vascular smooth muscle cells of large and small arteries mediate vasoconstriction, while H2 receptors located on vascular smooth muscle cells of arterioles mediate vasodilation (312, 403). In addition, H1 receptors located on the endothelium can stimulate NO release to produce coronary vasodilation (312, 586). To our knowledge, there are no studies demonstrating a role for endogenous histamine in regulation of coronary vasomotor tone in awake healthy animals or humans during physiological conditions either at rest or during exercise. B) BRADYKININ. Bradykinin, produced by conversion of kininogens by tissue and plasma kallikreins, plays an important role in inflammation, nociception, and possibly regulation of blood pressure and fluid balance. Bradykinin synthesized within the vascular wall (85, 438) can exert a potent vasodilator influence on coronary arterial vessels of all sizes via stimulation of B2 receptors to produce the endothelium-derived relaxing factors NO, prostacyclin, and endothelium-derived hyperpolarizing factor (EDHF) (578, 579). In addition, some vasodilation also occurs via B1 receptor stimulation, which appears to be NO-mediated (543), although this is substantially less than that produced via B2 receptors, likely because induction of B1 receptors principally occurs after tissue damage. Although there is some in vitro evidence for basal bradykinin release, obtained in isolated rat hearts (47), in vivo observations in awake healthy dogs failed to define a role for endogenous bradykinin in the regulation of coronary blood flow (460). Groves et al. (223) reported that infusion of the selective B2 receptor antagonist HOE 140 into a coronary artery of patients without significant coronary stenoses (⬍30% luminal narrowing) caused vasoconstriction with a decrease in epicardial coronary luminal area and a decrease of coronary blood flow. These observations indicate that both coronary conductance and resistance vessels were subject to a bradykinin-mediated tonic vasodilator influence. Since this study was performed in patients with recurrent chest pain, it is uncertain whether bradykinin contributes to the regulation of coronary vasomotor tone in the normal human heart. In summary, there is little evidence for a role of histamine or bradykinin in regulation of coronary blood 88 • JULY 2008 • www.prv.org 1032 DIRK J. DUNCKER AND ROBERT J. BACHE flow in the normal heart. It is likely that proinflammatory conditions, as are likely to occur in isolated heart preparations or in patients with coronary artery disease, will lead to an increased autacoid-mediated influence on coronary vasomotor. 4. Endothelium-derived vasoactive factors A) NO. The normal coronary endothelium can cause vasodilation as constitutive NO synthase acts on L-arginine to produce NO, which causes relaxation of vascular smooth muscle via an increase in cGMP and consequent activation of calcium-activated K⫹ channels (KCa) channels and possibly KATP channels (461, 578). Endothelial production of NO can be triggered by specific receptors (e.g., muscarinic, bradykinin, and histamine receptors) or by mechanical deformation resulting from shear forces or pulsatile strain caused by blood flow (200, 276). NOdependent mechanisms cause vasodilation of both epicardial arteries and coronary resistance vessels in response to increased blood flow in vitro (328) and contribute to coronary reactive hyperemia in vivo (7, 445, 618). The contribution of NO to maintenance of coronary blood flow has been studied by administering analogs of L-arginine that act as competitive inhibitors of the NO synthesis. In vivo studies in dogs have generally shown no change (7, 293, 445, 472, 529, 610, 618) or only a small decrease (56, 568) of coronary flow in response to inhibitors of NO synthesis during basal conditions. In both anesthetized (314) and awake swine (147), inhibition of NO synthesis resulted in a small decrease of coronary blood flow that was accompanied by increased oxygen extraction and a decrease in coronary venous oxygen tension. In the human heart, endogenous NO exerts a modest vasodilator influence on the coronary vessels under resting conditions (160, 360, 464). In contrast, in isolated non-blood-perfused rodent hearts (rats, guinea pigs, rabbits), inhibitors of NO synthesis generally cause marked coronary vasoconstriction (9, 49, 81, 332, 447, 528, 570). The discrepancy between the modest contribution of NO in in vivo blood-perfused hearts compared with isolated buffer-perfused rodent hearts is likely due to differences in experimental conditions. In buffer-perfused hearts, flow rates are very high (⬃6 ml䡠min⫺1 䡠g⫺1), favoring shear-mediated release of NO. In addition, the absence of blood (and therefore the NO scavenger hemoglobin) is likely to increase the biological half-life of NO in the isolated perfused hearts (42). Chilian and co-workers (300) reported that inhibition of NO synthesis with N-nitro-L-arginine methyl ester (L-NAME; 30 g䡠kg⫺1 䡠min⫺, intracoronary) constricted small coronary arteries (⬎100 m), but this was counterbalanced by vasodilation of arterioles (⬍100 m), indicating that compensatory vasomotor adjustments in dif- Physiol Rev • VOL ferent segments of the coronary vasculature occurred to maintain blood flow. These data imply that under resting conditions, the effects of NO synthase inhibition can be blunted by compensatory arteriolar dilation but suggest that this basal dilation of the coronary arterioles might limit further exercise-induced increases in coronary blood flow. There is evidence that during exercise coronary NO production is increased in the dog heart (56, 562), likely due to an increase in endothelial shear secondary to the increased coronary flow rates. Furthermore, NO release from erythrocytes could be stimulated in the canine heart during exercise in response to the decrease in intravascular oxygen tension (527, 536). Together these observations suggest that NO might contribute to the increase of coronary blood flow during exercise. However, blockade of NO production with intracoronary NG-nitro-L-arginine (L-NA) did not impair the ability to increase coronary flow during treadmill exercise in dogs (7, 56, 288, 568). In fact, coronary flow rates during exercise were slightly higher after L-NA, in parallel with a slight increase in myocardial oxygen consumption (7, 56, 288). The relation between myocardial oxygen consumption and coronary venous oxygen tension was not significantly altered by L-NA (Fig. 19), indicating that inhibition of NO production did not interfere with metabolic regulation of coronary vasomotor tone (7, 288). In swine, the small decrease in coronary venous oxygen tension that occurred after NO blockade under resting conditions was maintained during exercise (Fig. 19), indicating that the role of NO was not increased compared with resting conditions, and (similar to observations in the dog) that NO is not mandatory for the exercise-induced increase in coronary blood flow in swine (147, 393). It could be argued that NO released from erythrocytes could have contributed to exercise hyperemia in the presence of NO synthase inhibition. However, this mechanism is unlikely to be of importance in swine, in view of the lack of an exercise-induced decrease in coronary venous oxygen tension and saturation in this species. B) PROSTANOIDS. The coronary endothelium can metabolize arachidonic acid to produce prostacyclin and other vasodilator prostanoids (121, 331) that act to increase myocardial blood flow via an increase in cAMP resulting in opening KATP channels in coronary vascular smooth muscle (331). Prostanoids have been proposed to contribute to metabolic dilation of coronary resistance vessels in humans (135, 198), although this is not a consistent finding (159, 439). Inhibition of cyclooxygenase with indomethacin in a dose that caused marked blunting of the vasodilator response to intracoronary arachidonic acid caused no change in coronary blood flow during resting conditions and did not impair the increase in coronary flow in response to treadmill exercise in dogs (121) so that the relation between myocardial oxygen consumption and coronary venous oxygen tension remained unaffected (Fig. 19). 88 • JULY 2008 • www.prv.org CORONARY BLOOD FLOW FIG. 19. Top panels show the effect of NO synthase inhibition with N-nitro-L-arginine (L-NA, 20 mg/kg iv) on the relation between myocardial oxygen consumption and coronary venous oxygen tension in the left ventricles of dogs (7; left panel) and swine (147; right panel) during treadmill exercise. Bottom panels show the effect of cyclooxygenase inhibition with indomethacin [Indo; 5 mg/kg iv (121) or 10 mg/kg iv (396)] on the relation between myocardial oxygen consumption and coronary venous oxygen tension in the left ventricles of dogs (121; left panel) and swine (396; right panel) during treadmill exercise. Data are means ⫾ SE. *P ⬍ 0.05 vs. control. See text for further explanation. Interestingly, several studies have suggested that an interaction exists between NO and prostanoids in the canine coronary circulation. Inhibition of cyclooxygenase shortened the duration of reactive hyperemia in dogs treated with L-NAME, but not in control dogs (459). These findings indicate an increased contribution of prostanoids when NO synthase activity is blunted, and could explain why two of three clinical studies of patients with (minimal) coronary artery disease reported a role for vasodilator prostanoids (135, 198), whereas the single study in healthy human volunteers failed to observe a role (159). Merkus et al. (396) investigated the effects of blocking endogenous prostanoids with indomethacin on coronary resistance vessels, in exercising swine. To study possible interactions between NO and prostanoids, experiments were repeated after NO synthase blockade with L-NA. Indomethacin decreased coronary venous oxygen tension at rest and during exercise (Fig. 19). However, Physiol Rev • VOL 1033 prostanoids were not mandatory for the exercise-induced vasodilation, because coronary venous oxygen tension was not further altered by exercise in the presence of indomethacin. The contribution of prostanoids to the regulation of coronary vascular tone was not enhanced by inhibition of NO synthesis in swine (396). These findings suggest that in the porcine heart, prostanoids and NO do not act in a compensatory manner when one of these pathways is blocked. C) EDHF. Endothelium-derived relaxing factors other than prostacyclin and NO have been implicated in the endothelium-dependent vasodilation produced by acetylcholine and bradykinin. These factors have been named EDHFs, in view of their ability to hyperpolarize the vascular smooth muscle, via opening of KCa channels to produce vasodilation (463, 579). Several factors appear to participate in endothelium-derived hyperpolarization, including cytochrome P-450-dependent metabolites of arachidonic acid (463) and hydrogen peroxide (229, 495). The role of EDHF in coronary vasodilation in healthy humans or awake dogs at rest or during treadmill exercise has not been studied to date, although preliminary data in swine suggest that inhibition of cytochrome P-450 2C9 with sulfaphenazole had no effect on coronary vasomotor tone in swine (398). D) ENDOTHELIN. Endothelins are vasoactive peptides that are produced by coronary vascular endothelium. Three isoforms have been identified, of which endothelin-1 (ET) is the most abundant and biologically active isopeptide in the heart (246). ET is produced in endothelial cells by cleavage of its nonvasoactive precursors preproendothelin and big ET (487) and acts on ET receptors located both on the endothelium and vascular smooth muscle. Binding of ET to ETB receptors on the endothelium leads to production of NO and prostacyclin (190, 560), which induce vasodilation. In contrast, binding of ET to the ETA and ETB receptors on vascular smooth muscle leads to vasoconstriction (487). Administration of exogenous ET causes ETB-mediated vasodilation at low doses but ETA-mediated constriction at high doses, indicating that the ETB receptor on the endothelium is more sensitive to ET than the receptors on vascular smooth muscle (487). Endogenous levels of ET exert a coronary vasoconstrictor influence in vivo. Thus the mixed ETA/ ETB receptor antagonist tezosentan caused an increase in coronary venous oxygen tension and saturation both under resting conditions and during treadmill exercise, indicating a small vasoconstrictor influence (Fig. 20). In swine, the ET-mediated vasoconstrictor influence appears to be principally ETA mediated, as the ETA receptor antagonist EMD 122946 resulted in a similar response (395). In contrast, in the coronary circulation of patients with stable angina pectoris, the nonselective ET receptor antagonist bosentan increased artery diameter, but had no effect on coronary flow, indicating that endogenous ET 88 • JULY 2008 • www.prv.org 1034 DIRK J. DUNCKER AND ROBERT J. BACHE FIG. 20. Effect of mixed ETA/ETB endothelin receptor blockade with tezosentan [1 mg/kg iv (212), 2 mg/kg iv (548), or 3 mg/kg iv (395, 397)] on the relation between myocardial oxygen consumption and coronary venous oxygen tension and saturation in the left ventricles of dogs (212, 548; left panels) and swine (395, 397; middle panels) during treadmill exercise. Also shown are the effects of selective ETA receptor blockade with EMD 122946 (3 mg/kg iv) in swine (395, 397; right panels). Data are means ⫾ SE. *P ⬍ 0.05 vs. control. †P ⬍ 0.05 effect of ET receptor antagonist decreases at higher levels of myocardial oxygen consumption. See text for further explanation. does not contribute to regulation of coronary resistance vessel tone in humans (597). However, it should be noted that bosentan was administered intravenously and caused a 10% decrease in aortic blood pressure, which likely caused a decrease in myocardial oxygen demand that may have masked a direct coronary vasodilator effect. Interestingly, measurements of ET levels in blood yield concentrations in the picomolar range, while receptor sensitivities are in the nanomolar range, which is likely explained by abluminal secretion of ET (196). Consequently, plasma levels do not accurately represent actual interstitial levels of ET, although they likely reflect directional changes in interstitial levels. The finding that ET receptor antagonists cause coronary vasodilation in awake animals (212, 392, 395, 548) implies that receptor activation does occur despite the subpharmacological plasma levels. During exercise, the effect of either ETA or mixed ETA/ETB blockade on coronary vascular tone in swine decreased progressively with incremental levels of exercise (392, 395). A similar trend was observed with tezosentan in exercising dogs (212, 548), suggesting that the vasoconstrictor influence of ET was blunted during exercise (Fig. 20). This seemingly paradoxical finding is in accord with in vitro findings that the ET-vasoconstrictor influence on coronary arterioles is modified by the cardiomyocytes according to their metabolic status so that at Physiol Rev • VOL higher pacing rates myocytes inhibited the vasoconstrictor influence of ET (392). Merkus et al. (397) investigated possible mechanisms for the blunted ET influence during exercise and observed that following inhibition of either NO synthase or cyclooxygenase, the vasodilator response to tezosentan that was observed under resting conditions was now also maintained during exercise. Furthermore, when both NO synthase and cyclooxygenase were blocked, the vasodilator effect of tezosentan actually increased with increasing exercise intensity, implying that these vasodilator systems acted in concert to limit the vasoconstrictor influence exerted by endogenous ET (Fig. 21). E) SUMMARY AND INTEGRATION. NO and vasodilator prostanoids contribute to the regulation of coronary blood flow in swine, while in dogs a critical role for these endothelium-derived vasodilator substances is lacking. Importantly, in none of these species do NO or vasodilator prostanoids appear mandatory for the exercise-induced increases in coronary blood flow. Conversely, in swine and in dogs, ET exerts a small coronary vasoconstrictor influence under resting conditions which wanes with increasing exercise intensities so that it is virtually absent during heavy exercise. Both NO and prostanoids act in concert during exercise to blunt the coronary vasoconstrictor influence of ET. Finally, the role of EDHF in coronary blood flow regulation has not been studied in 88 • JULY 2008 • www.prv.org CORONARY BLOOD FLOW FIG. 21. Effect of combined NO synthase (L-NA, 20 mg/kg iv) and COX blockade (Indo, 10 mg/kg iv) on the responses to mixed ETA/ETB endothelin receptor blockade with tezosentan of the relation between myocardial oxygen consumption and coronary venous oxygen tension in the left ventricles of swine (397) during treadmill exercise. Data are means ⫾ SE. *P ⬍ 0.05 vs. control. †P ⬍ 0.05 effect of ET receptor antagonist decreases (left panels) or increases (right panels) at higher levels of myocardial oxygen consumption. ‡P ⬍ 0.05 effect of ET receptor antagonist was greater following L-NA and indomethacin. See text for further explanation. 1035 A) CARBON DIOXIDE AND PH. Case and colleagues (95, 96), using constant-flow perfused heart preparations to study the role of carbon dioxide in regulating coronary blood flow, observed that coronary vascular resistance correlated well with coronary venous carbon dioxide concentrations. Broten et al. (80) proposed that up to 40% of the increase in coronary blood flow produced by ventricular pacing could be explained by the synergistic interaction between myocardial oxygen and carbon dioxide tensions. The mechanism by which carbon dioxide dilates coronary arterioles is incompletely understood but could be the result of an acidosis-induced opening of KATP channels (292). Despite its attractiveness as a regulator of coronary resistance vessel tone, carbon dioxide is not a likely mediator of the exercise-induced coronary vasodilation, as coronary venous carbon dioxide tension or pH remain essentially unchanged during exercise (Fig. 22), in either dogs (568, 569) or swine (149, 393). B) ADENOSINE. Adenosine predominantly dilates arterioles ⬍100 m in diameter. Vessels of this size correspond to the site at which coronary metabolic regulation (306) and autoregulation occur (307). Adenosine has characteristics which suggest that it could be a messenger by which the coronary resistance vessels are regulated in response to changing myocardial metabolic needs (54, 168, 180). There are two pathways for adenosine production in the heart. Under normal conditions, adenosine is generated mainly via extracellular pathways: from interstitial AMP, via AMP 5⬘-ectonucleotidase, and from Sadenosylhomocysteine via S-adenosylhomocysteine hy- exercising animals or humans and awaits further clarification. In humans, a role for vasodilator prostanoids and particularly for NO is likely under resting conditions, but interpretation is hampered by the fact that most studies have been performed in patients with “minimal” coronary artery disease and hence are likely to have perturbations in endothelial function. Furthermore, in clinical studies, coronary blood flow measurements generally have not been corrected for alterations in myocardial oxygen demand, making interpretation difficult. Future clinical studies should relate coronary blood flow and coronary venous oxygen tension to myocardial oxygen consumption. 5. Metabolic messengers The accumulation of metabolic messengers, including carbon dioxide, H⫹, and adenosine, has been proposed to contribute to coronary vasodilation in response to an increase in myocardial metabolic activity. Physiol Rev • VOL FIG. 22. Arterial and coronary venous carbon dioxide tension and pH in exercising dogs (568, 569) and swine (149, 393). Data are means ⫾ SE. See text for further explanation. 88 • JULY 2008 • www.prv.org 1036 DIRK J. DUNCKER AND ROBERT J. BACHE drolase (54, 168). Under physiological conditions, the newly formed adenosine is salvaged by the cardiomyocytes for formation of AMP via adenosine kinase; these extracellular pathways of adenosine metabolism are largely independent of the metabolic state of the cardiomyocyte. However, if an increase in cardiac work causes myocardial metabolic requirements to exceed the rate of oxygen delivery, the intracellular pathway for adenosine production is activated. Thus, when the rate of ATP hydrolysis by the contractile apparatus exceeds the rate of resynthesis of ATP through oxidative phosphorylation, cytosolic free ADP increases. Adenylate kinase can then act on two molecules of ADP to form one molecule each of ATP and AMP. The resultant AMP can be catabolized by AMP 5⬘-nucleotidase to produce adenosine. When cytosolic adenosine concentrations increase from the normal level of 0.8 to ⬃2 M, adenosine is removed from the cell via degradation by adenosine deaminase or transported out of the cell into the interstitium via nucleoside transporters where it can act on the coronary arteriolar smooth muscle to cause vasodilation (54, 168). The pathway for adenosine production and release is responsive to the metabolic state of the cell and could thus serve as a metabolic messenger to cause vasodilation of the coronary arterioles. The mechanism of coronary arteriolar dilation by adenosine is not yet fully elucidated, but may involve stimulation of A1 receptors directly coupled to KATP channels and A2 receptor-mediated elevation of cAMP/protein kinase A (PKA) which produces vasodilation in part via opening of KATP channels (537). Data obtained in dogs do not support a role for adenosine in regulation of coronary blood flow during basal conditions. Thus, in anesthetized open-chest dogs (207, 241, 326, 494, 515) and in chronically instrumented awake dogs (28), augmenting adenosine degradation by intracoronary administration of adenosine deaminase (28, 241, 326, 494), or nonselective blockade of A1/A2 adenosine receptors with either aminophylline (207, 515) or 8-phenyltheophylline (8-PT) (28), did not cause a decrease in basal coronary blood flow or coronary venous oxygen tension (Fig. 23), although one study reported a small decrease in coronary venous oxygen tension (569). In contrast, data obtained in sedated closed-chest swine (204) and awake resting swine (149, 393) suggest that adenosine does contribute to maintenance of basal tone in this species. Thus intravenous administration of aminophylline (204) or 8-phenyltheophylline (149, 393) resulted in small increases in coronary vascular resistance and oxygen extraction, and a small decrease in coronary venous oxygen tension, indicating a slight mismatch between oxygen delivery and consumption (Fig. 23). One study in humans reported no effect of aminophylline on basal coronary blood flow (479), but other studies reported that intravenous theophylline produced small increases in coronary resistance and oxygen extraction and Physiol Rev • VOL FIG. 23. Integrated metabolic control of coronary vasomotor tone in dogs (left panels) and swine (right panels) at rest and during treadmill exercise. Shown are the effects of adenosine receptor blockade with 8-phenyltheophylline (8PT, 5 mg/kg iv; top panels), the effects of KATP channel blockade with glibenclamide [Glib, 50 g/kg/min ic (153) or 3 mg/kg iv (393)] and additional adenosine receptor blockade (middle panels), and the effects of NO synthase inhibition with N-nitro-L-arginine [L-NA, 1.5 mg/kg ic (288) or 20 mg/kg iv (393)] and additional adenosine receptor blockade and KATP channel blockade (bottom panels) on the relation between myocardial oxygen consumption (MV̇O2) and coronary venous oxygen tension (CVPO2) in the left ventricles. [Data in dogs are from Refs. 28, 153, 288. Swine data are from Merkus et al. (393).] Data are means ⫾ SE. *P ⬍ 0.05 effect of 8PT; †P ⬍ 0.05 effect of glibenclamide; ‡P ⬍ 0.05 effect of L-NA. See text for further explanation. 88 • JULY 2008 • www.prv.org CORONARY BLOOD FLOW decreases in coronary venous oxygen tension (157–159). In conclusion, although endogenous adenosine causes tonic vasodilation of the coronary arterioles in normal swine and possibly humans, this effect is small and does not represent a principal mechanism for maintaining coronary blood flow during basal conditions. This is also supported by observations that adenosine receptor blockade does not impair myocardial contractile performance or cause metabolic evidence of ischemia (28, 149, 616). Measurement of plasma and tissue levels of adenosine is difficult. Measurements in blood are hampered by rapid breakdown of adenosine (238, 337), while interstitial measurements with microdialysis techniques require insertion of permeable fibers into the myocardium, thereby causing tissue damage and an acute rise in adenosine levels (577). Notwithstanding these difficulties, several studies have reported that adenosine production by the heart is augmented during increased contractile work associated with exercise (22, 166, 391, 594), which was positively correlated with coronary blood flow. Although these observations appear consistent with an exerciseinduced increase in myocardial adenosine production, more recent studies have questioned the validity of the techniques to directly measure pericardial and interstitial adenosine measurements (see Ref. 180 for a critical in depth review), and several more recent studies reported that coronary venous and computed interstitial adenosine concentrations failed to increase significantly during exercise (568, 569). Demonstration of an essential role for an exerciseassociated increase in interstitial adenosine concentration in mediating the exercise-induced coronary vasodilation requires that interruption of the adenosine effect interferes with exercise-induced vasodilation. However, blockade of adenosine receptors with 8-phenyltheophylline or increasing adenosine catabolism with adenosine deaminase did not impair exercise-induced increases in coronary blood flow in dogs (28, 568), swine (149, 393), or humans (157–159) and did not change the relation between myocardial oxygen consumption and coronary venous oxygen tension (Fig. 23). These findings indicate that adenosine is not obligatory for the coronary vasodilation that occurs during exercise and could be interpreted to suggest that adenosine is either not important for control of resistance vessel tone under normal inflow conditions or that other vasodilator systems can compensate when adenosine is blocked. It has been suggested that blockade of adenosine receptors would result in increased myocardial interstitial levels of adenosine which might overcome the effects of the competitive adenosine receptor antagonists (390). However, several investigators have failed to find increased myocardial interstitial levels of adenosine following adenosine receptor blockade (569, 616). In summary, despite the attractiveness of the adenosine hypothesis, there is no evidence to support a role for Physiol Rev • VOL 1037 adenosine in exercise hyperemia in the normal heart of dogs, swine, or humans. C) ATP. ATP is a potent coronary vasodilator (147, 181) that is progressively released from red blood cells during decreases in oxygen tension (165) and may thus contribute to the regulation of skeletal muscle blood flow (164). A role for ATP as an oxygen sensor, and hence a possible flow regulator, has also been proposed for the coronary circulation. Thus, during treadmill exercise in dogs, coronary venous oxygen tension decreased from 19 to 13 mmHg, and this was accompanied by an increase in coronary venous plasma ATP level from 13 to 51 nM that was linearly related to the increase of coronary blood flow (174). Intravascular ATP produces vasodilation by acting on endothelial purinergic P2Y-receptors to increase NO production (164, 165, 387). In addition, ATP can produce vasodilation after its conversion to adenosine by stimulation of adenosine A2A receptors on vascular smooth muscle cells (421). Studies examining the effects of ATP receptor antagonists on coronary blood flow regulation during exercise are lacking. However, NO synthase inhibition either alone or in combination with adenosine receptor blockade failed to blunt the exercise-induced vasodilation in dogs (288) and swine (393). These observations do not support a critical role for ATP in exerciseinduced coronary vasodilation. 6. End effectors: K⫹ channels A) KATP CHANNELS. Vascular smooth muscle cells contain potassium channels that are sensitive to the intracellular energy charge (425, 537, 538). Opening of the KATP channels results in an outward flux of potassium that increases the membrane potential of the sarcolemma. This hyperpolarization closes voltage-dependent calcium channels, leading to a decreased influx of calcium, thereby causing vasodilation. Conversely, closing of KATP channels decreases smooth muscle membrane potential, thereby opening voltage-dependent calcium channels; increased calcium influx then results in vasoconstriction. The mechanism of regulation of KATP channel activity in the normal heart is still incompletely understood. Coronary arteriolar smooth muscle KATP channels are tonically active under conditions of normal arterial inflow despite the presence of physiological ATP concentrations (4 –5 mmol), whereas in in vitro studies KATP channels are inactivated at ATP concentrations well below 1 mmol (461). Other regulators of vascular KATP channel activity are prostacyclin, adenosine, and ß2-adrenoceptors that increase KATP channel activity via cAMP/PKA and NO that activates KATP channels via cGMP (461). Several of these vasodilator mechanisms are operative during normal arterial inflow and can be further activated during myocardial ischemia. In addition, there is substantial evidence for modulation of KATP channel activity by the metabolic state of the vascular 88 • JULY 2008 • www.prv.org 1038 DIRK J. DUNCKER AND ROBERT J. BACHE smooth cell, with an increase in ADP/ATP ratio near the sarcolemma, acidosis, and hypoxia all being potent activators of KATP channels directly (461). Thus, rather than the bulk cytosolic ATP concentration, it is likely that the ADP/ATP ratio in the subsarcolemmal microenvironment and vasodilator signaling pathways act in concert to regulate KATP channel activity and maintain coronary blood flow in the normal heart. The influence of KATP channels on coronary blood flow has been assessed using selective inhibitors of KATP channel activity such as glibenclamide. Intracoronary glibenclamide in doses of 10 –50 g䡠kg⫺1 䡠min⫺1 caused coronary vasoconstriction with a 20 –55% decrease in basal coronary blood flow in open-chest dogs (285, 496) or awake resting dogs (150, 153), resulting in reduced coronary venous oxygen tension at a given level of myocardial oxygen consumption (153) (Fig. 23). Systemic administration of glibenclamide to dogs in a dose of 1 mg/kg also resulted in a decrease in coronary venous oxygen tension (473). Similarly, in awake resting swine, glibenclamide (3 mg/kg iv) produced increases in coronary vascular resistance and decreases in coronary venous oxygen tension (145) (Fig. 23). The decrease in coronary blood flow produced by KATP channel blockade was associated with a decrease in regional systolic wall thickening (150, 153, 285). When coronary blood flow was restored to preglibenclamide levels with intracoronary nitroprusside (which by itself was devoid of any effect on systolic wall thickening), contractile performance recovered (150, 285), indicating that glibenclamide caused a primary decrease in coronary flow with a secondary decrease in contractile function. Furthermore, the decrease in coronary flow produced by intracoronary glibenclamide caused metabolic changes of ischemia, including a decrease in myocardial phosphocreatine and phosphorylation potential with an increase of inorganic phosphate and adenosine release (153, 496). This is an important observation, since it indicates that blockade of the endogenous vasodilator system associated with KATP channel activity can cause coronary vasoconstriction sufficient to result in myocardial ischemia. In humans, a low intracoronary dose of glibenclamide (40 g/min) resulted in a small decrease in resting blood flow (176) and blunted the pacing-induced increase in coronary blood flow by ⬃30% (175), suggesting that KATP channels contribute to coronary vasodilation during increased myocardial metabolic activity. KATP channel blockade also decreases coronary blood flow and coronary venous oxygen tension in normal dogs (150, 153, 473) and swine (145, 393) during treadmill exercise. However, the exercise-induced increase in coronary flow was unaffected so that the increase in oxygen extraction and the decrease in coronary venous oxygen tension were comparable to the changes under resting conditions (145, 150, 153, 393, 473). These findings could be interpreted Physiol Rev • VOL to suggest either that KATP channels are unimportant for exercise-induced coronary vasodilation, or that other mechanisms compensate when KATP channels are blocked. B) KCa CHANNELS. KCa channels are abundantly expressed in coronary vascular smooth muscle cells (74, 210). Upon activation, these channels hyperpolarize the cell membrane, thereby closing voltage-sensitive calcium channels to produce vasodilation. The KCa channel family consists of small-, intermediate-, and large-conductance channels (BKCa), all with different structural components and gating properties (229). The BKCa channel is the most prominent KCa channel in vascular smooth muscle so that small changes in open probability have a significant effect on the sarcolemmal membrane potential and therefore on vasomotor tone (74, 425, 490, 530). Membrane depolarization and increases in intracellular Ca2⫹ concentration are thought to be the main activators of KCa channels, thereby providing an important negative-feedback mechanism to moderate vasoconstrictor responses (74, 425). In addition, various protein kinases have been shown to modulate the activity of the KCa channels (381, 511). KCa channels are activated by phosphorylation through cGMP-dependent protein kinase (PKG; Refs. 549, 604) and PKA (406, 517), while protein kinase C (PKC) inhibits KCa channels (405, 407, 458). Many endogenous vasoactive substances exert their actions through these protein kinases, thereby modulating KCa channel activity and hence altering coronary resistance vessel tone. In general, vasodilator substances such as adenosine, EDHF, NO, norepinephrine, and H⫹ act through stimulation of PKA and PKG (74, 126, 381, 425), resulting in increased opening of KCa channels, while vasoconstrictor substances, such as endothelin and angiotensin II, decrease the opening of KCa channels in part via PKC activation (281, 358, 405). Hence, it is likely that many vasoactive substances act in concert to mediate the exercise-induced opening of KCa channels. The role of KCa channels has been investigated in anesthetized dogs. The KCa channel antagonists iberiotoxin (429), charybdotoxin (443), or tetraethylammonium (477) had no effect on coronary blood flow under basal conditions, which is in accord with the concept that in dogs KATP channels are the principal K⫹ channel involved in metabolic regulation of coronary vasomotor tone. In swine, tetraethylammonium administered intravenously in a dose that did not inhibit vasodilation by the KATP channel opener bimakalim produced a small decrease in coronary venous oxygen tension that was progressively amplified with increasing levels of exercise (Fig. 24), suggesting that KCa channels contribute to exercise-induced coronary vasodilation (399). The upstream vasodilator pathways that activate the KCa channels in swine remain to be determined, but are unlikely to involve adenosine, NO, and prostanoids, as these vasodilator substances ex- 88 • JULY 2008 • www.prv.org CORONARY BLOOD FLOW 1039 Voltage-dependent K⫹ channels constitute a diverse family of outwardly rectifying K⫹ channels present in the vascular smooth muscle sarcolemma (229). These channels are sensitive to membrane potential so that depolarization will induce opening of KV channels and thereby oppose vasoconstriction. In addition, these channels are sensitive to -adrenoceptor stimulation and other cAMP-mediated vasodilator responses (2, 363). Alterations in the oxidative state of the vasculature (termed “redox signaling”) most notably superoxide and H2O2 can also modulate KV channel activity (229). Thus Rogers et al. (477) recently reported that KV channels play a role in redox signaling-mediated regulation of coronary blood flow, as the KV channel antagonist 4-aminopyridine (4-AP) blocked coronary vasodilation produced by intracoronary H2O2. Furthermore, 4-AP caused a 54 ⫾ 10% decrease in basal blood flow, suggesting that KV channels play an important role in maintaining resting coronary blood flow. A possible role for KV channels in mediating the coronary vasodilation that occurs in response to exercise has not been studied. However, evidence for a role of H2O2 and KV channels in metabolic regulation of coronary blood flow was recently reported by Saitoh et al. (495), who showed in open-chest dogs that the decrease in coronary venous oxygen tension produced by intracoronary 4-AP increased progressively at higher levels of myocardial oxygen consumption produced by pacing or norepinephrine. C) Kv CHANNELS. FIG. 24. Effect of KCa channel blockade with tetraethylammonium (TEA, 20 mg/kg iv) on the relation between myocardial oxygen consumption and coronary venous oxygen tension in the left ventricles of swine (399) during treadmill exercise. Data are means ⫾ SE. *P ⬍ 0.05 vs. control; †P ⬍ 0.05 effect of TEA increases at higher levels of MV̇O2. See text for further explanation. ert a tonic influence on the coronary resistance vessels that is similar at rest and during exercise. A potential candidate is -adrenoceptor activation which has been shown to contribute to exercise-induced vasodilation (148) and is known to act, at least in part, via KCa channels (283, 517). Alternatively, the progressive withdrawal of the vasoconstrictor influence of ET (which is known to inhibit opening of KCa channels; Refs. 281, 407, 450) could contribute to activation of these channels during exercise (392, 395). Interestingly, in swine the effects of combined KATP and KCa channel blockade was not greater than the sum of the effects of KATP and KCa channel blockade alone (399), suggesting that these channels act in a linear additive, rather than a nonlinear