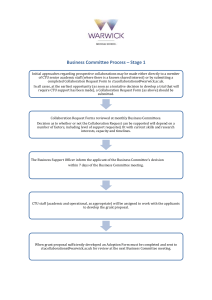
Document 13863235
advertisement

RAPS Protocol Version 5.0 22 September 2014 GENERAL INFORMATION This document was constructed using the University College London (UCL) Clinical Trials Unit (CTU) Protocol Template Version 1.0. It describes the RAPS trial, coordinated by the UCL CTU, and provides information about procedures for entering patients into it. The protocol should not be used as an aide-memoire or guide for the treatment of other patients. Every care has been taken in drafting this protocol, but corrections or amendments may be necessary. These will be circulated to the registered investigators in the trial, but sites entering patients for the first time are advised to contact the UCL CTU, London, UK, to confirm they have the most up-to-date version. COMPLIANCE The trial will be conducted in compliance with the approved protocol, the Declaration of Helsinki (version 2008), the principles of Good Clinical Practice (GCP) as laid down by Commission Directive 2005/28/EC with the implementation in national legislation in the UK by Statutory Instrument 2004/1031 and subsequent amendments, the UK Data Protection Act (DPA number: Z5886415), and the National Health Service (NHS) Research Governance Framework for Health and Social Care (RGF). SPONSOR UCL is the trial sponsor and has delegated responsibility for the overall management of the RAPS trial to the UCL CTU. Queries relating to UCL sponsorship of this trial should be addressed to the Director, UCL CTU or via the trial team. FUNDING Arthritis Research UK Arthritis Research UK is supporting this trial with regard to staff salaries and trial expenses, which are reduced as the UCL CTU is undertaking RAPS as a CTU development project. Bayer Bayer is supporting this trial by supplying without charge rivaroxaban for clinical trial use and financial support for specific pharmacovigilance functions. Local Clinical Research Network (LCRN) Funding for the Research Nurse input will be obtained from the LCRN. AUTHORISATIONS AND APPROVALS This trial was approved by NIHR Coordinated System for gaining NHS Permission (NIHR CSP) and is, therefore, part of the NIHR clinical research network portfolio. TRIAL REGISTRATION This trial will be registered with the International Standard Randomised Controlled Trial Number Register and the registration number will be added when provided. UCL CTU Page 2 RAPS Protocol Version 5.0 22 September 2014 TRIAL ADMINISTRATION Please direct all queries to the Trial Manager at the coordinating site in the first instance; clinical queries will be passed to the Chief Investigator via the Trial Manager. COORDINATING SITE UCL Clinical Trials Unit Switchboard: 020 7679 1975 Email: rapstrial@ucl.ac.uk Gower Street London WC1 6BT UCL CTU STAFF Trial Manager: Simon Clawson Tel: 020 7679 5669 Statistical oversight: Caroline Doré Tel: 020 7679 1824 Trial Statistician: Yvonne Sylvestre Tel: 020 3108 3939 Project Leader: Nicola Muirhead Tel: 020 3108 3263 Tel: 020 3447 7368 / 9456 (PA) CHIEF INVESTIGATOR Dr Hannah Cohen Consultant Haematologist and Honorary Email: Reader Dept of Haematology, University College London (UCL) Hospitals NHS Foundation Trust and Haemostasis Research Unit, UCL 1st Floor Central 250 Euston Road, London NW1 2PG UCL CTU hannah.cohen@uclh.nhs.uk Page 3 RAPS Protocol Version 5.0 22 September 2014 CO-INVESTIGATORS Professor Munther Khamashta Kings College London and Guy’s and St Thomas’ NHS Foundation Trust Lupus Research Unit, Kings College London and Consultant Physician Guys and St Thomas’ NHS Foundation Trust Westminster Bridge Road London, SE1 7EH Professor Beverley Hunt Professor of Thrombosis & Haemostasis Kings College London and Consultant Haematologist Guys and St Thomas’ NHS Foundation Trust 4th Floor, North Wing Westminster Bridge Road London, SE1 7EH Professor Samuel Machin Professor of Haematology Haemostasis Research Unit Haematology Dept University College London 1st Floor, 51 Chenies Mews London, WC1E 6HX UCL CTU Page 4 RAPS Protocol Version 5.0 22 September 2014 COLLABORATORS Dr Ian Mackie Haemostasis Research Unit Haematology Dept University College London 1st Floor, 51 Chenies Mews London, WC1E 6HX Dr Andrew Lawrie Haemostasis Research Unit Haematology Dept University College London 1st Floor, 51 Chenies Mews London, WC1E 6HX Professor David Isenberg University College London Hospitals NHS Foundation Trust and University College London Centre for Rheumatology UCL Division of Medicine 4th Floor, Rayne Building 5 University Street London WC1E 46F Professor Anisur Rahman University College London Hospitals NHS Foundation Trust and University College London Centre for Rheumatology UCL Division of Medicine 4th Floor, Rayne Building 5 University Street London WC1E 46F Dr Ian Giles University College London Hospitals NHS Foundation Trust and University College London Centre for Rheumatology UCL Division of Medicine 4th Floor, Rayne Building 5 University Street London WC1E 46F Dr John Ioannou University College London Hospitals NHS Foundation Trust and University College London Centre for Rheumatology UCL Division of Medicine 4th Floor, Rayne Building 5 University Street UCL CTU Page 5 RAPS Protocol Version 5.0 22 September 2014 London WC1E 46F Professor David D’Cruz Lupus Research Unit Guys and St Thomas’ NHS Foundation Trust and Kings College London Westminster Bridge Road London, SE1 7EH Dr Maria Cuadrado Lupus Research Unit Guys and St Thomas’ NHS Foundation Trust Westminster Bridge Road London, SE1 7EH Dr Maria Efthymiou RAPS trial post-doc Haemostasis Research Unit Haematology Dept University College London 1st Floor, 51 Chenies Mews London, WC1E 6HX Dr Deepa Arachchillage Haemostasis Research Unit Haematology Dept University College London 1st Floor, 51 Chenies Mews London, WC1E 6HX UCL CTU Page 6 RAPS Protocol Version 5.0 22 September 2014 CONTRACT RESEARCH ORGANISATION (CRO) – PRODUCTLIFE LTD ProductLife Ltd The Jeffreys Building St John’s Innovation Park Cowley Road Cambridge CB4 0WS UCL CTU Page 7 RAPS Protocol Version 5.0 22 September 2014 SUMMARY OF TRIAL SUMMARY INFORMATION TYPE SUMMARY DETAILS ACRONYM (or Short Title of Trial) Rivaroxaban in Antiphospholipid Syndrome (RAPS) Long Title of Trial A prospective randomised controlled phase II/III clinical trial of rivaroxaban versus warfarin in patients with thrombotic antiphospholipid syndrome, with or without SLE Version 5.0 Date 22 September 2014 UCL CTU ID 12/0033/CTU/IMM/001 ISRCTN # ISRCTN68222801 EudraCT # 2012-002345-38 CTA # 20363 REC # 12.SC.0566 Study Design Phase II/III randomised controlled non-inferiority clinical trial Type of Participants to be Studied Patients with thrombotic antiphospholipid syndrome (APS) Interventions to be Compared Study Hypothesis Primary Outcome Measure(s) Secondary Outcome Measure(s) UCL CTU Rivaroxaban versus warfarin, target International Normalised Ratio (INR) 2.5 (range 2.0-3.0) The study hypothesis is that the intensity of anticoagulation achieved in patients on rivaroxaban is not inferior to that obtained on warfarin. Intensity of anticoagulation will be assessed by the Endogenous Thrombin Potential (ETP) which is the area under the thrombin generation curve, a key parameter of the Thrombin Generation Test (TGT). The TGT is a global measure of anticoagulation which can assess the anticoagulant effects of both rivaroxaban and warfarin (drugs with very different modes of action on the coagulation mechanisms). Clinically, our hypothesis is that in patients with thrombotic APS, rivaroxaban could induce more predictable anticoagulation and therefore a greater sustained reduction in thrombin generation than would warfarin, with additional benefits to patients because there is no requirement for regular laboratory monitoring of anticoagulation. The primary outcome measure is the percentage change in ETP from randomisation to day 42. a) Efficacy: i) recurrent venous thromboembolism (VTE) alone; ii) a composite of recurrent VTE and other thrombotic events. iii) The thrombin generation curve will also be quantified in terms of: the lag-time; the time to peak; and peak thrombin concentration. iv) markers of in vivo coagulation activation: prothrombin fragment 1.2, thrombin-antithrombin complex and D-dimer Page 8 RAPS Protocol Version 5.0 22 September 2014 b) Safety: i) serious adverse events; ii) all bleeding events. c) Quality of life (QoL): EQ5D 5L. d) Laboratory assessment of compliance: i) anti-Xa assay in patients on rivaroxaban; ii) factor X amidolytic assay as an antiphospholipid antibodyindependent assessment of anticoagulation, in addition to an International Normalised Ratio (INR) in patients on warfarin, iii) percentage time in therapeutic range (TTR) between baseline and day 180 (ideally ±14 days) in patients on warfarin. Randomisation Patients will be randomised to either remain on warfarin or switch to rivaroxaban. Number of Participants to be Studied 116 patients will be randomised (Ratio 1:1) Duration Ancillary Studies/Substudies Each patient will have a six month treatment period and a final visit 30 days after the end of treatment for the trial. The planned recruitment period is 18 months and overall planned duration of the study is 44 months. Optional blood sample storage for translational laboratory research. Sponsor University College London Funders Arthritis Research UK and Bayer Plc. Trial Manager Simon Clawson Chief Investigator Dr Hannah Cohen UCL CTU Project Manager Nicola Muirhead UCL CTU Page 9 RAPS Protocol Version 5.0 22 September 2014 TRIAL SCHEMA Trial Entry, Randomisation and Treatment UCL CTU Page 10 RAPS Protocol Version 5.0 22 September 2014 TRIAL ASSESSMENT SCHEDULE Assessment (Procedure/activity) Screening Consent Medical History Demographics Patient review Height Weight BMI Blood pressure FBC U&E, CrCl LFTs Anti-DNA aPL 20 mL citrated blood sample for trial assessments (all patients) 20 mL blood sample for the translational research (optional) Pregnancy tests Current medication Document INRs Dispensation rivaroxaban Drug accountability of rivaroxaban Enquiry for bleeding symptoms Enquiry for recurrent thrombosis QoL questionnaire X X X X X X X X X X X X X X Baseline Visit 1 Day 42 (ideally 12days+14 days) Visit 2 Day 90 (ideally ±14days) Visit 3 Day 180 (ideally ±14days) Visit 4 Day 210 (ideally ±14days) X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X For full details on the screening and baseline procedures refer to section 3.5. For full details on the trial assessment schedule (visits) refer to section 6. Full details on collection and processing the trial assessment blood samples and the blood samples for translational research are found in Appendix C. UCL CTU Page 11 RAPS Protocol Version 5.0 22 September 2014 CONTENTS GENERAL INFORMATION ......................................................................................... 2 COMPLIANCE ................................................................................................................................... 2 SPONSOR ........................................................................................................................................ 2 FUNDING ........................................................................................................................................ 2 AUTHORISATIONS AND APPROVALS ....................................................................................................... 2 TRIAL REGISTRATION.......................................................................................................................... 2 TRIAL ADMINISTRATION ..................................................................................................................... 3 Coordinating Site ..................................................................................................................................................... 3 UCL CTU Staff ........................................................................................................................................................... 3 Chief Investigator..................................................................................................................................................... 3 Co-investigators ....................................................................................................................................................... 4 Collaborators ........................................................................................................................................................... 5 Contract Research Organisation (CRO) – ProductLife Ltd ....................................................................................... 7 SUMMARY OF TRIAL ................................................................................................ 8 TRIAL SCHEMA ....................................................................................................... 10 Trial Entry, Randomisation and Treatment ........................................................................................................... 10 TRIAL ASSESSMENT SCHEDULE ............................................................................... 11 CONTENTS ............................................................................................................. 12 ABBREVIATIONS .................................................................................................... 15 1 BACKGROUND........................................................................................... 21 1.1 1.2 1.3 1.4 1.5 DISEASE SETTING & CONTEXT OF STUDY ................................................................................ 22 WHAT EVIDENCE IS ALREADY AVAILABLE TO SUPPORT THE INTERVENTION ..................................... 23 RESEARCH LEADING TO THE PROPOSED TRIAL .......................................................................... 24 INVESTIGATIONAL MEDICINAL PRODUCT (IMP) ....................................................................... 25 RATIONALE & OBJECTIVES OF THE STUDY ............................................................................... 25 Rationale ................................................................................................................................................................ 25 Objectives .............................................................................................................................................................. 26 1.6 1.7 HYPOTHESIS TO BE TESTED.................................................................................................. 27 RISKS AND BENEFITS ......................................................................................................... 28 2 SELECTION OF SITES/CLINICIANS ............................................................... 31 2.1 SITE/INVESTIGATOR ELIGIBILITY CRITERIA ............................................................................... 31 2.1.1 2.1.2 2.1.3 PI's Qualifications & Agreements ......................................................................................................... 31 Adequate Resources ............................................................................................................................ 31 Site Assessment.................................................................................................................................... 32 2.2 APPROVAL AND ACTIVATION ............................................................................................... 