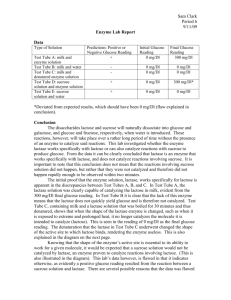
Enzymes Introduction:
advertisement

Enzymes Introduction: Lactose intolerance is a condition that affects millions of individuals. It is a condition that prevents an individual from metabolizing (breaking down) lactose. Lactose is a disaccharide that is composed of the monomers beta-D-galactose and beta-D-glucose. Lactase is an enzyme that is found in the small intestine, liver, and kidney of mammals. In the presence of lactase, lactose is cleaved and reduced to its subunits D-galactose and D-glucose, monosaccharides that can eventually be utilized as a source of energy in cells. Pharmaceutical advancements have resulted in the production of enzymatic supplements for people who have lactose intolerance. As their name implies, these solutions supplement, or provide, the enzyme to the individual who lacks it. In today’s experiment you will be using a solution of lactase to test the chemical and physiological properties of this particular enzyme. Furthermore, based on the results you obtain throughout the lab, you will attempt to determine if the lactase used was extracted from human cells or bacterial cells. General Enzymatic Terms: Enzyme: A protein that acts as a catalyst, lowering the activation energy needed for reactions to progress in cells. The reaction can still occur without the presence of the enzyme, but at a much slower rate. Activation Energy: The minimum amount of energy needed for a chemical reaction to occur, yielding products from a given set of reactants. Substrate(s): The reactant(s) of the reaction that is/are bound by the enzyme Active Site: A region of the enzyme where binding to substrate occurs. This site is flexible, and can reform to assist with the catalysis of the reaction of the substrates. Once the substrates have formed product(s) and have become unbound, the active site can return to its original shape. 1 Figure 1. The metabolism of lactose into galactose and glucose is facilitated by lactase. The Effect of Temperature on Enzymatic Activity The activity of every enzyme can be affected by alterations to its environment. In the following experiment you will test the effects that changes in temperature have on lactase. At extremely low temperatures, molecules move so slowly that reaction rates can be hampered even though the enzyme is still properly folded and active. At extremely high temperatures, bonds and interactions that exist between the amino acid side chains (R groups) in an enzyme become disrupted. The result is that the enzyme is incapable of maintaining its shape, and therefore unfolds, becoming inactive. This process of forcing a protein to lose is structure, and in most cases its functionality, is called protein denaturation. Although temperature can have negative effects on enzymatic activity, there is a specific temperature where the enzyme is most effective at catalyzing its specific reaction and its enzymatic activity is maximized. This temperature is known as the optimal temperature. Think about where and when lactose metabolism occurs in humans. You may need to do some internet research to determine this! Using this knowledge, write up the following hypothesis regarding the effect of temperature on the lactase reaction. Based on what I know about the effect of temperature on the enzymatic activity of lactase, if the lactase used in today’s lab was extracted from human cells, I hypothesize that __________________ _______________________________________________________________________________________. Based on what I know about the effect of temperature on the enzymatic activity of lactase, if the lactase used in today’s lab was extracted from bacterial cells, I hypothesize that _______________ _____________________________________________________________________________________. 2 Materials and Methods - Temperature experiment: 1. Label a microfuge tube or microfuge tubes with 0, 25, 40, 60, 80, or 100 (depending upon which temperature your instructor assigns to you). Each bench will evaluate one temperature. One bench will do 25 0C and 80 0C. 2. Using a plastic pipette, fill each tube up to the 0.5 line with lactase solution. The volume of the lactase solution is 500 µL (0.5 mL). 3. Place your tube in a water bath or a 100 mL beaker that matches each temperature and let it sit for 5 minutes. 4. Using an alternate plastic pipette, add milk to the tube, until the mixture of milk and lactase reaches the 1.0 line. At this point there is 1 mL of mixture in the tube: 500 µL of milk and 500 µL of lactase solution. If you removed the tube while performing this step, place it back into the appropriate water bath. 5. After ten minutes, place a glucose strip in each tube for one second, and then remove it and allow it to sit on your bench top for up to three minutes. At the end of 3 minutes compare the coloration of it to the chart provided and determine the amount of glucose in mg/dL. Record this value in Figure 2. 3 Results: 0° C Glucose (mg/dL) 25° C Glucose (mg/dL) 40° C Glucose (mg/dL) 60° C Glucose (mg/dL) 80° C Glucose (mg/dL) 100° C Glucose (mg/dL) 10 Minutes Figure 2. Enzymatic Activity of Lactase at varying temperatures based on Glucose production. Check your understanding of this experiment: 1. What is the substrate in the above experiment? What is the enzyme? Substrate: _______________________ Enzyme: _____________________ 2. What is the substrate source? _________________________________________________________________ 3. What is the independent variable in this experiment? The dependent variable? Independent: ___________________ Dependent: ___________________ 4. What are the products in this experiment? ______________________________________________________ 4 5. Please graph your data in Figure 3. Be sure to construct a curve that passes through all of your data points. Effect of Temperature on Lactase Activity at 10 Minutes Glucose (mg/dL) Temperature °C Figure 3. Enzymatic Activity of Lactase at varying temperatures based on Glucose production 6. What temperatures resulted in a loss of enzymatic activity? 