redundant, fashion (143, 565). KATP channels are activated directly by increases in intracellular ADP/ATP (425), while vasodilators such as adenosine and prostacyclin are thought to exert their action principally via cAMP/PKA-mediated opening of KATP channels (73, 425, 579). In contrast, KCa channels are activated directly by membrane depolarization and calcium (74, 358), while vasodilators like NO and atrial natriuretic peptide are considered to exert their action predominantly via cGMP/PKG-mediated opening of KCa channels (425, 549, 579). Although some overlap between the activation pathways appears to exist (74, 425), the separate intracellular signaling pathway involved in the activation of KATP and KCa channels may explain, at least in part, the observation that KATP and KCa channels act in an additive manner in the regulation of coronary resistance vessel tone in swine during exercise. Physiol Rev • VOL 7. Summary and integration of coronary vasodilator mechanisms Enhanced delivery of oxygen and metabolic substrate is essential for the cardiac response to an increased work load and involves a number of parallel mechanisms that contribute to coronary vasodilation. In the dog, blockade of any of these vasodilator mechanisms fails to blunt the increase in coronary blood flow in response to exercise, suggesting that adenosine, KATP channel opening, prostanoids, or NO are not mandatory for exerciseinduced coronary vasodilation, or that these redundant vasodilator mechanisms can compensate when one mechanism is blocked. A compensatory role for adenosine in the regulation of coronary blood flow when KATP channels are blocked was first demonstrated in dogs (153, 496) (Fig. 23). Following KATP channel blockade with intracoronary glibenclamide, which produced a 20% decrease in coronary blood flow and a 50% decrease in regional wall thickening (150, 153), adenosine receptor blockade resulted in a significant further decrease in coronary flow and regional wall thickening, in particular during exercise (153). In contrast, Richmond et al. (473) found that administration of an intravenous dose of 1 mg/kg glibenclamide, which had no effect on lactate extraction, did not affect coro- 88 • JULY 2008 • www.prv.org 1040 DIRK J. DUNCKER AND ROBERT J. BACHE nary venous adenosine concentrations (P ⫽ 0.11), either at rest or during exercise. In that study, the dose of glibenclamide may have been too low to fully block vascular KATP channels, as the authors stated that higher doses resulted in coronary flow oscillations, suggesting that larger doses did produce vasoconstriction, but this resulted in generation of an underdamped error signal that overcompensated for the constriction. The latter was shown by Samaha et al. (496) to be due to cyclic release of adenosine resulting from ATP breakdown, i.e., the presence of myocardial ischemia. In swine, the effect of simultaneous blockade of KATP channels and adenosine receptors on oxygen extraction and coronary venous oxygen tension was equal to the sum of the individual effects of 8-PT and glibenclamide (Fig. 23), indicating that loss of KATP channel activity was not compensated for by an increased adenosine-mediated vasodilator influence (393). These findings suggest that adenosine and KATP channels act in an additive fashion in swine. However, adenosine mediates its vasodilator effect on porcine coronary resistance vessels via both KATP channels (255) and Kv channels (250). It is therefore possible that following KATP channel blockade, adenosine levels increased sufficiently to maintain a vasodilator influence via Kv. Alternatively, it is possible that following adenosine receptor blockade, KATP channel activity was maintained via a compensatory increase of alternate mediators such as NO and prostacyclin (294, 512). In awake dogs, inhibition of NO synthase alone or in combination with adenosine receptor blockade did not affect the relation between oxygen consumption and coronary venous oxygen tension (288) (Fig. 23). However, combined blockade of adenosine receptors, NO synthase, and KATP channels markedly (50%) reduced coronary blood flow at rest and nearly abolished the exerciseinduced coronary vasodilation (288). Those findings suggest that metabolic dilation of canine coronary resistance vessels is regulated via a myriad of vasodilator systems that act in concert to match coronary blood flow to myocardial oxygen demand so that when one system fails, back-up systems ensure an adequate oxygen supply to the myocardium. The observation that only KATP channel blockade alone decreased coronary blood flow and caused myocardial ischemia suggests that this represents a principal coronary vasodilator pathway in the dog, with adenosine and NO primarily acting as back-up systems (153, 288). In contrast, Tune et al. (567) reported that while simultaneous blockade of adenosine receptors, KATP channels, and NO synthase caused coronary vasoconstriction in awake resting dogs, the triple blockade failed to blunt the exercise-induced coronary vasodilation. Consequently, these authors concluded that these mediators act in a linear additive fashion rather than a nonlinear redundant manner (565). Interpretation of the data and comparison with previous studies in dogs (150, Physiol Rev • VOL 153, 288) are difficult, however, due to differences in study design. One such difference is the use of a low dose of glibenclamide (1 mg/kg iv) that was selected to avoid reductions of coronary blood flow that resulted in myocardial ischemia (473, 567). However, this may have inadvertently resulted in incomplete KATP channel blockade, allowing coronary blood flow to increase during exercise. In swine, a high dose of glibenclamide (3 mg/kg iv), which caused signs of anaerobic myocardial metabolism and impaired left ventricular function (393), failed to enhance the vasoconstrictor response to adenosine receptor blockade and NO-synthase inhibition (Fig. 23), suggesting a vasomotor control design in the porcine heart in which vasodilator pathways act in a linear additive fashion (393). Simultaneous blockade of adenosine, KATP channels, and NO caused intense coronary vasoconstriction (forcing oxygen extraction to increase to over 90%) with signs of anaerobic metabolism and impaired left ventricular function under resting conditions (393). However, in contrast to the observations by Ishibashi et al. (288) in the dog heart, the responses of coronary blood flow and oxygen supply in swine to subsequent exercise were essentially unperturbed (393). Apparently, in swine, other vasodilator mechanisms that are not recruited under resting conditions can be recruited during exercise when NO, adenosine, and KATP channels are blocked. Such candidate mechanisms include -adrenergic feed-forward vasodilation, prostacyclin, EDHF, and H⫹. -Adrenergic vasodilation plays an important role in exercise-induced vasodilation in swine (148) and could have acted through opening of KCa channels (399). G. Coronary Blood Flow in the Exercise-Trained Heart Chronic endurance exercise leads to myocardial hypertrophy that can produce up to a 30% increase of relative left ventricular mass. Morphometric studies have shown that this hypertrophy includes proportionate increases of cardiac myocytes and coronary vasculature with no change in the proportion of extracellular collagen. Coronary vasodilation in response to endotheliumdependent and -independent vasodilators is normal in the physiologically hypertrophied heart so that vasodilator reserve is maintained or increased. Physical conditioning leads to adaptations in the myocardium that are aimed at increasing maximal cardiac output and maximal total body oxygen consumption. These adaptations also affect the major determinants of myocardial oxygen demand: heart rate, contractility, and left ventricular work (integrated systolic wall stress and shortening). Dynamic exercise training lowers heart rates at rest and at any given level of submaximal exercise, and this reduction is accompanied by parallel decreases in 88 • JULY 2008 • www.prv.org CORONARY BLOOD FLOW myocardial oxygen consumption (107, 258, 383, 588) and coronary blood flow (40, 258, 588). Myocardial contractility is difficult to assess in vivo but appears minimally affected by physical conditioning (87, 432, 492). Left ventricular systolic pressure is not significantly altered by dynamic exercise training in normal individuals but may decrease slightly in older or hypertensive subjects (509). Left ventricular end-diastolic internal diameter and left ventricular end-diastolic wall thickness increase in parallel so that their ratio is not significantly altered (161, 382, 508, 601). Consequently, left ventricular systolic wall stress is minimally affected by exercise training. Stroke volume increases in parallel with the increased enddiastolic volume so that muscle fiber shortening is maintained. As a result, ventricular work per gram of myocardium is minimally affected by dynamic exercise training. These findings indicate that myocardial oxygen demand is decreased at rest or at a given absolute level of exercise mainly because of the decrease in heart rate. In addition to reducing myocardial oxygen demands at rest and during submaximal exercise, physical conditioning results in coronary vascular adaptations that can increase the myocardial oxygen supply. Although there is no evidence to suggest that coronary flow rates limit oxidative metabolism in the normal heart even during maximal exercise, an increase in myocardial oxygen supply following exercise training could act to facilitate maximal cardiac performance (see sect. IIB3C). The oxygen supply can be increased by raising the arterial oxygencarrying capacity, by increasing oxygen extraction, or by increasing blood flow. The oxygen-carrying capacity of the blood is generally not significantly altered or may decrease (⬍10%) following exercise training (97). Myocardial oxygen extraction does increase slightly following exercise training (541, 588), but this is very modest since oxygen extraction is already near maximal even in the 1041 untrained state. Therefore, an improvement of oxygen supply must stem primarily from an increase of coronary blood flow. An enhanced ability to increase coronary blood flow can result from adaptations within the coronary vasculature or from a decrease in the extravascular compressive forces acting on the intramural coronary microvessels (Fig. 25). Coronary vascular adaptations in response to exercise training can be divided into structural (angiogenesis and vascular remodeling) and functional adaptations (alterations in vasomotor control) (338 –340). Functional adaptations can include changes in neurohumoral control mechanisms or changes in local vascular control mechanisms, i.e., metabolic, myogenic, and endothelial control of vasomotor tone. The following sections will consider each of these effects of exercise training. 1. Structural adaptations A) CORONARY ARTERIOLES. The effect of chronic treadmill exercise training on numerical density (i.e., the number of vessels per mm2) of coronary arterioles has been studied in domestic (77) and miniature (600, 601) swine (Table 2). Numerical density of arterioles, which were defined as vessels containing at least three layers of smooth muscle and with diameters between 35 and 75 m, was 40 – 60% greater in trained swine than in sedentary control animals (77, 601). White et al. (600) studied in more detail the adaptations of arterioles of varying sizes to exercise training up to 16 wk. The total cross-sectional area (m2 arterioles per mm2 of myocardium) of arterioles in the range of 20 –120 m increased significantly by 16 wk of training, with a greater increase in total area in arterioles of 20 – 40 m (40 – 60%) than in arterioles of 40 –120 m (15–30%). Interestingly, the mechanism of the cross-sectional area increase also varied depending on the diameter range of the arterioles. Thus, for 20- to 40-m arte- FIG. 25. Graph summarizing the structural and functional coronary microcirculatory adaptations to chronic exercise training. ACh, acetylcholine; M, muscarinic receptor; NE, norepinephrine; ␣1, ␣1-adrenergic receptor; ß2, ß2-adrenergic receptor. See text for further explanation. Physiol Rev • VOL 88 • JULY 2008 • www.prv.org Physiol Rev • VOL 88 • JULY 2008 • www.prv.org Swine M Swine M, F Swine M, F Y Y Y Y A Run Run Run Run Run Run Run Type 70–85% of max (⫹ sprints at 80–100% of max HR), 60 min/day, 5 days/wk, 10 wk 70–80% of max HR, 70 min/ day, 5 days/ wk, 16 wk 6.4–12.8 km/h, 10%; 60 min/ day, 5 days/ wk, 12 wk 10–20 km/h, 10– 20%; 75 min/ day, 5 days/ wk, 18 wk 70–85% of max HR (⫹sprints at 80–100% of max HR), 70 min/day, 5 days/wk, 12 wk 70–85% of max HR (⫹ sprints at 80-90% of max HR), 60 min/day, 5 days/wk, 10 wk ? Program Perfusion fixation LM/EM Perfusion fixation LM/EM LVW/BW 24% 1 VO2max 1, HREX 2 Perfusion fixation LM/EM Perfusion fixation EM Perfusion fixation EM EM Nuclear staining Histological Technique LVW 26% 1, LVW/BW 31% 1 LVW 29% 1, LVW/BW 15% 1 LVW 13% 1, LVW/BW 29% 1 LVW, LVW/BW 7 HW 22% 1, HW/BW ? LVW 9% 1, LVW/BW 7% 1 Cardiac Hypertrophy VO2max 1, SMVO2 1 VO2max 1 SMVO2 1 VO2max 1, HRREST 2 SMVO2 1, performance1 HRREST 2, performance 1 HRREST 2 Efficacy LV free wall LV free wall full thickness Subendocardium Subepicardium LV free wall full thickness Subendocardium Subepicardium LV free wall full thickness LV free wall full thickness LV free wall full thickness LV septum full thickness Heart Chamber 1 1 1 1 Arteriolar Density, mm⫺2 7* 2 2 1 7 7 7 7 1 Capillary Density, mm⫺2 M, male; F, female; Y, young; A, adult; “?” ⫽ not reported; HRREST, resting heart rate; HREX, heart rate during exercise; SMVO2, skeletal muscle oxidative capactity; VO2max, maximum total body oxygen consumption; LVW, left ventricular weight; LVW/BW, left ventricular to body weight ratio; HW, heart weight; HW/BW, heart weight-to-body weight ratio; LM, light microscopy; EM, electron microscopy; 1 ⫽ increase; 2 ⫽ decrease; and 7 ⫽ no change. *An increase was noted after 3 wk of training that had disappeared after 8 and 16 wk. White et al. (600) White et al. (601) Swine M, F Dog M, F A Dog M, F Laughlin and Tomanek (353) Breisch et al. (77) Y Dog ? Thörner (555) Wyatt and Mitchell (612) Age Training Exercise training and arteriolar and capillary densities in left ventricular myocardium in large animals Species, Sex 2. Investigators TABLE 1042 DIRK J. DUNCKER AND ROBERT J. BACHE CORONARY BLOOD FLOW rioles, the increase was principally the result of an increase in numerical density, whereas for arterioles with a diameter between 40 and 120 m, this was due to an increase in arteriolar diameter (600). B) CORONARY CAPILLARIES. Several investigators examined the effects of physical conditioning on the capillaryto-fiber ratio (number of capillaries per myocyte), the capillary numerical density (number of capillaries per mm2), or capillary surface density (total capillary surface area per myocardial tissue volume). Early studies in which open capillaries were identified by erythrocyte staining (452, 453) suggested an increase in capillary numerical density and exercise-induced cardiac hypertrophy in young guinea pigs following treadmill-exercise training. Similar results were obtained with hematoxylineosin staining in treadmill-exercise conditioned dogs (555). Capillary numerical density was found to be greater in wild than in domesticated rabbits or rats (589, 590), suggesting that greater physical activity of wild animals is associated with higher capillary density. However, it is not clear to what extent natural selection versus physical conditioning caused the increased capillary densities in the wild animals. In contrast, adult guinea pigs subjected to swimming (193) or young guinea pigs subjected to running (237) had decreased capillary numerical densities compared with sedentary controls, as the capillary-tofiber ratio failed to increase in the face of cardiac hypertrophy. Subsequent studies examined genetically similar rats trained by either swimming or treadmill exercise. In the rat, the response of myocardial capillary density is critically dependent on age. In young rats trained by swimming or running, exercise-induced capillary angiogenesis occurs as indicated by an increase in [3H]thymidine labeling of capillary endothelial cells (94, 572) and an increase in the capillary-to-fiber ratio (51, 60, 295, 362, 388, 558). Angiogenesis outweighed myocyte hypertrophy in most of these studies, resulting in increased capillary numerical density (13, 60, 295, 553, 558) or relative capillary surface area (388). In adult rats 3– 4 mo of age, and in old rats 6 –18 mo of age, exercise training was also associated with enhanced formation of capillaries (60, 295, 361, 371, 558, 572). However, in contrast to the young rats, in adult (60, 295, 558) and old rats (60, 558) angiogenesis usually did not outweigh cardiac hypertrophy, leaving capillary numerical density unchanged. Interestingly, in old rats, swimming increased the capillary-to-fiber ratio, but this occurred due to a loss of myocardial fibers (60). Evidence that supports a positive effect of exercise training on myocardial capillary density stems mainly from studies of young male rats trained by swimming (51, 60, 94, 295, 362, 388, 558). In contrast, studies of treadmill exercise-trained rats have reported both increases (13, 271, 295, 558) as well as no change in capillary density (11, 446, 552). A methodological concern is that several of the Physiol Rev • VOL 1043 rat studies reported histological data exclusively for the left ventricle (371, 388, 446, 558), while other studies reported data averaged for both ventricles (51, 60, 94, 295, 361, 362, 572). Anversa et al. (13) showed that a moderate treadmill exercise program increased capillary numerical density in the right but not the left ventricle. Following a more strenuous exercise program, capillary numerical density again did not change in the left ventricle (12), but actually decreased in the right ventricle (11, 14). These findings raise concern that combined analysis of tissue from both ventricles might obscure individual differences in capillarization induced by exercise training in either the left or the right ventricle. Most studies in larger animals such as dogs (341, 612, 613) or swine (77) have also failed to observe an increased capillary-to-fiber ratio (77, 341) or capillary numerical density (77, 341, 612, 613) following treadmill exercise training for at least 10 wk (Table 2). Interestingly, significant DNA labeling via tritium-labeled thymidine incorporation in dividing capillary cells and capillary sprouting were observed at 1, 3, and 8 wk of training but were no longer apparent at 16 wk of training (Fig. 26). In addition, capillary growth outweighed myocyte growth at the 3-wk time point, but capillary densities had returned to levels observed in sedentary swine by 8 wk of training (600). These results suggest that during the training program, capillary growth does occur and may even temporarily outgrow the increase in left ventricular mass that occurs early during the training program. However, with prolonged training, capillary growth is not in excess of, but rather commensurate with, the increase in left ventricular mass. C) SUMMARY AND INTEGRATION. Exercise training is associated with adaptations in the coronary microvasculature including increased arteriolar densities and/or diameters, which provide a morphometric basis for the observed increase in peak coronary blood flow rates in exercise trained animals (Fig. 25; see also sect. IIG4A). Most evidence that exercise training increases myocardial capillary density stems from studies of young male rats trained by swimming. In larger animals trained by treadmill exercise, the formation of new capillaries maintains capillary density at a level commensurate with the degree of myocardial hypertrophy. This does not imply a lack of effect of exercise on the formation of new capillaries, since in pathological forms of hypertrophy caused by hypertension or aortic stenosis capillary rarefaction often occurs (76, 154). 2. Adaptations of neurohumoral control Alterations in neurohumoral control of the coronary vasculature can result from altered central autonomic activity, changes in the number or affinity of receptors, or changes in postreceptor events. Several studies reported 88 • JULY 2008 • www.prv.org 1044 A D DIRK J. DUNCKER AND ROBERT J. BACHE C B E FIG. 26. Effects of exercise training in swine on DNA labeling in capillaries (A), sprouting of new capillaries (B), percent labeling of sprouts (C), capillary diameter (D), capillary density (E), and coronary transport reserve (CTR; F). Data are means ⫾ SE. *P ⬍ 0.05 vs. 0 wk time point (sedentary swine). See text for further explanation. [Data from White et al. (600).] F decreased circulating levels of catecholamines in exercise-trained humans or animals. These differences are most pronounced between comparable absolute levels of submaximal exercise before and after training, suggesting that sympathetic activity is decreased following training (59, 468, 509). Information concerning adaptations at the adrenergic receptor level is sparse and equivocal. Current evidence is controversial with reports indicating that myocardial -adrenergic receptor density and sensitivity is unchanged (240, 480, 606), or slightly decreased (38). Similarly, ␣-adrenergic receptor density has been reported to be either decreased (606) or increased (177) in rat myocardium following exercise training. An explanation for these discrepant findings is not readily found. Physical conditioning increases resting parasympathetic tone to the heart, and this is thought to arise from increased vagal nerve activity rather than changes at the muscarinic receptor level, since myocardial receptor density and sensitivity appear to be slightly decreased (63, 606) or unchanged (38, 177). It should be noted that all of these studies pertain to bulk left ventricular myocardium, containing a mixture of cardiomyocytes, fibroblasts, vascular cells, and nerve endings. Studies of the effects of exercise training on adrenergic and muscarinic receptor density and sensitivity in the coronary vasculature are lacking. A) ␣-ADRENERGIC CONTROL OF CORONARY RESISTANCE VESSEL TONE. Stone and co-workers (130, 235, 365) examined alterations in neurohumoral control in dogs subjected to daily treadmill exercise for 4 –5 wk. With trained dogs and untrained dogs exercising at submaximal exercise levels, Physiol Rev • VOL the nonselective ␣-adrenergic receptor antagonist phentolamine produced an increase in diastolic coronary blood flow. The increase in diastolic flow was significantly less in exercise-trained compared with untrained dogs. (365), suggesting that ␣-adrenergic vasconstrictor influence was lower in trained animals. In subsequent studies from the same laboratory, phentolamine produced significantly greater (235) or smaller (130) increases in mean coronary blood flow in partially trained dogs during submaximal exercise compared with sedentary animals. An explanation for these divergent results is not readily found, but in the latter study phentolamine produced identical increases in mean coronary flow in trained and sedentary dogs during submaximal exercise in the presence of 2-adrenoceptor blockade (130). In open-chest dogs, ␣1-adrenoceptor blockade caused a slightly greater increment of mean coronary blood flow in exercisetrained than in sedentary animals (339). Taken together, the studies suggest that exercise training maintains or slightly increases ␣-adrenergic tone in coronary resistance vessels during submaximal exercise. The finding of maintained or increased ␣-adrenergic tone during exercise despite lower circulating levels of catecholamines implies increased ␣-adrenergic receptor responsiveness. There is evidence in both peripheral (357, 376) and coronary vascular beds (129, 235) that resistance vessels undergo greater constriction in response to ␣1adrenergic stimulation after exercise training. Thus the decreases in coronary blood flow in awake dogs produced by intracoronary injections of the ␣1-adrenergic agonist phenylephrine or the nonselective agonist norepinephrine 88 • JULY 2008 • www.prv.org CORONARY BLOOD FLOW are enhanced following exercise training (129, 235). When dogs were exercised after left stellate ganglionectomy, the training-induced increase of ␣-adrenergic responsiveness was abolished, indicating the importance of intact sympathetic innervation for the coronary vascular adaptations to physical conditioning (129). Maintained or slightly increased ␣-adrenergic tone in coronary resistance vessels despite lower circulating catecholamines acts to limit “luxury perfusion” of the myocardium and to optimize capillary diffusion (see sect. IIG4B). This is supported by the observation that myocardial oxygen extraction is enhanced following exercise training (541, 588). B) -ADRENERGIC CONTROL OF CORONARY RESISTANCE VESSEL TONE. Interpretation of studies examining the effects of exercise training on -adrenergic tone in coronary resistance vessels is complicated by effects of -adrenergic blockade on the myocardium, which can mask the direct vascular effect of -adrenergic agents. Several investigators have observed similar coronary flow reductions in response to nonselective (235, 367), 1-selective (235, 367), or 2-selective (130) adrenoceptor blockade in exercise-trained dogs during submaximal exercise compared with sedentary dogs. 2-Adrenergic receptor responsiveness of coronary resistance vessels has been reported to be enhanced following exercise training (129, 242). Thus it appears that a decrease in sympathetic neuronal input during submaximal exercise is balanced by increased responsiveness of vascular -adrenergic receptors so that vascular 2-adrenergic activity is maintained. C) PARASYMPATHETIC CONTROL OF CORONARY RESISTANCE VESSEL TONE. Although vagal tone is increased by exercise training (197, 209), there is no evidence for altered parasympathetic control of coronary resistance vessel tone after exercise training. Thus the vasoconstrictor responses of isolated porcine arterioles 110 ⫾ 5 m in diameter to acetylcholine were similar in sedentary and exercise-trained swine (350). Furthermore, muscarinic receptor blockade had no effect on coronary blood flow in exercising dogs before or after exercise training (235), indicating that exercise training did not induce a parasympathetic influence in coronary resistance vessels during submaximal exercise with heart rates of 190 – 210 beats/min. D) SUMMARY AND INTEGRATION. The available data indicate that exercise training maintains or slightly increases ␣-adrenergic coronary tone and does not alter -adrenergic tone in coronary resistance vessels during submaximal exercise. Maintenance of adrenergic tone in the presence of lower circulating catecholamine levels appears to be due to increased receptor responsiveness to adrenergic stimulation. Finally, there is no evidence for altered parasympathetic control of coronary resistance vessel tone after exercise training. Physiol Rev • VOL 1045 3. Local coronary vascular control of resistance vessels A) METABOLIC CONTROL. Exercise training has generally been reported to result in slightly decreased coronary blood flow rates per gram of myocardium at rest and during submaximal exercise (258, 588). However, at similar levels of cardiac work, coronary blood flow is not altered by exercise training (235, 258, 365, 541, 588), suggesting minimal effect of exercise training on the coupling between myocardial metabolism and coronary blood flow. Von Restorff et al. (588) and Stone (541) reported a slight increase in myocardial oxygen extraction in exercise-trained dogs that was not sufficient to result in a measurable decrease in coronary blood flow at any submaximal heart rate. The slightly increased myocardial oxygen extraction during treadmill exercise likely reflects improved capillary blood flow distribution (see sect. IIG4B), but may be facilitated by increased myogenic tone (see sect. IIG3B) and/or ␣-adrenergic tone (see sect. IIG2A) in the coronary resistance vessels. The mechanisms involved in metabolic regulation of coronary blood flow likely include metabolic mediators released by the myocardium that act on K⫹ channels in the vascular smooth muscle. Although adenosine does not play an essential role in regulation of coronary blood flow under conditions of normal arterial inflow (28), exercise training was reported to increase resistance vessel sensitivity or maximal response to adenosine in dogs in vivo (129, 339, 353), and miniature swine in vivo (351), but not in swine resistance vessels in vitro (414). The reason for this difference is unclear. Heaps et al. (252) reported that exercise training had no effect on Kv or KCa channel function in arterioles from remote normally perfused myocardium in hearts with a chronic coronary artery occlusion. Conversely, Kv or KCa channel activity is increased in large-conductance arteries of exercise-trained swine and may act to facilitate metabolic vasodilation (69). The effects of exercise training on arteriolar K channel function in the normal heart remain to be determined. B) MYOGENIC CONTROL. The myogenic mechanism produces an increase in vasoconstrictor tone in response to increased stretch of the vascular smooth muscle. This response has been implicated in autoregulation and reactive hyperemia. Muller et al. (414) studied the effect of exercise training on the myogenic response of small coronary arteries of swine (75–150 m in diameter) in vitro. Active changes in vessel diameter measured in response to 10-mmHg increments of distending pressure were similar in small arteries from exercise-trained and sedentary swine for intraluminal pressures below 40 mmHg. However, for pressures above 40 mmHg, the myogenic response was significantly greater in vessels from exercisetrained than sedentary swine. The enhanced myogenic tone was shown to be due to calcium-dependent PKC 88 • JULY 2008 • www.prv.org 1046 DIRK J. DUNCKER AND ROBERT J. BACHE signaling in the vascular smooth muscle cells (324), which enhances voltage-gated calcium currents through L-type calcium channels in large arterioles (68). This increase in basal myogenic tone in arterioles from exercise-trained swine appears to be specific to stretch-mediated contractions. Thus neither the receptor-mediated vasoconstriction by acetylcholine and endothelin, nor the vasoconstriction in response to direct voltage-gated calcium channel activation by K⫹ and the L-type calcium channel agonist BAY K 8644 were altered by exercise training (350). The molecular basis for the exercise-induced alterations in intracellular calcium control in coronary arteriolar smooth muscle cells remains to be fully elucidated. On the basis of observations in epicardial arteries from exercise-trained animals, it could be speculated that the adaptation involves increased activity of voltage-gated and calcium-activated sarcolemmal K⫹ channels or adaptations at the level of the sarcoplasmic reticulum (70, 251, 340). However, in view of nonuniform effects of exercise training on coronary vascular smooth muscle throughout the coronary arterial tree, it is clear that these mechanisms that are involved in epicardial arteries await confirmation by observations in coronary arterioles. C) ENDOTHELIAL CONTROL. Bove and Dewey (66) observed that 8 wk of exercise training enhanced the increases in myocardial blood flow produced by serotonin in closed-chest anesthetized dogs. Since coronary resistance vessel dilation by serotonin is mediated through endothelial 5-HT receptors, the findings suggest that exercise training augmented endothelium-dependent resistance vessel dilation. Similarly, Muller et al. (414) reported that exercise training augmented vasorelaxation in response to the endothelium-dependent vasodilator bradykinin in isolated coronary arterioles (64 –157 m in diameter) from swine. Indomethacin decreased the vasodilator responses in both groups but did not alter the difference between the two groups. In contrast, NG-monomethyl-L-arginine (L-NMMA) inhibited the bradykinin-induced vasodilation to a greater extent in the exercisetrained group and eliminated the difference between the two groups, suggesting that exercise training enhances bradykinin-induced vasodilation through increased NO production (414). The observation that cytosolic copper/ zinc superoxide dismutase (SOD-1) was upregulated suggests that the increased endothelium-dependent vasodilator responses were, at least in part, the result of decreased quenching of NO by superoxide (491). However, the vasodilator response to sodium nitroprusside was not different between sedentary and exercise-trained swine, suggesting that exercise training also caused an increase in NO production. In support of this, Laughlin et al. (352) demonstrated increases in endothelial NO synthase content in the coronary arterioles of exercise-trained swine. D) SUMMARY AND INTEGRATION. The available data indicate that exercise training alters local control of coronary resisPhysiol Rev • VOL tance vessels (Fig. 25). Arterioles exhibit increased myogenic tone, which appears specific for stretch-induced contractions, as vasoconstrictor responses to various other agonists are not altered. The molecular basis for the increased myogenic tone is likely the result of a calcium-dependent PKC signaling-mediated alteration in voltage-gated calcium channel activity in response to stretch. It is presently unclear whether metabolic control mechanisms, including adenosine and K⫹ channel activity, are altered in coronary resistance vessels following exercise training. However, exercise training augments endothelium-dependent vasodilation throughout the coronary microcirculation. This enhanced responsiveness appears to be principally the result of an increased expression of NO synthase. 4. Integrated coronary vascular adaptations To document a beneficial effect of exercise training, it is necessary to show that the structural or functional adaptations of the different vessel segments have the potential to improve myocardial oxygen supply. Increases in maximal blood flow per gram of myocardium or capillary diffusion capacity are the two main determinants of oxygen supply in the normal heart. A) MAXIMAL CORONARY BLOOD FLOW. There is controversy as to whether maximal coronary blood flow is increased following physical conditioning, with investigators reporting either no change (40, 67, 77, 93, 110, 364, 506, 541, 619) or an increase (48, 86, 129, 344, 351, 366, 451, 535) in blood flow capacity. Several factors may contribute to the differing results, including differences in species, sex, and age of the experimental animals, as well as the type, intensity, and duration of the exercise-training protocol. However, the most important factor appears to be the method used to assess blood flow capacity. Thus studies in which increments in cardiac work load were used to increase blood flow have found no change (40, 67, 541) or increased blood flow capacity (366, 451). Studies in young rat hearts using hypoxia to dilate the coronary vasculature have also yielded divergent results, with increased blood flow in isolated buffer-perfused hearts from exercise-trained animals (48), but similar (619) or increased (535) flow rates in blood-perfused in situ hearts from exercise-trained rats. Laughlin et al. (344) reported an increase in peak reactive hyperemia flow following a 10-s coronary artery occlusion in exercise-trained dogs, whereas Stone (541) found no effect. Vasodilator stimuli like increased cardiac work, hypoxia, or a 10-s occlusion may not elicit maximal coronary vasodilation, nor is it certain that maximal coronary vasodilation was achieved when adenosine was infused intravenously (77, 93, 364). In contrast, when maximal vasodilation was achieved with intracoronary administration of adenosine, maximum coronary blood flow per gram of myocardium was generally increased following exercise training in swine 88 • JULY 2008 • www.prv.org CORONARY BLOOD FLOW (351, 600), dogs (339, 353), and rats (86) (Table 3). In only two studies in which maximal coronary vasodilation was documented and systemic hemodynamic variables were controlled (110, 506) was maximal blood flow not increased by exercise training. In the study of Scheel et al. (506), the size of the perfusion beds was not assessed, and coronary flow was expressed as milliliters per minute so that interanimal variability might have obscured a difference in flow between the trained and sedentary dogs. Conversely, DiCarlo (129) reported that maximal coronary flow expressed as milliliters per minute was enhanced by exercise training in dogs. The negative results obtained by Cohen (110) could have been influenced by differences between breeds (trained greyhounds versus sedentary mongrel dogs). In addition, four of the five studies that reported an increase in maximal blood flow rates documented a training effect (86, 129, 339, 351, 353), whereas in neither of the two negative studies was a training effect reported (110, 506) (Table 3). In summary, the weight of evidence suggests that physical conditioning increases maximal coronary blood flow per gram of myocardium when the exercise training program is of sufficient intensity. B) CAPILLARY DIFFUSION CAPACITY. Capillary exchange capacity is determined by capillary permeability and total capillary surface area. Although exercise training does not increase the capillary numerical density, an increase in the permeability-surface area product (PSA) could still result from optimization of the distribution of blood flow so that all capillaries are perfused close to their exchange capacity. This would then increase the effective capillary surface area without an increase in anatomical surface area. PSA can be determined using indicator dilution techniques that incorporate a diffusible test substance and an intravascular reference substance. Laughlin and associates have shown an increase in PSA in exercise-trained dogs (339, 343, 353) and miniature swine (351). When the PSA and morphometric measurements of capillarization were examined in the same hearts, exercise was found to increase PSA with no change in capillary numerical density (353). This suggests that the increase in PSA resulted from optimization of the distribution of blood flow, thereby increasing the effective surface area. In the maximally vasodilated bed, the PSA is a function of the coronary flow rate, possibly because the latter is associated with recruitment of more capillaries and hence microvascular exchange area (353). Although a higher PSA could be due to the higher maximal flow rates in trained animals when hearts are perfused at comparable coronary artery pressures, this is unlikely because a higher PSA was also observed when exercise-trained and sedentary animals were compared at similar flow rates by lowering coronary pressure in the trained animals (349). In summary, exercise training alters the distribution of coronary vascular resistance so that more capillaries are recruited, resulting in an increase in the PSA without a change in capillary numerical density. Physiol Rev • VOL 1047 5. Extravascular determinants of coronary blood flow Alterations of systemic hemodynamic variables resulting from exercise training affect not only myocardial oxygen demands but can also modulate extravascular compressive forces, which can influence coronary flow. For example, exercise training results in a lower heart rate at rest and during submaximal levels of exercise (18, 509). This relative bradycardia not only decreases myocardial oxygen demand (258) but, by reducing the time spent in systole, decreases the net impedance to blood flow produced by systolic compression of the intramural coronary vessels (151). Exercise training slightly improves indices of global left ventricular systolic and diastolic function, mainly because of altered ventricular dimensions and loading conditions (118), as intrinsic myocardial contractility is minimally affected (87, 432). Improvement of systolic and diastolic function has opposing effects on impedance of coronary blood flow and will tend to balance each other (151) with a minimal net effect on impedance to blood flow. Dynamic exercise training also increases left ventricular end-diastolic internal dimensions and wall thickness so that their ratio is not significantly altered (382, 601). Since left ventricular diastolic and systolic pressures are minimally affected by endurance exercise training in normal animals (40, 120, 601), it is likely that left ventricular diastolic and systolic wall stress are not altered by dynamic exercise training. The available data indicate that physical conditioning decreases extravascular compressive forces at rest and at comparable absolute levels of exercise, mainly because of a decrease in heart rate. III. CORONARY BLOOD FLOW DISTAL TO A CORONARY ARTERY OBSTRUCTION DURING EXERCISE A. Regulation of Coronary Blood Flow Distal to a Coronary Artery Stenosis 1. Effects of a coronary stenosis on myocardial blood flow during exercise Autoregulation is defined as the capacity to maintain constant blood flow in the face of a change in perfusion pressure (with constant metabolic needs). The vasomotor mechanisms that underlie autoregulation appear to include those involved in metabolic regulation but likely also involve the myogenic response. Normally, large epicardial “conductance” coronary arteries contribute little to total coronary resistance. However, autoregulation becomes clinically important when atherosclerosis causes narrowing of a large coronary artery that obliterates over 70% of the luminal cross-sectional area (50% diameter reduction). Such a stenosis results in a significant in- 88 • JULY 2008 • www.prv.org 3. Swine M, F White et al. (600) Physiol Rev • VOL 88 • JULY 2008 • www.prv.org Dog M, F Dog M, F Laughlin and Tomanek (353) DiCarlo et al. (129) A A A A A A A Y Age Run Run Run Run Run Run Run Swim Type 6.4–12.8 km/h, 0%; 75 min/day, 5 days/wk, 16–22 wk 70–80% of max HR, 70 min/day, 5 days/wk, 16 wk 5.8 km/h, 25% grade; 45 min/day, 7 days/wk, 6 wk 9.6 km/h, 10–20%; 75 min/day, 5 days/wk, 12–20 wk Greyhound dogs compared with mongrel dogs 9.6 km/h, 10–20%; 75 min/day, 5 days/wk, 12-20 wk 6.4–9.6 km/h, 20%; 5 days/wk, 4–5 wk 150 min/day, 5 days/wk, 8–10 wk Program Isolated bloodperfused heart LVW 7, LVW/BW 7 No* Awake Open-chest extracorporeal perfusion HW 7, HW/BW 7 SMVO2 1 ? Awake LVW 100% 1, LVW/BW 75% 1 Open-chest extracorporeal perfusion ? SMVO2 1 ? HW 18% 1, HW/BW 7 Open-chest extracorporeal perfusion LVW/BW 24% 1 VO2max 1, HREX 2 Open-chest extracorporeal perfusion Isolated bufferperfused heart Experimental Conditions SMVO2 1, HREX 2 ? 