32 3 SELECTION OF PATIENTS ........................................................................... 33 3.1 3.2 3.3 3.4 3.5 PATIENT INCLUSION CRITERIA .............................................................................................. 33 PATIENT EXCLUSION CRITERIA ............................................................................................. 33 NUMBER OF PATIENTS ....................................................................................................... 34 CO-ENROLMENT GUIDELINES ............................................................................................... 34 SCREENING PROCEDURES & PRE-RANDOMISATION INVESTIGATIONS............................................. 34 Screening ............................................................................................................................................................... 34 UCL CTU Page 12 RAPS Protocol Version 5.0 22 September 2014 4 REGULATION & RANDOMISATION............................................................. 36 4.1 4.2 4.3 RANDOMISATION PRACTICALITIES ........................................................................................ 36 RANDOMISATION CODES & UNBLINDING ............................................................................... 36 CO-ENROLMENT GUIDELINES ............................................................................................... 36 5 TREATMENT OF PATIENTS ......................................................................... 37 5.1 5.2 INTRODUCTION ................................................................................................................ 37 ARM A .......................................................................................................................... 37 5.2.1 5.2.2 Products ............................................................................................................................................... 37 Compliance & Adherence..................................................................................................................... 37 5.3 ARM B........................................................................................................................... 37 5.3.1 5.3.2 5.3.3 5.3.4 5.3.5 5.3.6 5.3.7 Products ............................................................................................................................................... 37 Treatment Schedule ............................................................................................................................. 37 Dispensing ............................................................................................................................................ 37 Dose Modifications, Interruptions & Discontinuations ........................................................................ 38 Stopping Drug Early .............................................................................................................................. 38 Accountability & Unused Drugs/Devices ............................................................................................. 38 Compliance & Adherence..................................................................................................................... 39 5.4 5.5 5.6 5.7 5.8 5.9 5.10 OVERDOSE OF TRIAL MEDICATION ........................................................................................ 39 UNBLINDING ................................................................................................................... 39 PROTOCOL TREATMENT DISCONTINUATION ............................................................................ 39 ACCOUNTABILITY & UNUSED DRUGS/DEVICES ........................................................................ 40 COMPLIANCE & ADHERENCE ............................................................................................... 40 TREATMENT DATA COLLECTION ........................................................................................... 40 NON-TRIAL TREATMENT ..................................................................................................... 40 5.10.1 5.10.2 5.10.3 5.10.4 Medications Permitted ........................................................................................................................ 40 Medications Not Permitted .................................................................................................................. 40 Medications to be Used With Caution ................................................................................................. 41 Treatment After Trial Event ................................................................................................................. 41 5.11 CO-ENROLMENT GUIDELINES ............................................................................................... 41 6 ASSESSMENTS & FOLLOW-UP .................................................................... 42 6.1 6.2 6.3 6.4 6.5 6.6 6.7 6.8 TRIAL ASSESSMENT SCHEDULE ............................................................................................. 42 PROCEDURES FOR ASSESSING EFFICACY .................................................................................. 44 PROCEDURES FOR ASSESSING SAFETY .................................................................................... 45 PROCEDURES FOR ASSESSING QUALITY OF LIFE ........................................................................ 45 OTHER ASSESSMENTS ........................................................................................................ 45 EARLY STOPPING OF FOLLOW-UP ......................................................................................... 45 PATIENT TRANSFERS.......................................................................................................... 46 LOSS TO FOLLOW-UP ......................................................................................................... 46 7 SAFETY REPORTING ................................................................................... 47 7.1 DEFINITIONS ................................................................................................................... 47 Table 7.1: Definitions ............................................................................................................................................. 47 Adverse Reactions ................................................................................................................................................. 48 Guidance for Adverse Event Inclusions & Exclusions ............................................................................................ 48 Disease-related Events .......................................................................................................................................... 48 7.2 INVESTIGATOR RESPONSIBILITIES .......................................................................................... 48 7.2.1 Investigator assessment ....................................................................................................................... 48 Seriousness 48 Severity or Grading of Adverse Events 49 Causality 49 UCL CTU Page 13 RAPS Protocol Version 5.0 22 September 2014 Table 7.2: Assigning Type of SAE Through Causality ............................................................................................. 49 Expectedness 49 Notification 50 7.2.2 Notification Procedure ......................................................................................................................... 50 7.3 7.4 Pregnancy UCL CTU RESPONSIBILITIES ................................................................................................ 50 OTHER NOTIFIABLE EVENTS ................................................................................................ 51 51 8 QUALITY ASSURANCE & CONTROL ............................................................ 52 8.1 8.2 8.3 RISK ASSESSMENT ............................................................................................................ 52 CENTRAL MONITORING AT UCL CTU .................................................................................... 52 ON-SITE MONITORING....................................................................................................... 52 8.3.1 8.3.2 Direct Access to Patient Records.......................................................................................................... 52 Confidentiality ...................................................................................................................................... 52 9 STATISTICAL CONSIDERATIONS ................................................................. 53 9.1 9.2 9.3 9.4 9.5 METHOD OF RANDOMISATION ............................................................................................ 53 OUTCOME MEASURES ....................................................................................................... 53 SAMPLE SIZE ................................................................................................................... 53 INTERIM MONITORING & ANALYSES ..................................................................................... 54 ANALYSIS PLAN (BRIEF) ..................................................................................................... 54 10 11 ANCILLARY STUDIES .................................................................................. 57 REGULATORY & ETHICAL ISSUES ................................................................ 58 11.1 COMPLIANCE ................................................................................................................... 58 11.1.1 11.1.2 11.1.3 Regulatory Compliance ........................................................................................................................ 58 Site Compliance .................................................................................................................................... 58 Data Collection & Retention ................................................................................................................ 58 11.2 ETHICAL CONDUCT OF THE STUDY ......................................................................................... 58 11.2.1 11.2.2 Ethical Considerations .......................................................................................................................... 58 Ethical Approvals .................................................................................................................................. 59 11.3 11.4 COMPETENT AUTHORITY APPROVALS .................................................................................... 59 OTHER APPROVALS ........................................................................................................... 59 12 13 14 INDEMNITY ............................................................................................... 60 FINANCE ................................................................................................... 61 OVERSIGHT & TRIAL COMMITTEES ............................................................ 62 14.1 14.2 14.3 14.4 TRIAL MANAGEMENT GROUP (TMG) ................................................................................... 62 TRIAL STEERING COMMITTEE (TSC) ...................................................................................... 62 INDEPENDENT DATA MONITORING COMMITTEE [IDMC] ........................................................... 62 ROLE OF STUDY SPONSOR .................................................................................................. 62 15 16 17 18 PUBLICATION ............................................................................................ 63 PROTOCOL AMENDMENTS ........................................................................ 64 REFERENCES .............................................................................................. 66 APPENDICES .............................................................................................. 72 UCL CTU Page 14 RAPS Protocol Version 5.0 22 September 2014 ABBREVIATIONS A&E Accident and Emergency ABPI Association of the British Pharmaceutical Industry AE Adverse event ALT Alanine transaminase ANA Antinuclear antibodies APS Antiphospholipid syndrome aPL Antiphospholipid antibodies AR Adverse reaction Bid Bis in die (twice a day) BCSH British Committee for Standards in Haematology BMI Body Mass Index BNF British National Formulary CF Consent Form CI Chief Investigator CI Confidence interval COM Clinical Operations Manager CPM Clinical Project Manager CrCl Creatinine Clearance CRF Case Report Form CRN Clinical Research Network CRO Contract Research Organisation CTA Clinical Trials Authorisation CTAAC Clinical Trials Awards and Advisory Committee CTIMP Clinical trial of an investigational medicinal product CTU Clinical Trials Unit DCF Data Clarification Form DH Department of Health DM Data Manager DNA Deoxyribonucleic acid DPA (UK) Data Protection Act DVT Deep vein thrombosis ECRIN European Clinical Research Infrastructure Network EFGCP European Forum for Good Clinical Practice UCL CTU Page 15 RAPS Protocol Version 5.0 22 September 2014 ELISA Enzyme-linked immunosorbent assay EMA European Medicines Agency ETP Endogenous Thrombin Potential EU European Union EudraCT European Union Drug Regulatory Agency Clinical Trial FBC Full blood count GCP Good Clinical Practice GP General Practitioner GPL IgG phospholipid units GSA Group-specific appendix HE Health economics HES Hospital Episodes Statistics HIV Human Immunodeficiency Virus IB Investigator Brochure IBW Ideal body weight ICH International Conference on Harmonisation IDMC Independent Data Monitoring Committee IMP Investigational medicinal product INR International Normalised Ratio IRAS Integrated Research Application System IRB Institutional Review Board ISRCTN International Standard Randomised Controlled Trial Number ITT Intention-to-treat LA Lupus anticoagulant LCRN Local Clinical Research Network LFTs Liver Function Tests LLN Lower Limit of Normal LMWH Low molecular weight heparin MPL IgM phospholipid units MedDRA Medical Dictionary for Regulatory Activities MHRA Medicines and Healthcare products Regulatory Agency MoU Memorandum of Understanding NHS National Health Service NHSCR National Health Service Central Register NHS-IC National Health Service Information Centre UCL CTU Page 16 RAPS Protocol Version 5.0 22 September 2014 NICE National Institute for Health and Clinical Excellence NIHR National Institute for Health Research NIHR CSP National Institute for Health Research Co-ordinated System for gaining NHS Permission NIMP Non-investigational-medicinal product NPSA National Patient Safety Agency OD Once daily PE Pulmonary Embolism PI Principal Investigator PIS Patient Information Sheet PK Pharmacokinetics PT Prothrombin time QMG Quality Management Group QoL Quality of life QP Qualified Person R&D Research and Development RCT Randomised controlled trial REC Research Ethics Committee RGF Research Governance Framework (for Health and Social Care) SAE Serious adverse event SAP Statistical Analysis Plan SAR Serious adverse reaction SD Standard deviation SLE Systemic lupus erythematosus SMT Senior Management Team SOP Standard operating procedure SPC Summary of Product Characteristics SSA Site-specific approval SSI Site-specific information SUSAR Suspected unexpected serious adverse reaction TGT Thrombin Generation Test TM Trial Manager TMF Trial Master File TMG Trial Management Group TMT Trial Management Team UCL CTU Page 17 RAPS Protocol Version 5.0 22 September 2014 ToR Terms of Reference TTR Time in Therapeutic Range TSC Trial Steering Committee UAR Unexpected adverse reaction UCL University College London UCL CTU University College London Clinical Trials Unit U&E Urea and electrolytes ULN Upper limit of normal VKA Vitamin K antagonist VTE Venous thromboembolism UCL CTU Page 18 RAPS Protocol Version 5.0 22 September 2014 GLOSSARY Adequate contraception Adequate contraception is defined as hormonal contraception or barrier method contraception. Sub-therapeutic anticoagulant therapy Patients who develop a new venous thromboembolic event while on warfarin with an INR (International Normalised ratio) of <2.0 (target INR 2.5, i.e. range 2.0-3.0) will be considered to have had the event when sub-therapeutically anticoagulated. The same would apply if the patient was on a dose of another anticoagulant (e.g. low molecular weight heparin) administered at less than the manufacturer’s recommended therapeutic dose for VTE at the time of the event. Thrombotic APS Thrombotic APS is defined as venous thromboembolism (VTE) associated with persistent positivity of one or more antiphospholipid antibodies (aPL; i.e. lupus anticoagulant, IgG and/or IgM anticardiolipin and/or anti beta 2 glycoprotein 1 antibodies at >40 GPL or MPL units or > the 99th percentile of normal). Persistent aPL positivity is defined as aPL present on at least two consecutive occasions at least 12 weeks apart as defined in the International (Sydney) Consensus Statement criteria. Clinical outcome events All thrombotic events, defined below, will be classified by the PI at the trial site. 1. Venous thromboembolism The diagnosis of symptomatic recurrent venous thromboembolism (VTE), i.e. deep-vein thrombosis (DVT) or non-fatal or fatal pulmonary embolism (PE) is based on the following: DVT is diagnosed objectively on venous doppler or duplex scanning or venography. PE is diagnosed objectively on computed tomography pulmonary angiogram (CTPA) or ventilation/perfusion (V/Q) lung scanning. Fatal PE: the diagnosis of fatal PE is based on objective diagnostic testing, autopsy, or death which cannot be attributed to a documented cause and for which PE cannot be ruled out (unexplained death). 