Writing Displacement Reactions REINFORCEMENT Goal

DATE:
C H A P T E R 6
REINFORCEMENT
NAME:
Writing Displacement
Reactions
Goal
• Practise writing single and double displacement reactions.
CLASS:
B L M 6 - 9
What to Do
Answer each question in the space provided.
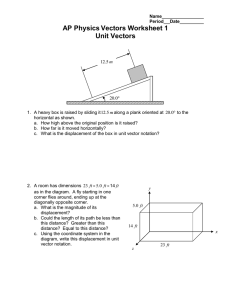
1. Write the two general forms of the equation for a single displacement reaction. Use the letters A and B for cations and the letters X and Y for anions.
_____________________________________________________________________________________________
_____________________________________________________________________________________________
2. Write the general form of the equation for a double displacement reaction. Use the letters A and B for cations and the letters X and Y for anions.
_____________________________________________________________________________________________
3. Complete each equation by writing the correct products. State whether the reaction is a single or double displacement reaction.
(a) CaO(s) Mg(s) →
Type of displacement reaction: _______________________________________
(b) NaOH(aq) HCl(aq) →
Type of displacement reaction: _______________________________________
(c) LiCl(aq) Na(s) →
Type of displacement reaction: _______________________________________
(d) Mg(s) H
2
O( l ) →
Type of displacement reaction: _______________________________________
(e) KOH(aq) HBr(aq) →
Type of displacement reaction: _______________________________________
(f) LiOH(aq) HF(aq) →
Type of displacement reaction: _______________________________________
(g) Ag
2
SO
4
(s) Cu(s) →
Type of displacement reaction: _______________________________________
186
Copyright © McGraw-Hill Ryerson Limited. Permission to reproduce this page is granted to the purchaser for use in her/his classroom only.