1; 23% 1, 1 35% 1 Cardiac Hypertrophy Male: HW 7, HW/BW 10% Female: HW HW/BW 24% HW ?, HW/BW Efficacy Ado-ic Ado-ic Ado-ic Ado-ic Ado-ic Ado-ic Ado-ic Ado-ic Method of Vasodilation Yes Yes Yes Yes Yes Yes Yes Yes Maximal Vasodilation CBF CBF/g CBF/g CBF/g CBF CBF/g CBF/g CBF/g Measured Variable 1 1 7 1 7 1 1 1 Maximum Blood Flow HR, heart rate; Ado, adenosine; ic, intracoronary; CBF/g, mean coronary blood flow normalized per gram of myocardium; ? , not reported.*Not reported in that study but based on previous observations from the same laboratory (Stone, 1980; Liang et al., 1984). Dog M, F Dog M, F Cohen (110) Laughlin (339) Dog M Swine F Laughlin et al. (351) Scheel et al. (506) Rat M, F Buttrick et al. (86) Species, Sex Training Exercise training and coronary blood flow capacity Investigators TABLE 1048 DIRK J. DUNCKER AND ROBERT J. BACHE CORONARY BLOOD FLOW crease in proximal resistance and causes a decrease in distal coronary perfusion pressure. In this situation, autoregulation can preserve basal coronary blood flow, but maximum coronary blood flow (as commonly measured using intracoronary administration of adenosine) is reduced (Fig. 27). Consequently, coronary flow reserve (the ratio of maximum to basal coronary flow) is attenuated. When a stenosis becomes sufficiently severe to reduce the poststenotic pressure below 40 mmHg during resting conditions, endogenous vasodilator reserve becomes exhausted and basal flow decreases, resulting in myocardial hypoperfusion (29, 92, 138). Autoregulatory reserve is not homogeneously distributed across the left ventricular wall. Thus Ball and Bache (36) showed that when dogs were exercised on a treadmill, progressive obstruction of a coronary artery with a hydraulic occluder resulted in a decrease in flow first in the subendocardial myocardium while flow to the subepicardial layers was reduced only when more severe levels of stenosis were applied (Fig. 28). This preferential decrease in subendocardial flow is the result of higher extravascular compressive forces in the subendocardium (274, 599), causing the lower limb of the autoregulation curve to be shifted to the right compared with that in the subepicardial layers (Fig. 27). This also explains the observation that intracoronary infusions of adenosine or dipyridamole (which have no effect on systemic hemodynamics), in the presence of a critical stenosis that has exhausted subendocardial flow reserve can result in “coronary epicardial steal” (29, 138). In this situation, adenosine will increase blood flow in subepicardium, but the resulting increase in coronary arterial inflow will increase the pressure gradient across the stenosis, thereby decreasing poststenotic perfusion pressure and hence subendocardial blood flow (point c to c⬘ in Fig. 27, top). When myocardial oxygen consumption increases during exercise, the autoregulatory plateau will be shifted upward, transposing the break point to the right (Fig. 27, bottom). During exercise, the increase in heart rate, which results in an increase in average myocardial tissue pressure (as relatively more time is spent in systole) shifts the pressure-flow relation to the right, particularly in the subendocardial layers. This explains why a subcritical coronary stenosis (that has no effect on resting coronary blood flow) can result in selective subendocardial hypoperfusion during exercise (point b to b⬘⬘ in bottom panel of Fig. 27). 2. Coronary vasomotor tone regulation distal to a stenosis: importance of functional anatomy of the coronary microcirculation Ischemia has generally been assumed to cause maximal vasodilation of the coronary microvasculature and to render these vessels unresponsive to vasoconstrictor Physiol Rev • VOL 1049 FIG. 27. Coronary pressure-flow relation in the subepicardial (Epi) and subendocardial (Endo) layers during autoregulation (auto) or during maximal coronary vasodilation with adenosine (max), at rest (top panel) and during exercise (bottom panel). Top panel shows myocardial blood flow under conditions of normal arterial inflow (a and a⬘), modest stenosis (b and b⬘), and severe stenosis (c and c⬘). Note that with increasing stenosis severity coronary perfusion distal to the stenosis decreases, thereby reducing the maximal achievable myocardial blood flow. In the case of the severe stenosis, infusion of adenosine will result in an increase in subepicardial flow that increases the pressure drop across the stenosis, thereby further decreasing poststenotic coronary pressure, thereby causing a decrease in subendocardial blood flow. Bottom panel shows myocardial blood flow under conditions of normal arterial inflow (a and a⬙) and modest stenosis (b and b⬙). Note that exercise causes an upward shift of the autoregulatory plateau. During exercise in the presence of a stenosis, the rightward shift of particularly the subendocardial pressure-maximal flow relation causes subendocardial flow to decrease while subepicardial blood flow shows a normal increase. See text for further explanation. 88 • JULY 2008 • www.prv.org 1050 DIRK J. DUNCKER AND ROBERT J. BACHE FIG. 28. Effect of a coronary artery stenosis on the transmural distribution of myocardial blood flow during treadmill exercise in dogs. Blood flows are shown at rest and during exercise with normal arterial inflow and in the presence of a modest (stenosis 1) and severe (stenosis 2) degree of stenosis. Note the progressive hypoperfusion from the outer- to the innermost layer. Data are means ⫾ SE. *P ⬍ 0.05 vs. corresponding exercise control. See text for further explanation. [Data from Ball and Bache (36).] stimuli. However, even during ischemia, the coronary resistance vessels retain some degree of vasomotor tone and can respond to vasoconstrictor stimuli. This finding has required reassessment of the location and function of the principal sites for resistance to blood flow in the coronary circulation. The coronary arterial vasculature has traditionally been divided into two discrete segments: arteries that offer little resistance to blood flow and do not participate in regulation of perfusion, and microvessels, which represent the major locus of resistance to flow. The resistance vessels have generally been treated as a homogeneous array of vessels, which act principally in response to local myocardial needs, but can also respond weakly to systemic vasomotor influences. The development of methods to directly visualize the coronary microvessels has demonstrated that this concept of a functionally homogeneous coronary microvascular circulation is an oversimplification (Figs. 29 and 30). Direct measurements of microvascular pressures in beating hearts have demonstrated that during basal conditions, up to 40% of total coronary resistance resides in small arteries between 100 and 400 m in diameter, and that during vasodilation these vessels contribute an even greater fraction of total coronary resistance (105, 380, 556). The importance of this finding is that although these small arteries contribute a substantial fraction of total coronary resistance and are capable of active vasomotion, they do not appear to be under local metabolic control. The finding that a substantial fraction of resistance resides at the Physiol Rev • VOL level of the small arteries may in part explain the finding that myocardial ischemia does not predictably cause maximal vasodilation of the coronary resistance vessels. Persistence of vasomotor tone in the coronary resistance vessels during myocardial ischemia can be ascribed (at least in part) to vasoconstrictor tone in these small arteries, which are not under metabolic control. In addition, there is evidence that even in coronary arterioles which are under metabolic control (⬍100 micron in diameter), vasoconstrictor influences can compete with metabolic vasodilator activity (103, 380, 556). A) LARGE EPICARDIAL ARTERIES. In the normal heart, the large arteries (⬎400 m in diameter) are truly conduit vessels that contribute ⬍5% of total coronary resistance (101, 105). When atherosclerosis or vasospasm decreases coronary artery cross-sectional area by ⬎70%, a substantial fraction of total resistance can reside in these large vessels, resulting in a pressure drop across the stenotic segment. In this situation, studies of the regulation of vasomotor tone of the coronary bed distal to a stenosis must take into account alterations in stenosis severity and changes in the pressure drop across the stenosis that result from changes in flow. For example, pharmacological agents that dilate large arteries can decrease the severity of a compliant stenosis, thereby improving blood flow. To eliminate effects of changes in stenosis severity, some investigators have employed experimental models in which a stenosis maintains a constant limited rate of arterial inflow. With a constant flow model, vasodilation of the resistance vessels causes a decrease in coronary perfusion pressure as the stenosis prevents flow from increasing in response to distal vasodilation. Since arterial inflow is constant, coronary pressure distal to the stenosis should reflect changes in vasomotor tone of the resistance vessels. A problem with the constant flow model is that at the low perfusion pressures distal to a coronary stenosis, blood flow is influenced not only by active vasomotion of the resistance vessels, but also by interaction between the intravascular distending pressure and the extravascular forces which act to collapse the thin-walled microvessels. If distal coronary pressure falls as the result of microvascular vasodilation while extravascular forces remain constant, then the extravascular forces will exert an increasing influence on blood flow. The influence of extravascular forces becomes progressively more important as distal coronary pressure decreases and is greater in diseased hearts in which left ventricular diastolic pressure is elevated or the rate of relaxation slowed. Because extravascular forces are highest in the subendocardium, a decrease in coronary pressure distal to a stenosis can cause passive redistribution of blood flow away from the subendocardium. The term passive redistribution is used since the redistribution of flow away from the subendocardium with decreasing perfusion pressure can occur independently of active vaso- 88 • JULY 2008 • www.prv.org CORONARY BLOOD FLOW FIG. 29. Schematic overview of the coronary microcirculation and the relative sensitivities of the various microvascular segments to pressure, flow, and metabolism. [Adapted from Tiefenbacher and Chilian (556).] motion. To avoid passive alterations in flow secondary to changes in the interaction between extravascular forces and intravascular perfusion pressure (especially at the low coronary pressures distal to a flow-limiting stenosis), experimental models have been used in which a stenosis maintains a constant distal coronary pressure so that only changes in vasomotor tone of the microvasculature result in changes in blood flow (25, 33, 92, 137, 140, 141, 144, 287, 289, 354 –356, 529). B) CORONARY COLLATERAL VESSELS. Collateral vessels that are sufficiently developed to provide an alternate parallel blood supply can complicate interpretation of measurements of blood flow in a myocardial region perfused by a stenotic coronary artery. Thus vasomotion of coronary collaterals can alter both the inflow of arterial blood and the poststenotic perfusion pressure in the terminal vascular bed (192, 317). However, if distal coronary pressure is maintained constant, changes in myocardial tissue blood flow reflect changes at the level of the intramural resistance vessels, irrespective of the source of blood flow (i.e., antegrade or via collateral vessels). Physiol Rev • VOL 1051 C) SMALL ARTERIES. Active vasomotor tone in arterial segments that are not under metabolic control but which contribute substantially to total coronary resistance have the potential to alter myocardial blood flow. Chilian and co-workers (101, 105) showed that under normal conditions up to 25% of total coronary resistance can reside in arterial vessels larger than 170 m in diameter, with as much as 40% in vessels larger than 100 m. Metabolic vasodilation and autoregulation occur predominantly in arterioles smaller than 100 m; under conditions of unimpeded coronary inflow, vasoconstriction of the small arteries can be compensated for by vasodilation of the arterioles (104, 299, 300). However, when hypoperfusion has already caused metabolic vasodilation of the arterioles, the ability to compensate for vasoconstriction of the small arteries is lost. Furthermore, in the presence of intense arteriolar vasodilation, an even greater fraction of total coronary resistance resides in the arteries; in this situation, vasoconstriction of small arteries can aggravate hypoperfusion. D) INTRAMURAL PENETRATING ARTERIES. The penetrating arteries represent a special group of small arteries that traverse the left ventricular wall to deliver blood from the epicardial arteries to the subendocardial microvasculature. The penetrating arteries range from 100 to 500 m in diameter, with most around 200 m (64, 169). At an aortic pressure of 100 mmHg, pressure in coronary arterioles 100 m in diameter was 80 mmHg in the subepicardium but only 60 mmHg in the subendocardium, indicating that a substantial pressure drop occurs across the penetrating arteries (99). In the presence of a coronary stenosis, which has caused the arterioles smaller than 100 m to undergo metabolic vasodilation, the penetrating arteries can pose an important additional source of resistance to subendocardial perfusion. In this situation, variations in tone in these vessels could directly alter blood flow to the subendocardium. E) ARTERIOLES. In the normal heart, a major fraction of coronary resistance resides in arterial segments ⬍100 m in diameter (arterioles). These arterioles are responsive to changing myocardial metabolic needs and are the site for metabolic vasodilation (306), autoregulation (307), and ischemic vasodilation (103, 307). Although the arterioles are the site of metabolic vasoregulation, there is evidence that vasomotor tone can persist in these vessels even in the presence of myocardial ischemia (103). 3. Mediators of coronary vasodilation distal to a stenosis A) ADENOSINE. In contrast to the normal heart where adenosine does not participate in regulation of coronary flow under physiological conditions, adenosine does contribute to coronary vasodilation when there is an insufficient supply of oxygen. During ischemia or hypoxia, ATP 88 • JULY 2008 • www.prv.org 1052 DIRK J. DUNCKER AND ROBERT J. BACHE FIG. 30. Schematic overview of various vasodilator (yellow text boxes) and vasoconstrictor (blue text boxes) influences in different (micro)vascular segments (epicardial artery, small intramural arteries class A and B, and arterioles) of the coronary arterial bed of the LV wall. TxA2, thromboxane A2 (receptor); 5HT, serotonin or 5-hydroxytryptamine (receptor); B2, bradykinin receptor subtype 2; ANG II, angiotensin II; ET, endothelin; ß1 and ß2, ß1- and ß2-adrenergic receptor; ␣1 and ␣2, ␣1- and ␣2-adrenergic receptors; NO, nitric oxide; PGI2, prostacyclin (receptor); EDHF, endothelium-derived hyperpolarizing factor; KATP, ATP-sensitive K⫹ channel; KCa, calcium-sensitive K⫹ channel; KV, voltage-sensitive K⫹ channel. breakdown leads to increased adenosine production and release from cardiac myocytes and possibly endothelial cells (39, 180, 510, 534). In this situation, adenosine does exert a significant vasodilator effect, since the increase in coronary flow in response to systemic or local myocardial hypoxia is attenuated following intracoronary administration of adenosine deaminase (205, 359, 400, 401, 595). Similarly, adenosine plays a role in ischemic coronary vasodilation. Thus intracoronary adenosine deaminase (28, 494), or adenosine receptor blockade with intravenous 8-phenyltheophylline (28) or aminophylline (204, 207, 515), caused a decrease in the total volume of excess flow during reactive hyperemia following a brief total coronary artery occlusion in open-chest and awake dogs. Laxson et al. (356) studied the contribution of adenosine to the resistance vessel dilation that occurs when a coronary artery stenosis results in myocardial hypoperfusion during exercise. During treadmill exercise in dogs, a hydraulic occluder around the proximal left circumflex coronary artery was partially inflated to produce a constant distal coronary pressure of 40 – 42 mmHg. Mean blood flow in the region perfused by the stenotic coronary artery was decreased to 1.25 ⫾ 0.20 compared with 2.63 ⫾ 0.25 ml䡠min⫺1 䡠g myocardium⫺1 in the normally perfused control region. Hypoperfusion was most severe in the subendocardium with a subendocardial/subepicardial (endo/epi) flow ratio of 0.37 ⫾ 0.07 versus 1.21 ⫾ 0.06 in the control region. To determine whether adenosine contributed to vasodilation of the resistance vessels in the ischemic region, exercise was repeated during blockade of adenosine receptors with 8-phenyltheophylline combined with intracoronary adenosine deaminase to augment Physiol Rev • VOL adenosine catabolism (390). While the stenosis maintained distal coronary pressure constant at 41 ⫾ 1 mmHg, adenosine blockade caused a further decrease in mean blood flow in the region perfused by the stenotic coronary artery to 0.92 ⫾ 0.13 ml䡠min⫺1 䡠g⫺1 (Fig. 31). Although there is evidence that adenosine production is positively correlated with the degree of hypoperfusion (128), the effect of adenosine was not greater in the subendocardium even though hypoperfusion was most severe in that region. The decrease in flow produced by adenosine blockade was associated with further deterioration of FIG. 31. Effect of adenosine receptor blockade with 8-phenyltheophylline (8PT, 5 mg/kg iv) and increased adenosine catabolism with adenosine deaminase (ADA; top panel) and effect of NO synthase inhibition with N-nitro-L-arginine (L-NA, 20 mg/kg iv; bottom panel) on blood flow distal to a coronary artery stenosis. Epi, subepicardium; OM, outer mid; IM, inner mid; Endo, subendocardium. Data are means ⫾ SE. *P ⬍ 0.05 vs. corresponding control. See text for further explanation. [Data from Laxson et al. (356) and Duncker and Bache (137).] 88 • JULY 2008 • www.prv.org CORONARY BLOOD FLOW systolic segment shortening in the hypoperfused region, indicating worsening of ischemia (356). These findings indicate that adenosine does contribute to vasodilation of the coronary resistance vessels when exercise in the presence of a flow-limiting stenosis results in myocardial ischemia. In summary, adenosine does not play a role in metabolic regulation of myocardial blood flow in the normal heart during physiological conditions. However, adenosine does contribute to coronary vasodilation under conditions of hypoxia or impaired arterial inflow, principally at the level of the arterioles ⬍100 m in diameter (300, 306). B) NO. NO production increases during hypoxia, suggesting that NO-dependent vasodilator mechanisms could have increased importance during ischemia (81, 447, 455). In accordance with this concept, Smith and Canty (529) observed that inhibition of NO synthesis with L-NAME had no effect on coronary flow at normal coronary pressures, but when coronary artery pressure was reduced below the autoregulatory range, coronary flow rates were lower after L-NAME than during control conditions. As a result, the lower limb of coronary pressure-flow relation during autoregulation was shifted toward higher coronary pressures after blockade of NO synthesis. We assessed the contribution of NO to the vasodilation of coronary resistance vessels that occurs during exercise in the presence of a flow-limiting coronary stenosis (Fig. 31). Dogs underwent moderate treadmill exercise while partial inflation of a hydraulic occluder created a stenosis that maintained distal coronary pressure at 55 ⫾ 2 mmHg (137). Inhibition of NO production with L-NA did not decrease coronary flow at rest or during exercise in the normally perfused control region (137). However, in the region perfused by the stenotic coronary artery, inhibition of NO synthesis decreased mean myocardial blood flow from 1.09 ⫾ 0.13 to 0.68 ⫾ 0.11 ml䡠min⫺1 䡠g⫺1 (P ⬍ 0.05). This reduction of blood flow (137, 529) could have resulted from vasoconstriction either in the small coronary arteries that are not under metabolic control (⬎100 m in diameter) or in arterioles that are under metabolic control (⬍100 m). Studies of isolated microvessels have demonstrated that basal release of NO does occur in canine coronary arterioles (40 – 80 m) but only in the presence of flow (328, 418). In the in vivo heart, basal NO release occurs in both arterioles (⬍100 m) and in resistance arteries (⬎100 m) (298, 299, 323), and vessels of both size dilate in response to acetylcholine (323). The observation by Jones et al. (299) that in the normal heart inhibition of NO production resulted in vasoconstriction of small arteries but (compensatory) vasodilation of the arterioles suggests that constriction of the small arteries was principally responsible for aggravation of hypoperfusion distal to a coronary artery stenosis. Thus, in the normal heart, vasoconstriction resulting from inhibition of NO production is comPhysiol Rev • VOL 1053 pensated for by metabolic vasodilator factors acting at the arteriolar level. However, when a coronary stenosis has already caused metabolic vasodilation of the arterioles, the ability to compensate for inhibition of NO production is lost. In this situation, NO inhibition can aggravate hypoperfusion by causing constriction of the resistance arteries. It is unclear whether constriction of the resistance vessels produced by inhibition of NO synthesis in hypoperfused myocardium is the result of unmasking of normal levels of NO release when other vasodilator mechanisms have been exhausted, or whether it is due to an actual increase of NO production in response to myocardial hypoperfusion. Impaired tissue oxygenation has been proposed to result in increased NO release from the endothelium (455) or erythrocytes (527, 536). Oxygen tensions are lower in the walls of arterioles and even small arteries than in the central aorta (136), suggesting that conditions exist that could allow the vascular wall to serve as a tissue oxygen sensor (454). Alternatively, decreases in oxygen tension can stimulate the release of NO from red blood cells (527, 536). There is evidence that the vasoconstrictor response to ␣1- and postjunctional ␣2adrenoceptor stimulation is augmented in hypoperfused myocardium distal to a coronary artery stenosis during exercise (355, 518). Since endothelial ␣2-adrenoceptors can stimulate NO production, sympathetic nervous system activation during exercise could potentially augment NO production distal to a flow-limiting stenosis. In this situation, inhibition of NO production would aggravate hypoperfusion by leaving ␣1- and ␣2-adrenergic coronary vasoconstriction unopposed. This is supported by the finding that coronary microvessel constriction in response to norepinephrine is enhanced following the administration of L-NA (298) and that after inhibition of NO synthesis exercise in the presence of a coronary stenosis unmasked an ␣2-mediated vasoconstrictor response (287). NO-dependent vasodilator responses are impaired in patients with atherosclerosis, hyperlipidemia (187), or hypertension (368, 442). The experimental evidence demonstrating that inhibition of NO production can aggravate myocardial hypoperfusion distal to a coronary artery stenosis supports the clinical observation that endothelial dysfunction of the coronary resistance vessels can render patients more vulnerable to hypoperfusion in myocardial regions perfused by a stenotic coronary artery (497, 622). C) PROSTAGLANDINS. In early studies, inhibitors of cyclooxygenase were reported to depress coronary reactive hyperemia and hypoxic coronary vasodilation (1, 3). However, these results were likely influenced by experimental preparations in which acute surgical trauma had caused activation of the prostaglandin system (437), since subsequent studies in intact animals have generally found little effect of these agents (269, 437). In anesthetized swine in which an intraluminal hollow plug was used to produce 88 • JULY 2008 • www.prv.org 1054 DIRK J. DUNCKER AND ROBERT J. BACHE an 80% reduction in coronary artery cross-sectional area, cyclooxygenase blockade with indomethacin did not alter coronary blood flow (489), suggesting that vasodilator prostaglandins do not contribute to resistance vessel dilation distal to a flow-limiting coronary stenosis. Conversely, in patients with coronary artery disease, inhibition of cyclooxygenase resulted in coronary vasoconstriction (135, 198), suggesting that vasodilator prostaglandins assume greater importance in regulation of coronary vasomotor control in chronic ischemia. However, in those studies, it cannot be excluded that vasoconstriction occurred not only in the distal microvasculature but also in proximal stenotic segments. D) KATP CHANNELS. Opening of KATP channels contributes to coronary vasodilation during reactive hyperemia (21, 150, 153) and hypoxia (124). Studies in which coronary microvessels were directly visualized using intravital microscopy in open-chest dogs demonstrated that KATP channels become progressively more activated as coronary artery pressure is decreased. Thus Komaru et al. (322) observed that superfusion of the subepicardial vasculature with the KATP blocker glibenclamide had no effect on arteriolar diameter (⬍100 m) at coronary artery pressures of 90 –100 mmHg, but abolished the arteriolar dilation that occurred in response to progressive reductions of coronary pressure. In contrast, small coronary artery (⬎100 m) diameter decreased as perfusion pressure (distending pressure) was decreased, and this was not modified by glibenclamide. These findings indicate that KATP channels in arterioles are important mediators of the vasodilator response that accounts for coronary autoregulation. In extracorporeally perfused dog hearts, intracoronary glibenclamide (10 g䡠kg⫺1 䡠min⫺1) had no effect on coronary flow when perfusion pressures were equal to or greater than aortic pressure (⬎100 mmHg), but caused a decrease in flow when pressures were below 80 mmHg (422). We studied coronary flow responses over a range of perfusion pressures produced by progressively inflating a hydraulic occluder in dogs running on a treadmill (heart rates ⬃200 beats/min) (152). KATP channel blockade with glibenclamide produced similar decreases in blood flow both at perfusion pressures below the autoregulatory range and at normal pressures, so that the absolute decrease in flow was similar at all coronary artery pressures, but the relative flow reduction became progressively greater as coronary pressure decreased (152). Furthermore, the selective KATP channel opener pinacidil increased coronary blood flow at normal perfusion pressures but not at pressures below the autoregulatory range (152), indicating that KATP channels became progressively activated as coronary pressures decreased, so that most of the channels were open when perfusion pressures reached the lower limit of autoregulation. Thus the available evidence indicates that KATP channels located in coronary arterioles (⬍100 m) contribute imporPhysiol Rev • VOL tantly to coronary metabolic vasodilation and autoregulation. E) KCA CHANNELS. A possible role for KCa channel activity in regulation of coronary vasomotor tone distal to a coronary artery stenosis during exercise has not been investigated to date. However, in extracorporeally perfused hearts of open-chest dogs, the KCa channel antagonist charybdotoxin had no effect on coronary flow during normal arterial inflow conditions, but caused a decrease of coronary flow during myocardial hypoperfusion at a constant coronary artery perfusion pressure of 42 ⫾ 2 mmHg (429). It cannot be determined from that study whether the constriction of resistance vessels produced by KCa channel blockade was the result of unmasking of normal KCa channel activity because other vasodilator mechanisms had been exhausted in the hypoperfused region, or whether it was due to an actual increase in KCa channel activity in response to myocardial hypoperfusion. F) SUMMARY AND INTEGRATION. The increased delivery of oxygen and metabolic substrate which is essential for the cardiac response to an increased work load involves a number of parallel mechanisms that produce coronary vasodilation. In the dog, blockade of any one of these vasodilator mechanisms fails to blunt the increase in coronary blood flow in response to exercise in the normal heart, suggesting that adenosine, KATP channel opening, prostacyclin, or NO either do not contribute to exerciseinduced coronary vasodilation or that redundancy of these vasodilator mechanisms allows compensation when one mechanism is blocked. In contrast, when exercise in the presence of a coronary artery stenosis has caused all vasodilator mechanisms to become activated, blockade of any of these vasodilator mechanisms aggravates myocardial hypoperfusion. 4. Is coronary vasodilation maximal in ischemic myocardium? Systolic wall stress and hence oxygen demands are highest in the subendocardial layers of the left ventricular wall. The need for greater blood flow in the subendocardium requires a transmural gradient of vasomotor tone, with vascular resistance being lower in the inner than in the outer left ventricular wall. Since extravascular compressive forces are greatest in the subendocardium, vasodilator reserve is exhausted first in the subendocardium when perfusion pressure falls (186, 273). As a result, vasodilator reserve can still exist in the subepicardial layers of the left ventricle at a time when reduced blood flow indicates loss of vasodilator reserve in the subendocardium (203, 206). Myocardial ischemia has traditionally been viewed to produce maximal vasodilation of the coronary resistance vessels and to override any competing vasoconstrictor influences. However, a number of investigators have demonstrated that subendocardial vasodila- 88 • JULY 2008 • www.prv.org CORONARY BLOOD FLOW tor reserve can exist distal to a coronary artery stenosis that results in subendocardial hypoperfusion (19, 91, 213, 218, 441). Thus, with coronary artery pressure distal to a stenosis maintained at a fixed level, intracoronary infusion of adenosine increased subendocardial blood flow by ⬎50%, sometimes with an improvement in myocardial contractile performance (441). The presence of vasodilator reserve in ischemic myocardial regions may have been the result of increased sympathetic drive in anesthetized open-chest animals, since a study in closed-chest sedated dogs failed to observe recruitable vasodilator reserve in ischemic myocardium (92). During treadmill exercise when sympathetic activity is high, intravenous infusion of the L-type Ca2⫹ channel antagonist nifedipine increased blood flow and contractile performance of ischemic myocardium perfused by a stenotic coronary artery or by collateral vessels (265). Laxson et al. (354) exercised dogs on a treadmill while a stenosis was produced with a hydraulic occluder. While distal coronary artery pressure was maintained constant at 43 ⫾ 2 mmHg, an intracoronary infusion of adenosine increased subendocardial blood flow by 50% (Fig. 32) and improved regional systolic segment shortening. In contrast to other studies in which vasodilator reserve was found in only the subepicardium (186, 203, 206, 273), vasodilator reserve was present in both the subendocardium and the subepicardium (354). It is important to note that in this study coronary artery pressure distal to the coronary stenosis was maintained constant to prevent passive redistribution of flow due to changes in poststenotic pressure, whereas distal coronary perfusion pressure was not controlled in the earlier studies (186, 203, 206, 273). These findings demonstrate that ⫺1 ⫺1 FIG. 32. Effect of adenosine (50 g 䡠 kg 䡠 min ic) (A) and the nitrovasodilator ITF 296 (2 g 䡠 kg⫺1 䡠 min⫺1 iv) (B) on blood flow distal to a coronary artery stenosis. Epi, subepicardium; OM, outer mid; IM, inner mid; Endo, subendocardium. Data are means ⫾ SE. Note that while adenosine produces similar increases in flow to all myocardial layers, ITF increases flow selectively to the subendocardial layers. *P ⬍ 0.05 vs. corresponding control. See text for further explanation. [Data from Laxson et al. (354) and Duncker et al. (144).] Physiol Rev • VOL 1055 vasodilator reserve can exist in coronary resistance vessels within hypoperfused myocardium. In in vivo studies directly observing subepicardial microvessels in openchest dogs, coronary arterioles (⬍100 m in diameter) dilated in response to progressive decreases in perfusion pressure while small arteries ⬎100 m showed either no change (103) or a decrease (307) in vessel diameter. At a coronary pressure of 40 mmHg, which produced a significant decrease in blood flow to the subepicardium, local intra-arterial infusion of adenosine caused dilation of the arterioles but not of the small arteries (103). These findings support the concept that vasodilator reserve can persist in coronary arterioles within acutely ischemic myocardium. Whereas adenosine is a potent vasodilator of coronary arterioles ⬍100 m in diameter (103, 140, 306), it is a weak dilator of arterial vessels ⬎100 m in diameter (103, 306). In contrast, nitrovasodilators are known to preferentially dilate arteries ⬎100 m in diameter (520, 607). To assess vasodilator reserve at the level of the small coronary arteries within acutely ischemic myocardium, we investigated the effects of intravenous nitroglycerin, isosorbide dinitrate, and the NO donors ITF 296 and ITF 1129 on blood flow distal to a coronary artery stenosis during treadmill exercise in dogs (144, 289). During normal arterial inflow, these agents had no effect on coronary blood flow either at rest or during exercise, which can be explained by the small artery dilation being countered by an autoregulatory increase in arteriolar tone to maintain blood flow commensurate with the needs of the myocardium (103, 300). In contrast, in the presence of a coronary artery stenosis that maintained distal perfusion pressure constant, the nitrovasodilators (Fig. 32) increased blood flow to the hypoperfused region, an effect that was most pronounced in the deeper myocardial layers. Improvement in blood flow to hypoperfused regions by nitrovasodilators could involve several mechanisms. First, in patients with an eccentric atherosclerotic narrowing of a coronary artery, nitrovasodilators can dilate the artery at the site of the stenosis (199, 466). However, in our experimental model, this effect was prevented by using an occluder that maintained a constant distal coronary perfusion. Second, nitrates cause venodilation, which can lower left ventricular diastolic pressure, thereby reducing extravascular forces that compress the intramyocardial vasculature. However, even nitrate doses that had no effect on left ventricular end-diastolic pressure or dimensions enhanced myocardial perfusion distal to the stenosis, indicating that a decrease of extravascular compressive forces could not account for the improved perfusion (144, 287). In the normal dog heart, intravascular pressure in coronary arterioles is substantially lower in the subendocardium than in the subepicardium (99), indicating that a significant pressure drop occurs across the penetrating arteries (diameter ⬃200 m) which traverse the left ven- 88 • JULY 2008 • www.prv.org 1056 DIRK J. DUNCKER AND ROBERT J. BACHE tricular wall to deliver blood to the subendocardium. Since nitrovasodilators are known to dilate vessels larger than 100 m in diameter (520, 607), vasodilation of the penetrating arteries by nitrovasodilators would preferentially enhance flow to the innermost myocardial layers. In the presence of a flow-limiting stenosis, the arterioles ⬍100 m have already undergone metabolic vasodilation so that a disproportionate fraction of resistance resides in the small arteries ⬎100 m, which includes the penetrating arteries. In this situation, dilation of the penetrating arteries by nitrovasodilators could increase blood flow to the inner layers of the left ventricle. Although the small arteries that supply the subepicardial microvasculature will also be dilated by nitrovasodilators, these arteries travel a much shorter distance (64, 169). Hence, their contribution to subepicardial resistance is less than the contribution of the penetrating arteries to subendocardial resistance. In summary, myocardial ischemia that occurs during exercise in the presence of a flow-limiting coronary artery stenosis does not cause maximal vasodilation of the coronary resistance vessels so that substantial vasodilator reserve exists in the terminal vascular bed of the hypoperfused region. This reserve can be recruited with both small artery dilators (e.g., nitrovasodilators) and arteriolar dilators (e.g., adenosine), indicating persistent vasomotor tone throughout the coronary microcirculation. 