2. Other thrombotic events a) Arterial thrombosis/thromboembolism Stroke is defined as a sudden focal neurologic deficit of presumed cerebrovascular etiology that persists beyond 24 hours and is not due to another identifiable cause. An event matching this definition but lasting less than 24 hours is considered to be a transient ischemic attack. The diagnosis of stroke is based on brain imaging (computed tomography or magnetic resonance imaging). Systemic embolism is defined as abrupt vascular insufficiency associated with clinical or radiological evidence of arterial occlusion in the absence of another likely mechanism (e.g. atherosclerosis, instrumentation, or trauma). Myocardial infarction (MI) is defined by typical symptoms, cardiac biomarker elevation and electrocardiogram changes, or confirmation at autopsy. Deaths are classified as either vascular (e.g. due to stroke, embolism, myocardial infarction, or arrhythmia) or non-vascular (e.g. malignancy or infection). UCL CTU Page 19 RAPS Protocol Version 5.0 22 September 2014 b) Microvascular thrombosis Thrombosis in the microvasculature, generally associated with evidence of organ dysfunction in patients with thrombotic APS, is diagnosed on histological examination of a tissue biopsy. Categorisation of bleeds: Major bleeding Major bleeding is defined as clinically overt bleeding associated with any of the following: fatal outcome, involvement of a critical anatomic site (intracranial, spinal, ocular, pericardial, articular, retroperitoneal, or intramuscular with compartment syndrome), fall in hemoglobin concentration of at least 20 g/L, transfusion of 2 or more units of red blood cells, or permanent disability. Clinically relevant non-major bleeding Clinically relevant non-major bleeding is defined as overt bleeding not meeting the criteria for major bleeding but associated with medical intervention, unscheduled contact (visit or telephone) with a physician, temporary interruption of study drug (i.e. delayed dosing), or associated with any other discomfort such as pain or impairment of activities of daily life. Minor bleeding Bleeding events that do not meet the criteria of major or clinically relevant non-major bleeding are defined as minor. Lack of Drug Effect (LODE) Non-serious adverse events (AE) will be considered due to LODE if the investigator explicitly states that the non-serious adverse event occurred due to the drug not working. A non-serious AE would not be considered to be due to LODE if e.g. there has been a medication error (insufficient dose was used, prescribed dosing regimen was not followed), handling error (instructions for use not followed, product stored incorrectly or used after expiry date) or there was a misuse or abuse of the product. UCL CTU Page 20 RAPS Protocol Version 5.0 22 September 2014 1 BACKGROUND Thrombotic antiphospholipid syndrome (APS) is a potentially fatal and devastating clinical disorder with the cardinal features being venous and/or arterial thromboembolism associated with persistent antiphospholipid antibodies (aPL). It constitutes a unique form of autoantibody mediated thrombophilia.1,2,3 aPL is detected in 20% of patients who have had a stroke aged below 50 years4 and in 24% of approximately 4500 patients with venous thromboembolism (VTE) (deep venous thrombosis (DVT) and/or pulmonary embolism (PE)) in whom thrombophilia testing was done.5 APS may occur alone, however, approximately 40% of patients with systemic lupus erythematosus (SLE) have aPL6 and 40% of these patients will develop thrombotic APS,7,8 which is considered to be a major adverse prognostic factor in patients with SLE.7 Particularly in patients with SLE, appropriate management of thrombotic APS is of key importance to minimise its deleterious clinical impact. The current mainstay of the treatment of venous and/or arterial thromboembolism in patients with APS is long-term anticoagulation with oral vitamin K antagonists (VKAs) such as warfarin. Prospective, randomised controlled trials suggest that high-intensity warfarin (target International Normalised Ratio (INR) 3.5, range 3.0 – 4.0, INR being the test used to monitor the anticoagulant effect of VKAs) is not superior to moderate-intensity warfarin (target INR 2.5, range 2.0 – 3.0) in the management of these patients,9,10 and the current recommendations based on these data are indefinite anticoagulation at a target INR of 2.5 for patients with APS, with or without SLE, presenting with a first VTE event, or a recurrent VTE event which occurred whilst off anticoagulation11,12. However, patients with recurrent thrombosis on VKAs were excluded from studies investigating anticoagulation intensity in patients with APS9,10 and those with APS and previous arterial events were poorly represented, and so the optimal intensity of anticoagulation with VKAs in these patient groups with APS is not established, although the consensus view is that they should be maintained at a target INR of >3.0.12 Unfortunately, treatment with VKAs is problematic. Warfarin, the most widely used VKA in the UK, has a slow onset of action of 3-5 days and a narrow therapeutic window. This, as well as its numerous drug and dietary interactions, and potential for variation of action with alcohol, intercurrent illness, exercise and smoking necessitates frequent monitoring of the INR, which is inconvenient and costly. Over-anticoagulation with warfarin is associated with a risk of bleeding (12% of patients suffer major bleeds and 0.25% die from bleeding) and under-anticoagulation is associated with recurrent thrombosis. As around 1 million people in the UK receive oral anticoagulant therapy, with the numbers increasing by approximately 10% each year,13 warfarinassociated bleeding represents a significant healthcare problem. VKAs present particular problems in patients with APS. First, VKA monitoring in patients with aPL can be complicated by the variable responsiveness of thromboplastin reagents (thromboplastin being the main reagent used in the INR test) to lupus anticoagulants (LA; one of the family of aPL) which may in turn potentially influence the validity of the prothrombin time–INR in monitoring oral VKA treatment in patients with APS. A multisite study of laboratory INR testing in patients with APS concluded that LA interference with the PT-INR measured with the majority of commercial thromboplastins is not enough to cause concern if insensitive thromboplastins, properly calibrated to assign them an instrument-specific International Sensitivity Index (ISI) are used. The investigators also suggested that new thromboplastins, especially those made of relipidated recombinant human tissue factor (rTF), should be checked to ensure that they are insensitive to the effects of aPL before they are used to monitor oral anticoagulant treatment in patients with APS.14 Whilst these UCL CTU Page 21 RAPS Protocol Version 5.0 22 September 2014 procedures are generally routine in specialist centres, they may not be as easily undertaken in other institutions, and thus as a result the INR may not accurately reflect the true level of anticoagulation. The variable responsiveness of aPL to LA can result in instability of the INR, which necessitates frequent anticoagulant monitoring with the attendant inconvenience to the patient, adverse impact on quality of life and increased costs. It may also be associated with thrombotic or bleeding complications. A systematic review reported that approximately 2.8% of APS patients on VKA had recurrent thrombotic events, and bleeding rates of up to 10% per year.15 Secondly, LA detection in patients on warfarin may be problematic because of the prolonged basal clotting time.16 This limits the ability to diagnose APS in patients on VKA and also monitoring of aPL status in those with an established diagnosis. The limitations of warfarin and other existing anticoagulants have driven a search for new agents. 1.1 DISEASE SETTING & CONTEXT OF STUDY The lives of patients with thrombotic APS, with or without SLE, are often blighted by the requirement to take warfarin with all its complications and need for frequent, often weekly, INR monitoring. It is often very difficult to establish the correct dose leading to major concern about excessive bleeding and/or recurrent thrombosis. Furthermore, warfarin interacts with many other drugs and dietary constituents, making the treatment of concomitant diseases, including SLE, problematic. The new oral anticoagulants are fixed-dose, and, unlike warfarin, have a predictable anticoagulant effect and do not require routine monitoring with coagulation tests. They offer a potential opportunity to revolutionize the lives of those patients on warfarin. Dabigatran etexilate (Pradaxa®; Boehringer Ingelheim), a direct thrombin inhibitor,17 and rivaroxaban (Xarelto®; Bayer HealthCare), a direct anti-Xa inhibitor and the first in a new class of drugs,18 are licensed in the UK and many other countries for the prevention of VTE following high risk lower limb orthopaedic surgery,19-25 and endorsed for these indications by the National Institute of Health and Clinical Excellence (NICE).26,27 In 2011 rivaroxaban was licensed for the treatment of deep vein thrombosis (DVT), prevention of recurrent DVT and pulmonary embolism (PE) following an acute DVT in adults and prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation, and in the same year dabigatran was licenced for the prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation.28-31 This could lead to dabigatran and rivaroxaban being established as therapeutic alternatives to warfarin and becoming the new standard of care for a wide range of indications, but without data being available on patients with thrombotic APS. The RAPS trial will include patients who have thrombotic APS, with or without SLE, currently on warfarin with a target INR 2.5 (range 2.0 - 3.0), who will be randomised to either continue on warfarin or switch to rivaroxaban. Inclusion of patients with and without SLE is appropriate for the following reasons: a) Many patients with ‘primary’ thrombotic APS have clinical features such as joint pain which in effect makes some of these patients ‘lupus like’. b) Our findings in ‘primary’ thrombotic APS will be applicable to all APS patients, i.e. with or without SLE. Of note here, we have previously established that there is no difference in the risk of recurrent thrombosis between patients with SLE- and non-SLE associated thrombotic APS.32 c) No difference in the effect of rivaroxaban would be expected between patients with SLEassociated thrombotic APS or thrombotic APS alone. The randomisation will be stratified by site and also by patient type (SLE/non-SLE), to ensure a balance of patients in each arm for each site and patient type. UCL CTU Page 22 RAPS Protocol Version 5.0 22 September 2014 1.2 WHAT EVIDENCE IS ALREADY AVAILABLE TO SUPPORT THE INTERVENTION Rivaroxaban and dabigatran etexilate are both fixed-dose orally administered agents which exert their anticoagulant effects within hours rather than days and, due to their predictable pharmacokinetics, do not require routine laboratory monitoring with coagulation tests. Following phase III international multisite trials in a total of about 21,500 patients, they were licensed in the UK and Europe for the prevention of VTE in adults undergoing elective total hip replacement or knee replacement;19-25 and in 2011, following phase III trials in over 44,000 patients, they were licenced as detailed in section 1.1.28-31 The phase III clinical trials using therapeutic fixed-dose rivaroxaban or dabigatran for medium and long-term indications suggest that they could offer an attractive alternative to warfarin in patients with thrombotic APS. The EINSTEIN-DVT international multisite randomised trial in 3,449 patients, which was open label, assigned patients with acute symptomatic DVT to either around 5 days of subcutaneous (SC) low molecular weight heparin (LMWH) followed by a VKA (warfarin or acenocoumarol) to maintain an INR of 2.0-3.0, or rivaroxaban given as 15 mg twice daily for the first three weeks followed by 20 mg daily. The primary efficacy outcome, the rate of first symptomatic VTE events, was similar in both groups, occurring in 2.1% of those on rivaroxaban compared with 3% of those on VKA. The safety profiles with the two regimens were also similar, a composite of major and other clinically relevant bleeding events being 8.1% in both treatment groups. In a parallel randomised double-blind, placebo-controlled, superiority trial (the EINSTEIN-Extension study published together with the Einstein-DVT trial in December 2010) in 1,196 patients, rivaroxaban 20mg daily for a duration of 6-12 months after completion of 6-12 months treatment with VKAs following acute VTE, was associated with a significantly lower rate of recurrent events (1.3% vs. 7.1%). Major bleeds were not significantly different between the rivaroxaban and placebo arms although there were more minor bleeds with rivaroxaban (5.4% vs. 1.2%).29 In a randomized, openlabel, event-driven, noninferiority trial involving 4832 patients who had acute symptomatic PE with or DVT, rivaroxaban (15 mg twice daily for 3 weeks, followed by 20 mg once daily) was compared with