7. Explain what most likely contributed to the loss of enzymatic activity at each temperature from question 5. 5 Conclusions: After comparing the effect of temperature on the production of glucose, I conclude that __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ _________________________________________________________________________________________. The Effect of pH on Enzymatic Activity Similar to temperature, pH can also affect the activity an enzyme. pH that is too high or too low can cause the enzyme to become denatured. Additionally, there exists an optimal pH where the enzyme’s activity and therefore efficiency will be maximized. Recall where and when lactose metabolism occurs in the human body. Think about the pH of various organs in the body. Formulate hypotheses about what you expect to happen in each of the following experiments. Based on what I know about the effect of pH on the enzymatic activity of lactase, if the lactase used in today’s lab was extracted from human cells, I hypothesize that__________________________________ _________________________________________________________________________________________. Based on what I know about the effect of pH on the enzymatic activity of lactase, if the lactase used in today’s lab was extracted from bacterial cells, I hypothesize that_________________________________ _________________________________________________________________________________________. 6 Materials and Methods- pH experiment: 1. Label seven microfuge tubes 2, 4, 6, 7, 8, 10, and 12. Each bench can do all pHs or one pH per bench, depending on time constraints. 2. Using a plastic pipette, fill each tube up to the 0.5 line with the appropriate pH buffer (2, 4, 5, 6, 7, 8, 10, or 12). The volume of pH buffer in each tube is now 500 µL. 3. Using a clean plastic pipette, add 3 drops milk to each tube. 4. Close the lid of each microfuge tube and invert each tube three times to mix the milk and pH buffer. 5. Using a clean plastic pipette, add 3 drops of lactase solution to each tube. 6. Close the lid of each microfuge tube and invert each tube three times to mix the milk, lactase and pH buffer. 7. Place all tubes in the 40° C water bath and incubate them for 10 minutes. 8. After 10 minutes, place a glucose strip in each tube for one second, remove it and let it sit on your bench top for thirty seconds. At the end of thirty seconds compare the coloration of it to the chart provided and determine the amount of glucose in mg/dL. Record this value in Figure 4. 7 Results: pH Glucose (mg/dL) Figure 4. Enzymatic Activity of Lactase at varying pHs based on Glucose production. Check your understanding of this experiment: 1. What is the substrate in the above experiment? What is the enzyme? Substrate:_________________ Enzyme:_________________ 2. What is the substrate source? ___________________________________________ 3. What is the independent variable in this experiment? The dependent variable? Independent:_______________ Dependent:_______________ 4. What are the products in this experiment? _____________________________________________ 8 5. Please graph your data in figure 5. Be sure to construct a curve that passes through all of your data points. Effect of pH on Lactase Activity Glucose (mg/dL) pH Figure 5. Graphical analysis depicting the effects of pH on lactase activity. 6. What pHs resulted in a loss of enzymatic activity? 7. Explain what most likely contributed to the loss of enzymatic activity at each pH from question 5. 9 Conclusions: After comparing the effect of pH on the production of glucose, I conclude that ____________________ __________________________________________________________________________________________ _________________________________________________________________________________________. Enzymatic Specificity The active site or active sites of every enzyme are composed of a particular array of amino acids, and, therefore, amino acid side chains. These amino acid side chains form chemical interactions with the substrate of the enzyme. Furthermore, the active site exhibits specificity for the substrate of the enzyme. This means that in theory, the enzyme should bind and catalyze the reaction for its specific substrate(s) better than with any other substrate(s). The following experiment will test the ability of lactase to specifically bind and interact with lactose compared to maltose. Figure 6. Structural comparison between Lactose and Maltose. Knowing that lactase is specific for __________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ _________________________________________________________________________________________. 10 Materials and Methods- Specificity Experiment: 1. Label one microfuge tube “Lactose” or “L” and label another tube “Maltose” or “M.” 2. Using a clean plastic pipette add milk up to the 0.5 line of the “Lactose” tube. The volume of milk in this tube is 500 µL. 3. Using a clean plastic pipette add maltose solution up to the 0.5 line of the “Maltose” tube. The volume of maltose in this tube is 500 µL. 4. Using a clean plastic pipette, add lactase solution to each tube, until the level of mixture in each tube comes up to the 1.0 line of the tube. Each tube now contains 1.0 mL of mixture: 500 µL of lactase and 500 µL of either milk or maltose. 5. Place both tubes in the 40° C water bath and incubate them for 10 minutes. 