5. Mediators of persistent vasomotor tone in ischemic myocardium A) ␣-ADRENERGIC CONTROL. ␣-Adrenergic vasoconstriction not only opposes metabolic coronary vasodilation during exercise in the normal heart, but also limits coronary vasodilation in regions of ischemic myocardium. Thus, when a coronary artery stenosis resulted in subendocardial underperfusion and impaired regional systolic segment shortening during treadmill exercise (heart rates ⬃200 beats/min), local blockade of ␣1-adrenoceptors with intracoronary prazosin significantly increased blood flow to the hypoperfused region (Fig. 33) and caused improvement of contractile performance with no change in coronary artery pressure distal to the stenosis (355). A similar increase in flow to the hypoperfused region was observed when prazosin was administered intravenously (227). Selective blockade of ␣2-adrenoceptors caused variable results with either no change (287, 355) or an increase (228, 518) in blood flow in the ischemic region. The differing results may be related to the severity of the coronary artery stenosis; ␣2-adrenoceptor activation appears to be of greater importance during more severe ischemia. In open-chest dogs, cardiac nerve stimulation caused resistance vessel constriction in myocardium perfused by a stenotic coronary artery, which appeared to be mediated by ␣2-adrenoceptors (264). Postjunctional ␣2-adrenergic Physiol Rev • VOL FIG. 33. Effect of ␣1-adrenergic receptor blockade with prazosin (A) and ␣2-adrenergic receptor blockade with idazoxan (B) on blood flow distal to a coronary artery stenosis. Epi, subepicardium; OM, outer mid; IM, inner mid; Endo, subendocardium. Data are means ⫾ SE. *P ⬍ 0.05 vs. corresponding control. See text for further explanation. [Data from Laxson et al. (355).] vasoconstriction in ischemic myocardial regions is opposed by simultaneous stimulation of endothelial ␣2-receptor-mediated release of NO. Thus, in the presence of a coronary stenosis which resulted in myocardial hypoperfusion during treadmill exercise, blockade of ␣2-adrenoceptors had no effect on blood flow in the ischemic region. However, after inhibition of NO production with L-NA, selective blockade of ␣2-adrenoceptors caused an increase in coronary flow (287). This indicates that ␣2adrenergic activation during exercise did exert a vasoconstrictor effect on the coronary resistance vessels, but this effect was opposed by simultaneous release of NO by the endothelium. When the NO effect was blocked, then the direct ␣2-adrenergic vasoconstrictor effect was revealed. These findings suggest that in patients with impaired endothelial function, unopposed ␣2-adrenergic constriction could contribute to hypoperfusion during exercise in myocardial regions perfused by a stenotic coronary artery (622). Feigl and colleagues (183, 282) proposed that adrenergic coronary vasoconstriction can augment subendocardial blood flow during exercise. However, ␣-adrenoceptor blockade caused a transmurally uniform increase of blood flow in myocardial regions perfused by a stenotic coronary artery (355), as well as in the pressure overloaded hypertrophied left ventricles of dogs (155), indicating transmurally uniform ␣-adrenergic coronary vasoconstriction. Furthermore, the relative vasoconstriction produced by ␣-adrenergic activity was greater during hypoperfusion (355) than during normal arterial inflow (31, 123), suggesting that hypoperfusion amplified the vasoconstrictor response. In agreement with this finding, Chilian (98) observed that while ␣1- and ␣2-adrenoceptor stimulation had no effect on vessels smaller than 100 m during normal arterial inflow, myocardial hypoperfusion 88 • JULY 2008 • www.prv.org CORONARY BLOOD FLOW resulted in unmasking of both ␣1- and ␣2-adrenoceptormediated vasoconstriction in these vessels. The results suggest that the capacity of the coronary arterioles to escape from vasoconstrictor influences becomes impaired at the low perfusion pressures that exist distal to a flow-limiting coronary artery stenosis. B) ANG II. The role of ANG II in the residual coronary vasomotor tone that exists distal to a coronary artery stenosis during exercise has not been investigated. However, in open-chest dogs with extracorporeal perfusion of the left anterior descending coronary artery, Kitakaze et al. (315) demonstrated that the AT1 receptor antagonist CV11974 improved coronary flow and regional segment systolic shortening in ischemic myocardium while coronary artery perfusion pressure was maintained constant. These findings suggest that endogenous ANG II can exert a vasoconstrictor influence on the coronary microcirculation of normal and ischemic myocardium via AT1 receptor activation. It is likely that the vasoconstriction produced by endogenous ANG II is in part due to increased norepinephrine release from the sympathetic nerve endings (493). C) ET. A role for ET in the residual coronary vasomotor tone that exists distal to a flow-limiting stenosis during exercise has not been investigated. Clozel and Sprecher (108) studied the influence of coronary perfusion pressure on the response of myocardial blood flow and wall motion to ET in extracorporeally perfused dog hearts. At a coronary artery pressure of 100 mmHg, intracoronary bolus injections of ET-1 in doses of 1 and 3 g produced transmurally homogeneous decreases of blood flow (up to 60%) and systolic segment shortening (up to 80%). A reduction of coronary artery pressure to 40 mmHg resulted in a 60% decrease of myocardial blood flow, which was associated with a 25% decrease of systolic segment shortening. At this perfusion pressure, ET-1 (1–3 g/kg) nearly abolished myocardial blood flow (85% flow reduction) and resulted in dyskinesis of the hypoperfused region. In contrast, intracoronary injections of ANG II were not enhanced by the hypoperfusion, suggesting that the increased vasoconstrictor response to ET-1 was not simply a dilution effect, i.e., a higher concentration as a result of a lower flow. The observation that the vasoconstrictor response to ET-1 was potentiated at lower perfusion pressures supports the concept that hypoperfusion can augment the response of the coronary microvasculature to vasoconstrictor influences. D) PLATELET PRODUCTS. Thromboxane A2 is a product of prostaglandin endoperoxide metabolism, which is liberated during platelet aggregation. Plasma levels of its metabolites, thromboxane B2 and urinary 2,3-dinorthromboxane B2, are increased in patients with unstable angina and in some patients with postinfarction angina (239, 270). Furthermore, transcardiac serotonin concentrations can be increased in patients with occlusive coronary arPhysiol Rev • VOL 1057 tery disease, especially those with irregular eccentric atherosclerotic lesions (575). These findings suggest the presence of intravascular platelet activation, which is in agreement with angiographic and angioscopic studies that have documented thrombus formation at the site of a ruptured atherosclerotic plaque (216, 526). Thromboxane A2 and serotonin can aggravate myocardial ischemia by accelerating platelet aggregation, thereby increasing the degree of local mechanical obstruction and causing platelet microemboli. In addition, these platelet products have the potential to aggravate ischemia by causing vasoconstriction. Thromboxane A2 constricts both small and large vessel segments; serotonin constricts epicardial arteries but dilates coronary resistance vessels (334, 380). These compounds exert both direct vascular smooth muscle constrictor and indirect endothelium-dependent dilator influences so that arterial vasoconstriction is enhanced when endothelial function is impaired (333, 335, 609). I) Serotonin. Serotonin (5-hydroxytryptamine, 5-HT) has the interesting property of causing vasodilation of coronary arterial vessels smaller than 100 m in diameter while simultaneously causing constriction of larger coronary artery segments (334). As a result of the microvascular dilation, serotonin causes an increase in coronary blood flow in the normal heart (65, 284, 334). The increase in coronary flow is endothelium dependent (605) and is mediated principally via NO (304, 564). During normal treadmill exercise, intracoronary infusion of serotonin into a coronary artery of dogs caused a doubling of coronary blood flow, even when exercise had already resulted in substantial vasodilation of the coronary resistance vessels (33). In contrast to the dilating effect of serotonin on microvessels, in vivo studies have demonstrated that serotonin caused 25– 45% reductions in crosssectional area of epicardial arteries 2–3 mm in diameter (284, 335). The arterial constriction produced by serotonin was enhanced by removal of the endothelium (335), since serotonin causes both direct (109, 117) and flowmediated (335) endothelium-dependent vasodilation, which opposes its direct vascular smooth muscle constrictor action. Serotonin-induced coronary artery and arteriolar vasodilation is mediated by endothelial 5-HT receptors (likely the 5-HT2B receptor, although a 5-HT1B/1D receptor may also be involved Ref. 280), while large epicardial artery constriction involves either 5-HT1B receptors (116, 284) or 5-HT2A receptors (75, 576) located on vascular smooth muscle cells. The effect of serotonin on coronary blood flow in the presence of an arterial stenosis has been studied in anesthetized and in awake animals. In open-chest dogs, Ichikawa et al. (284) employed a coronary artery stenosis that caused a 30-mmHg reduction in distal coronary artery pressure and a 15% decrease in coronary flow. Intracoronary infusion of serotonin, which produced a doubling of blood flow in the absence of a stenosis, caused a 20% 88 • JULY 2008 • www.prv.org 1058 DIRK J. DUNCKER AND ROBERT J. BACHE increase in blood flow in the presence of a stenosis. In contrast, Woodman (609) reported that an intracoronary bolus injection of serotonin, which caused a 70% increase in flow under normal inflow conditions in open-chest dogs, resulted in a 12% decrease of flow in the presence of a critical coronary stenosis. Ruocco et al. (488) failed to observe a significant effect of serotonin on blood flow or the transmural distribution of perfusion distal to an 80% coronary artery stenosis in closed-chest sedated swine. When we exercised dogs on a treadmill (heart rates ⬃190 beats/min), in the presence of a coronary artery stenosis which maintained a constant distal coronary pressure of 42 ⫾ 1 mmHg, serotonin tended to increase flow to the subepicardium but caused a significant decrease in flow to the subendocardium with no change in total blood flow to the ischemic region (Fig. 34) (33). It might be argued that the transmurally heterogeneous response to serotonin was the result of ischemia in the inner but not the outer layer so that autoregulatory reserve existed in the subepicardial arterioles but not in the subendocardium. However, blood flow in the poststenotic region was reduced in all myocardial layers, which would have caused metabolic arteriolar vasodilation. Moreover, earlier studies using a similar experimental model revealed that intracoronary adenosine produced an increase in blood flow which was essentially uniform in all transmural layers, suggesting that once endogenous metabolic vasodilator reserve is exhausted, vasodilator reserve in response to exogenous dilators is uniformly distributed across the left ventricular wall. Since both serotonin (334) and adenosine (103, 306) dilate isolated arterioles with diameters smaller than 100 m, serotonin might be expected to also increase flow in all transmural FIG. 34. Effect of serotonin (A) and TxA2 mimetic U46619 (B) on blood flow distal to a coronary artery stenosis. Epi, subepicardium; OM, outer mid; IM, inner mid; Endo, subendocardium. Note that while U46619 decreases flow similarly in all myocardial layers, serotonin decreases flow selectively in the subendocardial layers. Data are means ⫾ SE. See text for further explanation. [Data from Bache et al. (33) and Bache and Dai (25).] Physiol Rev • VOL layers. Although serotonin caused the expected increase in flow in the subepicardium, the stenosis unmasked an unexpected vasoconstrictor action of serotonin in the subendocardium. The results can be explained by vasoconstriction of the penetrating arteries by serotonin which would selectively increase resistance to blood flow to the subendocardium (334). In the normal heart, dilation of the resistance vessels by serotonin outweighs the effect of constriction of the penetrating arteries (33). However, in the presence of a flow-limiting coronary artery stenosis, which has caused the arterioles to undergo metabolic vasodilation, vasoconstriction of the penetrating arteries cannot be counterbalanced by additional arteriolar vasodilation. Although the arteries which supply the subepicardium would also be constricted by serotonin, these vessels travel a much shorter distance (64, 169), so that their contribution to subepicardial resistance is less important than the contribution of the penetrating arteries to subendocardial resistance. II) Thromboxane A2. Thromboxane A2 (TxA2) receptor activation (using the stable analog U46619) produces vasoconstriction of epicardial coronary arteries and coronary resistance vessels under conditions of normal coronary artery inflow (83, 380). In vivo studies failed to demonstrate a decrease in coronary flow in the normal heart in response to U46619, although a relatively low dose of the agonist was used (83, 488). Ruocco et al. (489) examined the interaction between endogenous prostacyclin and TxA2 in sedated swine in which an intraluminal hollow plug had been placed into the anterior descending coronary artery to cause an 80% decrease in luminal cross-sectional area. The stenosis resulted in a slight but significant decrease in myocardial blood flow with a mean distal coronary artery pressure of 78 mmHg. Infusion of U46619 caused a 20% further decrease in blood flow in the region perfused by the stenotic coronary artery with a change from lactate consumption to lactate production. Similar results were obtained after inhibition of cyclooxygenase activity with indomethacin, indicating that endogenous prostacyclin did not oppose the vasoconstriction produced by U46619. In the presence of a coronary artery stenosis that resulted in subendocardial hypoperfusion and ischemic contractile dysfunction in exercising dogs (heart rates ⬃190 beats/min), U46619 produced vasoconstriction that resulted in a 20 –25% further decrease in myocardial blood flow with aggravation of the contractile dysfunction (25, 141). Of particular interest was the observation that the dose of U46619 (0.01 g䡠kg⫺1 䡠min⫺1, intracoronary) decreased blood flow only in the presence of myocardial ischemia (Fig. 32) but not during unimpeded coronary arterial inflow (34). Thus, instead of opposing vasoconstriction, the decreased blood flow and ischemia produced by the coronary artery stenosis appeared to facilitate vasoconstriction by U46619. It is possible that the normal metabolic vasodilator mech- 88 • JULY 2008 • www.prv.org CORONARY BLOOD FLOW anisms are maximally activated in ischemic myocardium and therefore cannot respond to a further decrease in blood flow. TxA2 constricts coronary arterial microvessels of all sizes. In the presence of normal arterial inflow, constriction of the resistance arteries (⬎100 m) can be compensated for by metabolic vasodilation of the arterioles (104, 299, 300). However, when a coronary artery stenosis has already caused metabolic arteriolar vasodilation, the ability to compensate for vasoconstriction by thromboxane in segments larger than 100 m is lost. In agreement with this hypothesis, we observed that while endogenous adenosine contributed to coronary vasodilation during myocardial ischemia produced by exercise in the presence of a coronary artery stenosis, adenosine failed to attenuate the vasoconstriction produced by U46619 in hypoperfused myocardium (141). The inability of endogenous adenosine to attenuate the vasoconstrictor response in hypoperfused myocardium can be explained by differing vasoactive profiles of TxA2 and adenosine in coronary arterial segments of different sizes. Whereas adenosine predominantly dilates arterioles smaller than 100 m in diameter, thromboxane constricts coronary arterial vessels of all sizes (308, 380). Thus vasoconstriction by thromboxane of the resistance arteries ⬎100 m, which are minimally responsive to the vasodilator actions of adenosine, can aggravate hypoperfusion of ischemic myocardium. In addition, thromboxane has been shown to cause constriction in the arteriolar segments that are dilated by adenosine, so that competition between thromboxane and adenosine can also occur in vessels smaller than 100 m in diameter. Chilian and Layne (103) reported that even during severe hypoperfusion, exogenous adenosine caused further vasodilation of the coronary arterioles. This implies that residual vasomotor tone is present in ischemic myocardium and lends support to the hypothesis that vasoconstrictors can directly compete with endogenous vasodilator substances within a given vascular segment. 6. Integrated control of resistance vessel tone in ischemic myocardium In contrast to the classical concept that myocardial hypoperfusion would cause the coronary microvasculature distal to the stenosis to become unresponsive to vasoconstrictor influences, it has become clear that residual vasomotor tone is present in the coronary resistance vessels within myocardium that becomes ischemic during exercise in the presence of a coronary artery stenosis. The residual vasomotor tone can be the result of vasoconstrictor influences of ␣-adrenoceptor activity, the platelet products TxA2 and serotonin, and the neurohormones ANG II and endothelin. This vasoconstriction can occur in small arteries (⬎100 m) that account for a significant fraction of total coronary resistance but are Physiol Rev • VOL 1059 not under metabolic control (␣1-adrenoceptors, ANG II, TxA2, serotonin, endothelin), as well in arterioles (⬍100 m) which are under metabolic control (␣1- and ␣2-adrenoceptors, ANG II, TxA2, endothelin) (Fig. 35). These vasoconstrictor influences can compete with endogenous substances such as adenosine, bradykinin, NO, prostanoids, and EDHF which cause vasodilation through activation of KATP and KCa channels that are recruited during ischemia (Fig. 35). In support of this concept, clinical evidence is emerging that demonstrates residual coronary vasomotor tone in ischemic myocardium distal to a coronary artery stenosis (458, 497, 622). 7. Myocardial responses to chronic ischemia Ischemia elicits local vasodilator responses that result in decreased vascular resistance and an increase in the number of open capillaries within the ischemic region, thereby augmenting blood flow and minimizing the diffusion distance for oxygen and metabolic substrate (138, 277). In addition to these vascular effects, hypoperfusion (coronary hypotension) can elicit responses within the cardiac myocytes that act to decrease energy demands, thereby reducing metabolic markers of ischemia despite persistently decreased blood flow. Short-term changes of coronary blood flow (increases or decreases) can result in directionally similar changes of myocardial oxygen consumption, termed the Gregg effect (219). Sustained limitation of blood flow in myocardial regions perfused by a stenotic coronary artery or collateral vessels can result in a state termed “hibernation” in which contractile activity, and metabolic demands, are decreased to accommodate the persistently reduced perfusion. Myocardial hibernation has been observed both in patients with occlusive coronary artery disease (88, 467) and in experimental animal models (172, 389, 404). Although a comprehensive discussion of the myocardial responses to reduced blood flow is beyond the scope of this review, the ability of the myocardium to reduce contractile activity, and thus energy demands, in response to limited blood flow deserves brief comment. It should be mentioned that myocardial hibernation appears to be fundamentally different from “stunning,” in which a transient period of ischemia results in hypokinesis with normal myocardial blood flow rates, although it is likely that repetitive stunning can coexist with or lead to hibernation over time (89, 90, 554). Regions of hibernating myocardium in which blood flow is limited either by a proximal coronary stenosis or by collateral vessels have been reported to have resting blood flow rates 10 –20% less than remote normally perfused myocardium, with a parallel decrease in contractile function (88, 170, 171, 317, 462, 523, 561). The response to catecholamine stimulation is blunted in hibernating regions, providing some degree of protection against the development of demand-induced ischemia (171, 375). 88 • JULY 2008 • www.prv.org 1060 DIRK J. DUNCKER AND ROBERT J. BACHE FIG. 35. Schematic overview of various vasodilator (yellow text boxes) and vasoconstrictor (blue text boxes) influences in different microvascular segments (small intramural arteries class A and B, and arterioles) of the coronary arterial bed of the LV wall, during exercise in the presence of a proximal coronary artery stenosis. In solid line text boxes are shown the contribution to vasomotor control of endogenous vasoactive substances, whereas in dashed line text boxes the effects of exogenously administered vasoactive substances have been shown. In regular font are depicted the mechanisms that have been demonstrated in awake exercising animals; in italics are depicted those mechanisms that have been demonstrated in open-chest anesthetized animal preparations. TxA2, thromboxane A2; 5HT, serotonin or 5-hydroxytryptamine; AII, angiotensin II; ET, endothelin; ␣1 and ␣2, ␣1- and ␣2-adrenergic receptors; Ado, adenosine; NO, nitric oxide; KATP, ATP-sensitive K⫹ channel; KCa, calcium-sensitive K⫹ channel. There are few studies examining coronary responses to exercise in chronically hypoperfused regions. In a canine model of coronary artery occlusion with moderate collateral vessel development (blood flow in the collateralized region 15–20% less than in remote normally perfused myocardium), exercise caused an increase in blood flow to the collateral-dependent region (317, 462, 561). With the use of measurements of aortic pressure and pressure in the collateral-dependent artery, it was possible to separately calculate resistance of the collateral vessels and small vessel resistance in the collateralized region. Exercise did not cause dilation of the collateral vessels (transcollateral resistance did not decrease during exercise). Rather, the increase in blood flow during exercise resulted from vasodilation of the resistance vessels in the collateral-dependent region (462). Preservation of vasodilator capacity in the collateral zone demonstrates that the reduced resting blood flow and persistent vasomotor tone did not result from absence of vasodilator reserve. It appears rather that energy requirements were decreased, inasmuch as such relatively hypoperfused collateral-dependent regions do not demonstrate evidence for ischemia during resting conditions (172, 404). It should be emphasized that although alterations of both the supply and demand sides of the energy equation can contribute Physiol Rev • VOL to maintenance of metabolic equilibrium in the heart, during physiological conditions the predominant sequence is that changes in hemodynamic demands (as during exercise) result in changes in contractile work (and oxygen requirements) that drive parallel changes in blood flow. B. Regulation of Coronary Blood Flow to Collateral-Dependent Myocardium During Exercise Coronary artery occlusion can result in development of an effective intercoronary collateral circulation. If occlusion proceeds gradually, sufficient collateral vessel recruitment and growth may occur to allow progression to total arterial occlusion with little or no infarction of the dependent myocardium. In this situation, the collateral vessels are able to provide adequate arterial inflow to maintain myocardial integrity during resting conditions, but the ability to augment blood flow in response to exercise or other stress may be limited and develop only gradually. This section will consider studies of the coronary vascular system in the intact heart that contains a region of collateral-dependent myocardium. Emphasis 88 • JULY 2008 • www.prv.org CORONARY BLOOD FLOW will be placed on studies in which the response of the coronary collateral system is integrated into the overall response of the heart to exercise. 1. Coronary collateral blood flow during exercise Several investigators have examined the ability of blood flow in collateral-dependent myocardial regions to increase in response to exercise. These studies have commonly used the ameroid constrictor technique to induce collateral vessel growth (370). Coronary artery occlusion is produced by surgically implanting a ring of casein plastic material (“ameroid”) around a proximal coronary artery. The hygroscopic ameroid material slowly swells as it comes in contact with tissue fluid; external expansion is prevented by a stainless steel backing incorporated into the ring, so the inward expansion of the material causes progressive arterial narrowing. The arterial narrowing and accompanying inflammation generally results in total arterial occlusion within 3– 4 wk, often in association with local thrombus formation during the final stages of occlusion. Since occlusion occurs gradually, sufficient growth of collateral vessels generally occurs to allow progression to total occlusion with little or no infarct in dogs, although in swine, a species with negligible innate collateral vessels, some subendocardial necrosis is often present (433). Fedor et al. (179) measured myocardial blood flow with radioactive microspheres during moderate treadmill exercise 11–12 wk after placement of ameroid constrictors on both the right and left circumflex coronary arteries of adult dogs. During resting conditions, myocardial blood flow was similar in the normally perfused and collateral-dependent regions. During moderate treadmill exercise, blood flow in the collateral region increased normally in 6 of 11 dogs. In the remaining five animals, blood flow in the subepicardial half of the collateral-dependent region increased normally during exercise, but the increase in blood flow to the subendocardium was markedly impaired, resulting in a prominent decrease of the subendocardial-to-subepicardial flow ratio. Since collateral vessel development is a time-dependent process, the heterogeneous response to exercise might be related to differences in the rate of collateral vessel growth between animals. This question has been examined by measuring the exercise-induced increase in collateral blood flow relatively early (1 mo) and late (up to 8 mo) after coronary artery occlusion in dogs (32, 329). One month after ameroid placement, myocardial blood flow measured with microspheres during resting conditions was similar in the collateral-dependent and normally perfused regions, but the response of blood flow to exercise in the collateral-dependent region was highly variable (32). Approximately one-third of the animals had sufficient collateralization to allow a completely normal response of myocardial blood flow during both light and Physiol Rev • VOL 1061 heavy levels of exercise (group 1). In a second group of animals (group 2), mean myocardial blood flow was normal at rest and demonstrated a normal increase during light exercise, but a further increase in exercise intensity resulted in no further increase in mean blood flow. In the third group of animals (group 3), blood flow was normal at rest but failed to increase or underwent a subnormal increase even during light exercise with no additional change in response to a further increase of exercise intensity. Animals grouped according to the ability of mean blood flow in the collateral-dependent region to increase in response to exercise are shown in Figure 36. The distribution of blood flow across the left ventricular wall from epicardium to endocardium is shown for the collateral-dependent region; data from a group of normal animals undergoing similar exercise are shown for comparison. For animals in group 1, not only was the mean blood flow rate normal in the collateral-dependent region during both stages of exercise, but the transmural distribution of perfusion was also normal. For the animals in group 2, the normal increase in blood flow in response to light exercise was associated with a normal transmural distribution of perfusion. However, when the exercise level was further increased, mean flow failed to increase and a transmural redistribution of perfusion away from the subendocardium occurred. Finally, for the animals in group 3, any exercise resulted in a prominent redistribution of perfusion so that subepicardial flow increased, while blood flow to the inner half of the left ventricular wall did not change or decreased compared with resting values. Minipigs studied 4 –16 wk after ameroid placement on the left circumflex coronary artery had responses to exercise similar to those of the dogs in group 3 (481, 602). Exercise which increased heart rates to 245 beats/min caused a ⬃200% increase in blood flow in the normal region, while mean blood flow to the collateral-dependent region increased only ⬃50%. The subnormal increase in blood flow to the collateral-dependent region during exercise was associated with redistribution of perfusion away from the subendocardium, with the ratio of subendocardial/subepicardial flow falling to 0.39. The findings indicate that even in the pig, in which coronary collateralization is notoriously poor, there is some ability to increase blood flow to the collateral-dependent region during exercise relatively early after coronary occlusion. As in the dog, the limited blood flow in the collateraldependent region is delivered primarily to the outer myocardial layers, resulting in preferential underperfusion of the subendocardium. The transmural distribution of blood flow away from the subendocardium that occurs when the collateral vessels are unable to provide a normal increase in blood flow during exercise is similar to the subendocardial underperfusion that occurs when an arterial stenosis limits myocardial blood flow during exercise (36, 500). The ability to 88 • JULY 2008 • www.prv.org 1062 DIRK J. DUNCKER AND ROBERT J. BACHE FIG. 36. Myocardial blood flow to four transmural layers from epicardium to endocardium at rest and during two levels of treadmill exercise. Data are shown for 7 normal dogs (control) and for collateral-dependent myocardium 1 mo after placement of an ameroid constrictor on the left circumflex coronary artery of 14 dogs. Animals with coronary occlusion were divided into three groups. Group I represents animals with sufficient collateralization to allow a completely normal increase of myocardial blood flow during both levels of exercise. In group II, collateralization was sufficient to allow a normal increase in myocardial blood flow during light exercise, but a further increase in exercise intensity resulted in no further increase in mean blood flow. In group III, blood flow was normal at rest but underwent a subnormal increase during light exercise with no additional change during heavy exercise. Note that exercise caused a dramatic redistribution in myocardial blood flow from the inner to the outer layers during heavy exercise in group II, and during light and heavy exercise in group III. Data are means ⫾ SE. Dot inside symbol denotes significant (P ⬍ 0.05) differences from corresponding control. See text for further explanation. [Data from Bache and Schwartz (32).] maintain a normal transmural distribution of perfusion is likely related to the degree of vasodilation of the resistance vessels within the collateralized region. When collateral vessels are poorly developed, the resistance vessels in the collateral-dependent region must maintain a substantial degree of vasodilation even during resting conditions to compensate for the resistance of the collateral vessels. Furthermore, the poorly developed collateral vessels impose a limit beyond which blood flow cannot increase. When myocardial demands are below this limit, the resistance vessels are able to maintain a normal transmural distribution of perfusion in the collateral-dependent region. However, when myocardial demands exceed the capacity of the collateral vessels to deliver blood flow, intense vasodilation of the resistance vessels impairs their ability to maintain a normal transmural distribution of blood flow. The observed wide variation in the ability of the collateral vessels to increase blood flow in response to exercise could be due to intrinsic differences between animals in the ability to develop collateral vessels, or differences in the rate of collateral vessel development between animals. If the latter were true, then allowing additional time after coronary occlusion would permit all animals to develop sufficient collateralization to undergo a uniform increase in blood flow during exercise. To examine this hypothesis, myocardial blood flow was measured at rest and during two exercise stages 8 mo after implantation of an ameroid constrictor on the left circumflex coronary artery in dogs (329). Both the increase in mean myocardial blood flow and the transmural distribuPhysiol Rev • VOL tion of perfusion were normal at rest and during both levels of exercise in the collateral-dependent region. Similar findings were reported by Longhurst et al. (373) in dogs studied 2–3 mo after ameroid occlusion of the left circumflex coronary artery. These findings demonstrate that the marked heterogeneity of perfusion in the collateral-dependent region during exercise 1 mo after ameroid implantation did not result from differences in the intrinsic ability for ultimate collateral vessel development, but rather from differences in the rate of collateral vessel growth between animals. When sufficient time was allowed, all animals developed sufficient collateralization to permit a normal increase in blood flow in response to a moderate level of treadmill exercise. In summary, if the coronary collateral vessels can conduct sufficient arterial inflow to meet the needs of the dependent myocardium, then the volume and distribution of blood flow will be determined by the normal autoregulatory responses of the microvasculature in the dependent myocardium. However, if the collateral vessels cannot deliver sufficient arterial inflow to meet the needs of the dependent myocardium, then the volume of flow will be determined by the conductance of the collateral system and the transmural distribution of perfusion will be similar to that observed when flow is limited by an arterial stenosis. 