standard therapy with enoxaparin followed by an adjusted-dose vitamin K antagonist for 3, 6, or 12 months. Rivaroxaban was noninferior to standard therapy for the primary efficacy outcome of symptomatic recurrent VTE, with 50 events in the rivaroxaban group (2.1%) versus 44 events in the standard-therapy group (1.8%). The principal safety outcome, major or clinically relevant nonmajor bleeding, occurred in 10.3% of patients in the rivaroxaban group and 11.4% of those in the standardtherapy group. Major bleeding was observed in 26 patients (1.1%) in the rivaroxaban group and 52 patients (2.2%) in the standard-therapy group (P=0.003). Rates of other adverse events were similar in the two groups.33 In an international multisite randomised, double blind, non-inferiority trial in 1,274 patients with acute VTE (the RE-COVER trial) initially given parenteral anticoagulation with subcutaneous (SC) LMWH for 9 days, and oral dabigatran 150 mg twice daily versus warfarin to achieve a target INR of 2.5, the primary efficacy outcome, the six month incidence rate of recurrent symptomatic, objectively confirmed VTE and related deaths, was similar (2.4% and 2.1% respectively).28 The safety profile of dabigatran, including with regard to bleeding events, was similar to that of warfarin, with major bleeding episodes in 1.6% of patients assigned to dabigatran and 1.9% of patients assigned to warfarin. Other adverse effects, including acute coronary events, elevation of the alanine or aspartate transaminase exceeding three times the upper limit of normal, did not differ significantly in patients on dabigatran versus those on VKA. Patients on dabigatran in the RE-COVER trial had a significantly higher incidence of dyspepsia, 3.1%, compared with 0.7% in patients on warfarin.28 This may be explained by the increased acidity as a result of the tartaric acid core within dabigatran capsules. The findings in the RE-COVER trial were confirmed in RECOVER II which included a larger number of patients and more ethnic variability (with approximately 20% Asian patients).34 UCL CTU Page 23 RAPS Protocol Version 5.0 22 September 2014 In the international multisite RE-LY (Randomised Evaluation of Long-term Anticoagulation Therapy) non-inferiority trial of the efficacy and safety of dabigatran versus warfarin, 18,113 patients with atrial fibrillation were assigned to warfarin or dabigatran 110 or 150 mg twice daily. Dabigatran at both doses was as efficacious as warfarin, however in patients on the higher dose of 150 mg twice daily, dabigatran was associated with lower rates of stroke and systemic embolism but similar rates of major haemorrhage, although the incidence of intracranial haemorrhage was significantly lower in the dabigatran groups.30 A recent analysis of the efficacy and safety of dabigatran compared with warfarin at different levels of INR control in this study showed that for all vascular events, nonhaemorrhagic events and mortality, the advantages of dabigatran were greater at sites with poor INR control than those with good INR control35. These advantages of dabigatran therapy could be particularly beneficial in patients with thrombotic APS. In the ROCKET AF trial, rivaroxaban was demonstrated to be as efficacious as warfarin in the prevention of stroke and systemic embolisation in patients with atrial fibrillation and was also associated with similar rates of major haemorrhage but, as with dabigatran, significantly lower rates of intracranial haemorrhage compared with warfarin.31 1.3 RESEARCH LEADING TO THE PROPOSED TRIAL Systematic review of the literature has not identified any published studies of any of the new oral anticoagulants in patients with thrombotic APS, with or without SLE; there are no trials in this area registered on clinicaltrials.gov; and there are no other studies on-going, or, to the best of our knowledge, planned. The new generation of oral anticoagulants are a major advance as, unlike warfarin, they do not require regular monitoring, have no interactions with dietary constituents or alcohol, and have few reported drug interactions which affect anticoagulant intensity. These agents therefore would be expected to result in a major improvement in quality of life for patients. Among patients with acute VTE, approximately 10% have APS, and therefore it is likely that patients with APS were included in the study populations in the phase III clinical trials of rivaroxaban or dabigatran versus VKA in patients with VTE. However, aPL status was not documented in these trials.28,29 The question arises as to whether specific clinical trials are required in patients with thrombotic APS. Patients with APS inherently differ from other patients with VTE by virtue of their aPL, particularly LA and anti-prothrombin antibodies, which are known to interfere with a number of haemostatic mechanisms, and which could therefore modulate the actions of anticoagulants. VKAs exert their anticoagulant action by inhibition of the vitamin K dependent post-translational gammacarboxylation of procoagulant factors II, VII, IX and X. However, VKAs also reduce the activity of the major naturally occurring anticoagulant protein C/S system (both protein C and S are vitamin K dependent), which counterbalances the anticoagulant effects of VKAs. aPL not infrequently induce reduced protein C and S activity ex vivo36,37 which could increase this counterbalancing effect, and thus potentially shift the equilibrium further away from anticoagulation. An activated protein C (APC) resistant phenotype in the absence of factor V Leiden, demonstrated in a thrombin generation-based test, is seen in individuals with APS exhibiting LA, with a history of thrombotic events in these patients associated with a stronger resistance to the anticoagulant effects of APC.38 Rivaroxaban and dabigatran have very specific targets (factor Xa and the active site of thrombin respectively)17,18 and would not be expected to directly influence protein C and S activity. As a result, these new oral anticoagulants may have greater anticoagulant efficacy than warfarin in patients with APS. It is possible that aPL could directly interfere with the anticoagulant action of rivaroxaban or dabigatran, although this is unlikely as rivaroxaban and dabigatran are both small molecules with high affinity for their specific targets. The available phase III clinical trials of rivaroxaban or UCL CTU Page 24 RAPS Protocol Version 5.0 22 September 2014 dabigatran compared with VKA in the treatment of acute VTE may therefore not be applicable to patients with thrombotic APS where there remains an unmet need.28,29,34,39 Clinical trials are therefore needed to establish whether or not the new oral anticoagulants should become the standard of care for the treatment of these patients. Thrombin is a pivotal component of the haemostatic mechanism, with increased ex vivo thrombin generation a key marker of thrombogenic potential with predictive value for the development of recurrent VTE.40,41 Generation of thrombin via the tissue factor (TF) pathway is integral to the blood coagulation process, and thus, assessment of TF-triggered thrombin generation provides a useful means of studying the inhibitory actions of antithrombotic agents.42,43 The Thrombin Generation Test (TGT) provides information about the dynamics of ex vivo thrombin generation, with the thrombin generation curve described in terms of: the lag-time; the time to peak; peak thrombin concentration; and the area under the thrombin generation curve, known also as the Endogenous Thrombin Potential (ETP).43 Markers of in vivo coagulation activation, prothrombin fragment 1.2 (F1.2), thrombin-antithrombin complex (TAT) and D-dimer (a marker of activation of fibrinolysis secondary to coagulation activation), also provide information about an individual's thrombogenic potential,41,44-49 and anticoagulation reduces the levels of these markers.44 F1.2, as the activation peptide originating from the factor Xa-mediated activation of prothrombin, is a sensitive marker of anticoagulation intensity.50,51 Warfarin (in non-APS patients) at a target INR of 2.5 (range 2-3) has been shown to reduce the ETP by 30%-50% compared with the pre-warfarin result52 or normal controls.53 After activation of the TF pathway in healthy subjects, nanomolar concentrations of rivaroxaban significantly prolonged the initiation phase of thrombin generation and significantly reduced the rate of propagation of the thrombin phase. These effects led to a reduction in the total amount of thrombin generated and to a decrease in ETP.54 LMWHs have an indirect inhibitory effect on thrombin generation via the physiological anticoagulant antithrombin.55 In hip/knee replacement surgery, where there is an increase in thrombin generation, we have demonstrated that rivaroxaban inhibits thrombin generation more than does the LMWH dalteparin at 24 hours after surgery.56 The TGT, as a global measure of anticoagulation, can assess the anticoagulant effects of both rivaroxaban and warfarin (drugs with very different modes of action on the coagulation mechanisms). We and others have established that the ETP, a key parameter of the TGT, is an appropriate measure of the intensity of the anticoagulant effect in individuals on rivaroxaban.56,57 1.4 INVESTIGATIONAL MEDICINAL PRODUCT (IMP) Warfarin (generic) used as per standard care – the comparator Rivaroxaban as 20mg and 15mg film-coated tablets 1.5 RATIONALE & OBJECTIVES OF THE STUDY RATIONALE Approximately 10% of patients with acute VTE have APS, however, the presence of APS was not documented in the phase III trials of the new oral anticoagulants in patients with acute VTE. We are therefore keen to take advantage of what we strongly believe is a limited time-window to study the effects of these new drugs in patients with thrombotic APS in a controlled and optimal fashion. If we do not do the appropriate trial now, these drugs will be introduced piecemeal, and the situation is very likely to end up unsatisfactory both from a clinical and scientific viewpoint. Utilization of the window of opportunity that we now have to demonstrate a) non-inferiority in the intensity of the anticoagulant effect of rivaroxaban compared with that of warfarin in the TGT; and UCL CTU Page 25 RAPS Protocol Version 5.0 22 September 2014 b) absence of safety signals, c) improved quality of life, would provide sufficient supporting data to change practice for our patients, i.e. for rivaroxaban to become the standard of care for the treatment of patients with thrombotic APS, with or without SLE. OBJECTIVES We plan to compare the anticoagulant effect of the new fixed-dose anticoagulant drug rivaroxaban with that of warfarin in patients with thrombotic APS, with or without SLE. We will do this by assessment of the TGT, which as a global measure of anticoagulation can assess the anticoagulant effects of both rivaroxaban and warfarin (drugs with very different modes of action on the coagulation mechanisms). We and others have established that the ETP which is the area under the thrombin generation curve, a key parameter of the TGT, is an appropriate measure in individuals on rivaroxaban.56,57 We will also compare rates of bleeding and recurrent thrombosis, and compare quality of life in patients on rivaroxaban with those on warfarin. Primary aim The primary aim is to demonstrate that the intensity of anticoagulation achieved with rivaroxaban is not inferior to that of warfarin, by measurement of the dynamics of ex vivo thrombin generation using the TGT with the ETP as the key parameter, in patients with thrombotic APS with or without SLE. Secondary aims a) To compare efficacy outcomes defined as: i) recurrent VTE alone, and ii) a composite of recurrent VTE and other thrombotic events in patients on rivaroxaban versus those on warfarin; iii) The thrombin generation curve will also be quantified in terms of: the lag-time; the time to peak; and peak thrombin concentration; iv) markers of in vivo coagulation activation: prothrombin fragment 1.2, thrombinantithrombin complex and D-dimer. b) To compare serious adverse events and all bleeding events in patients on rivaroxaban versus those on warfarin; c) To compare quality of life (QoL) outcomes in patients on rivaroxaban versus those on warfarin. d) To assess compliance, concurrent with the TGT, an anti-Xa assay for rivaroxaban will be performed in patients on rivaroxaban and a factor X amidolytic assay will be performed in patients on warfarin as an antiphospholipid antibody-independent assessment of anticoagulation.58,59 Percentage time in therapeutic range (TTR) between baseline and day 180 will be calculated in patients on warfarin.60 aPL status at baseline aPL (lupus anticoagulant, IgG and IgM anticardiolipin and anti beta 2 glycoprotein 1 antibodies) will be assessed at baseline in all patients using established methods16,61-65, to define the aPL status of the patient population at the time of trial entry. The primary outcome measure will be assessed after six weeks, but the total treatment duration will be six months for the following reasons: a) It will take a number of weeks to re-establish patients on warfarin, and during this period they would require even more frequent INR monitoring, which we feel would be clinically unreasonable if UCL CTU Page 26 RAPS Protocol Version 5.0 22 September 2014 they had only received treatment for a brief period of six weeks. b) Six months of treatment should be long enough to identify any impact on the quality of life of patients randomised to rivaroxaban, as routine anticoagulation clinic visits for INR monitoring will no longer be required. c) A six month treatment duration will provide more opportunity to identify any clinical safety signals of rivaroxaban treatment. 