6. At the end of ten minutes, place a glucose strip in each tube for one second, remove it and allow it to sit on your bench top for thirty seconds. At the end of thirty seconds compare the coloration of it to the chart provided. Using the chart, determine the amount of glucose in mg/dL. Record this value in Figure 7. Results: Time in Minutes Lactose Tube (Glucose mg/dL) Maltose Tube (Glucose mg/dL) 10 Figure 7. Determining the specificity of Lactase in the presence of Lactose or Maltose. 11 Check your understanding of this experiment: 1. What is the substrate in the above experiment? What is the enzyme? Substrate: ____________________ Enzyme: ____________________ 2. What is the substrate source? _________________________________________________________ ___________________________________________________________________________________. 3. What is the independent variable in this experiment? The dependent variable? Independent: _________________ Dependent: __________________ 4. What are the products in this experiment? ______________________________________________ ___________________________________________________________________________________. Conclusions: ______________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ _________________________________________________________________________________________. Determining Cofactors of Enzymes Cofactors are non-protein molecules or ions that bind to proteins (usually enzymes). Furthermore, proteins that bind to cofactors require the cofactor to maintain biological activity. Metal ions, such as Ca++ and Mg++, are frequently present when cells are disrupted and are often introduced as contaminants in reagents and water. Metals can accelerate the formation of undesired disulfide bonds and can act as cofactors for proteases (enzymes responsible for hydrolyzing proteins). Chelating agents are often added to laboratory solutions to bind and remove metal ions from solution in order to slow undesired enzymatic reactions. The most common chelator is ethylenediaminetetraacetic acid (EDTA). In this experiment you will determine if lactase requires a cofactor. This experiment will test the role, if any, that metal ions have on the activity of lactase. If these ions are involved in the enzymatic activity of lactase, then they may either help or hinder lactase’s ability to increase the rate of lactose metabolism. Furthermore, EDTA removes these ions from the reaction. 12 Therefore, the presence of EDTA should remove any effect that these ions would have on the effectiveness of lactase in lowering the activation energy needed for lactose metabolism. Write a hypothesis that describes your prediction of the effect EDTA will have when added to a lactase mediated reaction. __________________________________________________________________ __________________________________________________________________________________________ _________________________________________________________________________________________. Materials and Methods- Cofactors: 1. Label one microfuge tube “Control” and label another one “EDTA.” 2. Add 0.5 M EDTA to the EDTA tube until the solution reaches the line between 1.0 and 1.5 lines on the tube. This tube now contains 1.25 mL of EDTA. 3. Add distilled water to the Control tube until the water reaches the line between 1.0 and 1.5 lines on the tube. This tube now contains 1.25 mL of water. 4. Add three drops of milk to each tube, invert the tube, and allow it to sit for 1 minute. 5. Add three drops of lactase solution to each tube. 6. Place both tubes in the 40° C water bath and leave them for 10 minutes. 7. After 10 minutes, place a glucose strip in each tube for one second, and then remove it and allow it to sit on your bench top for thirty seconds. At the end of thirty seconds compare the coloration of it to the chart provided. Using the chart, determine the amount of glucose in mg/dL. Record this value in Figure 8. Results: Control – Glucose (mg/dL) EDTA – Glucose (mg/dL) Figure 8. Utilizing the chelating agent EDTA to determine if Lactase activity is dependent upon a cofactor. Check your understanding of this experiment: 1. What is the substrate in the above experiment? _________________________________________. 2. What is the enzyme source? __________________________________________________________. 13 3. What is the independent variable in this experiment? The dependent variable? Independent: ____________________ Dependent: _____________________ 4. What are the products in this experiment? _____________________________________________. Conclusions: ______________________________________________________________________________ __________________________________________________________________________________________ __________________________________________________________________________________________ _________________________________________________________________________________________. Points for Discussion: 1. What is the optimal temperature for lactase? What do you theorize is occurring at the other temperatures? 2. What is the optimal pH for lactase? What do you theorize is occurring at the other pH values? 3. If you were working in a research lab, and your mentor asked you to create as much galactose and glucose as quickly as you could, please discuss the environmental conditions that would govern your experiment. Why would you use such conditions? 4. Was lactase specific for the substrate lactose? How do you know? (Justify your answer by comparing what you saw in your experiment to what you would have expected to see if the enzyme was vs. was not specific for lactose?) 14 5. As discussed previously, enzymes are normally very specific for their substrate. Can you think of a reason that this may be important? More specifically, suppose that lactase was not specific for lactose, and could instead also bind and catalyze the hydrolysis reaction of maltose. If this were the case, what would happen if abundant levels of lactose and maltose were present in a cell? 6. Did lactase activity depend upon the presence of a cofactor(s)? Please justify your answer. 