2. Influence of infarct in the collateral region A) COLLATERAL FLOW DURING RESTING CONDITIONS. Gradual application of a coronary occlusion to produce a region of 88 • JULY 2008 • www.prv.org CORONARY BLOOD FLOW collateral-dependent myocardium without infarct allows study of the collateral circulation uninfluenced by infarcted myocardium. In the clinical setting, however, collateral-dependent regions frequently include areas of infarcted myocardium. In this situation, blood flow to the collateral-dependent region is influenced not only by the structure and function of the collateral vessels, but also by abnormalities resulting from the presence of infarcted tissue. In both human subjects suffering acute myocardial infarction and experimental canine models of abrupt coronary occlusion, the area of infarcted myocardium is smaller than the region of tissue normally perfused by the coronary artery (“risk region”) (505). Survival of a portion of myocardium normally perfused by an occluded artery is dependent on delivery of a limited inflow of arterial blood by preexisting coronary collateral vessels. The fraction of myocardium surviving acute coronary occlusion is quantitatively related to the volume of collateral blood flow available early after coronary occlusion (476). Several reports have examined blood flow to collateral-dependent myocardium, which included regions of infarct studied ⬃2 wk after acute coronary artery occlusion (259 –261). Regional myocardial blood flow was measured with radioactive microspheres in multiple myocardial specimens; the percent infarct in each myocardial specimen was determined using quantitative microscopy. When blood flow to collateral-dependent myocardial specimens was plotted against percent infarct during resting conditions, an inverse linear relationship was found, with myocardial blood flow decreasing in direct proportion to the fraction of infarct within a myocardial specimen. B) COLLATERAL FLOW DURING EXERCISE. The above studies indicate that during resting conditions, blood flow to collateral-dependent regions that include infarct is proportional to the volume of residual viable myocardium. To examine whether the presence of infarct influences the response collateral flow during exercise, myocardial blood flow was measured during three levels of treadmill exercise (heart rates of 164, 205, and 242 beats/min) in dogs 2 wk after abrupt total occlusion of the left anterior descending coronary artery (261). The degree of infarcted myocardium importantly influenced the response of collateral flow during exercise. In specimens that contained ⬍50% infarct, blood flow during resting conditions was depressed in direct proportion to the degree of infarct, but the proportionate increase in blood flow in response to exercise was not different from a normally perfused myocardial region. Thus the presence of infarct did not impair the increase in blood flow in the collateral-dependent noninfarcted myocardium. However, when more than 50% of a myocardial specimen was occupied by infarct, not only was resting flow depressed, but the relative increase in flow during exercise was also reduced. It is likely that impairment of perfusion in regions containing ⬎50% infarct resulted from the high resistance to Physiol Rev • VOL 1063 blood flow offered by the poorly developed collateral vessels. Regions with the largest percent infarct were those with the sparsest native collateral vessels; even 2 wk after coronary occlusion, collateral vessel development was insufficient to allow a normal increase in flow to the residual viable myocardium during exercise. In addition, the presence of infarct might directly influence blood flow to the residual viable myocardium, or metabolic alterations in the residual myocardium (such as hibernation) might blunt the increase in oxygen demands during exercise (178). 3. Influence of collateral vessel tone Studies using isolated vessel segments and in vivo studies performed in open-chest animals have demonstrated that well-developed collateral vessels are capable of active vasomotion (243, 249). This suggests that collateral vessels do not behave as fixed conduits with a constant upper limit for conductance of blood to the dependent myocardium, but that vasomotor tone of collateral vessels can modulate the availability of blood to the dependent myocardium. This hypothesis was tested by infusion of arginine vasopressin in dogs in which a collateral-dependent myocardial region was produced using the repetitive coronary occlusion technique of Franklin et al. (195); this technique uses repeated 2-min coronary artery occlusions to stimulate collateral vessel development. An intra-arterial microcatheter was implanted distal to the occluder to allow measurement of the coronary pressure perfusing the collateralized region and calculation of collateral resistance (192). With the use of this model, distal occlusion pressures gradually increase as collateralization occurs; when the distal pressure increases to 35– 40 mmHg (⬃3 wk of repetitive 2-min occlusions), permanent coronary occlusion can be produced without causing infarct. Following permanent occlusion, distal coronary pressures increase even more rapidly so that within 1 wk mean distal pressure increases to ⬃75 mmHg. At this time, myocardial blood flow was measured with microspheres at rest and during treadmill exercise. After completion of control measurements, arginine vasopressin was infused (0. 01 mg䡠kg⫺1 䡠min⫺1 iv) and blood flow was again measured at rest and during exercise. Arginine vasopressin was used because it causes constriction of isolated segments of collateral vessels (243) but does not constrict epicardial coronary arteries, since constriction of arteries proximal to the origin of the collateral vessels could also impair collateral blood flow (243, 308). Myocardial blood flow in the normal and collateral-dependent regions are shown in Figure 37. During resting conditions, blood flow was slightly but significantly lower in the collateral zone than in the normal zone. Exercise increased blood flow in both the normal and collateraldependent regions, although the increase was subnormal in the collateralized region. When exercise was repeated 88 • JULY 2008 • www.prv.org 1064 DIRK J. DUNCKER AND ROBERT J. BACHE FIG. 37. Effect of vasopressin on myocardial blood flow to normally perfused and collateral-dependent left ventricular regions at rest and during treadmill exercise. During control conditions, exercise resulted in significant increases in blood flow in both the normal and collateraldependent regions, although the increase in flow was subnormal in the collateralized region. Infusion of vasopressin did not significantly alter the response of blood flow during exercise in the normally perfused region, but essentially abolished the increase in blood flow in response to exercise in the collateral-dependent region. Data are means ⫾ SE. *P ⬍ 0.05 vs. corresponding rest. †P ⬍ 0.05 vs. corresponding normal region. ‡P ⬍ 0.05 vs. corresponding control. See text for further explanation. [Data from Foreman et al. (192).] during vasopressin infusion, the increase in blood flow in the normal zone was not significantly different from control. However, vasopressin essentially abolished the increase in collateral zone blood flow in response to exercise. The impaired response of blood flow to exercise during vasopressin infusion resulted from vasoconstriction of both coronary collateral vessels and the resistance vessels in the collateral-dependent region. Thus transcollateral resistance (calculated as aortic pressure minus coronary artery pressure distal to the occlusion divided by total collateral blood flow) increased 48 ⫾ 14% in response to vasopressin, while small-vessel resistance (calculated as coronary artery pressure distal to the occlusion minus left ventricular end-diastolic pressure divided by total collateral blood flow) increased by 40 ⫾ 9%. In contrast, vasopressin did not increase coronary vascular resistance in the normally perfused region. The finding that vasopressin caused small-vessel constriction in the collateral-dependent region but not in the normal zone indicates that small-vessel responsiveness is altered in regions of myocardium perfused by collateral vessels. These results demonstrate that the coronary collateral vessels do not act as fixed conduits. Rather, active vasoconstriction of both collateral vessels and coronary resistance vessels can worsen hypoperfusion of the collateraldependent myocardium during exercise. It should be noted that there is no evidence that vasopressin normally limits collateral blood flow during exercise, since exercise does not cause a sufficient inPhysiol Rev • VOL crease in vasopressin levels to have a significant vasomotor effect. Rather, vasopressin was used as a convenient agent to test the general hypothesis that active constriction of coronary collateral vessels has the potential to limit perfusion of the dependent myocardium during exercise. Consequently, the dose of vasopressin was calculated to correspond to levels that cause collateral vasoconstriction in vitro without causing substantial systemic hemodynamic effects (243). As predicted, plasma levels increased to far above those during physiological conditions, being 2.1 ⫾ 0.64 pg/ml during control conditions and increasing to 98 ⫾ 34 pg/ml during vasopressin infusion. Individual vasopressin plasma levels were within the range reported to occur in response to smoking two cigarettes or during hemorrhage of moderate degree (208, 591). Thus, although plasma vasopressin levels are normally far less than would influence collateral flow during physiological conditions, it is possible that sufficient elevations of vasopressin levels can be achieved during pathological conditions to cause constriction of coronary collateral vessels in vivo. 4. Control of coronary blood flow in collateralized hearts A) ␣-ADRENERGIC CONTROL. ␣-Adrenergic vasoconstrictor influences can restrain the increase in coronary blood flow that occurs in response to the increased myocardial oxygen demands during exercise in the normal heart (31, 232) and in the presence of a coronary stenosis that results in myocardial hypoperfusion (82, 227, 297, 354, 518). A study was performed to determine whether ␣-adrenergic vasoconstriction also limits blood flow to collateral-dependent myocardium during exercise (260). Myocardial blood flow was measured at rest and during two levels of treadmill exercise in dogs 2 wk after acute coronary occlusion that produced a collateralized region that included a variable degree of subendocardial infarct. The increase in subendocardial blood flow in response to exercise was impaired in the collateral-dependent region, while subepicardial flow was not different from that in the remote normally perfused region. During resting conditions, selective ␣1-adrenergic blockade with prazosin caused no change in either mean blood flow or the transmural distribution of perfusion in either the normal or the collateral-dependent regions. However, at comparable levels of exercise, prazosin caused a 27% increase in mean blood flow in the normally perfused region and a 35% increase in mean flow in the collateral-dependent region (P ⬍ 0. 01). Prazosin caused increases in blood flow in all transmural layers in the collateral-dependent region from epicardium to endocardium, although the absolute increase in flow was most marked in the subepicardium. Since previous studies have failed to document ␣-adrenergic vasoconstriction in coronary collateral vessels stud- 88 • JULY 2008 • www.prv.org CORONARY BLOOD FLOW ied either in vitro or in vivo, it is unlikely that the increase in blood flow to the collateral-dependent region produced by prazosin was the result of interruption of ␣-adrenergic vasoconstriction in the collateral vessels (30, 243, 249). More likely, ␣-adrenergic blockade increased blood flow by interrupting vasomotor tone of the resistance vessels in the collateral-dependent myocardium. This is analogous to studies demonstrating that resistance vessels in regions distal to a flow-limiting coronary stenosis that results in myocardial ischemia during exercise also retain vasomotor tone (354). It appears that ␣-adrenergic vasoconstriction of the resistance vessels can also limit myocardial blood flow in region perfused by collateral vessels. B) -ADRENERGIC CONTROL. -Adrenergic stimulation has been found to cause relaxation of isolated rings of welldeveloped canine coronary collateral vessels (184). Furthermore, norepinephrine infusion caused a decrease in collateral resistance in dogs studied 2–3 mo after implantation of an ameroid constrictor on the left circumflex coronary artery (384). These findings of adrenergic collateral vessel dilation suggest that -adrenergic blockade might have the potential to decrease collateral flow. However, arterioles isolated from collateral-dependent myocardium are reported to have blunted ß-adrenergic responsiveness (522). The influence of ß-adrenergic activity on collateral flow was studied in dogs in which acute coronary occlusion had resulted in a collateral-dependent myocardial region containing infarct (259). During resting conditions, selective ß1-adrenergic blockade with timolol caused no change in blood flow to either the normally perfused or collateral-dependent myocardium, indicating minimal adrenergic activity at rest. However, during exercise, timolol decreased blood flow in both collateralized and remote regions, largely due to the negative chronotropic effect of ß-adrenergic blockade. To correct for changes resulting from alterations of myocardial oxygen demands, results were compared at similar rate-pressure products. At comparable rate-pressure products, timolol significantly decreased blood flow in both the subepicardium and subendocardium of the normally perfused region. In the collateral region, timolol caused a decrease of subepicardial blood flow comparable to that in the normally perfused region. However, in the subendocardium of the collateral region (in which blood flow during exercise was substantially less than normal), ß-adrenergic blockade did not cause a further reduction of blood flow. The effect of ß-adrenergic blockade on subepicardial flow could have resulted from vasoconstriction of either the collateral vessels or the resistance vessels in the collateral zone. To further examine this question, Traverse et al. (559) studied the effect of ß-adrenergic activity on collateral blood flow during treadmill exercise using the nonselective ß-blocker propranolol. Pressure in the coronary artery distal to the occlusion was measured to separate the effects of propranolol on collateral resistance and Physiol Rev • VOL 1065 small-vessel resistance in the collateral zone. Collateral vessel growth was stimulated with repetitive 2-min coronary occlusions so that myocardial infarction was avoided. During control exercise, blood flow in the collateral zone was 38 ⫾ 5% less than in the normal zone. At identical levels of exercise, with heart rate maintained constant by atrial pacing, propranolol decreased mean blood flow in the collateralized myocardium by ⬃20%; this occurred principally in the subepicardium with no significant effect on flow in the subendocardium. The decrease in collateral zone blood flow in response to propranolol resulted from a 40% increase in transcollateral resistance and a ⬃50% increase in small-vessel resistance in the collateral-dependent region. The decrease in subepicardial flow in the collateral region following ß-adrenergic blockade was proportionately similar to the decrease in flow in the normally perfused region. Thus the decrease in subepicardial blood flow in the collateral region in response to ß-adrenergic blockade resulted from vasoconstriction of the coronary resistance vessels with a smaller effect on the collateral vessels. In summary, adrenergic nervous system activity can influence blood flow to collateral-dependent myocardium during exercise. The available data suggest that the influence of the adrenergic nervous system on blood flow in the collateral zone results principally from altered vasomotor activity of the microvasculature within the collateral-dependent region rather than from direct vasomotor effects on the collateral vessels. C) NO. In vitro studies using isolated vessel rings have demonstrated that collateral vessels acquire a functionally competent endothelium and muscular media early in their development and are responsive to NO-dependent agonists, such as bradykinin and acetylcholine (5, 10, 188). Furthermore, inhibition of NO synthesis has been shown to decrease retrograde blood flow from a cannulated collateral-dependent coronary artery in anesthetized open-chest dogs (188) and to significantly increase collateral vessel vascular resistance in awake dogs (194), demonstrating that tonic production of NO contributes to maintenance of collateral vasodilation. Conversely, arterioles isolated from collateral-dependent myocardium demonstrate blunted endothelium-dependent vasodilator responses to acetylcholine and ADP in dogs (521), and ADP and bradykinin in swine (222, 519). To determine whether endogenous NO contributes to maintenance of blood flow in collateral-dependent myocardium, Traverse et al. (561) exercised dogs on a treadmill (heart rate 230 beats/min) before and after NO synthase inhibition with L-NA (20 mg/kg iv). During resting conditions, L-NA tended to decrease blood flow to the collateral region with no change in normal zone blood flow. During exercise, L-NA caused a decrease in mean blood flow to the collateral region (from 2.24 ⫾ 0.19 to 1.78 ⫾ 0.26 ml䡠min⫺1 䡠g myocardium⫺1 after L-NA, P ⬍ 0.05), which occurred in both 88 • JULY 2008 • www.prv.org 1066 DIRK J. DUNCKER AND ROBERT J. BACHE the subendo- and subepicardium (Fig. 38). This decrease resulted from a near doubling of the collateral vascular resistance, with a trend toward an increase in small vessel resistance in the collateral zone. Interestingly, L-NA also decreased blood flow to the normal myocardial region during exercise (from 2.99 ⫾ 0.24 to 2.45 ⫾ 0.28 ml䡠min⫺1 䡠g myocardium⫺1) as the result of a 44 ⫾ 13% increase in coronary vascular resistance. These findings indicate that, in contrast to the normal heart (7) or normal regions in hearts with an acute coronary artery stenosis (137), endogenous NO is important in maintaining flow to regions of normally perfused myocardium during exercise in a model of single-vessel coronary artery occlusion. In view of the observation that endogenous NO contributes to maintenance of perfusion in collateral-dependent myocardium, the question arises as to whether exogenous NO can increase blood flow during exercise in a collateralized region. Studies in open-chest animals with well-developed coronary collateral vessels demonstrated that nitroglycerin increased blood flow and improved contractile function in collateral-dependent myocardium (113, 173, 249). However, nitroglycerin did not increase blood flow to collateral-dependent myocardium of exercising dogs with a chronic coronary occlusion (462). The lack of effect of nitroglycerin on either coronary collateral resistance or small-vessel resistance in the collateral zone suggests that the endogenous NO system is already maximally recruited during exercise in the canine model of single-vessel coronary artery occlusion. This is supported by the observation that after inhibition of NO synthase with L-NA, nitroglycerin did increase blood flow to the collateral zone, and this was associated with an improvement of regional systolic wall thickening (317). This ob- servation suggests that in patients in whom dyslipidemia and atherosclerosis have resulted in endothelial dysfunction and impaired NO bioavailability, nitroglycerin would be able to increase collateral blood flow. D) PROSTANOIDS. Although coronary vessels are capable of synthesizing and responding to vasoactive products of arachidonic acid (423), physiological studies have failed to demonstrate an important role for the prostaglandin system in regulation of coronary blood flow in the normal canine heart. In contrast, in open-chest dogs in which chronic coronary artery occlusion had resulted in growth of collateral vessels, cyclooxygenase blockade caused a decrease in retrograde blood flow from the collateraldependent artery (4, 6), suggesting that basal release of prostaglandins maintains tonic vasodilation of coronary collateral vessels. However, interpretation of these findings is complicated by reports that tissue injury during acute surgical preparation of open-chest animal models can artificially increase basal prostaglandin synthesis by the heart. Altman et al. (8) addressed the role of prostaglandins in control of blood flow in collateral-dependent myocardium in chronically instrumented dogs, free from the effects of anesthesia and acute surgical trauma. Myocardial blood flow was measured with radioactive microspheres at rest and during treadmill exercise (heart rate 215 ⫾ 7 beats/min). Cyclooxygenase blockade with indomethacin (5 mg/kg iv) caused no change in heart rate or arterial pressure, and no change in blood flow in the normal zone or the collateral zone during resting conditions. However, during exercise, indomethacin caused a 42 ⫾ 10% increase in transcollateral resistance that was associated with a 27 ⫾ 11% decrease in subendocardial flow in the collateral zone (both P ⬍ 0.05), but no change FIG. 38. Effect of inhibition of NO synthase inhibition on left ventricular subendocardial and subepicardial blood flows in hearts with a collateral-dependent region due to chronic coronary artery occlusion and in hearts subjected to an acute coronary artery stenosis during treadmill exercise. Inhibition of NO synthase decreased blood in the normal as well as in the collateral-dependent regions. In contrast, while NO synthase inhibition decreased flow distal to a coronary artery stenosis, it had no effect on the normal region. Data are means ⫾ SE. *P ⬍ 0.05 vs. corresponding control. See text for further explanation. [Data from Traverse et al. (561) and Duncker and Bache (137).] Physiol Rev • VOL 88 • JULY 2008 • www.prv.org CORONARY BLOOD FLOW in normal zone blood flow. Indomethacin had no effect on small vessel resistance in the collateral zone so that the decrease in blood flow was mediated entirely by constriction of the collateral vessels. Thus, in the setting of chronic coronary occlusion, the cyclooxygenase system exerts a vasodilator influence on collateral vessels that becomes apparent during exercise. E) ADENOSINE. Acadesine (5-AICA riboside) is an adenosine-regulating agent that increases adenosine production. In acutely ischemic myocardium, increased production of adenosine via activation of 5⬘-ectonucleotidase (278) can exert cardioprotective effects (225). Consequently, a study was performed to determine whether acadesine would improve blood flow to collateral-dependent myocardium during exercise (290). In dogs with moderately well-developed coronary collateral vessels but a subnormal increase in blood flow in the collateral zone during exercise, acadesine increased blood flow in the collateral-dependent region during exercise by 24 ⫾ 5% with no change in the transmural distribution of perfusion. The increase in collateral zone blood flow produced by acadesine resulted from a 25% decrease transcollateral resistance and a 20% decrease in smallvessel resistance in the collateral region. Acadesine also increased normal zone blood flow in the collateralized dogs but had no effect on coronary flow in normal dogs. These findings suggest that adenosine metabolism is al- 1067 tered not only in the collateral-dependent region but also in the remote region of hearts with a chronic coronary artery occlusion. The importance of endogenous adenosine for the regulation of coronary blood flow in the collateralized heart during exercise has not been studied to date. F) SUMMARY AND INTEGRATION. The coronary collateral system embodies a dynamic network of interarterial vessels that can undergo both long- and short-term adjustments that are capable of modulating blood flow to the dependent myocardium. Long-term adjustments including recruitment and growth of collateral vessels in response to arterial occlusion are time dependent and determine the maximum blood flow rates available to the collateraldependent vascular bed during exercise. Rapid short-term adjustments result from active vasomotor activity of the collateral vessels. Mature coronary collateral vessels are responsive to vasodilators such as nitroglycerin (113, 173, 249) and atrial natriuretic peptide (191), to vasoconstrictors such as vasopressin (192) and ANG II (243, 249), and to the platelet products serotonin and TxA2 (611) (Fig. 39). During exercise, ß-adrenergic activity and endotheliumderived NO and prostanoids exert vasodilator influences on coronary collateral vessels. Importantly, alterations in collateral vasomotor tone, e.g., by exogenous vasopressin, inhibition of endogenous NO or prostanoid production, or increasing local adenosine production FIG. 39. Schematic overview of various vasodilator (yellow text boxes) and vasoconstrictor (blue text boxes) influences in collateral arteries (and epicardial conduit vessels) and distal coronary microcirculation (small intramural arteries class A and B and arterioles) of the coronary arterial bed of the LV wall, during exercise in the presence of a chronic coronary artery occlusion. In solid line text boxes are shown the contribution to vasomotor control of endogenous vasoactive substances, whereas in dashed line text boxes the effects of exogenous administration of vasoactive substances have been shown. ß1 and ß2, ß1- and ß2-adrenergic receptor; NO, nitric oxide; Ado, adenosine (AICA-riboside); NTG, nitroglycerin; PGI2, prostacyclin; ␣1, ␣1-adrenergic receptor; VP, vasopressin. See text for further explanation. Physiol Rev • VOL 88 • JULY 2008 • www.prv.org 1068 DIRK J. DUNCKER AND ROBERT J. BACHE can modify collateral conductance, thereby influencing the blood supply to the dependent myocardium. In addition, vasomotor activity in the resistance vessels of the collateral perfused vascular bed can influence the volume and distribution of blood flow within the collateral zone (Fig. 39). Finally, there is evidence that vasomotor control of resistance vessels in the normally perfused regions ofcollateralized hearts is altered compared with normal hearts (290, 561), indicating that the coronary vascular adaptations in hearts with a coronary occlusion involve alterations in resistance vessels at a global as well as a regional level (532). These observations caution against using vessels from the remote “normally perfused” myocardial region as control vessels. C. Exercise Training and the Coronary Collateral Circulation Exercise training has emerged as an intervention for primary and secondary prevention of coronary artery disease (340, 369). The mechanisms that have been proposed to contribute to the beneficial effects of exercise include regression of atherosclerosis, formation of collaterals (arteriogenesis), development of new vessels (angiogenesis/ vasculogenesis), and improvement of endothelial function. For an overview of the effects of exercise training in patients with coronary artery disease and potential mechanisms, the reader is referred to a recent article by Linke et al. (369). Here we focus on the effects of exercise training on the collateral circulation and the coronary microcirculation within collateral-dependent myocardium. 1. Coronary collateral blood flow A) NATIVE COLLATERAL VESSELS IN THE NORMAL HEART. The effect of exercise training on collateral function in hearts with a normal coronary circulation has been assessed by measuring retrograde flow from the cannulated collateraldependent coronary artery opened to atmospheric pressure (84, 114, 506) or by measurement of collateral blood flow with radioactive microspheres (112, 131, 319, 321, 501, 507) (Table 4). Studies comparing collateral function in trained and sedentary control groups at the end of a training period have produced exclusively negative results (84, 114, 131, 321, 501, 506, 507). To compensate for the substantial interanimal differences in native collaterals present in the dog, Knight and Stone (319) measured collateral blood flow in chronically instrumented dogs before and after exercise training; collateral blood flow was found to increase. Cohen (112) also observed a tendency for collateral flow to increase in chronically instrumented dogs following exercise training, but a similar increase was also observed in sedentary animals, suggest- Physiol Rev • VOL ing that the chronic instrumentation procedure stimulated the growth of coronary collateral vessels independent of exercise training. These findings indicate that physical conditioning does not enhance native collateral blood flow in the normal heart. B) EXERCISE TRAINING WITH A CORONARY STENOSIS OR OCCLUSION. There is considerable interest in whether chronic exercise is capable of stimulating development of coronary collateral vessels in patients with occlusive coronary artery disease. Human studies using angiography to assess the collateral vasculature in exercise-trained patients with coronary artery disease have generally yielded negative results (195, 428). These studies are limited to anatomic assessment of coronary collateral vessels without measurement of collateral blood flow. In contrast, Belardinelli et al. (50) reported a beneficial effect of 8 wk of moderate exercise training on collateral-dependent myocardial perfusion, as assessed by thallium uptake in patients with ischemic cardiomyopathy. Eckstein (156) was the first to report a beneficial effect of exercise training on collateral formation in dogs with a coronary artery stenosis (Table 5). The increase in retrograde blood flow from the cannulated collateral-dependent artery produced by exercise training was most striking in the presence of a mild stenosis that resulted in minimal collateral formation in the sedentary animals, suggesting that exercise produced ischemia which then acted to stimulate collateral vessel growth. Cohen et al. (115) reported similar results in chronically instrumented dogs. Thus, while exercise training under normal inflow conditions does not stimulate the formation of coronary collaterals, these studies suggest that chronic exercise resulting in or aggravating ischemia in a myocardial region distal to a coronary artery stenosis can stimulate collateral vessel growth. Studies of the effect of exercise training on collateral formation in response to a progressive coronary artery occlusion have yielded equivocal results (Table 5). Heaton et al. (254) and Neill and Oxendine (424) reported no differences between sedentary and trained dogs in collateral blood flow measured 6 – 8 wk after placement of an ameroid constrictor on a coronary artery, although a slight improvement in the ENDO/EPI blood flow ratio in the collateralized region was observed after exercise training (254). Exercise training may have failed to exert an effect because the stimulus for collateral formation was nearly maximal even in the sedentary animals. Thus blood flow to the collateral-dependent myocardium in sedentary dogs was not different from flow in the normal myocardium, so that exercise training may not have induced ischemia in these well-collateralized hearts (254, 424). In contrast, in swine, blood flow in the collateraldependent region was ⬍50% of blood flow in the normal myocardial segment during exercise, so that exerciseinduced ischemia might have further stimulated collateral 88 • JULY 2008 • www.prv.org 4. Physiol Rev • VOL 88 • JULY 2008 • www.prv.org Y Y Rat M A Swine M, F Swine M, F A Y Dog M Dog M, F A Dog M, F A Y ? Age 1.6 km/h, 15%; 60 min/day, 5 days/ wk, 12–24 wk 8–16 km/h, 0%; 90 min/day, 5 days/ wk, 4–6 wk 6.4–9.6 km/h, 20%; 75 min/day, 5 days/wk, 12 wk 5.8 km/h, 25%; 45 min/day, 7 days/ wk, 6 wk 6.5–9.6 km/h, 20%; 45 min/day, 5 days/wk, 4 wk 6.4–9.6 km/h, 20%; 75 min/day, 5 days/wk, 12 wk 6.4–9.6 km/h, 20%; 75 min/day, 5 days/wk, 12 wk 6 km/h, 0%; 60 min/day, 5 days/ wk, 10 mo 5.5 km/h, 0%; 40– 60 min/day, 5 days/wk, 8 wk Program ? Efficacy Open chest Open chest LVW 20%1, LVW/BW ? LVW 7, LVW/BW 16%1 SMVO2 1 Open chest Open chest HREX 2 ? Awake, rest Awake, rest Open chest extracorporeal perfusion Isolated bloodperfused heart Open chest Experimental Conditions HW 7, HW/BW 7 No b Noa HW 7, HW/BW 7 LVW 7, LVW/BW 7 ? Cardiac Hypertrophy HRREST 2 HRREST 2 HREX 2 ? ? HREX2, SMVO2 1 Running Training Sedentary Trained Sedentary Trained Sedentary Trained Sedentary Trained Sedentary Trained Trained Sedentary Trained Sedentary Exercise Sedentary Exercise Study Groups 7 7 3.2 3.1 5.0 4.1 Retrograde Flow, ml/min 1.58 1.55 1.48 Before Training 6.25 4.93 0.95 0.79 0.47 0.43 0.99 1.70 2.06 1.44 1.01 1.08 After Training Normal Zone Flow, ml 䡠 min⫺1 䡠 g⫺1 0.35 0.26 0.71 Before Training 0.39 0.43 0.21 0.31 0.06 0.05 0.26 0.24 0.61* 0.49* 1.03* 0.33 0.19 After Training Collateral Zone Flow, ml 䡠 min⫺1 䡠 g⫺1 Collateral zone/Normal zone myocardial blood flow ratios were measured with radioactive microspheres. a,bNot measured in that study but based on previous observations from the b same laboratory (aStone, 1980; aLiang et al., 1984; Cohen, 1978). *P ⬍ 0.05, after training vs. before training. †P ⬍ 0.05, exercise group vs. sedentary group. Scheffer and Verdouw (507) Koerner and Terjung (321) Dodd-o and Gwirtz (131) Sanders et al. (501) Knight and Stone (319) Cohen (112) Dog M Dog M Dog M, F Species, Sex Effects of exercise training on native collateral blood flow in animals with normal arterial inflow Scheel et al. (506) Burt and Jackson (84) Cohen et al. (114) Investigators TABLE CORONARY BLOOD FLOW 1069 Physiol Rev • VOL 88 • JULY 2008 • www.prv.org Dog M, F Swine M, F Swine M, F Schaper et al. (504) Bloor et al. (61)a Roth etbal. (482) Y A Y A ? A Y ? 6–8 km/h, 5%; 60 min/day, 5 days/wk, 5 mo 5 km/h, ⱖ5%; 30–50 min/day, 5 days/wk, 5 wk 6 km/h, 22%; 60 min/day, 5 days/wk, 4 wk 5.8 km/h, 25%; 45 min/day, 5 days/wk, 8 wk 8 km/h, 15%; 30 min/day, 5 days/wk, 5 wk 9.6 km/h, 5%; 60 min/day, 5 days/wk, 6 wk 7.5 km/h, 30%; 70 min/day, 5 days/wk, 6–8 wk 6.4–9.6 km/h, 20%; 75 min/day, 5 days/wk, 12 wk Program 4 wk postplacement 2 wk postplacement 2 wk postplacement 3 mo postplacement 1 wk postplacement 2 wk postplacement 2 wk postplacement 1 wk postplacement Start Experimental Conditions Open chest extracorporeal perfusion Rest Rest Exercise Exercise HW 7, HW/BW 7 Noc Fixed Coronary Artery Stenosis Hypertrophy Isolated bloodperfused heart; perfusion pressure 100 mmHg, maximal vasodilation Isolated bloodperfused heart; perfusion pressure 80 mmHg, maximal vasodilation Anesthetized, open chest Rest Rest Exercise Exercise HW 7, HW/BW 7 LVW 7, LVW/BW ? LVW 7, LVW/BW 7 HREX 2 HRREST 2 HREX 2 Rest Rest Exercise Exercise Rest Rest Pacing, 250 beats/min Pacing, 250 beats/min Open chest LVW 7, LVW/BW 7 HW 7, HW/BW 7 ? ? ? HREX 2 Progressive Coronary Artery Occlusion HREX 2 ? Efficacy Sedentary Trained Sedentary Trained Sedentary Trained Sedentary Trained Sedentary Trained Sedentary Trained Sedentary Trained Sedentary Trained Sedentary Trained Sedentary Trained Sedentary Trained Sedentary Trained Sedentary Trained Study Groups 17 35† 128 225† 34 50† Retrograde Flow, ml/min 1.33 1.08 1.79 1.79 1.28 1.16 3.30 2.65 Before Training 0.48 0.48 3.75 3.40 1.53 0.92 1.58 1.76 1.29 1.29 1.11 1.10 1.67 1.29 3.38 4.21 After Training Normal Zone 0.92 1.05 0.47 0.53 1.29 1.04 1.50 1.43 0.42 0.41 0.68 0.83 Before Training 1.01 1.11 0.48 0.72* 0.29 0.35† 1.75 1.65 1.23 0.88 1.54 1.65 1.28 1.22 0.82 0.81 0.58 0.70* 0.95 1.90* After Training Collateral Zone Normal zone flows and collateral zone flows were measured with radioactive microspheres. aIn the study by Bloor et al. (61), a fixed stenosis was initially implanted; however, at the time of autopsy the stenosis had progressed into a total occlusion in all animals. bIn the study of Roth et al. (482), absolute flows were not presented; the flow data in the collateral-dependent zone column represent collateral/normal zone ratios. cNot measured in that study but based on previous observations from the same laboratory (Cohen, 1978). *P ⬍ 0.05, after training vs. before training. †P ⬍ 0.05, exercise group vs. sedentary group. Dog M Scheel et al. (506) Neill and Dog Oxendine M, F (424) Dog M, F Dog M Cohen et al. (115) Heaton et al. (254) Dog M, F Eckstein (156) Age Running-Training Effects of exercise training on collateral blood flow in animals with impeded coronary arterial inflow Species, Sex, 5. Investigators TABLE 1070 DIRK J. DUNCKER AND ROBERT J. BACHE CORONARY BLOOD FLOW 1071 growth (61, 482). Moreover, differences in collateral blood flow between exercised and sedentary dogs might not have been detected because maximal resistance vessel dilation was not achieved, i.e., maximal collateral conductance was not assessed (254, 424). This could explain why retrograde blood flow (which is not affected by resistance vessel tone) was increased in trained dogs (424, 506). However, Schaper et al. (504) measured collateral blood flow in isolated dog hearts perfused with blood at constant pressure during maximal coronary resistance vessel dilation with adenosine and observed no effect of exercise training on maximum collateral blood flow. Maximum blood flow in the collateral-dependent region was 45% of the maximal flow in the normal myocardium of the sedentary animals, which makes it likely that blood flow to the occluded area was sufficient to minimize ischemia during exercise training. In none of the above studies was an attempt made to maximally dilate the coronary collateral vessels, e.g., with nitroglycerin, so that differences in collateral vessel tone could have influenced the measured collateral blood flow rates (249). C) SUMMARY AND INTEGRATION. Exercise does not stimulate growth of coronary collateral vessels in the normal heart. However, if exercise produces ischemia, which would be absent or minimal under resting conditions, there is evidence that collateral growth can be enhanced. Finally, when there is ischemia even under resting conditions, exercise may have only a modest additional effect. However, the concept that exercise-induced ischemia can enhance collateral vessel growth is not supported by two studies in which -adrenergic blockade with propranolol was used to minimize the occurrence of myocardial ischemia during gradual coronary occlusion with an ameroid constrictor. In these studies, propranolol did not impair the rate of collateral formation either in swine (546) or dogs (111). These findings suggest that other factors such as the pressure gradient between vascular beds which determines the flow rate and therefore the shear stress on the collateral vessel endothelium may be more important than ischemia in stimulating growth of collateral vessels. duced by vascular endothelial growth factor (VEGF165) in these vessels was enhanced by exercise training, and this was mediated principally via increased NO bioavailability. Increased vascular responsiveness to VEGF165 could also act to promote angiogenesis in the collateral zone. Interestingly, exercise training had no effect on bradykinininduced vasodilation of arterioles isolated from the remote normally perfused region of collateralized hearts (222), which contrasts with observations in arterioles obtained from exercise-trained normal hearts (414). The authors proposed that adaptive responses in the nonoccluded arterial beds (which are the source of collateral flow to the occluded territory) likely modify the response to exercise training (222). These observations exemplify the importance of including control vessels from normal hearts, rather than simply using vessels from the remote myogenic zone as controls. Exercise training also increases basal myogenic tone in collateral-dependent arterioles, similar to the effect of exercise training on arterioles in normal hearts (413). This increase in basal tone is associated with augmented vasodilator influences exerted by increased NO production and KV channel activity (252). In summary, exercise-induced adaptations of coronary resistance vessels within a collateral-dependent ventricular region consist of simultaneously increased basal tone and increased vasodilator influences, including increased NO production and K⫹ channel activity. These microvascular adaptations may provide a greater intrinsic capacity of local vascular control mechanisms to regulate blood flow to collateral-dependent myocardium and thereby contribute to the improved perfusion observed after exercise training. 