1.6 HYPOTHESIS TO BE TESTED The RAPS trial is designed as a non-inferiority trial, to demonstrate that the intensity of anticoagulation in patients on rivaroxaban is not inferior to that obtained with warfarin as assessed by the ETP, with the non-inferiority margin based on the inter site assay variability of test performance and on clinical relevance. Clinically, our hypothesis is that in patients with thrombotic APS, with or without SLE, rivaroxaban can induce more predictable anticoagulation and therefore a greater sustained reduction in thrombin generation than would warfarin, with additional benefits to patients because of the lack of requirement for frequent laboratory monitoring of anticoagulation. The TGT is a global measure of anticoagulation which can assess the anticoagulant effects of both rivaroxaban and warfarin (drugs with very different modes of action on the coagulation mechanisms). The ETP is a key parameter of the TGT which we and others have established to be an appropriate measure in individuals on rivaroxaban.56,57 The primary outcome measure is the percentage change in ETP from randomisation to Day 42. The time point of Day 42 (first trial visit post-randomisation), was chosen as the therapeutic effect of rivaroxaban would be expected to be stable after 30 days of rivaroxaban therapy and would not be influenced by any residual warfarin, based on the biological half-lives of the vitamin K-dependent coagulation factors which range from 2-5 hours (factor VII) up to 72 hours (factor II).66 We also hypothesize that the percentage change from baseline in ETP could be lower (i.e. a greater anticoagulant effect) in the rivaroxaban group compared with the warfarin group in patients with thrombotic APS because: i) Rivaroxaban will be prescribed as a fixed-dose with a predictable anticoagulant effect, which does not require monitoring; conversely, warfarin displays an inherent variability of anticoagulant effect; ii) Rivaroxaban has a specific target (factor Xa) and, unlike warfarin, should not directly influence protein C and S activity and therefore may have greater anticoagulant efficacy in patients with thrombotic APS than warfarin; and iii) It is unlikely that aPL would interfere with the anticoagulant action of rivaroxaban as it is a small molecule with a high affinity for its specific target; conversely, in patients on warfarin, because aPL can induce reduced protein C and S activity, the equilibrium can be shifted further away from anticoagulation. If we can demonstrate i) that the anticoagulant effect of rivaroxaban is not inferior to that of warfarin using the TGT; and ii) absence of any adverse effects that cause concern with regard to the use of rivaroxaban, we believe that this would provide sufficient supporting information to change practice for our patients, i.e. to make rivaroxaban the standard of care for the treatment of patients with thrombotic APS, with or without SLE. UCL CTU Page 27 RAPS Protocol Version 5.0 22 September 2014 1.7 RISKS AND BENEFITS Rivaroxaban was licensed in the UK and Europe for VTE thromboprophylaxis in major lower limb orthopaedic surgery in 2008, and was subsequently approved by NICE for this indication.27 The Phase III trials of rivaroxaban in patients with VTE and atrial fibrillation showed an acceptable side effect profile for rivaroxaban.29,31,33 In an orthopaedic study of 26 patients at UCLH/UCL receiving extended duration prophylactic dose rivaroxaban (10 mg daily) for up to six weeks, no patient experienced any problems with rivaroxaban.67 Benefits 1) Warfarin has an erratic anticoagulant effect in patients with APS who therefore require frequent blood tests for INR monitoring. These blood tests are inconvenient for patients and reduce their quality of life. Rivaroxaban is given as fixed-dose tablets once daily and, unlike warfarin, does not require monitoring because it has a predictable anticoagulant effect. 2) Warfarin has numerous interactions with other drugs and dietary constituents and interacts with alcohol, which interferes with the anticoagulant effect of warfarin and can result in over- or under-anticoagulation with the potential for bleeding or thrombotic events respectively. These interactions also necessitate more frequent INR monitoring. In contrast to warfarin, rivaroxaban has no reported interactions with food or alcohol and has few drug interactions, and is therefore potentially safer and also more convenient for the patient, which would be expected to improve quality of life. 3) Although the new oral anticoagulants have not been specifically trialled in thrombotic APS patients, they have been widely used in large multinational Phase III trials for the prophylaxis and treatment of DVT and PE, the prevention of stroke in patients with atrial fibrillation, and for acute coronary syndrome. To date we estimate that over 100,000 patients with these conditions have been assessed and no major safety issues have emerged. UCL CTU Page 28 RAPS Protocol Version 5.0 22 September 2014 Risks of rivaroxaban and actions to be taken to minimise and manage these risks 1) All patients randomised to rivaroxaban will be given a Patient Information Sheet (PIS) about rivaroxaban and will be given opportunities at trial visits to discuss any specific issues pertaining to this. 2) All patients randomised will be given a Patient Card with contact details for the Research Nurse and the investigators for advice both in and out of hours and when the Research Nurse is on leave. 3) The trial visit schedule (detailed in section 6.1) will enable face-to-face contact with patients to discuss any concerns. Patients will be advised to contact the Research Nurse to report any concerns about the treatment between trial visits. 4) The management of warfarin reversal for routine or emergency procedures will be according to the hospital’s local guidelines based on national guidelines11 for bridging anticoagulation/emergency reversal. Patients on rivaroxaban will be supplied with the contact telephone number for a healthcare professional to use to gain information about what to do about rivaroxaban in the event of routine or emergency procedures. Clinical advice, based on a mutually agreed guideline, will also be available from the trial investigators. 5) All patients who experience bleeding should seek medical attention as per advice given by their local anticoagulation clinic. Patients should report to hospital immediately in the event of severe or persistent bleeding, and contact their trial site immediately. 6) Patients randomised to warfarin who experience bleeding will be managed according to the hospital’s local guidelines based on national guidelines11. 7) Patients randomised to rivaroxaban will be supplied with the contact telephone number for a healthcare professional to use to gain information on what to do about rivaroxaban in the event of bleeding, based on the site and severity of the bleed. They should be managed according to the hospital’s local guidelines in line with the manufacturer’s Summary of Product Characteristics (SPC). Clinical advice, based on local practice, will also be available from the trial investigators. 8) Patients who experience symptoms suggestive of recurrent thrombosis, VTE or arterial, will be asked to attend hospital and also to contact their trial site immediately. In patients on rivaroxaban, the rivaroxaban may be stopped based on clinical judgement. If the rivaroxaban is stopped the patient will be commenced initially on therapeutic dose subcutaneous LMWH with warfarin restarted to achieve a higher target INR range of 3.5 (3.0-4.0)11, and with overlap between LMWH and warfarin until the INR is in the target range. A similar approach will be undertaken in patients on warfarin. Recommencement of rivaroxaban will be considered if clinically appropriate. 9) Patients will be asked to contact their trial site immediately about any serious adverse events (SAEs). 10) We will undertake pregnancy tests in all women of childbearing age (pre-menopausal) prior to entry to the trial and at three and six months for both treatment arms and women will be strongly advised to avoid pregnancy in the PIS. Warfarin is teratogenic, particularly if administered between weeks six and 12 of gestation, and therefore all women of childbearing age are routinely counselled and given advice on obtaining effective contraception when warfarin is commenced, and also advised to contact their trial site staff as soon as possible in the event of pregnancy so that the warfarin can be switched to LMWH (which does not cross the placenta). Although there are no adequate data on the risks of rivaroxaban in pregnant women, studies in animals have shown that rivaroxaban crosses the placenta and is linked with UCL CTU Page 29 RAPS Protocol Version 5.0 22 September 2014 reproductive toxicity. It is also secreted into animal milk.18 Therefore, the use of rivaroxaban in pregnancy and breast feeding is contraindicated. Women of childbearing age randomised to rivaroxaban will also be given advice on obtaining effective contraception when rivaroxaban is commenced, and advised, should they unexpectedly conceive on rivaroxaban, to contact their trial site staff as soon as possible so that early review by a haematologist and obstetrician, to discuss the potential implications in pregnancy, can be organised. 11) As the patients will be self-dosing it is only possible to reduce the risk of overdose. Patients will be informed of the correct dose of the drug at trial entry and their GP will be informed, in all cases, that they are on the trial. The correct dose is also stated on the patient information sheet, which the patient keeps, and the patient card. In the event of an overdose the use of activated charcoal to reduce absorption may be considered, for both warfarin and rivaroxaban. Bleeding in patients on warfarin as a result of overdose will be managed according to the hospital’s local guidelines based on national guidelines.11 Bleeding in patients on rivaroxaban as a result of overdose will be managed according to the hospital’s local practice in line with the manufacturer’s SPC. Clinical advice, based on local practice, will also be available from the trial investigators. UCL CTU Page 30 RAPS Protocol Version 5.0 22 September 2014 2 SELECTION OF SITES/CLINICIANS The trial sponsor has overall responsibility for site and investigator selection and has delegated this activity to UCL CTU. 2.1 SITE/INVESTIGATOR ELIGIBILITY CRITERIA Once a site has been identified as satisfying the eligibility criteria, the trial team will provide the site with a copy of this protocol, a trial summary and the SPC. To participate in the RAPS trial, investigators and clinical trial sites must fulfil a set of basic criteria that have been agreed by the RAPS Trial Management Group (TMG) and are defined below. Sites where a previous serious protocol breach has occurred will be visited, thoroughly reviewed, and staff retrained before allowing further participants to enter the trial. Those sites that meet the criteria will be issued with the RAPS master file documentation for their Site-specific assessment (SSA) and UCL CTU site assessment documents. Sites must complete the RAPS site assessment documents (Section 2.1.3) at the same time as applying for their SSA. 2.1.1 PI'S QUALIFICATIONS & AGREEMENTS 1. The investigators should be qualified by education, training, and experience to assume responsibility for the proper conduct of the trial at their site and should provide evidence of such qualifications through an up-to-date curriculum vitae and/or other relevant documentation requested by the Sponsor, the REC and/or the regulatory authority. 2. The investigator should be thoroughly familiar with the appropriate use of the investigational product, as described in the protocol, in the current SPC, in the product information and in other information sources provided by the Sponsor. 3. The investigator should be aware of, and should comply with, the principles of GCP as given in the protocol compliance statement and the applicable regulatory requirements. A record of GCP training should be accessible for all investigators. 4. The investigator/site should permit monitoring and auditing by the Sponsor, and inspection by the appropriate regulatory authority. 5. The investigator should maintain a delegation log of appropriately-qualified persons to whom the investigator has delegated significant trial-related duties. 6. The investigator should sign a site agreement, which verifies that the site is willing and able to comply with the requirements of the trial. 2.1.2 ADEQUATE RESOURCES 1. The investigator should be able to demonstrate the potential for recruiting the required number of suitable patients within the agreed recruitment period (that is, the investigator regularly treats the target population). UCL CTU Page 31 RAPS Protocol Version 5.0 22 September 2014 2. The investigator should have sufficient time to properly conduct and complete the trial within the agreed trial period. 3. The investigator should have available an adequate number of qualified staff and adequate facilities for the foreseen duration of the trial to conduct the trial properly and safely. 4. The investigator should ensure that all persons assisting with the trial are adequately informed about the protocol, the investigational product, and their trial-related duties and functions. 5. The site should have sufficient data management resources to allow prompt data return to the UCL CTU. Sites that have previously participated in UCL CTU-coordinated trials should have a proven track record of good data return. 2.1.3 SITE ASSESSMENT Each selected clinical trial site must complete the RAPS site assessment process which includes completing the Site agreement, Signature and Delegation of Responsibilities Log, and staff contact details. The Site agreement confirms that the site is willing, and able to comply with the requirements of the trial. This will be signed by the Principal Investigator (PI) at the site. In addition and in compliance with the principles of GCP, all site staff participating in the trial must complete the Signature and Delegation of Responsibilities Log and forward this to the UCL CTU. The UCL CTU must be notified of any changes to trial personnel and/or their responsibilities. An up-to-date copy of this log must be stored in the Investigator Site File (ISF) at the site and Trial Master File (TMF) at the UCL CTU. 2.2 APPROVAL AND ACTIVATION The Clinical Trial Authorisation (CTA) for the trial requires that the Medicines and Healthcare products Regulatory Agency (MHRA) is supplied with the names and addresses of all participating site Principal Investigators. Trial staff at the UCL CTU will perform this task; hence it is vital to receive full contact details for all investigators prior to their entering patients. On receipt of the above documents, UCL CTU will send written confirmation to the PI. The trial manager or delegate will notify the PI in writing of the plans for site initiation. 1. The site should conduct the trial in compliance with the protocol as agreed by the Sponsor and by the regulatory authority and which was given favourable opinion by the REC. 2. The PI or delegate should document and explain any deviation from the approved protocol, and communicate this with the trial team at the UCL CTU. A list of activated sites may be obtained from the Trial Manager. UCL CTU Page 32 RAPS Protocol Version 5.0 22 September 2014 3 SELECTION OF PATIENTS There will be NO EXCEPTIONS (waivers) to eligibility requirements at the time of randomisation. Questions about eligibility criteria should be addressed PRIOR to attempting to randomise the participant. The eligibility criteria for this trial have been carefully considered and are the standards used to ensure that only medically appropriate patients are entered. Patients not meeting the criteria should not join the study for their safety and to ensure that the study results can be used to make treatment decisions for other patients with similar diseases. It is therefore important that no exceptions be made to these eligibility criteria. Participants will be considered eligible for enrolment in this trial if they fulfil all the inclusion criteria and none of the exclusion criteria as defined below. 3.1 PATIENT INCLUSION CRITERIA 1) Patients with thrombotic APS68, with or without SLE, who have had either a single episode of VTE whilst not on anticoagulation or recurrent episode(s) which occurred whilst off anticoagulation or on sub-therapeutic anticoagulant therapy 2) Patients with a target INR of 2.5 (range 2.0 – 3.0) 3) Treated with warfarin for a minimum period of three months since last VTE 4) Female patients must be using adequate contraception with the exception of postmenopausal or sterilised women 3.2 PATIENT EXCLUSION CRITERIA 1) Previous arterial thrombotic events due to APS 2) Recurrent venous thromboembolic events whilst on warfarin at a therapeutic INR of 2.0 – 3.0 3) Pregnant or lactating women 4) Severe renal impairment (creatinine clearance (calculated using the Cockcroft & Gault formula (Appendix A)) < 30 mL/min (i.e. 29 mL/min or less) 5) Liver function tests ALT > 2 x ULN 6) Cirrhotic patients with Child Pugh B or C 7) Thrombocytopenia (platelets < 75 x 109/L) 8) Non-compliance on warfarin (based on clinical assessment) 9) Patients on azole antifungals (e.g. ketoconazole, itraconazole, voriconazole and posaconazole) UCL CTU Page 33 RAPS Protocol Version 5.0 22 September 2014 10) Patients on Human Immunodeficiency Virus (HIV) protease inhibitors (e.g. ritonavir) 11) Patients on strong CYP3A4 inducers (e.g. rifampicin, phenytoin, carbamazepine, phenobarbital or St. John's Wort) 12) Patients on dronedarone 13) Patients less than 18 years of age 14) Refusal to consent to the site informing GP and healthcare professional responsible for anticoagulation care, of participation 3.3 NUMBER OF PATIENTS A total of 116 patients will be randomised using random permuted blocks for treatment allocation and to maintain balanced numbers in the two groups. 