7. Based on the data that you obtained from the lab, would you hypothesize that the lactase used was extracted from human or bacterial cells? Justify your answer 15 Statistical Analysis Before submitting a scientific paper for review, or before making a claim that they have discovered a new cure for a terminal disease, scientists must validate their claims by demonstrating that they have indeed made a statistically significant discovery. Statistical analysis utilizes statistical formulas and techniques to analyze the significance of a set of data and therefore the validity of conclusions made based on that data. Using this analysis allows scientists to determine if individual data sets are similar or demonstrate significant differences. Below are some terms that you will need to know in order to effectively interpret the results from this specificity test and the cofactors test of your enzyme. Probability: A probability is a numerical indication of likelihood, similar to a percent chance. All probabilities are between 0 and 1, with probability zero (0 percent chance) indicating an event that is impossible, and a probability of 1 (100 percent chance) indicating an event that is certain to occur. Thus, very small probabilities such as 0.01 indicate rare or unlikely events, while very large probabilities such as 0.90 indicate highly likely events. Null Hypothesis (A=B): A null hypothesis states that there will be no difference between the results of two separate variables: A and B. Specifically, in regards to the enzyme experiment, the null hypothesis states that lactase will not bind preferentially, or more specifically, to maltose or lactose. All calculations in a hypothesis test are made with the assumption that the null hypothesis is true. If the null hypothesis is true, then we will not witness a great difference between the amounts of product (glucose) produced when the substrate is varied. Some difference is expected due to random factors, so before a null hypothesis can be rejected, there must be a significantly large difference between glucose produced from maltose vs. lactose. Alternate Hypothesis (A≠B): The alternate hypothesis states that there will be a difference between the results of two separate variables: A and B. In regards to the enzyme experiment, the alternate hypothesis states that lactase will indeed bind more preferentially, or specifically, to maltose or lactose. While this is a generalized alternate hypothesis, your hypothesis should be more specific. Using the knowledge that you have gained from the information in this lab, you should be able to state that lactase will be more specific for one of the two substrates. Remember, before supporting an alternate hypothesis, there must be a significantly large difference between the glucose measurements. t-Test: A t-test is a statistical test that is used to test the validity of the null hypotheses (A=B) for 2 different sets of data. An excel spreadsheet has been created that will calculate the t-value of your sets of data. Furthermore, this t-value can be used to calculate a p-value, which in turn will be used to determine whether the results are statistically significant or not. 16 p-value: The p-value is a probability used to determine whether or not the null hypothesis can be rejected, and, therefore, if the alternate hypothesis can be supported. First, we should know what the p-value measures. Recall, in a hypothesis test, we always assume that the null hypothesis is true. If it is really true that A=B, then we expect very little difference in glucose production from maltose vs. lactose, and we calculate the p-value as the likelihood (or probability) of obtaining experimental data with a difference between A and B as large as that witnessed in the experimental data. Large p-values: High probability indicates that the difference observed is small and could be due to random chance. When a p-value is large, we have witnessed only a small difference which is what is most likely to happen when the null hypothesis is true. Thus, when a p-value is large, we do not have evidence of a statistically large difference, and we should not reject the null hypothesis. Small p-values: An extremely low p-value indicates that the difference is not likely to be due to random chance, but is instead due to an actual difference between the two substrates (in this case). When a p-value is extremely small, we have witnessed a very large difference that would be very unlikely to occur if the null hypothesis was true. Low p-values provide evidence of a statistically large difference. This leaves a lingering question: what is an acceptably small p-value that could be used to reject the null hypothesis? The scientific community generally settles on a p-value of less than 0.05 (5%) as an acceptable probability with which to reject the null hypothesis. This means that to declare two data sets “significantly different,” there must be less than a 5% chance one could obtain that data and it *not* be representative of a significant difference in the two variables. This leads to the second defining characteristic of the p- value: the “probability” or p-value of a statistical hypothesis test tells the percent chance that the difference in results obtained from the experiment were due only to random chance (not a real or significant difference), but the conclusion we draw (that the difference is real and significant) is in error. The lower the p-value, the less likely it is that the results are due to error. You will use the data that you have obtained in this lab to make statements regarding your hypotheses and the conclusions you can draw from the results obtained. 17