2. Vasomotor control of coronary resistance vessels in collateral-dependent myocardium REFERENCES The effects of exercise training on coronary resistance vessel vasomotor control have been studied exclusively in swine instrumented with a chronic ameroid occlusion. Exercise training improved endothelium-dependent vasodilation (221) and adenosine-induced vasodilation (253) in epicardial arteries within the collateral-dependent region. Furthermore, Griffin et al. (222) reported that exercise training restored endothelium-dependent vasodilation in response to bradykinin in porcine arterioles isolated from collateral-dependent myocardium. Similarly, Fogarty et al. (189) reported that the vasodilation proPhysiol Rev • VOL ACKNOWLEDGMENTS Address for reprint requests and other correspondence: D. J. Duncker, Div. of Experimental Cardiology, Dept. of Cardiology, Thoraxcenter, Erasmus University Medical Center, Rotterdam, PO Box 2040, 3000 CA Rotterdam, The Netherlands (e-mail: d.duncker@erasmusmc.nl). 1. Afonso S, Bandow GT, Rowe GG. Indomethacin and the prostaglandin hypothesis of coronary blood flow regulation. J Physiol 241: 299 –308, 1974. 2. Aiello EA, Malcolm AT, Walsh MP, Cole WC. Beta-adrenoceptor activation and PKA regulate delayed rectifier K⫹ channels of vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 275: H448 –H459, 1998. 3. Alexander RW, Kent KM, Pisano JJ, Keiser HR, Cooper T. Regulation of postocclusive hyperemia by endogenously synthesized prostaglandins in the dog heart. J Clin Invest 55: 1174 –1181, 1975. 4. Altman J, Dulas D, Bache RJ. Effect of cyclooxygenase blockade on blood flow through well-developed coronary collateral vessels. Circ Res 70: 1091–1098, 1992. 5. Altman J, Dulas D, Pavek T, Laxson DD, Homans DC, Bache RJ. Endothelial function in well-developed canine coronary collat- 88 • JULY 2008 • www.prv.org 1072 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. DIRK J. DUNCKER AND ROBERT J. BACHE eral vessels. Am J Physiol Heart Circ Physiol 264: H567–H572, 1993. Altman JD, Dulas D, Pavek T, Bache RJ. Effect of aspirin on coronary collateral blood flow. Circulation 87: 583–589, 1993. Altman JD, Kinn J, Duncker DJ, Bache RJ. Effect of inhibition of nitric oxide formation on coronary blood flow during exercise in the dog. Cardiovasc Res 28: 119 –124, 1994. Altman JD, Klassen CL, Bache RJ. Cyclooxygenase blockade limits blood flow to collateral-dependent myocardium during exercise. Cardiovasc Res 30: 697–704, 1995. Amezcua JL, Palmer RM, de Souza BM, Moncada S. Nitric oxide synthesized from L-arginine regulates vascular tone in the coronary circulation of the rabbit. Br J Pharmacol 97: 1119 –1124, 1989. Angus JA, Ward JE, Smolich JJ, McPherson GA. Reactivity of canine isolated epicardial collateral coronary arteries. Relation to vessel structure. Circ Res 69: 1340 –1352, 1991. Anversa P, Beghi C, Levicky V, McDonald SL, Kikkawa Y. Morphometry of right ventricular hypertrophy induced by strenuous exercise in rat. Am J Physiol Heart Circ Physiol 243: H856 – H861, 1982. Anversa P, Beghi C, Levicky V, McDonald SL, Kikkawa Y, Olivetti G. Effects of strenuous exercise on the quantitative morphology of left ventricular myocardium in the rat. J Mol Cell Cardiol 17: 587–595, 1985. Anversa P, Levicky V, Beghi C, McDonald SL, Kikkawa Y. Morphometry of exercise-induced right ventricular hypertrophy in the rat. Circ Res 52: 57– 64, 1983. Anversa P, Ricci R, Olivetti G. Effects of exercise on the capillary vasculature of the rat heart. Circulation 75: I12–18, 1987. Archie JP Jr. Transmural distribution of intrinsic and transmitted left ventricular diastolic intramyocardial pressure in dogs. Cardiovasc Res 12: 255–262, 1978. Armstrong RB, Delp MD, Goljan EF, Laughlin MH. Distribution of blood flow in muscles of miniature swine during exercise. J Appl Physiol 62: 1285–1298, 1987. Armstrong RB, Essen-Gustavsson B, Hoppeler H, Jones JH, Kayar SR, Laughlin MH, Lindholm A, Longworth KE, Taylor CR, Weibel ER. O2 delivery at VO2max and oxidative capacity in muscles of standardbred horses. J Appl Physiol 73: 2274 –2282, 1992. Astrand PO, Rodahl K. Textbook of work physiology. In: Textbook of Work Physiology. New York: McGraw-Hill, 1977, p. 681. Aversano T, Becker LC. Persistence of coronary vasodilator reserve despite functionally significant flow reduction. Am J Physiol Heart Circ Physiol 248: H403–H411, 1985. Aversano T, Klocke FJ, Mates RE, Canty JM Jr. Preloadinduced alterations in capacitance-free diastolic pressure-flow relationship. Am J Physiol Heart Circ Physiol 246: H410 –H417, 1984. Aversano T, Ouyang P, Silverman H. Blockade of the ATPsensitive potassium channel modulates reactive hyperemia in the canine coronary circulation. Circ Res 69: 618 – 622, 1991. Bacchus AN, Ely SW, Knabb RM, Rubio R, Berne RM. Adenosine and coronary blood flow in conscious dogs during normal physiological stimuli. Am J Physiol Heart Circ Physiol 243: H628 – H633, 1982. Bache RJ, Cobb FR. Effect of maximal coronary vasodilation on transmural myocardial perfusion during tachycardia in the awake dog. Circ Res 41: 648 – 653, 1977. Bache RJ, Dai XZ. Myocardial oxygen consumption during exercise in the presence of left ventricular hypertrophy secondary to supravalvular aortic stenosis. J Am Coll Cardiol 15: 1157–1164, 1990. Bache RJ, Dai XZ. The thromboxane A2 mimetic U46619 worsens canine myocardial hypoperfusion during exercise in the presence of a coronary artery stenosis. Cardiovasc Res 26: 351–356, 1992. Bache RJ, Dai XZ, Alyono D, Vrobel TR, Homans DC. Myocardial blood flow during exercise in dogs with left ventricular hypertrophy produced by aortic banding and perinephritic hypertension. Circulation 76: 835– 842, 1987. Bache RJ, Dai XZ, Herzog CA, Schwartz JS. Effects of nonselective and selective alpha 1-adrenergic blockade on coronary blood flow during exercise. Circ Res 61: II36 – 41, 1987. Physiol Rev • VOL 28. Bache RJ, Dai XZ, Schwartz JS, Homans DC. Role of adenosine in coronary vasodilation during exercise. Circ Res 62: 846 – 853, 1988. 29. Bache RJ, Duncker DJ. Coronary steal. Acc Cur J Rev 9 –12, 1994. 30. Bache RJ, Foreman B, Hautamaa PV. Response of canine coronary collateral vessels to ergonovine and alpha-adrenergic stimulation. Am J Physiol Heart Circ Physiol 261: H1019 –H1025, 1991. 31. Bache RJ, Homans DC, Schwartz JS, Dai XZ. Differences in the effects of alpha-1 adrenergic blockade with prazosin and indoramin on coronary blood flow during exercise. J Pharmacol Exp Ther 245: 232–237, 1988. 32. Bache RJ, Schwartz JS. Myocardial blood flow during exercise after gradual coronary occlusion in the dog. Am J Physiol Heart Circ Physiol 245: H131–H138, 1983. 33. Bache RJ, Stark RP, Duncker DJ. Serotonin selectively aggravates subendocardial ischemia distal to a coronary artery stenosis during exercise. Circulation 86: 1559 –1565, 1992. 34. Bache RJ, Vrobel TR, Ring WS, Emery RW, Andersen RW. Regional myocardial blood flow during exercise in dogs with chronic left ventricular hypertrophy. Circ Res 48: 76 – 87, 1981. 35. Baldwin KM. Effects of chronic exercise on biochemical and functional properties of the heart. Med Sci Sports Exercise 17: 522–528, 1985. 36. Ball RM, Bache RJ. Distribution of myocardial blood flow in the exercising dog with restricted coronary artery inflow. Circ Res 38: 60 – 66, 1976. 37. Ball RM, Bache RJ, Cobb FR, Greenfield JC Jr. Regional myocardial blood flow during graded treadmill exercise in the dog. J Clin Invest 55: 43– 49, 1975. 38. Barbier J, Reland S, Ville N, Rannou-Bekono F, Wong S, Carre F. The effects of exercise training on myocardial adrenergic and muscarinic receptors. Clin Auton Res 16: 61– 65, 2006. 39. Bardenheuer H, Schrader J. Supply-to-demand ratio for oxygen determines formation of adenosine by the heart. Am J Physiol Heart Circ Physiol 250: H173–H180, 1986. 40. Barnard RJ, Duncan HW, Baldwin KM, Grimditch G, Buckberg GD. Effects of intensive exercise training on myocardial performance and coronary blood flow. J Appl Physiol 49: 444 – 449, 1980. 41. Barnard RJ, Duncan HW, Livesay JJ, Buckberg GD. Coronary vasodilator reserve and flow distribution during near-maximal exercise in dogs. J Appl Physiol 43: 988 –992, 1977. 42. Bassenge E, Busse R. Endothelial modulation of coronary tone. Prog Cardiovasc Dis 30: 349 –380, 1988. 43. Bassenge E, Kucharczyk M, Holtz J, Stoian D. Treadmill exercise in dogs under adrenergic blockade: adaptation of coronary and systemic hemodynamics. Pflügers Arch 332: 40 –55, 1972. 44. Bauman RP, Rembert JC, Greenfield JC Jr. Regional blood flow in canine atria during exercise. Am J Physiol Heart Circ Physiol 265: H629 –H632, 1993. 45. Bauman RP, Rembert JC, Greenfield JC Jr. Regional vascular reserve in canine atria and ventricles during rest and exercise. Am J Physiol Heart Circ Physiol 269: H1578 –H1582, 1995. 46. Baumgart D, Ehring T, Kowallik P, Guth BD, Krajcar M, Heusch G. Impact of alpha-adrenergic coronary vasoconstriction on the transmural myocardial blood flow distribution during humoral and neuronal adrenergic activation. Circ Res 73: 869 – 886, 1993. 47. Baumgarten CR, Linz W, Kunkel G, Scholkens BA, Wiemer G. Ramiprilat increases bradykinin outflow from isolated hearts of rat. Br J Pharmacol 108: 293–295, 1993. 48. Baur TS, Brodowicz GR, Lamb DR. Indomethacin suppresses the coronary flow response to hypoxia in exercise trained and sedentary rats. Cardiovasc Res 24: 733–736, 1990. 49. Baydoun AR, Woodward B. Effects of bradykinin in the rat isolated perfused heart: role of kinin receptors and endotheliumderived relaxing factor. Br J Pharmacol 103: 1829 –1833, 1991. 50. Belardinelli R, Georgiou D, Ginzton L, Cianci G, Purcaro A. Effects of moderate exercise training on thallium uptake and contractile response to low-dose dobutamine of dysfunctional myocardium in patients with ischemic cardiomyopathy. Circulation 97: 553–561, 1998. 88 • JULY 2008 • www.prv.org CORONARY BLOOD FLOW 51. Bell RD, Rasmussen RL. Exercise and the myocardial capillaryfiber ratio during growth. Growth 38: 237–244, 1974. 52. Bellamy RF. Diastolic coronary artery pressure-flow relations in the dog. Circ Res 43: 92–101, 1978. 53. Berne RM, Rubio R. Coronary circulation In: Handbook of Physiology. The Cardiavascular System. The Heart. Bethesda, MD: Am. Physiol. Soc., 1979, sect. 2, vol. I, chapt. 25, p. 873–952. 54. Berne RM, Rubio R. Regulation of coronary blood flow. Adv Cardiol 12: 303–317, 1974. 55. Bernstein D. Exercise assessment of transgenic models of human cardiovascular disease. Physiol Gen 13: 217–226, 2003. 56. Bernstein RD, Ochoa FY, Xu X, Forfia P, Shen W, Thompson CI, Hintze TH. Function and production of nitric oxide in the coronary circulation of the conscious dog during exercise. Circ Res 79: 840 – 848, 1996. 57. Binak K, Harmanci N, Sirmaci N, Ataman N, Ogan H. Oxygen extraction rate of the myocardium at rest and on exercise in various conditions. Br Heart J 29: 422– 427, 1967. 58. Blinks JR, Endoh M. Modification of myofibrillar responsiveness to Ca2⫹ as an inotropic mechanism. Circulation 73: III85–98, 1986. 59. Blomqvist CG, Saltin B. Cardiovascular adaptations to physical training. Annu Rev Physiol 45: 169 –189, 1983. 60. Bloor CM, Leon AS. Interaction of age and exercise on the heart and its blood supply. Lab Invest 22: 160 –165, 1970. 61. Bloor CM, White FC, Sanders TM. Effects of exercise on collateral development in myocardial ischemia in pigs. J Appl Physiol 56: 656 – 665, 1984. 62. Boerth RC, Covell JW, Seagren SC, Pool PE. High-energy phosphate concentrations in dog myocardium during stress. Am J Physiol 216: 1103–1106, 1969. 63. Bolter CP, Hughson RL, Critz JB. Intrinsic rate and cholinergic sensitivity of isolated atria from trained and sedentary rats. Proc Soc Exp Biol Med 144: 364 –367, 1973. 64. Boucek RJ, Morales AR, Romanelli R, Judkins MP. Coronary artery branch arteries and collateral circulation: the vascular supply to specialized areas of the heart. In: Coronary Artery Disease. Baltimore, MD: Williams & Wilkins, 1983, p. 1296 –1305. 65. Bove AA, Dewey JD. Effects of serotonin and histamine on proximal and distal coronary vasculature in dogs: comparison with alpha-adrenergic stimulation. Am J Cardiol 52: 1333–1339, 1983. 66. Bove AA, Dewey JD. Proximal coronary vasomotor reactivity after exercise training in dogs. Circulation 71: 620 – 625, 1985. 67. Bove AA, Hultgren PB, Ritzer TF, Carey RA. Myocardial blood flow and hemodynamic responses to exercise training in dogs. J Appl Physiol 46: 571–578, 1979. 68. Bowles DK, Hu Q, Laughlin MH, Sturek M. Exercise training increases L-type calcium current density in coronary smooth muscle. Am J Physiol Heart Circ Physiol 275: H2159 –H2169, 1998. 69. Bowles DK, Laughlin MH, Sturek M. Exercise training increases K⫹-channel contribution to regulation of coronary arterial tone. J Appl Physiol 84: 1225–1233, 1998. 70. Bowles DK, Wamhoff BR. Coronary smooth muscle adaptation to exercise: does it play a role in cardioprotection? Acta Physiol Scand 178: 117–121, 2003. 71. Brandi G, McGregor M. Intramural pressure in the left ventricle of the dog. Cardiovasc Res 3: 472– 475, 1969. 72. Braunwald E, Ross J Jr. Control of cardiac performance. In: Handbook of Physiology. The Cardiovascular System. The Heart. Bethesda, MD: Am. Physiol. Soc., 1979, sect. 2, vol. I, chapt. 15, p. 533–580. 73. Brayden JE. Functional roles of KATP channels in vascular smooth muscle. Clin Exp Pharmacol Physiol 29: 312–316, 2002. 74. Brayden JE. Potassium channels in vascular smooth muscle. Clin Exp Pharmacol Physiol 23: 1069 –1076, 1996. 75. Brazenor RM, Angus JA. Actions of serotonin antagonists on dog coronary artery. Eur J Pharmacol 81: 569 –576, 1982. 76. Breisch EA, White FC, Nimmo LE, Bloor CM. Cardiac vasculature and flow during pressure-overload hypertrophy. Am J Physiol Heart Circ Physiol 251: H1031–H1037, 1986. 77. Breisch EA, White FC, Nimmo LE, McKirnan MD, Bloor CM. Exercise-induced cardiac hypertrophy: a correlation of blood flow and microvasculature. J Appl Physiol 60: 1259 –1267, 1986. Physiol Rev • VOL 1073 78. Britman NA, Levine HJ. Contractile element work: a major determinant of myocardial oxygen consumption. J Clin Invest 43: 1397–1408, 1964. 79. Broten TP, Miyashiro JK, Moncada S, Feigl EO. Role of endothelium-derived relaxing factor in parasympathetic coronary vasodilation. Am J Physiol Heart Circ Physiol 262: H1579 –H1584, 1992. 80. Broten TP, Romson JL, Fullerton DA, Van Winkle DM, Feigl EO. Synergistic action of myocardial oxygen and carbon dioxide in controlling coronary blood flow. Circ Res 68: 531–542, 1991. 81. Brown IP, Thompson CI, Belloni FL. Role of nitric oxide in hypoxic coronary vasodilatation in isolated perfused guinea pig heart. Am J Physiol Heart Circ Physiol 264: H821–H829, 1993. 82. Buffington CW, Feigl EO. Adrenergic coronary vasoconstriction in the presence of coronary stenosis in the dog. Circ Res 48: 416 – 423, 1981. 83. Burke SE, Lefer AM, Nicolaou KC, Smith GM, Smith JB. Responsiveness of platelets and coronary arteries from different species to synthetic thromboxane and prostaglandin endoperoxide analogues. Br J Pharmacol 78: 287–292, 1983. 84. Burt JJ, Jackson R. The effects of physical exercise on the coronary collateral circulation of dogs. J Sports Med Phys Fitness 5: 203–206, 1965. 85. Busse R, Lamontagne D. Endothelium-derived bradykinin is responsible for the increase in calcium produced by angiotensinconverting enzyme inhibitors in human endothelial cells. NaunynSchmiedebergs Arch Pharmacol 344: 126 –129, 1991. 86. Buttrick PM, Levite HA, Schaible TF, Ciambrone G, Scheuer J. Early increases in coronary vascular reserve in exercised rats are independent of cardiac hypertrophy. J Appl Physiol 59: 1861– 1865, 1985. 87. Buttrick PM, Scheuer J. Physiologic, biochemical, coronary adaptation to exercise conditioning. Cardiol Clin 5: 259 –270, 1987. 88. Camici PG, Rimoldi OE. Myocardial blood flow in patients with hibernating myocardium. Cardiovasc Res 57: 302–311, 2003. 89. Canty JM Jr, Fallavollita JA. Chronic hibernation and chronic stunning: a continuum. J Nucl Cardiol 7: 509 –527, 2000. 90. Canty JM Jr, Fallavollita JA. Hibernating myocardium. J Nucl Cardiol 12: 104 –119, 2005. 91. Canty JM Jr, Klocke FJ. Reduced regional myocardial perfusion in the presence of pharmacologic vasodilator reserve. Circulation 71: 370 –377, 1985. 92. Canty JM Jr, Smith TP, Jr. Adenosine-recruitable flow reserve is absent during myocardial ischemia in unanesthetized dogs studied in the basal state. Circ Res 76: 1079 –1087, 1995. 93. Carey RA, Santamore WP, Michele JJ, Bove AA. Effects of endurance training on coronary resistance in dogs. Med Sci Sports Exerc 15: 355–359, 1983. 94. Carlsson S, Ljungqvist A, Tornling G, Unge G. The myocardial capillary vasculature in repeated physical exercise. An experimental investigation in the rat. Acta Pathol Microbiol Scand 86: 117– 119, 1978. 95. Case RB, Felix A, Wachter M, Kyriakidis G, Castellana F. Relative effect of CO2 on canine coronary vascular resistance. Circ Res 42: 410 – 418, 1978. 96. Case RB, Greenberg H. The response of canine coronary vascular resistance to local alterations in coronary arterial PCO2. Circ Res 39: 558 –566, 1976. 97. Cerretelli P, Di Prampero PE. Gas exchange in exercise. In: Handbook of Physiology. The Respiratory System. Gas Exchange. Bethesda: Am. Physiol. Soc., 1987, sect. 3, vol. IV. 98. Chilian WM. Functional distribution of alpha 1- and alpha 2-adrenergic receptors in the coronary microcirculation. Circulation 84: 2108 –2122, 1991. 99. Chilian WM. Microvascular pressures and resistances in the left ventricular subepicardium and subendocardium. Circ Res 69: 561– 570, 1991. 100. Chilian WM, Boatwright RB, Shoji T, Griggs DM Jr. Evidence against significant resting sympathetic coronary vasoconstrictor tone in the conscious dog. Circ Res 49: 866 – 876, 1981. 101. Chilian WM, Eastham CL, Marcus ML. Microvascular distribution of coronary vascular resistance in beating left ventricle. Am J Physiol Heart Circ Physiol 251: H779 –H788, 1986. 88 • JULY 2008 • www.prv.org 1074 DIRK J. DUNCKER AND ROBERT J. BACHE 102. Chilian WM, Harrison DG, Haws CW, Snyder WD, Marcus ML. Adrenergic coronary tone during submaximal exercise in the dog is produced by circulating catecholamines. Evidence for adrenergic denervation supersensitivity in the myocardium but not in coronary vessels. Circ Res 58: 68 – 82, 1986. 103. Chilian WM, Layne SM. Coronary microvascular responses to reductions in perfusion pressure. Evidence for persistent arteriolar vasomotor tone during coronary hypoperfusion. Circ Res 66: 1227– 1238, 1990. 104. Chilian WM, Layne SM, Eastham CL, Marcus ML. Heterogeneous microvascular coronary alpha-adrenergic vasoconstriction. Circ Res 64: 376 –388, 1989. 105. Chilian WM, Layne SM, Klausner EC, Eastham CL, Marcus ML. Redistribution of coronary microvascular resistance produced by dipyridamole. Am J Physiol Heart Circ Physiol 256: H383–H390, 1989. 106. Clair MJ, Krombach RS, Hendrick JW, Houck WV, Hebbar L, Kribbs SB, Rios G, Whitebread S, Mukherjee R, de Gasparo M, Spinale FG. AT1 angiotensin II receptor inhibition in pacinginduced heart failure: effects on left ventricular performance and regional blood flow patterns. J Card Fail 4: 311–323, 1998. 107. Clausen JP, Larsen OA, Trap-Jensen J. Physical training in the management of coronary artery disease. Circulation 40: 143–154, 1969. 108. Clozel JP, Sprecher U. Influence of low perfusion pressure on effect of endothelin on coronary vascular bed. Am J Physiol Heart Circ Physiol 260: H893–H901, 1991. 109. Cocks TM, Angus JA. Endothelium-dependent relaxation of coronary arteries by noradrenaline and serotonin. Nature 305: 627– 630, 1983. 110. Cohen MV. Coronary vascular reserve in the greyhound with left ventricular hypertrophy. Cardiovasc Res 20: 182–194, 1986. 111. Cohen MV. Lack of effect of propranolol on canine coronary collateral development during progressive coronary stenosis and occlusion. Cardiovasc Res 27: 249 –254, 1993. 112. Cohen MV. Training in dogs with normal coronary arteries: lack of effect on collateral development. Cardiovasc Res 24: 121–128, 1990. 113. Cohen MV, Downey JM, Sonnenblick EH, Kirk ES. The effects of nitroglycerin on coronary collaterals and myocardial contractility. J Clin Invest 52: 2836 –2847, 1973. 114. Cohen MV, Yipintsoi T, Malhotra A, Penpargkul S, Scheuer J. Effect of exercise on collateral development in dogs with normal coronary arteries. J Appl Physiol 45: 797– 805, 1978. 115. Cohen MV, Yipintsoi T, Scheuer J. Coronary collateral stimulation by exercise in dogs with stenotic coronary arteries. J Appl Physiol 52: 664 – 671, 1982. 116. Cohen RA. Contractions of isolated canine coronary arteries resistant to S2-serotonergic blockade. J Pharmacol Exp Ther 237: 548 –552, 1986. 117. Cohen RA, Shepherd JT, Vanhoutte PM. 5-Hydroxytryptamine can mediate endothelium-dependent relaxation of coronary arteries. Am J Physiol Heart Circ Physiol 245: H1077–H1080, 1983. 118. Colan SD. Mechanics of left ventricular systolic and diastolic function in physiologic hypertrophy of the athlete heart. Cardiol Clin 10: 227–240, 1992. 119. Cowan CL, McKenzie JE. Cholinergic regulation of resting coronary blood flow in domestic swine. Am J Physiol Heart Circ Physiol 259: H109 –H115, 1990. 120. Cutilletta AF, Edmiston K, Dowell RT. Effect of a mild exercise program on myocardial function and the development of hypertrophy. J Appl Physiol 46: 354 –360, 1979. 121. Dai XZ, Bache RJ. Effect of indomethacin on coronary blood flow during graded treadmill exercise in the dog. Am J Physiol Heart Circ Physiol 247: H452–H458, 1984. 122. Dai XZ, Herzog CA, Schwartz JS, Bache RJ. coronary blood flow during exercise following nonselective and selective alpha 1-adrenergic blockade with indoramin. J Cardiovasc Pharmacol 8: 574 –581, 1986. 123. Dai XZ, Sublett E, Lindstrom P, Schwartz JS, Homans DC, Bache RJ. Coronary flow during exercise after selective alpha 1and alpha 2-adrenergic blockade. Am J Physiol Heart Circ Physiol 256: H1148 –H1155, 1989. Physiol Rev • VOL 124. Daut J, Maier-Rudolph W, von Beckerath N, Mehrke G, Gunther K, Goedel-Meinen L. Hypoxic dilation of coronary arteries is mediated by ATP-sensitive potassium channels. Science 247: 1341–1344, 1990. 125. De Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev 52: 415– 472, 2000. 126. Dellsperger KC. Potassium channels and the coronary circulation. Clin Exp Pharmacol Physiol 23: 1096 –1101, 1996. 127. Dempsey JA, Hanson PG, Henderson KS. Exercise-induced arterial hypoxaemia in healthy human subjects at sea level. J Physiol 355: 161–175, 1984. 128. Deussen A, Borst M, Kroll K, Schrader J. Formation of Sadenosylhomocysteine in the heart. II. A sensitive index for regional myocardial underperfusion. Circ Res 63: 250 –261, 1988. 129. DiCarlo SE, Blair RW, Bishop VS, Stone HL. Daily exercise enhances coronary resistance vessel sensitivity to pharmacological activation. J Appl Physiol 66: 421– 428, 1989. 130. DiCarlo SE, Blair RW, Bishop VS, Stone HL. Role of beta 2-adrenergic receptors on coronary resistance during exercise. J Appl Physiol 64: 2287–2293, 1988. 131. Dodd-o JM, Gwirtz PA. Cardiac response to acute coronary artery occlusion in exercise-trained dogs. Med Sci Sports Exerc 24: 1245–1251, 1992. 132. Dodd-o JM, Gwirtz PA. Coronary alpha 1-adrenergic constrictor tone varies with intensity of exercise. Med Sci Sports Exerc 28: 62–71, 1996. 133. Donald DE. Myocardial performance after excision of the extrinsic cardiac nerves in the dog. Circ Res 34: 417– 424, 1974. 134. Downey JM, Kirk ES. Inhibition of coronary blood flow by a vascular waterfall mechanism. Circ Res 36: 753–760, 1975. 135. Duffy SJ, Castle SF, Harper RW, Meredith IT. Contribution of vasodilator prostanoids and nitric oxide to resting flow, metabolic vasodilation, flow-mediated dilation in human coronary circulation. Circulation 100: 1951–1957, 1999. 136. Duling BR, Berne RM. Longitudinal gradients in periarteriolar oxygen tension. A possible mechanism for the participation of oxygen in local regulation of blood flow. Circ Res 27: 669 – 678, 1970. 137. Duncker DJ, Bache RJ. Inhibition of nitric oxide production aggravates myocardial hypoperfusion during exercise in the presence of a coronary artery stenosis. Circ Res 74: 629 – 640, 1994. 138. Duncker DJ, Bache RJ. Regulation of coronary vasomotor tone under normal conditions and during acute myocardial hypoperfusion. Pharmacol Ther 86: 87–110, 2000. 139. Duncker DJ, Haitsma DB, Liem DA, Verdouw PD, Merkus D. Exercise unmasks autonomic dysfunction in swine with a recent myocardial infarction. Cardiovasc Res 65: 889 – 896, 2005. 140. Duncker DJ, Ishibashi Y, Bache RJ. Effect of treadmill exercise on transmural distribution of blood flow in hypertrophied left ventricle. Am J Physiol Heart Circ Physiol 275: H1274 –H1282, 1998. 141. Duncker DJ, Laxson DD, Lindstrom P, Bache RJ. Endogenous adenosine and coronary vasoconstriction in hypoperfused myocardium during exercise. Cardiovasc Res 27: 1592–1597, 1993. 142. Duncker DJ, McFalls EO, Krams R, Verdouw PD. Pressuremaximal coronary flow relationship in regionally stunned porcine myocardium. Am J Physiol Heart Circ Physiol 262: H1744 –H1751, 1992. 143. Duncker DJ, Merkus D. Acute adaptations of the coronary circulation to exercise. Cell Biochem Biophys 43: 17–35, 2005. 144. Duncker DJ, Mizrahi J, Bache RJ. Nitrovasodilators ITF 296 and isosorbide dinitrate exert antiischemic activity by dilating coronary penetrating arteries. J Cardiovasc Pharmacol 25: 823– 832, 1995. 145. Duncker DJ, Oei HH, Hu F, Stubenitsky R, Verdouw PD. Role of KATP channels in regulation of systemic, pulmonary, coronary vasomotor tone in exercising swine. Am J Physiol Heart Circ Physiol 280: H22–H33, 2001. 146. Duncker DJ, Saxena PR, Verdouw PD. The effects of nisoldipine alone and in combination with beta-adrenoceptor blockade on systemic haemodynamics and myocardial performance in conscious pigs. Eur Heart J 8: 1332–1339, 1987. 88 • JULY 2008 • www.prv.org CORONARY BLOOD FLOW 147. Duncker DJ, Stubenitsky R, Tonino PA, Verdouw PD. Nitric oxide contributes to the regulation of vasomotor tone but does not modulate O2-consumption in exercising swine. Cardiovasc Res 47: 738 –748, 2000. 148. Duncker DJ, Stubenitsky R, Verdouw PD. Autonomic control of vasomotion in the porcine coronary circulation during treadmill exercise: evidence for feed-forward beta-adrenergic control. Circ Res 82: 1312–1322, 1998. 149. Duncker DJ, Stubenitsky R, Verdouw PD. Role of adenosine in the regulation of coronary blood flow in swine at rest and during treadmill exercise. Am J Physiol Heart Circ Physiol 275: H1663– H1672, 1998. 150. Duncker DJ, Van Zon NS, Altman JD, Pavek TJ, Bache RJ. Role of KATP channels in coronary vasodilation during exercise. Circulation 88: 1245–1253, 1993. 151. Duncker DJ, Van Zon NS, Crampton M, Herrlinger S, Homans DC, Bache RJ. Coronary pressure-flow relationship and exercise: contributions of heart rate, contractility, alpha 1-adrenergic tone. Am J Physiol Heart Circ Physiol 266: H795–H810, 1994. ⫹ 152. Duncker DJ, van Zon NS, Ishibashi Y, Bache RJ. Role of KATP channels and adenosine in the regulation of coronary blood flow during exercise with normal and restricted coronary blood flow. J Clin Invest 97: 996 –1009, 1996. 153. Duncker DJ, van Zon NS, Pavek TJ, Herrlinger SK, Bache RJ. Endogenous adenosine mediates coronary vasodilation during ex⫹ ercise after K(ATP) channel blockade. J Clin Invest 95: 285–295, 1995. 154. Duncker DJ, Zhang J, Bache RJ. Coronary pressure-flow relation in left ventricular hypertrophy. Importance of changes in back pressure versus changes in minimum resistance. Circ Res 72: 579 – 587, 1993. 155. Duncker DJ, Zhang J, Crampton MJ, Bache RJ. Alpha 1-adrenergic tone does not influence the transmural distribution of myocardial blood flow during exercise in dogs with pressure overload left ventricular hypertrophy. Basic Res Cardiol 90: 73– 83, 1995. 156. Eckstein RW. Effect of exercise and coronary artery narrowing on coronary collateral circulation. Circ Res 5: 230 –235, 1957. 157. Edlund A, Conradsson T, Sollevi A. A role for adenosine in coronary vasoregulation in man. Effects of theophylline and enprofylline. Clin Physiol 15: 623– 636, 1995. 158. Edlund A, Sollevi A. Theophylline increases coronary vascular tone in humans: evidence for a role of endogenous adenosine in flow regulation. Acta Physiol Scand 155: 303–311, 1995. 159. Edlund A, Sollevi A, Wennmalm A. The role of adenosine and prostacyclin in coronary flow regulation in healthy man. Acta Physiol Scand 135: 39 – 46, 1989. 160. Egashira K, Katsuda Y, Mohri M, Kuga T, Tagawa T, Kubota T, Hirakawa Y, Takeshita A. Role of endothelium-derived nitric oxide in coronary vasodilatation induced by pacing tachycardia in humans. Circ Res 79: 331–335, 1996. 161. Ehsani AA, Hagberg JM, Hickson RC. Rapid changes in left ventricular dimensions and mass in response to physical conditioning and deconditioning. Am J Cardiol 42: 52–56, 1978. 162. Ekstrom-Jodal B, Haggendal E, Malmberg R, Svedmyr N. The effect of adrenergic receptor blockade on coronary circulation in man during work. Acta Med Scand 191: 245–248, 1972. 163. Ellis AK, Klocke FJ. Effects of preload on the transmural distribution of perfusion and pressure-flow relationships in the canine coronary vascular bed. Circ Res 46: 68 –77, 1980. 164. Ellsworth ML. Red blood cell-derived ATP as a regulator of skeletal muscle perfusion. Med Sci Sports Exerc 36: 35– 41, 2004. 165. Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol Heart Circ Physiol 269: H2155–H2161, 1995. 166. Ely SW, Knabb RM, Bacchus AN, Rubio R, Berne RM. Measurements of coronary plasma and pericardial infusate adenosine concentrations during exercise in conscious dog: relationship to myocardial oxygen consumption and coronary blood flow. J Mol Cell Cardiol 15: 673– 683, 1983. 167. Engelhardt WV. Cardiovascular effects of exercise and training in horses. Adv Vet Sci Comp Med 7: 173–205, 1977. Physiol Rev • VOL 1075 168. Engler RL. Adenosine an autocoid. In: Scientific Foundations, edited by Fozzard HA, Haber E, Katz AM, and Morgan HE. New York: Raven, 1991, p. 1745–1765. 169. Estes EH Jr, Entman ML, Dixon HB 2nd, Hackel DB. The vascular supply of the left ventricular wall Anatomic observations, plus a hypothesis regarding acute events in coronary artery disease. Am Heart J 71: 58 – 67, 1966. 170. Fallavollita JA, Logue M, Canty JM Jr. Stability of hibernating myocardium in pigs with a chronic left anterior descending coronary artery stenosis: absence of progressive fibrosis in the setting of stable reductions in flow, function and coronary flow reserve. J Am Coll Cardiol 37: 1989 –1995, 2001. 171. Fallavollita JA, Malm BJ, Canty JM Jr. Hibernating myocardium retains metabolic and contractile reserve despite regional reductions in flow, function, oxygen consumption at rest. Circ Res 92: 48 –55, 2003. 172. Fallavollita JA, Perry BJ, Canty JM Jr. 18F-2-deoxyglucose deposition and regional flow in pigs with chronically dysfunctional myocardium. Evidence for transmural variations in chronic hibernating myocardium. Circulation 95: 1900 –1909, 1997. 173. Fam WM, McGregor M. Effect of coronary vasodilator drugs on retrograde flow in areas of chronic myocardial ischemia. Circ Res 15: 355–364, 1964. 174. Farias M 3rd, Gorman MW, Savage MV, Feigl EO. Plasma ATP during exercise: possible role in regulation of coronary blood flow. Am J Physiol Heart Circ Physiol 288: H1586 –H1590, 2005. 175. Farouque HM, Worthley SG, Meredith IT. Effect of ATP-sensitive potassium channel inhibition on coronary metabolic vasodilation in humans. Arterioscler Thromb Vasc Biol 24: 905–910, 2004. 176. Farouque HM, Worthley SG, Meredith IT, Skyrme-Jones RA, Zhang MJ. Effect of ATP-sensitive potassium channel inhibition on resting coronary vascular responses in humans. Circ Res 90: 231–236, 2002. 177. Favret F, Henderson KK, Clancy RL, Richalet JP, Gonzalez NC. Exercise training alters the effect of chronic hypoxia on myocardial adrenergic and muscarinic receptor number. J Appl Physiol 91: 1283–1288, 2001. 178. Fedele FA, Gewirtz H, Capone RJ, Sharaf B, Most AS. Metabolic response to prolonged reduction of myocardial blood flow distal to a severe coronary artery stenosis. Circulation 78: 729 – 735, 1988. 179. Fedor JM, Rembert JC, McIntosh DM, Greenfield JC Jr. Effects of exercise- and pacing-induced tachycardia on coronary collateral flow in the awake dog. Circ Res 46: 214 –220, 1980. 180. Feigl EO. Berne’s adenosine hypothesis of coronary blood flow control. Am J Physiol Heart Circ Physiol 287: H1891–H1894, 2004. 181. Feigl EO. Coronary physiology. Physiol Rev 63: 1–205, 1983. 182. Feigl EO. Neural control of coronary blood flow. J Vasc Res 35: 85–92, 1998. 183. Feigl EO. The paradox of adrenergic coronary vasoconstriction. Circulation 76: 737–745, 1987. 184. Feldman RD, Christy JP, Paul ST, Harrison DG. Beta-adrenergic receptors on canine coronary collateral vessels: characterization and function. Am J Physiol Heart Circ Physiol 257: H1634 – H1639, 1989. 185. Flaim SF, Minteer WJ, Clark DP, Zelis R. Cardiovascular response to acute aquatic and treadmill exercise in the untrained rat. J Appl Physiol 46: 302–308, 1979. 186. Flameng W, Wusten B, Schaper W. On the distribution of myocardial flow. Part II: Effects of arterial stenosis and vasodilation. Basic Res Cardiol 69: 435– 446, 1974. 187. Flavahan NA. Atherosclerosis or lipoprotein-induced endothelial dysfunction. Potential mechanisms underlying reduction in EDRF/ nitric oxide activity. Circulation 85: 1927–1938, 1992. 188. Flynn NM, Kenny D, Pelc LR, Warltier DC, Bosnjak ZJ, Kampine JP. Endothelium-dependent vasodilation of canine coronary collateral vessels. Am J Physiol Heart Circ Physiol 261: H1797–H1801, 1991. 189. Fogarty JA, Muller-Delp JM, Delp MD, Mattox ML, Laughlin MH, Parker JL. Exercise training enhances vasodilation responses to vascular endothelial growth factor in porcine coronary arterioles exposed to chronic coronary occlusion. Circulation 109: 664 – 670, 2004. 88 • JULY 2008 • www.prv.org 1076 DIRK J. DUNCKER AND ROBERT J. BACHE 190. Folta A, Joshua IG, Webb RC. Dilator actions of endothelin in coronary resistance vessels and the abdominal aorta of the guinea pig. Life Sci 45: 2627–2635, 1989. 191. Foreman B, Dai XZ, Homans DC, Laxson DD, Bache RJ. Effect of atrial natriuretic peptide on coronary collateral blood flow. Circ Res 65: 1671–1678, 1989. 192. Foreman BW, Dai XZ, Bache RJ. Vasoconstriction of canine coronary collateral vessels with vasopressin limits blood flow to collateral-dependent myocardium during exercise. Circ Res 69: 657– 664, 1991. 193. Frank A. Experimentelle herzhypertrophie. Z Ges Exp Med 115, 1950. 194. Frank MW, Harris KR, Ahlin KA, Klocke FJ. Endotheliumderived relaxing factor (nitric oxide) has a tonic vasodilating action on coronary collateral vessels. J Am Coll Cardiol 27: 658 – 663, 1996. 195. Franklin BA. Exercise training and coronary collateral circulation. Med Sci Sports Exerc 23: 648 – 653, 1991. 196. Frelin C, Guedin D. Why are circulating concentrations of endothelin-1 so low? Cardiovasc Res 28: 1613–1622, 1994. 197. Frick MH, Elovainio RO, Somer T. The mechanism of bradycardia evoked by physical training. Cardiologia 51: 46 –54, 1967. 198. Friedman PL, Brown EJ Jr, Gunther S, Alexander RW, Barry WH, Mudge GH Jr, Grossman W. Coronary vasoconstrictor effect of indomethacin in patients with coronary-artery disease. N Engl J Med 305: 1171–1175, 1981. 199. Fujita M, Yamanishi K, Miwa K, Inoko M. Variable responses of highly stenotic coronary arteries and collateral circulation to intracoronary injection of nitroglycerin in patients with chronic effort angina. Coron Artery Dis 5: 525–529, 1994. 200. Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376, 1980. 201. Furusho N, Araki H, Sakaino N, Nishi K, Miyauchi Y. Effects of perivascular nerve stimulation on the flow rate in isolated epicardial coronary arteries of pigs. Eur J Pharmacol 154: 79 – 84, 1988. 202. Furuya F, Yoshitaka H, Morita H, Hosomi H. Neural, humoral, metabolic control of coronary vascular resistance during exercise. Jpn J Physiol 42: 117–130, 1992. 203. Gallagher KP, Folts JD, Shebuski RJ, Rankin JH, Rowe GG. Subepicardial vasodilator reserve in the presence of critical coronary stenosis in dogs. Am J Cardiol 46: 67–73, 1980. 204. Gewirtz H, Olsson RA, Brautigan DL, Brown PR, Most AS. Adenosine’s role in regulating basal coronary arteriolar tone. Am J Physiol Heart Circ Physiol 250: H1030 –H1036, 1986. 205. Gewirtz H, Olsson RA, Most AS. Role of adenosine in mediating the coronary vasodilative response to acute hypoxia. Cardiovasc Res 21: 81– 89, 1987. 206. Gewirtz H, Williams DO, Ohley WH, Most AS. Influence of coronary vasodilation on the transmural distribution of myocardial blood flow distal to a severe fixed coronary artery stenosis. Am Heart J 106: 674 – 680, 1983. 207. Giles RW, Wilcken DE. Reactive hyperaemia in the dog heart: inter-relations between adenosine, ATP, aminophylline and the effect of indomethacin. Cardiovasc Res 11: 113–121, 1977. 208. Goetz KL, Wang BC, Sundet WD. Comparative effects of cardiac receptors and sinoaortic baroreceptors on elevations of plasma vasopressin and renin activity elicited by haemorrhage. J Physiol 79: 440 – 445, 1984. 209. Goldsmith RL, Bloomfield DM, Rosenwinkel ET. Exercise and autonomic function. Coron Artery Dis 11: 129 –135, 2000. 