3.4 CO-ENROLMENT GUIDELINES Co-enrolment in previous or future trials is considered in Section 4.3. 3.5 SCREENING PROCEDURES & PRE-RANDOMISATION INVESTIGATIONS Written informed consent to enter the trial and be randomised must be obtained from each patient, after explanation of the aims, methods, benefits and potential hazards of the trial and BEFORE any trial-specific procedures are performed or any blood is taken for the trial. It must be made completely and unambiguously clear that the patient is free to refuse to participate in all or any aspect of the trial, at any time and for any reason, without incurring any penalty or affecting their treatment. Signed consent forms must be kept by the investigator and documented in the CRF and a copy given to the patient. Upon consent, a letter should be sent to the general practitioner informing him/her of the trial and the participant's involvement in it. SCREENING The patient must have the following pre-randomisation assessments. These must be done no more than 30 days prior to randomisation (with exceptions: anti-DNA and aPL): Consent Medical history Demographics Patient review Height Weight Body mass index (BMI) UCL CTU Page 34 RAPS Protocol Version 5.0 22 September 2014 Blood pressure (BP) Full blood count (FBC) Urea and electrolytes (U&E) and creatinine clearance (CrCl) (to be calculated using the Cockcroft & Gault formula (Appendix A)) Liver function tests (LFTs) to include ALT + Anti-DNA ++ aPL International Normalised Ratio (INR) Current medication Enquiry for bleeding Enquiry for recurrent thrombosis The patient will also attend a routine specialist clinic visit at the time of screening. + Results of anti-DNA done routinely at the trial sites within three months of (prior to or at the time of) the screening visit, with the date of testing, to be documented in the CRF (i.e. testing for antiDNA is not required within 30 days prior to randomisation). ++ All patients will have been diagnosed with thrombotic antiphospholipid syndrome (APS), prior to trial entry. Results of antiphospholipid antibodies (aPL) establishing a diagnosis of thrombotic APS, with the dates when the tests were performed routinely at the trial sites, to be documented in the CRF (i.e. testing for aPL is not required within 30 days prior to randomisation). aPL will also be assessed at baseline in all patients to define the aPL status of the patient population at the time of trial entry. Pre-randomisation (baseline) assessments – post patient consent Pregnancy test INR Current medication documentation Document INRs for the three month period prior to randomisation Enquiry for bleeding Enquiry for recurrent thrombosis QoL assessment Note: Repeat the following tests if the screening was more than 14 days prior to randomisation: Full blood count (FBC) Urea and electrolytes (U&E) and creatinine clearance (CrCl) (to be calculated using the Cockcroft & Gault formula (Appendix A)) Liver function tests (LFTs) to include ALT UCL CTU Page 35 RAPS Protocol Version 5.0 22 September 2014 4 4.1 REGULATION & RANDOMISATION RANDOMISATION PRACTICALITIES Randomisations should be done via the CTU randomisation website (details below). If there are any difficulties in accessing this website they should call the number below. Further details on the process of randomisation can be found in Section 9.1. RANDOMISATIONS To randomise access website: https://www.sealedenvelope.com/uclctu/ In case of difficulties call UCL CTU, Monday to Friday 09:00 to 17:00hrs: Tel: 07823536715 A manual randomisation process will be set up to cover any instances when the main electronic system is not working. This will be detailed in the trial Working Instructions. 4.2 RANDOMISATION CODES & UNBLINDING Randomisation codes and unblinding are considered in Section 5.5. 4.3 CO-ENROLMENT GUIDELINES No co-enrolment into interventional clinical trials is allowed in the RAPS trial during the period of intervention. However, co-enrolment in another interventional trial is permissible two days (2 x maximum half-life) after the last administration of the rivaroxaban. There is no prohibition on co-enrolment into observational studies. UCL CTU Page 36 RAPS Protocol Version 5.0 22 September 2014 5 TREATMENT OF PATIENTSINTRODUCTION This is a pragmatic trial comparing the standard care for patients with thrombotic APS, which is warfarin anticoagulation, with rivaroxaban, a new fixed-dose anticoagulant drug. 5.2 ARM A This is the control arm where the treatment of patients with warfarin will remain unchanged. Warfarin will be prescribed and dispensed in accordance with national guidance (NPSA Safer Practice Notice 1869 and BCSH11) and warfarin anticoagulant monitoring will be undertaken by the patient’s usual anticoagulation clinic, which may be based at one of the trial sites or locally to the patient, either within a hospital or primary care anticoagulation clinic setting, or by patient self-monitoring under medical supervision. 5.2.1 PRODUCTS Warfarin 5.2.2 COMPLIANCE & ADHERENCE Compliance with warfarin treatment schedule is assessed by a laboratory assay for the INR and amidolytic factor X, which measure the anticoagulant effect of warfarin with the latter providing an aPL-independent assessment of anticoagulation. Percentage time in therapeutic range (TTR) between baseline and day 180 will also be calculated in patients on warfarin. 5.3 ARM B Patients randomised to rivaroxaban may have an INR above the upper limit of the target therapeutic range of 2.0 – 3.0 at the time of randomisation. These patients need to have an INR of ≤3 before starting rivaroxaban (please see below for details). 5.3.1 PRODUCTS Rivaroxaban 20mg and 15mg film-coated tablets 5.3.2 TREATMENT SCHEDULE Patients without renal impairment (creatinine clearance ≥ 50 mL/min) should take one 20mg tablet orally once daily in the morning, with food to maximise absorption, for six months18. Patients with moderate renal impairment (creatinine clearance of 30 – 49 mL/min) will be prescribed either one 20mg tablet or one 15mg tablet to be taken orally once daily in the morning, with food, for six months depending on local clinical care and following the SPC. If the patient is prescribed 15mg and subsequent testing shows that the patient’s creatinine clearance is ≥50 mL/min, the patient’s dose of rivaroxaban should generally be increased to one 20mg tablet once daily in the morning depending on local clinical care and following the SPC. 5.3.3 DISPENSING Dispensing will be in blister packs, with each pack containing 28 tablets. Sufficient packs of rivaroxaban will be dispensed to the patient to ensure that they have sufficient tablets between the scheduled assessment visits. For pragmatic reasons, as some patients live remote from the trial sites, it may be necessary for the rivaroxaban to be dispensed to the patient with instructions to not start UCL CTU Page 37 RAPS Protocol Version 5.0 22 September 2014 taking the tablets until their INR is ≤3 (please see below for details). This will mean that they will be monitored by their local anticoagulant clinic until their INR is ≤3. In such cases the patient will remain in daily telephone contact with the trial Research Nurse who will inform the patient when to start taking the study drug. Regimen for patients converting from warfarin to rivaroxaban: For patients randomised to rivaroxaban, the warfarin should be stopped on the day of conversion from warfarin to rivaroxaban If the INR is ≤2.5, the rivaroxaban should be started the following morning If the INR is 2.6 to ≤3, the rivaroxaban should be started 2 days later in the morning If the INR is >3, the INR should be rechecked as required and the rivaroxaban should be started, as a morning dose: o when the INR is 2.6 to ≤3, the rivaroxaban should be started 2 days later in the morning o when the INR is ≤2.5, the rivaroxaban should be started the following morning Note: when converting a patient from warfarin to rivaroxaban, the INR will be falsely elevated after dosing. Therefore, the INR should not be used as a measure of anticoagulant activity of rivaroxaban. 5.3.4 DOSE MODIFICATIONS, INTERRUPTIONS & DISCONTINUATIONS Dose modifications are required for patients on rivaroxaban with renal impairment. With changes in the creatinine clearance the following dose modification should be made: Creatinine clearance 30 – 49 mL/min, if the patient is on 20mg rivaroxaban once daily the clinician should consider changing to 15mg as per local clinical care and following the SPC Creatinine clearance ≥50 mL/min, if the patient is on 15mg rivaroxaban once daily they should be changed to 20mg rivaroxaban once daily Creatinine clearance of ≤29 mL/min, if the patient is on rivaroxaban they should be converted to warfarin It may be necessary to temporarily stop or interrupt treatment with rivaroxaban for patients who have bleeding events or bridging anticoagulation for procedures (routine or emergency). Supplementary information documents have been produced for these procedures. Copies of these documents should be present in the trial site file. Any bleeding event should be reported on the appropriate CRF and serious bleeding events should also be reported on a SAE report, see section 7 for details. If it is necessary to switch a patient from rivaroxaban to warfarin, this should be carried out according to local clinical care and following the SPC. Situations where the rivaroxaban should be discontinued are stated in section 5.6. 5.3.5 STOPPING DRUG EARLY If a patient requires treatment with one of the drugs specified as not being permitted in section 5.10.2 it is recommended that the patient is taken off rivaroxaban and reverts back to warfarin treatment. 5.3.6 ACCOUNTABILITY & UNUSED DRUGS/DEVICES The dispensing pharmacist at the site will keep accountability logs of the number of tablets prescribed and returned, and for the destruction of any returned rivaroxaban. At the end of the UCL CTU Page 38 RAPS Protocol Version 5.0 22 September 2014 trial, unused rivaroxaban will either be destroyed or taken into general hospital stock, as agreed by the drug provider and the MHRA. 5.3.7 COMPLIANCE & ADHERENCE Compliance will be tested by a laboratory assay for anti-factor Xa, which measures the anticoagulant effect of rivaroxaban. 5.4 OVERDOSE OF TRIAL MEDICATION Full details on the effects of an overdose of rivaroxaban and the management of such an overdose can be found under section 4.9 of the SPC for rivaroxaban. 5.5 UNBLINDING This trial will not be blinded or masked. We believe that it would be potentially unsafe to do so for the following reasons: 1) Uncertainty about the intensity of the anticoagulant effect of warfarin and increased instability of the INR in patients with thrombotic APS on warfarin necessitates frequent anticoagulant monitoring at variable time intervals which cannot be predetermined, therefore a regular trial schedule for anticoagulant monitoring would not be expected to be effective. 2) In patients on warfarin, the INR result has to be acted upon promptly (within a few hours), with computerized assisted dosing by an experienced individual who has access to the patient's previous warfarin dosing history and thus, knowledge of the individual patient's sensitivity to changes in warfarin dose. Dosing in a blinded fashion would be likely to result in suboptimal dosing management decisions. 3) Should patients experience bleeding, it is important to know immediately whether they are on warfarin or rivaroxaban as the management of bleeding differs. 5.6 PROTOCOL TREATMENT DISCONTINUATION In consenting to the trial, patients are consenting to trial treatment, trial follow-up and data collection. However, an individual patient may stop treatment early or be stopped early for any of the following reasons: Serious adverse events (SAE) Thrombotic event* Any change in the patient’s condition that justifies the discontinuation of treatment in the clinician’s opinion Withdrawal of consent for treatment by the patient * Patients who experience symptoms suggestive of recurrent thrombosis, VTE or arterial, should be managed as detailed in Section 1.7. An individual must be stopped early for the following reasons: Unacceptable toxicity UCL CTU Page 39 RAPS Protocol Version 5.0 22 September 2014 Unacceptable SAE Pregnancy As the patient’s participation in the trial is entirely voluntary, they may choose to discontinue the trial treatment at any time without penalty or loss of benefits to which they are otherwise entitled. Although the patient is not required to give a reason for discontinuing their trial treatment, a reasonable effort should be made to establish this reason while fully respecting the patient's rights. Patients should remain in the trial for the purpose of follow-up and data analysis (unless the patient withdraws their consent from all stages of the trial). If a patient is withdrawn from follow-up, refer to Section 6.6. Data will be kept and included for patients who stop follow-up early. 5.7 ACCOUNTABILITY & UNUSED DRUGS/DEVICES Refer to section 5.3.5 for accountability of rivaroxaban. 5.8 COMPLIANCE & ADHERENCE Refer to sections 5.2.2 for warfarin and 5.3.7 for rivaroxaban, for the respective processes for assessing compliance and adherence. 5.9 TREATMENT DATA COLLECTION Refer to the follow-up assessment schedule in section 6 assessments & follow-up. 5.10 NON-TRIAL TREATMENT 5.10.1 MEDICATIONS PERMITTED All non-contraindicated medications that the investigator responsible for the patients care feels are appropriate are permitted in the trial. Refer to sections 5.10.2 for medications not permitted and to 5.10.3 for medications to be used with caution. 5.10.2 MEDICATIONS NOT PERMITTED The use of azole antifungals (such as ketoconazole, itraconazole, voriconazole and posaconazole) and HIV protease inhibitors (such as ritonavir), which increase rivaroxaban levels, are not permitted, while the patient is on the rivaroxaban. This is because these agents are all strong inhibitors of both CYP3A4 and P-gp and concurrent use with rivaroxaban is not recommended due to the increased risk of bleeding.18 The use of strong CYP3A4 inducers (rifampicin, phenytoin, carbamazepine, phenobarbital or St. John's Wort) may lead to reduced rivaroxaban plasma concentrations and are not permitted, while the patient is on rivaroxaban.18 Avoid co-administration with dronedarone for patients on rivaroxaban. UCL CTU Page 40 RAPS Protocol Version 5.0 22 September 2014 If it is in the interest of patient’s best care that these are prescribed, it is recommended that the patient is taken off rivaroxaban and reverts to appropriate anticoagulation. 