210. Gollasch M, Ried C, Bychkov R, Luft FC, Haller H. K⫹ currents in human coronary artery vascular smooth muscle cells. Circ Res 78: 676 – 688, 1996. 211. Gorlin R, Krasnow N, Levine HJ, Messer JV. Effect of exercise on cardiac performance in human subjects with minimal heart disease. Am J Cardiol 13: 293–300, 1964. 212. Gorman MW, Farias M, 3rd Richmond KN, Tune JD, Feigl EO. Role of endothelin in alpha-adrenoceptor coronary vasoconstriction. Am J Physiol Heart Circ Physiol 288: H1937–H1942, 2005. 213. Gorman MW, Sparks HV Jr. Progressive coronary vasoconstriction during relative ischemia in canine myocardium. Circ Res 51: 411– 420, 1982. Physiol Rev • VOL 214. Gorman MW, Tune JD, Richmond KN, Feigl EO. Feedforward sympathetic coronary vasodilation in exercising dogs. J Appl Physiol 89: 1892–1902, 2000. 215. Gorman MW, Tune JD, Richmond KN, Feigl EO. Quantitative analysis of feedforward sympathetic coronary vasodilation in exercising dogs. J Appl Physiol 89: 1903–1911, 2000. 216. Gotoh K, Minamino T, Katoh O, Hamano Y, Fukui S, Hori M, Kusuoka H, Mishima M, Inoue M, Kamada T. The role of intracoronary thrombus in unstable angina: angiographic assessment and thrombolytic therapy during ongoing anginal attacks. Circulation 77: 526 –534, 1988. 217. Graham TP Jr, Covell JW, Sonnenblick EH, Ross J Jr, Braunwald E. Control of myocardial oxygen consumption: relative influence of contractile state and tension development. J Clin Invest 47: 375–385, 1968. 218. Grattan MT, Hanley FL, Stevens MB, Hoffman JI. Transmural coronary flow reserve patterns in dogs. Am J Physiol Heart Circ Physiol 250: H276 –H283, 1986. 219. Gregg DE. Relationship between coronary flow and metabolic changes. Cardiology 56: 291–301, 1971. 220. Gregg DE, Khouri EM, Donald DE, Lowensohn HS, Pasyk S. Coronary circulation in the conscious dog with cardiac neural ablation. Circ Res 31: 129 –144, 1972. 221. Griffin KL, Laughlin MH, Parker JL. Exercise training improves endothelium-mediated vasorelaxation after chronic coronary occlusion. J Appl Physiol 87: 1948 –1956, 1999. 222. Griffin KL, Woodman CR, Price EM, Laughlin MH, Parker JL. Endothelium-mediated relaxation of porcine collateral-dependent arterioles is improved by exercise training. Circulation 104: 1393– 1398, 2001. 223. Groves P, Kurz S, Just H, Drexler H. Role of endogenous bradykinin in human coronary vasomotor control. Circulation 92: 3424 –3430, 1995. 224. Grubbstrom J, Berglund B, Kaijser L. Myocardial blood flow and lactate metabolism at rest and during exercise with reduced arterial oxygen content. Acta Physiol Scand 142: 467– 474, 1991. 225. Gruber HE, Hoffer ME, McAllister DR, Laikind PK, Lane TA, Schmid-Schoenbein GW, Engler RL. Increased adenosine concentration in blood from ischemic myocardium by AICA riboside. Effects on flow, granulocytes, and injury. Circulation 80: 1400 – 1411, 1989. 226. Gute D, Fraga C, Laughlin MH, Amann JF. Regional changes in capillary supply in skeletal muscle of high-intensity endurancetrained rats. J Appl Physiol 81: 619 – 626, 1996. 227. Guth BD, Miura T, Thaulow E, Heusch G, Ross J Jr. Alpha 1-adrenergic blockade reduces exercise-induced regional myocardial ischemia in dogs. Basic Res Cardiol 88: 282–296, 1993. 228. Guth BD, Thaulow E, Heusch G, Seitelberger R, Ross J Jr. Alpha-adrenergic regulation of myocardial performance in the exercising dog: evidence for both presynaptic alpha 1- and alpha 2-adrenoceptors. Basic Res Cardiol 85 Suppl 1: 131–141, 1990. 229. Gutterman DD, Miura H, Liu Y. Redox modulation of vascular tone: focus of potassium channel mechanisms of dilation. Arterioscler Thromb Vasc Biol 25: 671– 678, 2005. 230. Gwirtz PA, Dodd-o JM, Brandt MA, Jones CE. Augmentation of coronary flow improves myocardial function in exercise. J Cardiovasc Pharmacol 15: 752–758, 1990. 231. Gwirtz PA, Mass HJ, Strader JR, Jones CE. Coronary and cardiac responses to exercise after chronic ventricular sympathectomy. Med Sci Sports Exerc 20: 126 –135, 1988. 232. Gwirtz PA, Overn SP, Mass HJ, Jones CE. Alpha 1-adrenergic constriction limits coronary flow and cardiac function in running dogs. Am J Physiol Heart Circ Physiol 250: H1117–H1126, 1986. 233. Gwirtz PA, Stone HL. Coronary blood flow and myocardial oxygen consumption after alpha adrenergic blockade during submaximal exercise. J Pharmacol Exp Ther 217: 92–98, 1981. 234. Gwirtz PA, Stone HL. coronary blood flow changes following activation of adrenergic receptors in the conscious dog. Am J Physiol Heart Circ Physiol 243: H13–H19, 1982. 235. Gwirtz PA, Stone HL. Coronary vascular response to adrenergic stimulation in exercise-conditioned dogs. J Appl Physiol 57: 315– 320, 1984. 88 • JULY 2008 • www.prv.org CORONARY BLOOD FLOW 236. Haitsma DB, Bac D, Raja N, Boomsma F, Verdouw PD, Duncker DJ. Minimal impairment of myocardial blood flow responses to exercise in the remodeled left ventricle early after myocardial infarction, despite significant hemodynamic and neurohumoral alterations. Cardiovasc Res 52: 417– 428, 2001. 237. Hakkila J. Studies on the myocardial capillary concentration in cardiac hypertrophy due to training; an experimental study with guinea pigs. Ann Med Exp Biol Fenn 33: 1– 82, 1955. 238. Hamm CW, Kupper W, Bredehorst R, Hilz H, Bleifeld W. Quantitation of coronary venous adenosine in patients: limitations evaluated by radioimmunoassay. Cardiovasc Res 22: 236 –243, 1988. 239. Hamm CW, Lorenz RL, Bleifeld W, Kupper W, Wober W, Weber PC. Biochemical evidence of platelet activation in patients with persistent unstable angina. J Am Coll Cardiol 10: 998 –1006, 1987. 240. Hammond HK, White FC, Brunton LL, Longhurst JC. Association of decreased myocardial beta-receptors and chronotropic response to isoproterenol and exercise in pigs following chronic dynamic exercise. Circ Res 60: 720 –726, 1987. 241. Hanley FL, Grattan MT, Stevens MB, Hoffman JI. Role of adenosine in coronary autoregulation. Am J Physiol Heart Circ Physiol 250: H558 –H566, 1986. 242. Harri MN. Physical training under the influence of beta-blockade in rats. II. Effects on vascular reactivity. Eur J Appl Physiol Occup Physiol 42: 151–157, 1979. 243. Harrison DG, Chilian WM, Marcus ML. Absence of functioning alpha-adrenergic receptors in mature canine coronary collaterals. Circ Res 59: 133–142, 1986. 244. Harrison MH. Effects on thermal stress and exercise on blood volume in humans. Physiol Rev 65: 149 –209, 1985. 245. Hart BJ, Bian X, Gwirtz PA, Setty S, Downey HF. Right ventricular oxygen supply/demand balance in exercising dogs. Am J Physiol Heart Circ Physiol 281: H823–H830, 2001. 246. Hasdai D, Kornowski R, Battler A. Endothelin and myocardial ischemia. Cardiovasc Drugs Ther 8: 589 –599, 1994. 247. Hastings AB, White FC, Sanders TM, Bloor CM. Comparative physiological responses to exercise stress. J Appl Physiol 52: 1077– 1083, 1982. 248. Hata H, Egashira K, Fukai T, Ohara Y, Kasuya H, Takahashi T, Takeshita A. The role of endothelium-derived nitric oxide in acetylcholine-induced coronary vasoconstriction in closed-chest pigs. Coron Artery Dis 4: 891– 898, 1993. 249. Hautamaa PV, Dai XZ, Homans DC, Bache RJ. Vasomotor activity of moderately well-developed canine coronary collateral circulation. Am J Physiol Heart Circ Physiol 256: H890 –H897, 1989. 250. Heaps CL, Bowles DK. Gender-specific K⫹-channel contribution to adenosine-induced relaxation in coronary arterioles. J Appl Physiol 92: 550 –558, 2002. 251. Heaps CL, Bowles DK, Sturek M, Laughlin MH, Parker JL. Enhanced L-type Ca2⫹ channel current density in coronary smooth muscle of exercise-trained pigs is compensated to limit myoplasmic free Ca2⫹ accumulation. J Physiol 528: 435– 445, 2000. 252. Heaps CL, Mattox ML, Kelly KA, Meininger CJ, Parker JL. Exercise training increases basal tone in arterioles distal to chronic coronary occlusion. Am J Physiol Heart Circ Physiol 290: H1128 – H1135, 2006. 253. Heaps CL, Sturek M, Rapps JA, Laughlin MH, Parker JL. Exercise training restores adenosine-induced relaxation in coronary arteries distal to chronic occlusion. Am J Physiol Heart Circ Physiol 278: H1984 –H1992, 2000. 254. Heaton WH, Marr KC, Capurro NL, Goldstein RE, Epstein SE. Beneficial effect of physical training on blood flow to myocardium perfused by chronic collaterals in the exercising dog. Circulation 57: 575–581, 1978. 255. Hein TW, Kuo L. cAMP-independent dilation of coronary arterioles to adenosine: role of nitric oxide, G proteins, KATP channels. Circ Res 85: 634 – 642, 1999. 256. Hein TW, Zhang C, Wang W, Kuo L. Heterogeneous beta2-adrenoceptor expression and dilation in coronary arterioles across the left ventricular wall. Circulation 110: 2708 –2712, 2004. Physiol Rev • VOL 1077 257. Heineman FW, Grayson J. Transmural distribution of intramyocardial pressure measured by micropipette technique. Am J Physiol Heart Circ Physiol 249: H1216 –H1223, 1985. 258. Heiss HW, Barmeyer J, Wink K, Hell G, Cerny FJ, Keul J, Reindell H. Studies on the regulation of myocardial blood flow in man. I. Training effects on blood flow and metabolism of the healthy heart at rest and during standardized heavy exercise. Basic Res Cardiol 71: 658 – 675, 1976. 259. Herzog CA, Aeppli DP, Bache RJ. Effect of beta-adrenergic blockade with timolol on myocardial blood flow during exercise after myocardial infarction in the dog. J Am Coll Cardiol 4: 1174 – 1183, 1984. 260. Herzog CA, Dai XZ, Bache RJ. Effect of alpha 1-adrenergic blockade on myocardial blood flow during exercise after myocardial infarction. Am J Physiol Heart Circ Physiol 261: H280 –H286, 1991. 261. Hess DS, Bache RJ. Regional myocardial blood flow during graded treadmill exercise following circumflex coronary artery occlusion in the dog. Circ Res 47: 59 – 68, 1980. 262. Hess DS, Bache RJ. Transmural distribution of myocardial blood flow during systole in the awake dog. Circ Res 38: 5–15, 1976. 263. Heusch G, Baumgart D, Camici P, Chilian W, Gregorini L, Hess O, Indolfi C, Rimoldi O. Alpha-adrenergic coronary vasoconstriction and myocardial ischemia in humans. Circulation 101: 689 – 694, 2000. 264. Heusch G, Deussen A. The effects of cardiac sympathetic nerve stimulation on perfusion of stenotic coronary arteries in the dog. Circ Res 53: 8 –15, 1983. 265. Heusch G, Guth BD, Seitelberger R, Ross J Jr. Attenuation of exercise-induced myocardial ischemia in dogs with recruitment of coronary vasodilator reserve by nifedipine. Circulation 75: 482– 490, 1987. 266. Heyndrickx GR, Muylaert P, Pannier JL. ␣-Adrenergic control of oxygen delivery to myocardium during exercise in conscious dogs. Am J Physiol Heart Circ Physiol 242: H805–H809, 1982. 267. Heyndrickx GR, Pannier JL, Muylaert P, Mabilde C, Leusen I. Alteration in myocardial oxygen balance during exercise after betaadrenergic blockade in dogs. J Appl Physiol 49: 28 –33, 1980. 268. Heyndrickx GR, Vilaine JP, Moerman EJ, Leusen I. Role of prejunctional alpha 2-adrenergic receptors in the regulation of myocardial performance during exercise in conscious dogs. Circ Res 54: 683– 693, 1984. 269. Hintze TH, Kaley G. Prostaglandins and the control of blood flow in the canine myocardium. Circ Res 40: 313–320, 1977. 270. Hirsh PD, Hillis LD, Campbell WB, Firth BG, Willerson JT. Release of prostaglandins and thromboxane into the coronary circulation in patients with ischemic heart disease. N Engl J Med 304: 685– 691, 1981. 271. Ho KW, Roy RR, Taylor JF, Heusner WW, Van Huss WD. Differential effects of running and weight-lifting on the rat coronary arterial tree. Med Sci Sports Exerc 15: 472– 477, 1983. 272. Hodgson JM, Cohen MD, Szentpetery S, Thames MD. Effects of regional alpha- and beta-blockade on resting and hyperemic coronary blood flow in conscious, unstressed humans. Circulation 79: 797– 809, 1989. 273. Hoffman JI. Transmural myocardial perfusion. Prog Cardiovasc Dis 29: 429 – 464, 1987. 274. Hoffman JI, Spaan JA. Pressure-flow relations in coronary circulation. Physiol Rev 70: 331–390, 1990. 275. Holmberg S, Serzysko W, Varnauskas E. Coronary circulation during heavy exercise in control subjects and patients with coronary heart disease. Acta Med Scand 190: 465– 480, 1971. 276. Holtz J, Giesler M, Bassenge E. Two dilatory mechanisms of anti-anginal drugs on epicardial coronary arteries in vivo: indirect, flow-dependent, endothelium-mediated dilation and direct smooth muscle relaxation. Z Kardiol 72 Suppl 3: 98 –106, 1983. 277. Honig CR, Frierson JL, Gayeski TE. Anatomical determinants of O2 flux density at coronary capillaries. Am J Physiol Heart Circ Physiol 256: H375–H382, 1989. 278. Hori M, Kitakaze M, Takashima S, Morioka T, Sato H, Minamino T, Node K, Komamura K, Inoue M, Kamada T. AICA riboside improves myocardial ischemia in coronary microemboli- 88 • JULY 2008 • www.prv.org 1078 279. 280. 281. 282. 283. 284. 285. 286. 287. 288. 289. 290. 291. 292. 293. 294. 295. 296. 297. 298. DIRK J. DUNCKER AND ROBERT J. BACHE zation in dogs. Am J Physiol Heart Circ Physiol 267: H1483–H1495, 1994. Horwitz LD, Atkins JM, Leshin SJ. Role of the Frank-Starling mechanism in exercise. Circ Res 31: 868 – 875, 1972. Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol Rev 46: 157–203, 1994. Hu SL, Kim HS, Jeng AY. Dual action of endothelin-1 on the Ca2⫹-activated K⫹ channel in smooth muscle cells of porcine coronary artery. Eur J Pharmacol 194: 31–36, 1991. Huang AH, Feigl EO. Adrenergic coronary vasoconstriction helps maintain uniform transmural blood flow distribution during exercise. Circ Res 62: 286 –298, 1988. Huang Y, Kwok KH. Effects of putative K⫹ channel blockers on beta-adrenoceptor-mediated vasorelaxation of rat mesenteric artery. J Cardiovasc Pharmacol 29: 515–519, 1997. Ichikawa Y, Yokoyama M, Akita H, Fukuzaki H. Constriction of a large coronary artery contributes to serotonin-induced myocardial ischemia in the dog with pliable coronary stenosis. J Am Coll Cardiol 14: 449 – 459, 1989. Imamura Y, Tomoike H, Narishige T, Takahashi T, Kasuya H, Takeshita A. Glibenclamide decreases basal coronary blood flow in anesthetized dogs. Am J Physiol Heart Circ Physiol 263: H399 – H404, 1992. Ingermann RL. Vertebrate hemoglobins. In: Handbook of Physiology. Comparative Physiology. Bethesda, MD: Am. Physiol. Soc., 1997, p. 358 – 408. Ishibashi Y, Duncker DJ, Bache RJ. Endogenous nitric oxide masks alpha 2-adrenergic coronary vasoconstriction during exercise in the ischemic heart. Circ Res 80: 196 –207, 1997. Ishibashi Y, Duncker DJ, Zhang J, Bache RJ. ATP-sensitive K⫹ channels, adenosine, and nitric oxide-mediated mechanisms account for coronary vasodilation during exercise. Circ Res 82: 346 – 359, 1998. Ishibashi Y, Mizrahi J, Duncker DJ, Bache RJ. The nitric oxide donor ITF 1129 augments subendocardial blood flow during exercise-induced myocardial ischemia. J Cardiovasc Pharmacol 30: 374 –382, 1997. Ishibashi Y, Quebbeman BB, Duncker DJ, Klassen C, Bache RJ. Acadesine increases blood flow in the collateralized heart during exercise. J Cardiovasc Pharmacol 32: 552–561, 1998. Ishibashi Y, Zhang J, Duncker DJ, Klassen C, Pavek T, Bache RJ. Coronary hyperperfusion augments myocardial oxygen consumption. Am J Physiol Heart Circ Physiol 271: H1384 –H1393, 1996. Ishizaka H, Kuo L. Acidosis-induced coronary arteriolar dilation is mediated by ATP-sensitive potassium channels in vascular smooth muscle. Circ Res 78: 50 –57, 1996. Ishizaka H, Okumura K, Yamabe H, Tsuchiya T, Yasue H. Endothelium-derived nitric oxide as a mediator of acetylcholineinduced coronary vasodilation in dogs. J Cardiovasc Pharmacol 18: 665– 669, 1991. Jackson WF, Konig A, Dambacher T, Busse R. Prostacyclininduced vasodilation in rabbit heart is mediated by ATP-sensitive potassium channels. Am J Physiol Heart Circ Physiol 264: H238 – H243, 1993. Jacobs TB, Bell RD, McClements JD. Exercise, age and the development of the myocardial vasculature. Growth 48: 148 –157, 1984. Johnson RLJ, Heigenhauser GJF, Hsia CCW, Jones NL, Wagner PD. Determinants of gas exchange and acid-base balance during exercise. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am. Physiol. Soc., 1996, p. 515–584. Jones CE, Liang IY, Maulsby MR. Cardiac and coronary effects of prazosin and phenoxybenzamine during coronary hypotension. J Pharmacol Exp Ther 236: 204 –211, 1986. Jones CJ, DeFily DV, Patterson JL, Chilian WM. Endotheliumdependent relaxation competes with alpha 1- and alpha 2-adrenergic constriction in the canine epicardial coronary microcirculation. Circulation 87: 1264 –1274, 1993. Physiol Rev • VOL 299. Jones CJ, Kuo L, Davis MJ, Chilian WM. Regulation of coronary blood flow: coordination of heterogeneous control mechanisms in vascular microdomains. Cardiovasc Res 29: 585–596, 1995. 300. Jones CJ, Kuo L, Davis MJ, DeFily DV, Chilian WM. Role of nitric oxide in the coronary microvascular responses to adenosine and increased metabolic demand. Circulation 91: 1807–1813, 1995. 301. Jones JH, Longworth KE, Lindholm A, Conley KE, Karas RH, Kayar SR, Taylor CR. Oxygen transport during exercise in large mammals. I. Adaptive variation in oxygen demand. J Appl Physiol 67: 862– 870, 1989. 302. Jorgensen CR, Gobel FL, Taylor HL, Wang Y. Myocardial blood flow and oxygen consumption during exercise. Ann NY Acad Sci 301: 213–223, 1977. 303. Jorgensen CR, Wang K, Wang Y, Gobel FL, Nelson RR, Taylor H. Effect of propranolol on myocardial oxygen consumption and its hemodynamic correlates during upright exercise. Circulation 48: 1173–1182, 1973. 304. Kadokami T, Egashira K, Kuwata K, Fukumoto Y, Kozai T, Yasutake H, Kuga T, Shimokawa H, Sueishi K, Takeshita A. Altered serotonin receptor subtypes mediate coronary microvascular hyperreactivity in pigs with chronic inhibition of nitric oxide synthesis. Circulation 94: 182–189, 1996. 305. Kajiya F, Yada T, Matsumoto T, Goto M, Ogasawara Y. Intramyocardial influences on blood flow distributions in the myocardial wall. Ann Biomed Eng 28: 897–902, 2000. 306. Kanatsuka H, Lamping KG, Eastham CL, Dellsperger KC, Marcus ML. Comparison of the effects of increased myocardial oxygen consumption and adenosine on the coronary microvascular resistance. Circ Res 65: 1296 –1305, 1989. 307. Kanatsuka H, Lamping KG, Eastham CL, Marcus ML. Heterogeneous changes in epimyocardial microvascular size during graded coronary stenosis. Evidence of the microvascular site for autoregulation. Circ Res 66: 389 –396, 1990. 308. Katusic ZS, Shepherd JT, Vanhoutte PM. Vasopressin causes endothelium-dependent relaxations of the canine basilar artery. Circ Res 55: 575–579, 1984. 309. Katz SA, Feigl EO. Systole has little effect on diastolic coronary artery blood flow. Circ Res 62: 443– 451, 1988. 310. Kawai H, Stevens SY, Liang CS. Renin-angiotensin system inhibition on noradrenergic nerve terminal function in pacing-induced heart failure. Am J Physiol Heart Circ Physiol 279: H3012–H3019, 2000. 311. Kaye MP, Brynjolfsson GG, Geis WP. Chemical epicardiectomy. A method of myocardial denervaion. Cardiologia 53: 139 –149, 1968. 312. Kern MJ. Histaminergic modulation of coronary vascular resistance: are we missing a therapeutic adjunct for the treatment of myocardial ischemia? J Am Coll Cardiol 17: 346 –347, 1991. 313. Khouri EM, Gregg DE, Rayford CR. Effect of exercise on cardiac output, left coronary flow and myocardial metabolism in the unanesthetized dog. Circ Res 17: 427– 437, 1965. 314. Kirkeboen KA, Naess PA, Offstad J, Ilebekk A. Effects of regional inhibition of nitric oxide synthesis in intact porcine hearts. Am J Physiol Heart Circ Physiol 266: H1516 –H1527, 1994. 315. Kitakaze M, Minamino T, Node K, Komamura K, Shinozaki Y, Mori H, Kosaka H, Inoue M, Hori M, Kamada T. Beneficial effects of inhibition of angiotensin-converting enzyme on ischemic myocardium during coronary hypoperfusion in dogs. Circulation 92: 950 –961, 1995. 316. Kitamura K, Jorgensen CR, Gobel FL, Taylor HL, Wang Y. Hemodynamic correlates of myocardial oxygen consumption during upright exercise. J Appl Physiol 32: 516 –522, 1972. 317. Klassen CL, Traverse JH, Bache RJ. Nitroglycerin dilates coronary collateral vessels during exercise after blockade of endogenous NO production. Am J Physiol Heart Circ Physiol 277: H918 – H923, 1999. 318. Klocke FJ, Mates RE, Canty JM Jr, Ellis AK. Coronary pressure-flow relationships Controversial issues and probable implications. Circ Res 56: 310 –323, 1985. 319. Knight DR, Stone HL. Alteration of ischemic cardiac function in normal heart by daily exercise. J Appl Physiol 55: 52– 60, 1983. 88 • JULY 2008 • www.prv.org CORONARY BLOOD FLOW 320. Koch-Weser J, Blinks JR. The influence of the interval between beats on myocardial contractility. Pharmacol Rev 15: 601– 652, 1963. 321. Koerner JE, Terjung RL. Effect of physical training on coronary collateral circulation of the rat. J Appl Physiol 52: 376 –387, 1982. 322. Komaru T, Lamping KG, Eastham CL, Dellsperger KC. Role of ATP-sensitive potassium channels in coronary microvascular autoregulatory responses. Circ Res 69: 1146 –1151, 1991. 323. Komaru T, Lamping KG, Eastham CL, Harrison DG, Marcus ML, Dellsperger KC. Effect of an arginine analogue on acetylcholine-induced coronary microvascular dilatation in dogs. Am J Physiol Heart Circ Physiol 261: H2001–H2007, 1991. 324. Korzick DH, Laughlin MH, Bowles DK. Alterations in PKC signaling underlie enhanced myogenic tone in exercise-trained porcine coronary resistance arteries. J Appl Physiol 96: 1425–1432, 2004. 325. Krams R, Sipkema P, Zegers J, Westerhof N. Contractility is the main determinant of coronary systolic flow impediment. Am J Physiol Heart Circ Physiol 257: H1936 –H1944, 1989. 326. Kroll K, Feigl EO. Adenosine is unimportant in controlling coronary blood flow in unstressed dog hearts. Am J Physiol Heart Circ Physiol 249: H1176 –H1187, 1985. 327. Krombach RS, Clair MJ, Hendrick JW, Mukherjee R, Houck WV, Hebbar L, Kribbs SB, Dodd MG, Spinale FG. Amlodipine therapy in congestive heart failure: hemodynamic and neurohormonal effects at rest and after treadmill exercise. Am J Cardiol 84: 3L–15L, 1999. 328. Kuo L, Chilian WM, Davis MJ. Interaction of pressure- and flow-induced responses in porcine coronary resistance vessels. Am J Physiol Heart Circ Physiol 261: H1706 –H1715, 1991. 329. Lambert PR, Hess DS, Bache RJ. Effect of exercise on perfusion of collateral-dependent myocardium in dogs with chronic coronary artery occlusion. J Clin Invest 59: 1–7, 1977. 330. Lameris TW, de Zeeuw S, Duncker DJ, Alberts G, Boomsma F, Verdouw PD, van den Meiracker AH. Exogenous angiotensin II does not facilitate norepinephrine release in the heart. Hypertension 40: 491– 497, 2002. 331. Lamontagne D, Konig A, Bassenge E, Busse R. Prostacyclin and nitric oxide contribute to the vasodilator action of acetylcholine and bradykinin in the intact rabbit coronary bed. J Cardiovasc Pharmacol 20: 652– 657, 1992. 332. Lamontagne D, Pohl U, Busse R. NG-nitro-L-arginine antagonizes endothelium-dependent dilator responses by inhibiting endothelium-derived relaxing factor release in the isolated rabbit heart. Pflügers Arch 418: 266 –270, 1991. 333. Lamping KG, Dole WP. Flow-mediated dilation attenuates constriction of large coronary arteries to serotonin. Am J Physiol Heart Circ Physiol 255: H1317–H1324, 1988. 334. Lamping KG, Kanatsuka H, Eastham CL, Chilian WM, Marcus ML. Nonuniform vasomotor responses of the coronary microcirculation to serotonin and vasopressin. Circ Res 65: 343–351, 1989. 335. Lamping KG, Marcus ML, Dole WP. Removal of the endothelium potentiates canine large coronary artery constrictor responses to 5-hydroxytryptamine in vivo. Circ Res 57: 46 –54, 1985. 336. Langer SZ. Sixth gaddum memorial lecture, National Institute for Medical Research, Mill Hill, January 1977. Presynaptic receptors and their role in the regulation of transmitter release. Br J Pharmacol 60: 481– 497, 1977. 337. Lasley RD, Hegge JO, Noble MA, Mentzer RM Jr. Comparison of interstitial fluid and coronary venous adenosine levels in in vivo porcine myocardium. J Mol Cell Cardiol 30: 1137–1147, 1998. 338. Laughlin MH. Coronary transport reserve in normal dogs. J Appl Physiol 57: 551–561, 1984. 339. Laughlin MH. Effects of exercise training on coronary transport capacity. J Appl Physiol 58: 468 – 476, 1985. 340. Laughlin MH, Joseph B. Wolfe Memorial lecture. Physical activity in prevention and treatment of coronary disease: the battle line is in exercise vascular cell biology. Med Sci Sports Exerc 36: 352–362, 2004. 341. Laughlin MH. Skeletal muscle blood flow capacity: role of muscle pump in exercise hyperemia. Am J Physiol Heart Circ Physiol 253: H993–H1004, 1987. Physiol Rev • VOL 1079 342. Laughlin MH, Burns JW, Fanton J, Ripperger J, Peterson DF. coronary blood flow reserve during ⫹Gz stress and treadmill exercise in miniature swine. J Appl Physiol 64: 2589 –2596, 1988. 343. Laughlin MH, Diana JN. Myocardial transcapillary exchange in the hypertrophied heart of the dog. Am J Physiol 229: 838 – 846, 1975. 344. Laughlin MH, Diana JN, Tipton CM. Effects of exercise training on coronary reactive hyperemia and blood flow in the dog. J Appl Physiol 45: 604 – 610, 1978. 345. Laughlin MH, Klabunde RE, Delp MD, Armstrong RB. Effects of dipyridamole on muscle blood flow in exercising miniature swine. Am J Physiol Heart Circ Physiol 257: H1507–H1515, 1989. 346. Laughlin MH, Korthuis RJ, Duncker DJ, Bache RJ. Regulation of blood flow to cardiac and skeletal muscle during exercise. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am. Physiol. Soc., 1996, p. 705–769. 347. Laughlin MH, Korthuis RJ, Sexton WL, Armstrong RB. Regional muscle blood flow capacity and exercise hyperemia in highintensity trained rats. J Appl Physiol 64: 2420 –2427, 1988. 348. Laughlin MH, McAllister RM. Exercise training-induced coronary vascular adaptation. J Appl Physiol 73: 2209 –2225, 1992. 349. Laughlin MH, Mohrman SJ, Armstrong RB. Muscular blood flow distribution patterns in the hindlimb of swimming rats. Am J Physiol Heart Circ Physiol 246: H398 –H403, 1984. 350. Laughlin MH, Muller JM. Vasoconstrictor responses of coronary resistance arteries in exercise-trained pigs. J Appl Physiol 84: 884 – 889, 1998. 351. Laughlin MH, Overholser KA, Bhatte MJ. Exercise training increases coronary transport reserve in miniature swine. J Appl Physiol 67: 1140 –1149, 1989. 352. Laughlin MH, Pollock JS, Amann JF, Hollis ML, Woodman CR, Price EM. Training induces nonuniform increases in eNOS content along the coronary arterial tree. J Appl Physiol 90: 501– 510, 2001. 353. Laughlin MH, Tomanek RJ. Myocardial capillarity and maximal capillary diffusion capacity in exercise-trained dogs. J Appl Physiol 63: 1481–1486, 1987. 354. Laxson DD, Dai XZ, Homans DC, Bache RJ. Coronary vasodilator reserve in ischemic myocardium of the exercising dog. Circulation 85: 313–322, 1992. 355. Laxson DD, Dai XZ, Homans DC, Bache RJ. The role of alpha 1and alpha 2-adrenergic receptors in mediation of coronary vasoconstriction in hypoperfused ischemic myocardium during exercise. Circ Res 65: 1688 –1697, 1989. 356. Laxson DD, Homans DC, Bache RJ. Inhibition of adenosinemediated coronary vasodilation exacerbates myocardial ischemia during exercise. Am J Physiol Heart Circ Physiol 265: H1471– H1477, 1993. 357. LeBlanc J, Boulay M, Dulac S, Jobin M, Labrie A, RousseauMigneron S. Metabolic and cardiovascular responses to norepinephrine in trained and nontrained human subjects. J Appl Physiol 42: 166 –173, 1977. 358. Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology 21: 69 –78, 2006. 359. Lee SC, Mallet RT, Shizukuda Y, Williams AG Jr, Downey HF. Canine coronary vasodepressor responses to hypoxia are attenuated but not abolished by 8-phenyltheophylline. Am J Physiol Heart Circ Physiol 262: H955–960, 1992. 360. Lefroy DC, Crake T, Uren NG, Davies GJ, Maseri A. Effect of inhibition of nitric oxide synthesis on epicardial coronary artery caliber and coronary blood flow in humans. Circulation 88: 43–54, 1993. 361. Leon AS, Bloor CM. The effect of complete and partial deconditioning on exercise-induced cardiovascular changes in the rat. Adv Cardiol 18: 81–92, 1976. 362. Leon AS, Bloor CM. Effects of exercise and its cessation on the heart and its blood supply. J Appl Physiol 24: 485– 490, 1968. 363. Li H, Chai Q, Gutterman DD, Liu Y. Elevated glucose impairs cAMP-mediated dilation by reducing Kv channel activity in rat small coronary smooth muscle cells. Am J Physiol Heart Circ Physiol 285: H1213–H1219, 2003. 88 • JULY 2008 • www.prv.org 1080 DIRK J. DUNCKER AND ROBERT J. BACHE 364. Liang IY, Hamra M, Stone HL. Maximum coronary blood flow and minimum coronary resistance in exercise-trained dogs. J Appl Physiol 56: 641– 647, 1984. 365. Liang IY, Stone HL. Changes in diastolic coronary resistance during submaximal exercise in conditioned dogs. J Appl Physiol 54: 1057–1062, 1983. 366. Liang IY, Stone HL. Effect of exercise conditioning on coronary resistance. J Appl Physiol 53: 631– 636, 1982. 367. Liang IY, Stone HL, Gwirtz PA. Effect of beta 1-receptor blockade on coronary resistance in partially trained dogs. Med Sci Sports Exerc 19: 382–388, 1987. 368. Linder L, Kiowski W, Buhler FR, Luscher TF. Indirect evidence for release of endothelium-derived relaxing factor in human forearm circulation in vivo. Blunted response in essential hypertension. Circulation 81: 1762–1767, 1990. 369. Linke A, Erbs S, Hambrecht R. Exercise and the coronary circulation-alterations and adaptations in coronary artery disease. Prog Cardiovasc Dis 48: 270 –284, 2006. 370. Litvak J, Siderides LE, Vineberg AM. The experimental production of coronary artery insufficiency and occlusion. Am Heart J 53: 505–518, 1957. 371. Ljungqvist A, Unge G. Capillary proliferative activity in myocardium and skeletal muscle of exercised rats. J Appl Physiol 43: 306 –307, 1977. 372. Lombardo TA, Rose L, Taeschler M, Tuluy S, Bing RJ. The effect of exercise on coronary blood flow, myocardial oxygen consumption and cardiac efficiency in man. Circulation 7: 71–78, 1953. 373. Longhurst JC, Motohara S, Atkins JM, Ordway GA. Function of mature coronary collateral vessels and cardiac performance in the exercising dog. J Appl Physiol 59: 392– 400, 1985. 374. Lopez JJ, Lorell BH, Ingelfinger JR, Weinberg EO, Schunkert H, Diamant D, Tang SS. Distribution and function of cardiac angiotensin AT1- and AT2-receptor subtypes in hypertrophied rat hearts. Am J Physiol Heart Circ Physiol 267: H844 –H852, 1994. 375. Malm BJ, Suzuki G, Canty JM, Fallavollita JA. Variability of contractile reserve in hibernating myocardium: dependence on the method of inotropic stimulation. Cardiovasc Res 56: 422– 432, 2002. 376. Mann SJ, Krakoff LR, Felton K, Yeager K. Cardiovascular responses to infused epinephrine: effect of the state of physical conditioning. J Cardiovasc Pharmacol 6: 339 –343, 1984. 377. Manohar M. Left ventricular oxygen extraction during submaximal and maximal exertion in ponies. J Physiol 404: 547–556, 1988. 378. Manohar M. Transmural coronary vasodilator reserve and flow distribution during maximal exercise in normal and splenectomized ponies. J Physiol 387: 425– 440, 1987. 379. Manohar M, Goetz TE, Hutchens E, Coney E. Atrial and ventricular myocardial blood flows in horses at rest and during exercise. Am J Vet Res 55: 1464 –1469, 1994. 380. Marcus ML, Chilian WM, Kanatsuka H, Dellsperger KC, Eastham CL, Lamping KG. Understanding the coronary circulation through studies at the microvascular level. Circulation 82: 1–7, 1990. 381. Marijic J, Toro L. Voltage and calcium-activated K⫹ channels of coronary smooth muscle. In: Heart Physiology and Pathophysiology, edited by Sperelakis N, Kurachi Y, Terzic A, Cohen MV. San Diego, CA: Academic, 2001. 382. Maron BJ. Structural features of the athlete heart as defined by echocardiography. J Am Coll Cardiol 7: 190 –203, 1986. 383. Martin WH 3rd, Spina RJ, Korte E, Ogawa T. Effects of chronic and acute exercise on cardiovascular beta-adrenergic responses. J Appl Physiol 71: 1523–1528, 1991. 384. Maruoka Y, McKirnan MD, Engler RL, Longhurst JC. Functional significance of alpha-adrenergic receptors in mature coronary collateral circulation of dogs. Am J Physiol Heart Circ Physiol 253: H582–H590, 1987. 385. Marzilli M, Goldstein S, Sabbah HN, Lee T, Stein PD. Modulating effect of regional myocardial performance on local myocardial perfusion in the dog. Circ Res 45: 634 – 641, 1979. 386. Mass H, Gwirtz PA. Myocardial flow and function after regional beta-blockade in exercising dogs. Med Sci Sports Exerc 19: 443– 450, 1987. Physiol Rev • VOL 387. Matsumoto T, Nakane T, Chiba S. Pharmacological analysis of responses to ATP in the isolated and perfused canine coronary artery. Eur J Pharmacol 334: 173–180, 1997. 388. McElroy CL, Gissen SA, Fishbein MC. Exercise-induced reduction in myocardial infarct size after coronary artery occlusion in the rat. Circulation 57: 958 –962, 1978. 389. McFalls EO, Baldwin D, Palmer B, Marx D, Jaimes D, Ward HB. Regional glucose uptake within hypoperfused swine myocardium as measured by positron emission tomography. Am J Physiol Heart Circ Physiol 272: H343–H349, 1997. 390. McKenzie JE, Steffen RP, Haddy FJ. Effect of theophylline on adenosine production in the canine myocardium. Am J Physiol Heart Circ Physiol 252: H204 –H210, 1987. 391. McKenzie JE, Steffen RP, Haddy FJ. Relationships between adenosine and coronary resistance in conscious exercising dogs. Am J Physiol Heart Circ Physiol 242: H24 –H29, 1982. 392. Merkus D, Duncker DJ, Chilian WM. Metabolic regulation of coronary vascular tone: role of endothelin-1. Am J Physiol Heart Circ Physiol 283: H1915–H1921, 2002. 393. Merkus D, Haitsma DB, Fung TY, Assen YJ, Verdouw PD, Duncker DJ. coronary blood flow regulation in exercising swine involves parallel rather than redundant vasodilator pathways. Am J Physiol Heart Circ Physiol 285: H424 –H433, 2003. 394. Merkus D, Haitsma DB, Sorop O, Boomsma F, Debeer VJ, Lamers JM, Verdouw PD, Duncker DJ. Coronary vasoconstrictor influence of angiotensin II is reduced in remodeled myocardium after myocardial infarction. Am J Physiol Heart Circ Physiol 291: H2082–H2089, 2006. 395. Merkus D, Houweling B, Mirza A, Boomsma F, van den Meiracker AH, Duncker DJ. Contribution of endothelin and its receptors to the regulation of vascular tone during exercise is different in the systemic, coronary and pulmonary circulation. Cardiovasc Res 59: 745–754, 2003. 396. Merkus D, Houweling B, Zarbanoui A, Duncker DJ. Interaction between prostanoids and nitric oxide in regulation of systemic, pulmonary, and coronary vascular tone in exercising swine. Am J Physiol Heart Circ Physiol 286: H1114 –H1123, 2004. 397. Merkus D, Sorop O, Houweling B, Boomsma F, van den Meiracker AH, Duncker DJ. NO and prostanoids blunt the endothelin-mediated coronary vasoconstrictor influence in exercising swine. Am J Physiol Heart Circ Physiol 291: H2075–H2081, 2006. 398. Merkus D, Sorop O, Houweling B, Duncker DJ. Metabolites of cytochrome P450 2C9 are not essential for the regulation of coronary vasomotor tone in swine (Abstract). FASEB J 20: A1399, 2006. 399. Merkus D, Sorop O, Houweling B, Hoogteijling BA, Duncker ⫹ DJ. KCa channels contribute to exercise-induced coronary vasodilation in swine. Am J Physiol Heart Circ Physiol 291: 2090 –H2097, 2006. 400. Merrill GF, Downey HF, Jones CE. Adenosine deaminase attenuates canine coronary vasodilation during systemic hypoxia. Am J Physiol Heart Circ Physiol 250: H579 –H583, 1986. 401. Merrill GF, Haddy FJ, Dabney JM. Adenosine, theophylline, perfusate pH in the isolated, perfused guinea pig heart. Circ Res 42: 225–229, 1978. 402. Messer JV, Wagman RJ, Levine HJ, Neill WA, Krasnow N, Gorlin R. Patterns of human myocardial oxygen extraction during rest and exercise. J Clin Invest 41: 725–742, 1962. 403. Miller WL, Bove AA. Differential H1- and H2-receptor-mediated histamine responses of canine epicardial conductance and distal resistance coronary vessels. Circ Res 62: 226 –232, 1988. 404. Mills I, Fallon JT, Wrenn D, Sasken H, Gray W, Bier J, Levine D, Berman S, Gilson M, Gewirtz H. Adaptive responses of coronary circulation and myocardium to chronic reduction in perfusion pressure and flow. Am J Physiol Heart Circ Physiol 266: H447–H457, 1994. 405. Minami K, Fukuzawa K, Nakaya Y. Protein kinase C inhibits the Ca2⫹-activated K⫹ channel of cultured porcine coronary artery smooth muscle cells. Biochem Biophys Res Commun 190: 263–269, 1993. 406. Minami K, Fukuzawa K, Nakaya Y, Zeng XR, Inoue I. Mechanism of activation of the Ca2⫹-activated K⫹ channel by cyclic AMP in cultured porcine coronary artery smooth muscle cells. Life Sci 53: 1129 –1135, 1993. 88 • JULY 2008 • www.prv.org CORONARY BLOOD FLOW 407. Minami K, Hirata Y, Tokumura A, Nakaya Y, Fukuzawa K. Protein kinase C-independent inhibition of the Ca2⫹-activated K⫹ channel by angiotensin II and endothelin-1. Biochem Pharmacol 49: 1051–1056, 1995. 408. Miyashiro JK, Feigl EO. Feedforward control of coronary blood flow via coronary beta-receptor stimulation. Circ Res 73: 252–263, 1993. 409. Miyashiro JK, Feigl EO. A model of combined feedforward and feedback control of coronary blood flow. Am J Physiol Heart Circ Physiol 268: H895–H908, 1995. 410. Mohrman DE, Feigl EO. Competition between sympathetic vasoconstriction and metabolic vasodilation in the canine coronary circulation. Circ Res 42: 79 – 86, 1978. 411. Moreland RS. Regulation of smooth muscle contraction. In: Regulation of Smooth Muscle Contraction. New York: Plenum, 1991. 412. Morita K, Mori H, Tsujioka K, Kimura A, Ogasawara Y, Goto M, Hiramatsu O, Kajiya F, Feigl EO. Alpha-adrenergic vasoconstriction reduces systolic retrograde coronary blood flow. Am J Physiol Heart Circ Physiol 273: H2746 –H2755, 1997. 413. Muller JM, Myers PR, Laughlin MH. Exercise training alters myogenic responses in porcine coronary resistance arteries. J Appl Physiol 75: 2677–2682, 1993. 414. Muller JM, Myers PR, Laughlin MH. Vasodilator responses of coronary resistance arteries of exercise-trained pigs. Circulation 89: 2308 –2314, 1994. 415. Mundie TG, Januszkiewicz AJ, Ripple GR. Effects of epinephrine, phenoxybenzamine, propranolol on maximal exercise in sheep. Lab Anim Sci 42: 486 – 490, 1992. 416. Murphree SS, Saffitz JE. Delineation of the distribution of betaadrenergic receptor subtypes in canine myocardium. Circ Res 63: 117–125, 1988. 417. Murray PA, Vatner SF. alpha-Adrenoceptor attenuation of the coronary vascular response to severe exercise in the conscious dog. Circ Res 45: 654 – 660, 1979. 418. Myers PR, Banitt PF, Guerra R Jr, Harrison DG. Characteristics of canine coronary resistance arteries: importance of endothelium. Am J Physiol Heart Circ Physiol 257: H603–H610, 1989. 419. Myers PR, Katwa LC, Tanner M, Morrow C, Guarda E, Parker JL. Effects of angiotensin II on canine and porcine coronary epicardial and resistance arteries. J Vasc Res 31: 338 –346, 1994. 