5.10.3 MEDICATIONS TO BE USED WITH CAUTION Caution should be taken prescribing antiplatelet agents e.g. aspirin, clopidogrel and dipyridamole, and also fluconazole, although their use during the trial is not prohibited. If fluconazole is required in trial patients, which is unlikely, these patients are likely to be in hospital and will be closely monitored. Rivaroxaban should be used with caution in patients with renal impairment who are concomitantly receiving other medicinal products, which increase rivaroxaban plasma concentrations (e.g. clarithromycin, erythromycin, fluconazole. Refer to the SPC for further information and discuss with the CI). 5.10.4 TREATMENT AFTER TRIAL EVENT Treatment will be at the discretion of the responsible physician. 5.11 CO-ENROLMENT GUIDELINES Co-enrolment in previous or future trials is considered in Section 4.3. UCL CTU Page 41 RAPS Protocol Version 5.0 22 September 2014 6 ASSESSMENTS & FOLLOW-UP All patients entered into this trial are under routine specialist long-term follow up and clinical surveillance by the clinical co-applicants and collaborators at the trial sites, and this will continue long-term after the trial has been completed 6.1 TRIAL ASSESSMENT SCHEDULE The trial visit schedule will enable face-to-face contact with patients to discuss any concerns. Patients will be advised to contact the Research Nurse to report any concerns about the treatment between trial visits. Routinely, patients with thrombotic APS and with thrombotic SLE are seen in clinic on average every three months. Where possible, trial visits will be scheduled to coincide with routine follow up and trial participation will entail approximately three additional hospital visits for each patient in this trial. Patients on warfarin require additional visits, which may be weekly, for monitoring of anticoagulation (managed at either the trial site or locally). Unlike patients on warfarin, patients on the new fixed-dose oral anticoagulants do not need routine anticoagulant monitoring because of the predictable anticoagulant effect of these agents, and so patients on rivaroxaban will not need to attend anticoagulation clinics during the trial period. At each routine specialist clinic visit, routine clinical assessment will include assessment with regard to potential effects relating to the efficacy and safety of anticoagulation: bleeding, recurrent thrombosis (VTE or arterial) and SAEs. Samples for research laboratory investigations will be obtained from routine blood draws at these routine specialist clinic visits where possible. At each routine specialist clinic visit, patients will have routine laboratory assessment as clinically indicated: full blood count (FBC), U&E, LFTs and INR/coagulation screen. aPL and anti-DNA are checked every six to 12 months. Additional laboratory or other tests will be performed as clinically indicated. At the end of the trial treatment period of six months patients will be offered appropriate anticoagulant care. UCL CTU Page 42 RAPS Protocol Version 5.0 22 September 2014 Visit 0 (Baseline) - Post randomisation procedure Blood collection 20 mL citrated blood for TGT, INR, amidolytic factor X and aPL 20 mL blood for the translational research (see section 10); this is optional and only applicable to patients who have consented to this part of the trial. This sample will be collected at the same time as the trial sample. Visit 1 – Day 42 after randomisation (ideally -12 days +14 days). The patient must have been on rivaroxaban for a minimum of 30 days before this visit. Blood collection 20 mL of citrated blood for TGT and anti-Xa (rivaroxaban patients)/INR, amidolytic factor X (warfarin patients). For patients on rivaroxaban the blood samples should preferably be collected 2–4 hours after tablet intake when the maximum concentration (Cmax) of rivaroxaban will be present. 20 mL blood for the translational research (section 10), this is optional and only applicable to patients who have consented to this part of the trial. This sample will be collected at the same time as the trial sample. Documentation of medication Document INRs since last trial visit Drug accountability of rivaroxaban Enquiry for bleeding Enquiry for recurrent thrombosis Visit 2 – Day 90 after randomisation (ideally ±14 days)* Full blood count U&E, LFTs Pregnancy test Documentation of medication Document INRs since last trial visit Drug accountability of rivaroxaban Enquiry for bleeding Enquiry for recurrent thrombosis Visit 3 – Day 180 after randomisation (ideally ±14 days)* – end of trial treatment Weight Body mass index Blood pressure Full blood count - as clinically indicated U&E, LFTs - as clinically indicated Pregnancy test Documentation of medication Document INRs since last trial visit Drug accountability of rivaroxaban Enquiry for bleeding Enquiry for recurrent thrombosis Quality of life assessment UCL CTU Page 43 RAPS Protocol Version 5.0 22 September 2014 Visit 4 – Day 210 after randomisation (ideally ±14 days) – 30 days after end of trial treatment Documentation of medication Enquiry for bleeding Enquiry for recurrent thrombosis *Note: Trial visits should be scheduled to coincide with routine specialist clinic visits where possible. 6.2 PROCEDURES FOR ASSESSING EFFICACY The primary aim is to compare the thrombogenic potential, by measurement of the dynamics of ex vivo thrombin generation using the TGT with the key parameter the ETP, the area under the thrombin generation curve, which we and others have established to be an appropriate measure in individuals on rivaroxaban.56,57 The primary outcome measure is the percentage change in the ETP from randomisation to Day 42. The time point of Day 42 (first trial visit following randomisation), was chosen as the therapeutic effect of rivaroxaban would be expected to be stable after 30 days of treatment, and rivaroxaban treatment must begin within a maximum of 10 days from randomisation. The ETP at Day 42 would not be influenced by any residual warfarin effect, based on the biological half-lives of the vitamin K-dependent coagulation factors which range from two to five hours (factor VII) up to 72 hours (factor II).66 The TGT will be performed using the Calibrated Automated Thrombogram (CAT) system (Thrombinoscope BV, Maastricht, The Netherlands) as described by Hemker43. In order to reduce intra-assay variation, paired samples for each patient will be tested at the same time. Despite the wide application of TGT in thrombotic disorders, literature searches of peer-reviewed journals failed to produce data relating to the assessment of thrombin generation in patients with APS on warfarin or rivaroxaban. We and others have previously assessed thrombin generation in warfarinised patients using a trigger reagent that gives a reaction concentration of 5 pmol/L tissue factor and 4 µmol/L phospholipids.70,71 Secondary laboratory outcome measures of efficacy are also being determined to provide a comprehensive assessment of ex vivo thrombin generation and in vivo coagulation activation. The thrombin generation curve will therefore also be quantified in terms of: the lag-time; the time to peak; and peak thrombin concentration.43 In addition, markers of in vivo coagulation activation, F1.2, TAT and D-dimer (a marker of activation of fibrinolysis secondary to coagulation activation), will provide supplementary information about thrombogenic potential;41,44-49 and F1.2 will also provide supplementary information about anticoagulation intensity.50,51 Symptoms suggestive of recurrent VTE will be evaluated according to the usual diagnostic methods (see Glossary). All recurrent thrombotic events (VTE or arterial) should be reported immediately to the trial site, and the trial investigators will advise on anticoagulant management as detailed in 1.7 (risks: point 7). Patients who present with symptoms suggestive of stroke, myocardial infarction or arterial thrombosis at other sites, generally present as a clinical emergency and clinical and diagnostic information will be obtained retrospectively from the site at which they have been managed. Clinical efficacy outcomes will be defined as: (i) recurrent VTE alone; and (ii) a composite of recurrent VTE and other thrombotic events. UCL CTU Page 44 RAPS Protocol Version 5.0 22 September 2014 6.3 PROCEDURES FOR ASSESSING SAFETY Results of liver function tests at 90 days and as clinically indicated and any other SAEs since the previous visit will be routinely assessed at each follow up visit. A bleeding event will be classified using established criteria as major if it is associated with a fall in the haemoglobin level of at least 20 g/L, results in the need for a transfusion of two or more units of red cells, involves a critical site, or is fatal.29,72 Less severe bleeding will be classified as clinically relevant non-major bleeding29 and bleeds not falling in these categories will be classed as minor bleeding. 6.4 PROCEDURES FOR ASSESSING QUALITY OF LIFE QoL will be evaluated via the EQ 5D 5L questionnaire73 in all patients at baseline (time of randomisation) and at visit three (180 days post randomisation after completion of the six months trial treatment period). Patients with thrombotic APS on warfarin require frequent INR monitoring blood tests, which may be weekly, to monitor the anticoagulant effect of warfarin. In contrast, patients on the new fixed-dose oral anticoagulant rivaroxaban do not need anticoagulant monitoring because of its predictable anticoagulant effect, and so patients randomised to switch to rivaroxaban will not need to attend anticoagulation clinics during the trial period. We anticipate that the reduction in the need for frequent anticoagulant monitoring will have a major impact on the quality of life of patients randomised to rivaroxaban. We recognise that the EQ 5D 5L with five dimensions each with five levels and visual analogue score is not an ideal instrument to assess QoL in patients on this trial. However, there is no other validated questionnaire for this situation, and we believe that the EQ 5D 5L might pick up differences, particularly in the question on ‘usual activities’ and the visual analogue score. 6.5 OTHER ASSESSMENTS An anti-Xa assay (to assess compliance) in patients on rivaroxaban at Day 42 , and an INR and factor X amidolytic assay as an antiphospholipid antibody-independent assessment of anticoagulation in patients on warfarin at baseline and at Day 42, will be performed by established methods.58,59 The percentage time in therapeutic range (TTR) in patients on warfarin will be calculated using the method described by Rosendaal et al.60 6.6 EARLY STOPPING OF FOLLOW-UP If a patient chooses to discontinue their trial treatment, they should be encouraged not to leave the whole trial and to continue with their visit schedule as planned. Where possible the patient should be encouraged to attend all their visits, but if that is not possible they should be encouraged to attend at least the 42 day and 180 day visits as these are the most important. If they do not wish to remain on trial follow-up, however, their decision must be respected and the patient may ask to be withdrawn from the trial completely. The UCL CTU should be informed of this in writing using the appropriate documentation. Patients stopping early have a negative impact on a trial's conclusions. If the medical data collected during the patient’s participation in the trial are kept for research and analysis purposes, they can be anonymised if necessary. Consent for future use of stored samples already collected can be refused when leaving the trial early (but this should be discouraged and should follow a discussion). UCL CTU Page 45 RAPS Protocol Version 5.0 22 September 2014 Patients may change their minds about stopping trial follow-up at any time and re-consent to participation in the trial. Patients who stop trial follow-up early will not be replaced. 6.7 PATIENT TRANSFERS If a patient moves from the area, every effort should be made for the patient to be seen at another participating trial site. With the patient’s consent a copy of the patient’s CRFs should be provided to the new site and the patient will need to sign a new consent form. Once this has been done, the new site will take over responsibility for the patient; until this has been done, responsibility for the patient lies with the original site. 6.8 LOSS TO FOLLOW-UP In the EINSTEIN Phase III open-label, randomised, non-inferiority study that compared oral rivaroxaban with warfarin in patients with acute DVT, premature discontinuation of treatment occurred in 11.3% of patients in the rivaroxaban arm and 14.2% in the warfarin arm. The reasons for this were: adverse events 4.3% and 3.9%; consent withdrawn 2.0% and 3.9%; and 0.9% and 1.0% of patients were lost to follow up in the rivaroxaban and warfarin arms respectively. In patients who were continued on rivaroxaban, versus placebo, beyond the initial treatment period, premature discontinuation of rivaroxaban occurred in 12.6%, with the reasons: adverse events in 6.5%, consent withdrawn in 3.7% and 1.0% were lost to follow up29. Based on our experience with the first 84 patients recruited, 2 patients have been lost to follow-up or withdrawn before the first trial visit post randomisation and a pair of ETP values has not been obtained in 5 patients, we conservatively allow for 12% non evaluable patients. It is expected that the loss to follow-up in RAPS trial, due to patients withdrawing consent, will be less than in the EINSTEIN trial. In the EINSTEIN trial patients were recruited post thrombotic event and were not routinely being followed up at the sites, whereas the RAPS patient population are routinely seen by the sites on a regular basis and therefore know the clinicians and nurses who will care for them in the trial. UCL CTU Page 46 RAPS Protocol Version 5.0 22 September 2014 7 SAFETY REPORTING Investigators and sponsors are required to follow specific procedures when notifying and reporting adverse events or reactions in clinical trials. These procedures are described in this section of the protocol. Section 7.1 lists definitions, Section 7.2 gives details of the investigator responsibilities and Section 7.3 provides information on ProductLife Ltd responsibilities who have been contracted by the UCL CTU to undertake the clinical safety and pharmacovigilance aspect of the RAPS trial. 