420. Namdar M, Koepfli P, Grathwohl R, Siegrist PT, Klainguti M, Schepis T, Delaloye R, Wyss CA, Fleischmann SP, Gaemperli O, Kaufmann PA. Caffeine decreases exercise-induced myocardial flow reserve. J Am Coll Cardiol 47: 405– 410, 2006. 421. Nanto S, Kitakaze M, Takano Y, Hori M, Nagata S. Intracoronary administration of adenosine triphosphate increases myocardial adenosine levels and coronary blood flow in man. Jpn Circ J 61: 836 – 842, 1997. 422. Narishige T, Egashira K, Akatsuka Y, Katsuda Y, Numaguchi K, Sakata M, Takeshita A. Glibenclamide, a putative ATP-sensitive K⫹ channel blocker, inhibits coronary autoregulation in anesthetized dogs. Circ Res 73: 771–776, 1993. 423. Needleman P, Kaley G. Cardiac and coronary prostaglandin synthesis and function. N Engl J Med 298: 1122–1128, 1978. 424. Neill WA, Oxendine JM. Exercise can promote coronary collateral development without improving perfusion of ischemic myocardium. Circulation 60: 1513–1519, 1979. 425. Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol Cell Physiol 268: C799 –C822, 1995. 426. Nelson RR, Gobel FL, Jorgensen CR, Wang K, Wang Y, Taylor HL. Hemodynamic predictors of myocardial oxygen consumption during static and dynamic exercise. Circulation 50: 1179 –1189, 1974. 427. Neumann T, Heusch G. Myocardial, skeletal muscle, renal blood flow during exercise in conscious dogs with heart failure. Am J Physiol Heart Circ Physiol 273: H2452–H2457, 1997. 428. Niebauer J, Hambrecht R, Marburger C, Hauer K, Velich T, von Hodenberg E, Schlierf G, Kubler W, Schuler G. Impact of intensive physical exercise and low-fat diet on collateral vessel formation in stable angina pectoris and angiographically confirmed coronary artery disease. Am J Cardiol 76: 771–775, 1995. Physiol Rev • VOL 1081 429. Node K, Kitakaze M, Kosaka H, Minamino T, Hori M. Bradykinin mediation of Ca2⫹-activated K⫹ channels regulates coronary blood flow in ischemic myocardium. Circulation 95: 1560 –1567, 1997. 430. Norton KI, Delp MD, Duan C, Warren JA, Armstrong RB. Hemodynamic responses during exercise at and above V̇O2max in swine. J Appl Physiol 69: 1587–1593, 1990. 431. Norton KI, Delp MD, Jones MT, Duan C, Dengel DR, Armstrong RB. Distribution of blood flow during exercise after blood volume expansion in swine. J Appl Physiol 69: 1578 –1586, 1990. 432. Nutter DO, Fuller EO. The role of isolated cardiac muscle preparations in the study of training effects of the heart. Med Sci Sports 9: 239 –245, 1977. 433. O’Konski MS, White FC, Longhurst J, Roth D, Bloor CM. Ameroid constriction of the proximal left circumflex coronary artery in swine. A model of limited coronary collateral circulation. Am J Cardiovasc Pathol 1: 69 –77, 1987. 434. O’Leary DS, Rossi NF, Churchill PC. Substantial cardiac parasympathetic activity exists during heavy dynamic exercise in dogs. Am J Physiol Heart Circ Physiol 273: H2135–H2140, 1997. 435. Oikawa Y, Maehara K, Saito T, Tamagawa K, Maruyama Y. Attenuation of angiotensin II-mediated coronary vasoconstriction and vasodilatory action of angiotensin-converting enzyme inhibitor in pacing-induced heart failure in dogs. J Am Coll Cardiol 38: 1188 –1194, 2001. 436. Opie LH, Lopaschuk GD. Fuels: aerobic and anaerobic metabolism. In: Heart Physiology, From Cell to Circulation, edited by Weinberg RW, Bersin J, Aversa F. Philadelphia, PA: Lippincot Williams & Wilkins, 2004, p. 306 –354. 437. Owen TL, Ehrhart IC, Weidner WJ, Scott JB, Haddy FJ. Effects of indomethacin on local blood flow regulation in canine heart and kidney. Proc Soc Exp Biol Med 149: 871– 876, 1975. 438. Oza NB, Schwartz JH, Goud HD, Levinsky NG. Rat aortic smooth muscle cells in culture express kallikrein, kininogen, bradykininase activity. J Clin Invest 85: 597– 600, 1990. 439. Pacold I, Hwang MH, Lawless CE, Diamond P, Scanlon PJ, Loeb HS. Effects of indomethacin on coronary hemodynamics, myocardial metabolism and anginal threshold in coronary artery disease. Am J Cardiol 57: 912–915, 1986. 440. Pagny JY, Peronnet F, Beliveau L, Sestier F, Nadeau R. Systemic and regional blood flows during graded treadmill exercise in dogs. J Physiol 81: 368 –373, 1986. 441. Pantely GA, Bristow JD, Swenson LJ, Ladley HD, Johnson WB, Anselone CG. Incomplete coronary vasodilation during myocardial ischemia in swine. Am J Physiol Heart Circ Physiol 249: H638 –H647, 1985. 442. Panza JA, Quyyumi AA, Brush JE Jr, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med 323: 22–27, 1990. 443. Paolocci N, Pagliaro P, Isoda T, Saavedra FW, Kass DA. Role of calcium-sensitive K⫹ channels and nitric oxide in in vivo coronary vasodilation from enhanced perfusion pulsatility. Circulation 103: 119 –124, 2001. 444. Parent R, Leblanc N, Lavallee M. Nitroglycerin reduces myocardial oxygen consumption during exercise despite vascular tolerance. Am J Physiol Heart Circ Physiol 290: H1226 –H1234, 2006. 445. Parent R, Pare R, Lavallee M. Contribution of nitric oxide to dilation of resistance coronary vessels in conscious dogs. Am J Physiol Heart Circ Physiol 262: H10 –H16, 1992. 446. Parizkova J, Wachtlova M, Soukupova M. The impact of different motor activity on body composition, density of capillaries and fibers in the heart and soleus muscles, cell’s migration in vitro in male rats. Int Z Angew Physiol 30: 207–216, 1972. 447. Park KH, Rubin LE, Gross SS, Levi R. Nitric oxide is a mediator of hypoxic coronary vasodilatation. Relation to adenosine and cyclooxygenase-derived metabolites. Circ Res 71: 992–1001, 1992. 448. Parks CM, Manohar M. Transmural coronary vasodilator reserve and flow distribution during severe exercise in ponies. J Appl Physiol 54: 1641–1652, 1983. 449. Pelc LR, Gross GJ, Warltier DC. Preferential increase in subendocardial perfusion produced by endothelium-dependent vasodilators. Circulation 76: 191–200, 1987. 88 • JULY 2008 • www.prv.org 1082 DIRK J. DUNCKER AND ROBERT J. BACHE 450. Peng W, Michael JR, Hoidal JR, Karwande SV, Farrukh IS. ET-1 modulates KCa-channel activity and arterial tension in normoxic and hypoxic human pulmonary vasculature. Am J Physiol Lung Cell Mol Physiol 275: L729 –L739, 1998. 451. Penpargkul S, Scheuer J. The effect of physical training upon the mechanical and metabolic performance of the rat heart. J Clin Invest 49: 1859 –1868, 1970. 452. Petren T, Sjöstrand T, Sylven B. Der influss des trainings auf die haufigkeit der capillaren in herz- und skeltenmuskulatur. Arbeitsphysiologie 9: 376 –386, 1930. 453. Petren T, Sylven B. Weitere untersuchungen uber den Einfluss des Trainings auf die Kapillarisierung der Herzmuskulator. Morphol Jahrb 80: 439 – 444, 1937. 454. Pohl U. Endothelial cells as part of a vascular oxygen-sensing system: hypoxia-induced release of autacoids. Experientia 46: 1175–1179, 1990. 455. Pohl U, Busse R. Hypoxia stimulates release of endotheliumderived relaxant factor. Am J Physiol Heart Circ Physiol 256: H1595–H1600, 1989. 456. Poliner LR, Dehmer GJ, Lewis SE, Parkey RW, Blomqvist CG, Willerson JT. Left ventricular performance in normal subjects: a comparison of the responses to exercise in the upright and supine positions. Circulation 62: 528 –534, 1980. 457. Prasad A, Halcox JP, Waclawiw MA, Quyyumi AA. Angiotensin type 1 receptor antagonism reverses abnormal coronary vasomotion in atherosclerosis. J Am Coll Cardiol 38: 1089 –1095, 2001. 458. Pupita G, Maseri A, Kaski JC, Galassi AR, Gavrielides S, Davies G, Crea F. Myocardial ischemia caused by distal coronaryartery constriction in stable angina pectoris. N Engl J Med 323: 514 –520, 1990. 459. Puybasset L, Bea ML, Ghaleh B, Giudicelli JF, Berdeaux A. Coronary and systemic hemodynamic effects of sustained inhibition of nitric oxide synthesis in conscious dogs. Evidence for cross talk between nitric oxide and cyclooxygenase in coronary vessels. Circ Res 79: 343–357, 1996. 460. Puybasset L, Giudicelli JF, Berdeaux A. Coronary effects of exogenous and endogenous bradykinin in conscious dogs. Fundam Clin Pharmacol 11: 322–330, 1997. 461. Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev 77: 1165–1232, 1997. 462. Quebbemann BB, Klassen CL, Bache RJ. Nitroglycerin fails to dilate coronary collateral vessels during exercise. J Cardiovasc Pharmacol 31: 821– 827, 1998. 463. Quilley J, Fulton D, McGiff JC. Hyperpolarizing factors. Biochem Pharmacol 54: 1059 –1070, 1997. 464. Quyyumi AA, Dakak N, Andrews NP, Husain S, Arora S, Gilligan DM, Panza JA, Cannon RO 3rd. Nitric oxide activity in the human coronary circulation. Impact of risk factors for coronary atherosclerosis. J Clin Invest 95: 1747–1755, 1995. 465. Raff WK, Kosche F, Lochner W. Extravascular coronary resistance and its relation to microcirculation. Influence of heart rate, end-diastolic pressure and maximal rate of rise of intraventricular pressure. Am J Cardiol 29: 598 – 603, 1972. 466. Rafflenbeul W, Lichtlen PR. Quantitative coronary angiography: evidence of a sustained increase in vascular smooth muscle tone in coronary artery stenoses. Z Kardiol 72 Suppl 3: 87–91, 1983. 467. Rahimtoola SH. The hibernating myocardium in ischaemia and congestive heart failure. Eur Heart J 14 Suppl A: 22–26, 1993. 468. Raven PB, Rohm-Young D, Blomqvist CG. Physical fitness and cardiovascular response to lower body negative pressure. J Appl Physiol 56: 138 –144, 1984. 469. Regan T, Timmis G, Gray M, Binak K, Hellems HK. Myocardial oxygen consumption during exercise in fasting and lipemic subjects. J Clin Invest 40: 624 – 630, 1961. 470. Rembert JC, Boyd LM, Watkinson WP, Greenfield JC Jr. Effect of adenosine on transmural myocardial blood flow distribution in the awake dog. Am J Physiol Heart Circ Physiol 239: H7–H13, 1980. 471. Richalet JP, Soulard C, Nitenberg A, Teisseire B, de Bovee J, Seroussi S. Myocardial oxygen extraction and oxygen-hemoglobin equilibrium curve during moderate exercise. Eur J Appl Physiol Occup Physiol 47: 27–39, 1981. Physiol Rev • VOL 472. Richard V, Berdeaux A, la Rochelle CD, Giudicelli JF. Regional coronary haemodynamic effects of two inhibitors of nitric oxide synthesis in anaesthetized, open-chest dogs. Br J Pharmacol 104: 59 – 64, 1991. ⫹ 473. Richmond KN, Tune JD, Gorman MW, Feigl EO. Role of KATP channels and adenosine in the control of coronary blood flow during exercise. J Appl Physiol 89: 529 –536, 2000. 474. Riggs A. Factors in the evolution of hemoglobin function. Federation Proc 35: 2115–2118, 1976. 475. Rigol M, Heras M, Solanes N, Epelde F, Roig E, Perez-Villa F, Roque M, Sanz G. Enalaprilat, losartan and LU 135252 in coronary blood flow regulation. Eur J Clin Invest 33: 363–369, 2003. 476. Rivas F, Cobb FR, Bache RJ, Greenfield JC Jr. Relationship between blood flow to ischemic regions and extent of myocardial infarction. Serial measurement of blood flow to ischemic regions in dogs. Circ Res 38: 439 – 447, 1976. 477. Rogers PA, Dick GM, Knudson JD, Focardi M, Bratz IN, Swafford AN Jr, Saitoh S, Tune JD, Chilian WM. H2O2-induced redox sensitive coronary vasodilation is mediated by 4-aminopyridine-sensitive K⫹ channels. Am J Physiol Heart Circ Physiol 291: H2473–H2482, 2006. 478. Rogers PJ, Miller TD, Bauer BA, Brum JM, Bove AA, Vanhoutte PM. Exercise training and responsiveness of isolated coronary arteries. J Appl Physiol 71: 2346 –2351, 1991. 479. Rossen JD, Oskarsson H, Minor RL Jr, Talman CL, Winniford MD. Effect of adenosine antagonism on metabolically mediated coronary vasodilation in humans. J Am Coll Cardiol 23: 1421–1426, 1994. 480. Roth DA, White CD, Podolin DA, Mazzeo RS. Alterations in myocardial signal transduction due to aging and chronic dynamic exercise. J Appl Physiol 84: 177–184, 1998. 481. Roth DM, Maruoka Y, Rogers J, White FC, Longhurst JC, Bloor CM. Development of coronary collateral circulation in left circumflex Ameroid-occluded swine myocardium. Am J Physiol Heart Circ Physiol 253: H1279 –H1288, 1987. 482. Roth DM, White FC, Nichols ML, Dobbs SL, Longhurst JC, Bloor CM. Effect of long-term exercise on regional myocardial function and coronary collateral development after gradual coronary artery occlusion in pigs. Circulation 82: 1778 –1789, 1990. 483. Rowell LB. Active neurogenic vasodilation in man. In: Vasodilation. New York: Raven, 1981, p. 1–17. 484. Rowell LB. Cardiovascular adaptations to chronic physical activity and inactivity. In: Human Circulation. New York: Oxford Univ. Press, 1986, p. 257–286. 485. Rowell LB. Ideas about control of skeletal and cardiac muscle blood flow (1876 –2003): cycles of revision and new vision. J Appl Physiol 97: 384 –392, 2004. 486. Rowell LB, Saltin B, Kiens B, Christensen NJ. Is peak quadriceps blood flow in humans even higher during exercise with hypoxemia? Am J Physiol Heart Circ Physiol 251: H1038 –H1044, 1986. 487. Rubanyi GM, Polokoff MA. Endothelins: molecular biology, biochemistry, pharmacology, physiology, pathophysiology. Pharmacol Rev 46: 325– 415, 1994. 488. Ruocco NA Jr, Most AS, Sasken H, Steiner M, Gewirtz H. Influence of serotonin on myocardial blood flow in the presence and absence of a coronary arterial stenosis: observations in domestic swine. Proc Soc Exp Biol Med 187: 416 – 424, 1988. 489. Ruocco NA, Most AS, Sasken H, Steiner M, Gewirtz H. Role of endogenous prostacyclin in myocardial blood flow regulation distal to a severe coronary stenosis. Cardiovasc Res 22: 511–519, 1988. 490. Rusch NJ, Liu Y, Pleyte KA. Mechanisms for regulation of arterial tone by Ca2⫹-dependent K⫹ channels in hypertension. Clin Exp Pharmacol Physiol 23: 1077–1081, 1996. 491. Rush JW, Laughlin MH, Woodman CR, Price EM. SOD-1 expression in pig coronary arterioles is increased by exercise training. Am J Physiol Heart Circ Physiol 279: H2068 –H2076, 2000. 492. Sable DL, Brammell HL, Sheehan MW, Nies AS, Gerber J, Horwitz LD. Attenuation of exercise conditioning by beta-adrenergic blockade. Circulation 65: 679 – 684, 1982. 493. Saino A, Pomidossi G, Perondi R, Valentini R, Rimini A, Di Francesco L, Mancia G. Intracoronary angiotensin II potentiates 88 • JULY 2008 • www.prv.org CORONARY BLOOD FLOW 494. 495. 496. 497. 498. 499. 500. 501. 502. 503. 504. 505. 506. 507. 508. 509. 510. 511. 512. 513. 514. coronary sympathetic vasoconstriction in humans. Circulation 96: 148 –153, 1997. Saito D, Steinhart CR, Nixon DG, Olsson RA. Intracoronary adenosine deaminase reduces canine myocardial reactive hyperemia. Circ Res 49: 1262–1267, 1981. Saitoh S, Zhang C, Tune JD, Potter B, Kiyooka T, Rogers PA, Knudson JD, Dick GM, Swafford A, Chilian WM. Hydrogen peroxide: a feed-forward dilator that couples myocardial metabolism to coronary blood flow. Arterioscler Thromb Vasc Biol 26: 2614 –2621, 2006. Samaha FF, Heineman FW, Ince C, Fleming J, Balaban RS. ATP-sensitive potassium channel is essential to maintain basal coronary vascular tone in vivo. Am J Physiol Cell Physiol 262: C1220 –C1227, 1992. Sambuceti G, Marzilli M, Marraccini P, Schneider-Eicke J, Gliozheni E, Parodi O, L’Abbate A. Coronary vasoconstriction during myocardial ischemia induced by rises in metabolic demand in patients with coronary artery disease. Circulation 95: 2652– 2659, 1997. Sanders M, White FC, Bloor CM. Cardiovascular responses of dogs and pigs exposed to similar physiological stress. Comp Biochem Physiol 58: 365–370, 1977. Sanders M, White FC, Bloor CM. Myocardial blood flow distribution in miniature pigs during exercise. Basic Res Cardiol 72: 326 –331, 1977. Sanders M, White FC, Peterson TM, Bloor CM. Characteristics of coronary blood flow and transmural distribution in miniature pigs. Am J Physiol Heart Circ Physiol 235: H601–H609, 1978. Sanders M, White FC, Peterson TM, Bloor CM. Effects of endurance exercise on coronary collateral blood flow in miniature swine. Am J Physiol Heart Circ Physiol 234: H614 –H619, 1978. Sato N, Shen YT, Kiuchi K, Shannon RP, Vatner SF. Splenic contraction-induced increases in arterial O2 reduce requirement for CBF in conscious dogs. Am J Physiol Heart Circ Physiol 269: H491–H503, 1995. Satoh S, Maruyama Y, Watanabe J, Keitoku M, Hangai K, Takishima T. Coronary zero flow pressure and intramyocardial pressure in transiently arrested heart. Cardiovasc Res 24: 358 –363, 1990. Schaper W. Influence of physical exercise on coronary collateral blood flow in chronic experimental two-vessel occlusion. Circulation 65: 905–912, 1982. Schaper W, Remijsen P, Xhonneux R. The size of myocardial infarction after experimental coronary artery ligation. Z Kreislaufforsch 58: 904 –909, 1969. Scheel KW, Ingram LA, Wilson JL. Effects of exercise on the coronary and collateral vasculature of beagles with and without coronary occlusion. Circ Res 48: 523–530, 1981. Scheffer MG, Verdouw PD. Decreased incidence of ventricular fibrillation after an acute coronary artery ligation in exercised pigs. Basic Res Cardiol 78: 298 –309, 1983. Scheuer J, Stezoski SW. Effect of physical training on the mechanical and metabolic response of the rat heart to hypoxia. Circ Res 30: 418 – 429, 1972. Scheuer J, Tipton CM. Cardiovascular adaptations to physical training. Annu Rev Physiol 39: 221–251, 1977. Schrader J, Baumann G, Gerlach E. Adenosine as inhibitor of myocardial effects of catecholamines. Pflügers Arch 372: 29 –35, 1977. Schubert R, Nelson MT. Protein kinases: tuners of the BKCa channel in smooth muscle. Trends Pharmacol Sci 22: 505–512, 2001. Schubert R, Serebryakov VN, Mewes H, Hopp HH. Iloprost dilates rat small arteries: role of KATP- and KCa channel activation by cAMP-dependent protein kinase. Am J Physiol Heart Circ Physiol 272: H1147–H1156, 1997. Schuijt MP, Basdew M, van Veghel R, de Vries R, Saxena PR, Schoemaker RG, Danser AH. AT2 receptor-mediated vasodilation in the heart: effect of myocardial infarction. Am J Physiol Heart Circ Physiol Heart Circ Physiol 281: H2590 –2596, 2001. Schulz R, Oudiz RJ, Guth BD, Heusch G. Minimal alpha 1- and alpha 2-adrenoceptor-mediated coronary vasoconstriction in the Physiol Rev • VOL 515. 516. 517. 518. 519. 520. 521. 522. 523. 524. 525. 526. 527. 528. 529. 530. 531. 532. 533. 534. 1083 anaesthetized swine. Naunyn-Schmiedebergs Arch Pharmacol 342: 422– 428, 1990. Schutz W, Zimpfer M, Raberger G. Effect of aminophylline on coronary reactive hyperaemia following brief and long occlusion periods. Cardiovasc Res 11: 507–511, 1977. Schwartz PJ, Stone HL. Effects of unilateral stellectomy upon cardiac performance during exercise in dogs. Circ Res 44: 637– 645, 1979. Scornik FS, Codina J, Birnbaumer L, Toro L. Modulation of coronary smooth muscle KCa channels by Gs␣ independent of phosphorylation by protein kinase A. Am J Physiol Heart Circ Physiol 265: H1460 –H1465, 1993. Seitelberger R, Guth BD, Heusch G, Lee JD, Katayama K, Ross J Jr. Intracoronary alpha 2-adrenergic receptor blockade attenuates ischemia in conscious dogs during exercise. Circ Res 62: 436 – 442, 1988. Sellke FW, Kagaya Y, Johnson RG, Shafique T, Schoen FJ, Grossman W, Weintraub RM. Endothelial modulation of porcine coronary microcirculation perfused via immature collaterals. Am J Physiol Heart Circ Physiol 262: H1669 –H1675, 1992. Sellke FW, Myers PR, Bates JN, Harrison DG. Influence of vessel size on the sensitivity of porcine coronary microvessels to nitroglycerin. Am J Physiol Heart Circ Physiol 258: H515–H520, 1990. Sellke FW, Quillen JE, Brooks LA, Harrison DG. Endothelial modulation of the coronary vasculature in vessels perfused via mature collaterals. Circulation 81: 1938 –1947, 1990. Sellke FW, Wang SY, Friedman M, Dai HB, Harada K, Lopez JJ, Simons M. Beta-adrenergic modulation of the collateral-dependent coronary microcirculation. J Surg Res 59: 185–190, 1995. Selvanayagam JB, Jerosch-Herold M, Porto I, Sheridan D, Cheng AS, Petersen SE, Searle N, Channon KM, Banning AP, Neubauer S. Resting myocardial blood flow is impaired in hibernating myocardium: a magnetic resonance study of quantitative perfusion assessment. Circulation 112: 3289 –3296, 2005. Setty S, Sun W, Tune JD. Coronary blood flow regulation in the prediabetic metabolic syndrome. Basic Res Cardiol 98: 416 – 423, 2003. Shen W, Ochoa M, Xu X, Wang J, Hintze TH. Role of EDRF/NO in parasympathetic coronary vasodilation following carotid chemoreflex activation in conscious dogs. Am J Physiol Heart Circ Physiol 267: H605–H613, 1994. Sherman CT, Litvack F, Grundfest W, Lee M, Hickey A, Chaux A, Kass R, Blanche C, Matloff J, Morgenstern L, et al. Coronary angioscopy in patients with unstable angina pectoris. N Engl J Med 315: 913–919, 1986. Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol 67: 99 –145, 2005. Smith RE, Palmer RM, Bucknall CA, Moncada S. Role of nitric oxide synthesis in the regulation of coronary vascular tone in the isolated perfused rabbit heart. Cardiovasc Res 26: 508 –512, 1992. Smith TP Jr, Canty JM, Jr. Modulation of coronary autoregulatory responses by nitric oxide Evidence for flow-dependent resistance adjustments in conscious dogs. Circ Res 73: 232–240, 1993. Sobey CG. Potassium channel function in vascular disease. Arterioscler Thromb Vasc Biol 21: 28 –38, 2001. Sonnenblick EH, Braunwald E, Williams JF Jr, Glick G. Effects of exercise on myocardial force-velocity relations in intact unanesthetized man: relative roles of changes in heart rate, sympathetic activity, ventricular dimensions. J Clin Invest 44: 2051– 2062, 1965. Sorop O, Merkus D, de Beer VJ, Houweling B, Pistea A, McFalls EO, Boomsma F, van Beusekom HM, van der Giessen WJ, VanBavel E, Duncker DJ. Functional and structural adaptations of coronary microvessels distal to a chronic coronary artery stenosis. Circ Res 102: 795– 803, 2008. Spaan JA. Coronary diastolic pressure-flow relation and zero flow pressure explained on the basis of intramyocardial compliance. Circ Res 56: 293–309, 1985. Sparks HV Jr, Bardenheuer H. Regulation of adenosine formation by the heart. Circ Res 58: 193–201, 1986. 88 • JULY 2008 • www.prv.org 1084 DIRK J. DUNCKER AND ROBERT J. BACHE 535. Spear KL, Koerner JE, Terjung RL. coronary blood flow in physically trained rats. Cardiovasc Res 12: 135–143, 1978. 536. Stamler JS, Jia L, Eu JP, McMahon TJ, Demchenko IT, Bonaventura J, Gernert K, Piantadosi CA. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science 276: 2034 –2037, 1997. 537. Standen NB, Quayle JM. K⫹ channel modulation in arterial smooth muscle. Acta Physiol Scand 164: 549 –557, 1998. 538. Standen NB, Quayle JM, Davies NW, Brayden JE, Huang Y, Nelson MT. Hyperpolarizing vasodilators activate ATP-sensitive K⫹ channels in arterial smooth muscle. Science 245: 177–180, 1989. 539. Stebbins CL, Symons JD. Role of angiotensin II in hemodynamic responses to dynamic exercise in miniswine. J Appl Physiol 78: 185–190, 1995. 540. Stebbins CL, Symons JD. Vasopressin contributes to the cardiovascular response to dynamic exercise. Am J Physiol Heart Circ Physiol 264: H1701–H1707, 1993. 541. Stone HL. Coronary flow, myocardial oxygen consumption, and exercise training in dogs. J Appl Physiol 49: 759 –768, 1980. 542. Strader JR, Gwirtz PA, Jones CE. Comparative effects of alpha-1 and alpha-2 adrenoceptors in modulation of coronary flow during exercise. J Pharmacol Exp Ther 246: 772–778, 1988. 543. Su JB, Houel R, Heloire F, Barbe F, Beverelli F, Sambin L, Castaigne A, Berdeaux A, Crozatier B, Hittinger L. Stimulation of bradykinin B1 receptors induces vasodilation in conductance and resistance coronary vessels in conscious dogs: comparison with B2 receptor stimulation. Circulation 101: 1848 –1853, 2000. 544. Suga H. Cardiac energetics: from E(max) to pressure-volume area. Clin Exp Pharmacol Physiol 30: 580 –585, 2003. 545. Suga H. Ventricular energetics. Physiol Rev 70: 247–277, 1990. 546. Symons JD, Pitsillides KF, Longhurst JC. Chronic reduction of myocardial ischemia does not attenuate coronary collateral development in miniswine. Circulation 86: 660 – 671, 1992. 547. Symons JD, Stebbins CL. Effects of angiotensin II receptor blockade during exercise: comparison of losartan and saralasin. J Cardiovasc Pharmacol 28: 223–231, 1996. 548. Takamura M, Parent R, Cernacek P, Lavallee M. Influence of dual ETA/ETB-receptor blockade on coronary responses to treadmill exercise in dogs. J Appl Physiol 89: 2041–2048, 2000. 549. Taniguchi J, Furukawa KI, Shigekawa M. Maxi K⫹ channels are stimulated by cyclic guanosine monophosphate-dependent protein kinase in canine coronary artery smooth muscle cells. Pflügers Arch 423: 167–172, 1993. 550. Teisman AC, Westerink BH, van Veldhuisen DJ, Scholtens E, de Zeeuw D, van Gilst WH. Direct interaction between the sympathetic and renin-angiotensin system in myocardial tissue: a microdialysis study in anaesthetised rats. J Auton Nerv Syst 78: 117–121, 2000. 551. Terrados N, Mizuno M, Andersen H. Reduction in maximal oxygen uptake at low altitudes: role of training status and lung function. Clin Physiol 5 Suppl 3: 75–79, 1985. 552. Tharp GD, Wagner CT. Chronic exercise and cardiac vascularization. Eur J Appl Physiol Occup Physiol 48: 97–104, 1982. 553. Thomas DP. Effects of acute and chronic exercise on myocardial ultrastructure. Med Sci Sports Exerc 17: 546 –553, 1985. 554. Thomas SA, Fallavollita JA, Suzuki G, Borgers M, Canty JM Jr. Dissociation of regional adaptations to ischemia and global myolysis in an accelerated Swine model of chronic hibernating myocardium. Circ Res 91: 970 –977, 2002. 555. Thörner W. Trainingsversuche an Hunden. III. Histologische Beobachtungen und Herz- und Skeletmuskeln. Arbeitphysiologie 8: 359 –370, 1935. 556. Tiefenbacher CP, Chilian WM. Heterogeneity of coronary vasomotion. Basic Res Cardiol 93: 446 – 454, 1998. 557. Timmermans PB, Wong PC, Chiu AT, Herblin WF, Benfield P, Carini DJ, Lee RJ, Wexler RR, Saye JA, Smith RD. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev 45: 205–251, 1993. 558. Tomanek RJ. Effects of age and exercise on the extent of the myocardial capillary bed. Anat Rec 167: 55– 62, 1970. 559. Traverse JH, Altman JD, Kinn J, Duncker DJ, Bache RJ. Effect of beta-adrenergic receptor blockade on blood flow to colPhysiol Rev • VOL 560. 561. 562. 563. 564. 565. 566. 567. 568. 569. 570. 571. 572. 573. 574. 575. 576. 577. 578. 579. 580. lateral-dependent myocardium during exercise. Circulation 91: 1560 –1567, 1995. Traverse JH, Judd D, Bache RJ. Dose-dependent effect of endothelin-1 on blood flow to normal and collateral-dependent myocardium. Circulation 93: 558 –566, 1996. Traverse JH, Kinn JW, Klassen C, Duncker DJ, Bache RJ. Nitric oxide inhibition impairs blood flow during exercise in hearts with a collateral-dependent myocardial region. J Am Coll Cardiol 31: 67–74, 1998. Traverse JH, Wang YL, Du R, Nelson D, Lindstrom P, Archer SL, Gong G, Bache RJ. Coronary nitric oxide production in response to exercise and endothelium-dependent agonists. Circulation 101: 2526 –2531, 2000. Trimble J, Downey J. Contribution of myocardial contractility to myocardial perfusion. Am J Physiol Heart Circ Physiol 236: H121– H126, 1979. Tschudi M, Richard V, Buhler FR, Luscher TF. Importance of endothelium-derived nitric oxide in porcine coronary resistance arteries. Am J Physiol Heart Circ Physiol 260: H13–H20, 1991. Tune JD, Gorman MW, Feigl EO. Matching coronary blood flow to myocardial oxygen consumption. J Appl Physiol 97: 404 – 415, 2004. Tune JD, Richmond KN, Gorman MW, Feigl EO. Control of coronary blood flow during exercise. Exp Biol Med 227: 238 –250, 2002. ⫹ Tune JD, Richmond KN, Gorman MW, Feigl EO. KATP channels, nitric oxide, adenosine are not required for local metabolic coronary vasodilation. Am J Physiol Heart Circ Physiol 280: H868 – H875, 2001. Tune JD, Richmond KN, Gorman MW, Feigl EO. Role of nitric oxide and adenosine in control of coronary blood flow in exercising dogs. Circulation 101: 2942–2948, 2000. Tune JD, Richmond KN, Gorman MW, Olsson RA, Feigl EO. Adenosine is not responsible for local metabolic control of coronary blood flow in dogs during exercise. Am J Physiol Heart Circ Physiol 278: H74 –H84, 2000. Ueeda M, Silvia SK, Olsson RA. Nitric oxide modulates coronary autoregulation in the guinea pig. Circ Res 70: 1296 –1303, 1992. Uhlig PN, Baer RW, Vlahakes GJ, Hanley FL, Messina LM, Hoffman JI. Arterial and venous coronary pressure-flow relations in anesthetized dogs. Evidence for a vascular waterfall in epicardial coronary veins. Circ Res 55: 238 –248, 1984. Unge G, Carlsson S, Ljungqvist A, Tornling G, Adolfsson J. The proliferative activity of myocardial capillary wall cells in variously aged swimming-exercised rats. Acta Pathol Microbiol Scand 87: 15–17, 1979. Utley J, Carlson EL, Hoffman JI, Martinez HM, Buckberg GD. Total and regional myocardial blood flow measurements with 25 micron, 15 micron, 9 micron, filtered 1–10 micron diameter microspheres and antipyrine in dogs and sheep. Circ Res 34: 391– 405, 1974. Van Citters RL, Franklin DL. Cardiovascular performance of Alaska sled dogs during exercise. Circ Res 24: 33– 42, 1969. Van den Berg EK, Schmitz JM, Benedict CR, Malloy CR, Willerson JT, Dehmer GJ. Transcardiac serotonin concentration is increased in selected patients with limiting angina and complex coronary lesion morphology. Circulation 79: 116 –124, 1989. Van Nueten JM, Janssen PA, Van Beek J, Xhonneux R, Verbeuren TJ, Vanhoutte PM. Vascular effects of ketanserin (R 41 468), a novel antagonist of 5-HT2 serotonergic receptors. J Pharmacol Exp Ther 218: 217–230, 1981. Van Wylen DG, Willis J, Sodhi J, Weiss RJ, Lasley RD, Mentzer RM Jr. Cardiac microdialysis to estimate interstitial adenosine and coronary blood flow. Am J Physiol Heart Circ Physiol 258: H1642–H1649, 1990. Vanhoutte PM. Endothelial dysfunction and inhibition of converting enzyme. Eur Heart J 19 Suppl J: J7–15, 1998. Vanhoutte PM. Endothelium-dependent hyperpolarizations: the history. Pharmacol Res 49: 503–508, 2004. Vatner SF, Braunwald E. Cardiovascular control mechanisms in the conscious state. N Engl J Med 293: 970 –976, 1975. 88 • JULY 2008 • www.prv.org CORONARY BLOOD FLOW 581. Vatner SF, Franklin D, Higgins CB, Patrick T, Braunwald E. Left ventricular response to severe exertion in untethered dogs. J Clin Invest 51: 3052–3060, 1972. 582. Vatner SF, Higgins CB, Franklin D, Braunwald E. Role of tachycardia in mediating the coronary hemodynamic response to severe exercise. J Appl Physiol 32: 380 –385, 1972. 583. Vatner SF, Higgins CB, Millard RW, Franklin D. Role of the spleen in the peripheral vascular response to severe exercise in untethered dogs. Cardiovasc Res 8: 276 –282, 1974. 584. Vatner SF, Pagani M. Cardiovascular adjustments to exercise: hemodynamics and mechanisms. Prog Cardiovasc Dis 19: 91–108, 1976. 585. Versluis JP, Heslinga JW, Sipkema P, Westerhof N. Microvascular pressure measurement reveals a coronary vascular waterfall in arterioles larger than 110 microm. Am J Physiol Heart Circ Physiol 281: H1913–H1918, 2001. 586. Vigorito C, Giordano A, De Caprio L, Vitale DF, Maurea N, Silvestri P, Tuccillo B, Ferrara N, Marone G, Rengo F. Effects of histamine on coronary hemodynamics in humans: role of H1 and H2 receptors. J Am Coll Cardiol 10: 1207–1213, 1987. 587. Von Restorff W, Hofling B, Holtz J, Bassenge E. Effect of increased blood fluidity through hemodilution on coronary circulation at rest and during exercise in dogs. Pflügers Arch 357: 15–24, 1975. 588. Von Restorff W, Holtz J, Bassenge E. Exercise induced augmentation of myocardial oxygen extraction in spite of normal coronary dilatory capacity in dogs. Pflügers Arch 372: 181–185, 1977. 589. Wachtlova M, Rakusan K, Poupa O. The coronary terminal vascular bed in the heart of the hare (Lepus europeus) and the rabbit (Oryctolagus domesticus). Physiol Bohemoslov 14: 328 –331, 1965. 590. Wachtlova M, Rakusan K, Roth Z, Poupa O. The terminal vascular bed of the myocardium in the wild rat (Rattus norvegicus) and the laboratory rat (Rattus norvegicus lab). Physiol Bohemoslov 16: 548 –554, 1967. 591. Waeber B, Schaller MD, Nussberger J, Bussien JP, Hofbauer KG, Brunner HR. Skin blood flow reduction induced by cigarette smoking: role of vasopressin. Am J Physiol Heart Circ Physiol 247: H895–H901, 1984. 592. Watanabe J, Maruyama Y, Satoh S, Keitoku M, Takishima T. Effects of the pericardium on the diastolic left coronary pressureflow relationship in the isolated dog heart. Circulation 75: 670 – 675, 1987. 593. Watanabe T, Harumi K, Akutsu Y, Yamanaka H, Michihata T, Okazaki O, Katagiri T. Relation between exercise-induced myocardial ischemia as assessed by nitrogen-13 ammonia positron emission tomography and QT interval behavior in patients with right bundle branch block. Am J Cardiol 81: 816 – 821, 1998. 594. Watkinson WP, Foley DH, Rubio R, Berne RM. Myocardial adenosine formation with increased cardiac performance in the dog. Am J Physiol Heart Circ Physiol 236: H13–H21, 1979. 595. Wei HM, Kang YH, Merrill GF. Coronary vasodilation during global myocardial hypoxia: effects of adenosine deaminase. Am J Physiol Heart Circ Physiol 254: H1004 –H1009, 1988. 596. Weiss HR. Regional oxygen consumption and supply in the dog heart: effect of atrial pacing. Am J Physiol Heart Circ Physiol 236: H231–H237, 1979. 597. Wenzel RR, Fleisch M, Shaw S, Noll G, Kaufmann U, Schmitt R, Jones CR, Clozel M, Meier B, Luscher TF. Hemodynamic and coronary effects of the endothelin antagonist bosentan in patients with coronary artery disease. Circulation 98: 2235–2240, 1998. 598. Westby J, Birkeland S, Rynning SE, Myking OL, Lekven J, Grong K. Alpha-adrenergic vasoconstriction in normal and hypoperfused myocardium during sympathetic nerve stimulation. Am J Physiol Heart Circ Physiol 263: H1682–H1688, 1992. 599. Westerhof N, Boer C, Lamberts RR, Sipkema P. Cross-talk between cardiac muscle and coronary vasculature. Physiol Rev 86: 1263–1308, 2006. 600. White FC, Bloor CM, McKirnan MD, Carroll SM. Exercise training in swine promotes growth of arteriolar bed and capillary angiogenesis in heart. J Appl Physiol 85: 1160 –1168, 1998. Physiol Rev • VOL 1085 601. White FC, McKirnan MD, Breisch EA, Guth BD, Liu YM, Bloor CM. Adaptation of the left ventricle to exercise-induced hypertrophy. J Appl Physiol 62: 1097–1110, 1987. 602. White FC, Roth DM, Bloor CM. Coronary collateral reserve during exercise induced ischemia in swine. Basic Res Cardiol 84: 42–54, 1989. 603. White FC, Sanders M, Bloor CM. Coronary reserve at maximal heart rate in the exercising swine. Cardiac Rehab 1: 31–39, 1981. 604. White RE, Kryman JP, El-Mowafy AM, Han G, Carrier GO. cAMP-dependent vasodilators cross-activate the cGMP-dependent protein kinase to stimulate BK(Ca) channel activity in coronary artery smooth muscle cells. Circ Res 86: 897–905, 2000. 605. Wiest E, Trach V, Dammgen J. Removal of endothelial function in coronary resistance vessels by saponin. Basic Res Cardiol 84: 469 – 478, 1989. 606. Williams RS. Role of receptor mechanisms in the adaptive response to habitual exercise. Am J Cardiol 55: 68D-73D, 1985. 607. Winbury MM, Howe BB, Hefner MA. Effect of nitrates and other coronary dilators on large and small coronary vessels: an hypothesis for the mechanism of action of nitrates. J Pharmacol Exp Ther 168: 70 –95, 1969. 608. Wolfson S, Gorlin R. Cardiovascular pharmacology of propranolol in man. Circulation 40: 501–511, 1969. 609. Woodman OL. Enhanced coronary vasoconstrictor responses to 5-hydroxytryptamine in the presence of a coronary artery stenosis in anaesthetized dogs. Br J Pharmacol 100: 153–157, 1990. 610. Woodman OL, Dusting GJ. N-nitro-L-arginine causes coronary vasoconstriction and inhibits endothelium-dependent vasodilatation in anaesthetized greyhounds. Br J Pharmacol 103: 1407–1410, 1991. 611. Wright L, Homans DC, Laxson DD, Dai XZ, Bache RJ. Effect of serotonin and thromboxane A2 on blood flow through moderately well developed coronary collateral vessels. J Am Coll Cardiol 19: 687– 693, 1992. 612. Wyatt HL, Mitchell J. Influences of physical conditioning and deconditioning on coronary vasculature of dogs. J Appl Physiol 45: 619 – 625, 1978. 613. Wyatt HL, Mitchell JH. Influences of physical training on the heart of dogs. Circ Res 35: 883– 889, 1974. 614. Wyss CA, Koepfli P, Fretz G, Seebauer M, Schirlo C, Kaufmann PA. Influence of altitude exposure on coronary flow reserve. Circulation 108: 1202–1207, 2003. 615. Wyss CA, Koepfli P, Mikolajczyk K, Burger C, von Schulthess GK, Kaufmann PA. Bicycle exercise stress in PET for assessment of coronary flow reserve: repeatability and comparison with adenosine stress. J Nucl Med 44: 146 –154, 2003. 616. Yada T, Richmond KN, Van Bibber R, Kroll K, Feigl EO. Role of adenosine in local metabolic coronary vasodilation. Am J Physiol Heart Circ Physiol 276: H1425–H1433, 1999. 617. Yaku H, Slinker BK, Mochizuki T, Lorell BH, LeWinter MM. Use of 2,3-butanedione monoxime to estimate nonmechanical VO2 in rabbit hearts. Am J Physiol Heart Circ Physiol 265: H834 –H842, 1993. 618. Yamabe H, Okumura K, Ishizaka H, Tsuchiya T, Yasue H. Role of endothelium-derived nitric oxide in myocardial reactive hyperemia. Am J Physiol Heart Circ Physiol 263: H8 –H14, 1992. 619. Yipintsoi T, Rosenkrantz J, Codini MA, Scheuer J. Myocardial blood flow responses to acute hypoxia and volume loading in physically trained rats. Cardiovasc Res 14: 50 –57, 1980. 620. Yonekura S, Watanabe N, Caffrey JL, Gaugl JF, Downey HF. Mechanism of attenuated pressure-flow autoregulation in right coronary circulation of dogs. Circ Res 60: 133–141, 1987. 621. Yu Y, Tune JD, Downey HF. Elevated right atrial pressure does not reduce collateral blood flow to ischemic myocardium. Am J Physiol Heart Circ Physiol 273: H2296 –H2303, 1997. 622. Zeiher AM, Krause T, Schachinger V, Minners J, Moser E. Impaired endothelium-dependent vasodilation of coronary resistance vessels is associated with exercise-induced myocardial ischemia. Circulation 91: 2345–2352, 1995. 623. Zhang C, Hein TW, Wang W, Kuo L. Divergent roles of angiotensin II AT1 and AT2 receptors in modulating coronary microvascular function. Circ Res 92: 322–329, 2003. 88 • JULY 2008 • www.prv.org 1086 DIRK J. DUNCKER AND ROBERT J. BACHE 624. Zhang C, Knudson JD, Setty S, Araiza A, Dincer UD, Kuo L, Tune JD. Coronary arteriolar vasoconstriction to angiotensin II is augmented in prediabetic metabolic syndrome via activation of AT1 receptors. Am J Physiol Heart Circ Physiol 288: H2154 – H2162, 2005. 625. Zhang J, Path G, Homans DC, Chepuri V, Merkle H, Hendrich K, Meyn MM, Bache RJ, Ugurbil K, From AHL. Bioenergetic and functional consequences of dobutamine infusion in Physiol Rev • VOL tachycardic, blood flow limited myocardium. Circulation 86, 1992. 626. Zong P, Sun W, Setty S, Tune JD, Downey HF. Alpha-adrenergic vasoconstrictor tone limits right coronary blood flow in exercising dogs. Exp Biol Med 229: 312–322, 2004. 627. Zong P, Tune JD, Downey HF. Mechanisms of oxygen demand/ supply balance in the right ventricle. Exp Biol Med 230: 507–519, 2005. 88 • JULY 2008 • www.prv.org