7.1 DEFINITIONS The definitions of the EU Directive 2001/20/EC Article 2 based on ICH GCP apply to this trial protocol. These definitions are given in Table 7.1. TABLE 7.1: DEFINITIONS TABLE DEFINITION Adverse Event (AE) Any untoward medical occurrence in a patient or clinical trial subject to whom a medicinal product has been administered including occurrences that are not necessarily caused by or related to that product. Adverse Reaction (AR) Any untoward and unintended response to an investigational medicinal product related to any dose administered. Unexpected Adverse Reaction (UAR) An adverse reaction, the nature or severity of which is not consistent with the information about the medicinal product in question set out in the Summary of Product Characteristics (SPC) or Investigator Brochure (IB) for that product. Serious Adverse Event (SAE) or Serious Adverse Reaction (SAR) or Suspected Unexpected Serious Adverse Reaction (SUSAR) Respectively any adverse event, adverse reaction or unexpected adverse reaction that: Results in death Is life-threatening* Requires hospitalisation or prolongation of existing hospitalisation** Results in persistent or significant disability or incapacity Consists of a congenital anomaly or birth defect Is another important medical condition*** *The term ‘life-threatening’ in the definition of ‘serious’ refers to an event in which the patient is at risk of death at the time of the event; it does not refer to an event that hypothetically might cause death if it were more severe, for example, a silent myocardial infarction. **Hospitalisation is defined as an inpatient admission, regardless of length of stay, even if the hospitalisation is a precautionary measure for continued observation. Hospitalisations for a pre-existing condition (including elective procedures that have not worsened) do not constitute an SAE. *** Medical judgement should be exercised in deciding whether an AE or AR is serious in other situations. The following should also be considered serious: important AEs or ARs that are not immediately lifethreatening or do not result in death or hospitalisation but may jeopardise the subject or may require UCL CTU Page 47 RAPS Protocol Version 5.0 22 September 2014 intervention to prevent one of the other outcomes listed in the definition above; for example, a secondary malignancy, an allergic bronchospasm requiring intensive emergency treatment, seizures or blood dyscrasias that do not result in hospitalisation or development of drug dependency. An investigational medicinal product is defined as the tested investigational medicinal product and the comparators used in the study. (EU guidance ENTR/CT 3, April 2006 revision). ADVERSE REACTIONS Adverse reactions include any untoward and unintended response to drugs. GUIDANCE FOR ADVERSE EVENT INCLUSIONS & EXCLUSIONS Adverse Events Include: An exacerbation of a pre-existing illness An increase in frequency or intensity of a pre-existing episodic event or condition A condition (even though it may have been present prior to the start of the trial) detected after trial drug administration Continuous persistent disease or a symptom present at baseline that worsens following administration of the study treatment Adverse Events Do Not Include: Medical or surgical procedures; the condition that leads to the procedure is the adverse event Pre-existing disease or a condition present before treatment that does not worsen Hospitalisations where no untoward or unintended response has occurred, e.g. elective cosmetic surgery, social admissions Overdose of medication without signs or symptoms DISEASE-RELATED EVENTS Due to the systemic nature of SLE, events can occur in any system. Events related to SLE can range from a skin rash to joint pain, headaches, fever etc. APS in the absence of SLE is also associated with a variety of clinical manifestations affecting various systems. Adverse events (with the exception of bleeding, thrombotic events and adverse events due to lack of drug effect) and adverse reactions do not need to be recorded for the RAPS trial. 7.2 INVESTIGATOR RESPONSIBILITIES All SAEs and SARs should be notified to ProductLife Ltd within 24 hours of the investigator becoming aware of the event. 7.2.1 INVESTIGATOR ASSESSMENT Seriousness When an AE or AR occurs, the investigator responsible for the care of the patient must first assess whether or not the event is serious using the definition given in Table 7.1. If the event is serious, then an SAE Form must be completed and ProductLife Ltd notified within 24 hours. UCL CTU Page 48 RAPS Protocol Version 5.0 22 September 2014 Severity or Grading of Adverse Events The severity of all SAEs and SARs in this trial should be graded using the CTCAE toxicity gradings version 4.0. A copy of this grading system should be located in the ISF. Causality The investigator must assess the causality of all serious events or reactions in relation to the trial therapy using the definitions in Table 7.2. There are five categories: unrelated, unlikely, possible, probable, and definitely related. If the causality assessment is unrelated or unlikely to be related, the event is classified as an SAE. If the causality is assessed as possible, probable or definitely related, then the event is classified as an SAR. TABLE 7.2: ASSIGNING TYPE OF SAE THROUGH CAUSALITY RELATIONSHIP DESCRIPTION Event Type Unrelated There is no evidence of any causal relationship Unrelated SAE Unlikely There is little evidence to suggest that there is a causal Unrelated SAE relationship (for example, the event did not occur within a reasonable time after administration of the trial medication). There is another reasonable explanation for the event (for example, the patient’s clinical condition, other concomitant treatment). Possible There is some evidence to suggest a causal relationship (for SAR example, because the event occurs within a reasonable time after administration of the trial medication). However, the influence of other factors may have contributed to the event (for example, the patient’s clinical condition, other concomitant treatments). Probable There is evidence to suggest a causal relationship and the influence of other factors is unlikely. SAR Definitely There is clear evidence to suggest a causal relationship and other possible contributing factors can be ruled out. SAR If an SAE is considered to be related to trial treatment and drug is stopped or the dose modified, refer to Section 5.2.4. Expectedness If there is at least a possible involvement of the trial treatment (or comparator), the investigator must assess the expectedness of the event. An unexpected serious adverse reaction is one not previously reported in the current SPC or one that is more frequent or more severe than previously reported. The definition of an unexpected adverse reaction (UAR) is given in Table 7.1. Please see Appendix B for a list of expected toxicities associated with the drugs being used in this trial. If a SAR is assessed as being unexpected, it becomes a SUSAR. UCL CTU Page 49 RAPS Protocol Version 5.0 22 September 2014 Notification ProductLife Ltd should be notified of all SAEs within 24 hours of the investigator becoming aware of the event. Investigators should notify ProductLife Ltd of all SAEs occurring from the time of randomisation until 30 days after the last protocol treatment administration. SARs and SUSARs must be notified to ProductLife Ltd until trial closure. Any subsequent events that may be attributed to treatment should be reported to the MHRA using the yellow card system. 7.2.2 NOTIFICATION PROCEDURE 1. The SAE Form must be completed by the investigator (the consultant named on the Signature List and Delegation of Responsibilities Log who is responsible for the patient’s care), with due care being paid to the grading, causality and expectedness of the event as outlined above. In the absence of the responsible investigator, the form should be completed and signed by a member of the site trial team and faxed or emailed as appropriate. The responsible investigator should subsequently check the SAE Form, make changes as appropriate, sign and then re-fax to ProductLife Ltd as soon as possible. The initial report must be followed by detailed, written reports as appropriate. The minimum criteria required for reporting an SAE are the trial number and date of birth, name of investigator reporting and why the event is considered serious. SAE REPORTING Within 24 hours of becoming aware of an SAE, please fax a completed SAE form to ProductLife Ltd on: Fax: +44 (0) 1223 413 689 or email to safety@verius.co.uk 2. Follow-up: patients must be followed up until clinical recovery is complete and laboratory results have returned to normal or baseline, or until the event has stabilised. Follow-up should continue after completion of protocol treatment if necessary. A further SAE Form, indicated as ‘Follow-up’ should be completed and faxed to ProductLife as information becomes available. Extra, annotated information and/or copies of test results may be provided separately. The patient must be identified by trial number, date of birth and initials only. The patient’s name should not be used on any correspondence and should be deleted from any test results. 3. Staff should follow their institution’s procedure for local notification requirements. 7.3 UCL CTU RESPONSIBILITIES Medically-qualified staff at ProductLife Ltd will review all SAE reports received. The causality assessment given by the local investigator at the hospital cannot be overruled; in the case of disagreement, both opinions will be provided in any subsequent reports. On behalf of the UCL CTU, ProductLife Ltd will review the assessment of expectedness and if, based on the wider knowledge of the reference material for the rivaroxaban or comparator and after discussion with the Chief Investigator (CI) may overrule the Investigator assessment of expectedness for the purposes of onward reporting. UCL CTU Page 50 RAPS Protocol Version 5.0 22 September 2014 ProductLife Ltd as a CRO is undertaking the clinical safety and pharmacovigilance duties for the RAPS trial on behalf of the sponsor. ProductLife Ltd is responsible for the reporting of SUSARs and other SARs to the regulatory authorities and the research ethics committees, as appropriate. Fatal and life-threatening SUSARs must be reported to the competent authorities within seven days of ProductLife Ltd becoming aware of the event; other SUSARs must be reported within 15 days. The responsibility of producing the Development Safety Update Report (DSUR) remains with UCL CTU. Bayer plc will keep UCL CTU informed of any safety issues associated with rivaroxaban that arise during the course of the trial and UCL CTU will report these issues to the trial sites and investigators. ProductLife Ltd, will submit line listing of SARs and SUSARs to Competent Authorities (Regulatory Authority and Ethics Committee), on behalf of the sponsor. Bayer plc will also be notified of all reportable (serious and unexpected and drug-related/unknown relationship) events. UCL CTU will also provide Bayer with a copy of the DSURs (line-listing of SARs and SUSARs). 7.4 OTHER NOTIFIABLE EVENTS Pregnancy Female patients who are not postmenopausal or sterilised should be on adequate contraception for inclusion into the trial. However, if pregnancies do occur during the trial they should be reported on a RAPS SAE report to ProductLife within 24 hours of the investigator becoming aware of the pregnancy. UCL CTU Page 51 RAPS Protocol Version 5.0 22 September 2014 8 8.1 QUALITY ASSURANCE & CONTROL RISK ASSESSMENT The Quality Assurance (QA) and Quality Control (QC) considerations have been based on a formal Risk Assessment, which acknowledges the risks associated with the conduct of the trial and proposals of how to mitigate them with QA and QC processes. QA includes all the planned and systematic actions established to ensure the trial is performed and data generated, documented and/or recorded and reported in compliance with the principles of GCP and applicable regulatory requirements. QC includes the operational techniques and activities done within the QA system to verify that the requirements for quality of the trial-related activities are fulfilled. This Risk Assessment has been reviewed by the Quality Management Group (QMG) and has led to the development of a Quality Management Plan (QMP), which will be kept in the TMF. 8.2 CENTRAL MONITORING AT UCL CTU UCL CTU staff will review CRFs for errors and missing values. The clinical database will be used to monitor the validity of the data with validation checks and data compliance reports. Other essential trial issues, events and outputs will be detailed in the Monitoring and Quality Management Plan that is based on the trial-specific Risk Assessment. 8.3 ON-SITE MONITORING The frequency, type and intensity of routine monitoring and the requirements for triggered monitoring will be detailed in the Monitoring and Quality Management Plan. This plan will also detail the procedures for review and sign-off of monitoring reports. 8.3.1 DIRECT ACCESS TO PATIENT RECORDS Participating investigators should agree to allow trial-related monitoring, including audits, ethics committee review and regulatory inspections by providing direct access to source data and documents as required. Patients’ consent for this must be obtained. 8.3.2 CONFIDENTIALITY UK DPA will be followed in this trial. UCL CTU Page 52 RAPS Protocol Version 5.0 22 September 2014 9 9.1 STATISTICAL CONSIDERATIONS METHOD OF RANDOMISATION 1) Patients will be prospectively randomised to either remain on warfarin or switch to rivaroxaban. 2) Randomisation will be stratified by site and patient type (SLE/non-SLE), to ensure a balance of patients in each arm in each site and patient type. 3) Random permuted blocks will be used to randomly allocate patients to treatment groups within each stratum. 4) Blocked randomisation with randomly varying block length will be used to ensure that the numbers in each group are balanced throughout the trial. 5) Randomisation will be carried out using the SealedEnvelope randomisation system with manual back up being supplied by the UCL CTU. 9.2 OUTCOME MEASURES Primary outcome measure The primary outcome measure is the percentage change in the ETP from randomisation to Day 42. Secondary outcome measures a) Efficacy outcomes Efficacy outcomes will be defined as (i) recurrent VTE alone; and ii) a composite of recurrent VTE and other thrombotic events. The thrombin generation curve will be quantified in terms of: the lag-time; the time to peak; peak thrombin concentration. Markers of in vivo coagulation activation: prothrombin fragment 1.2, thrombin-antithrombin complex and D-dimer b) SAEs and bleeding events Results of liver function tests will be assessed at baseline, day 90 and as clinically indicated. SAEs since the previous visit will be routinely assessed at each follow up visit. Bleeding will be categorised as: major, clinically relevant non-major, minor. c) Quality of life QoL will be evaluated via the EQ 5D 5L questionnaire in all patients at baseline and after completion of the six months trial treatment period. d) Laboratory measures of compliance An anti-Xa assay, in patients on rivaroxaban at Day 42, and an INR and factor X amidolytic assay, in patients on warfarin at baseline and at Day 42, will be performed by established methods. Percentage time in therapeutic range (TTR) between baseline and day 180 will be calculated in patients on warfarin. 9.3 SAMPLE SIZE Primary outcome RAPS is designed as a non-inferiority trial, to demonstrate that the intensity of anticoagulation in patients on rivaroxaban is not inferior to that obtained with warfarin as assessed by the ETP, with UCL CTU Page 53 RAPS Protocol Version 5.0 22 September 2014 the non-inferiority margin based on the inter site assay variability of test performance and on clinical relevance. The primary outcome measure is the percentage change in ETP from randomisation to Day 42. The time point of Day 42 (first trial visit post-randomisation), was chosen as the therapeutic effect of rivaroxaban would be expected to be stable after a minimum of 30 days of rivaroxaban therapy and would not be influenced by any residual warfarin, based on the biological half-lives of the vitamin K-dependent coagulation factors. Rivaroxaban would be regarded as non-inferior to warfarin if the percentage change in ETP is not more than 20% higher (i.e. less anticoagulant effect) than that for warfarin. This non-inferiority limit of 20% is based on the inter site assay variability of test performance74 and on clinical relevance. The null hypothesis (H0) is that rivaroxaban is inferior to warfarin with respect to the mean percentage change in ETP at six weeks: H0: μR - μW ≥ 20% The alternative hypothesis (H1) is that rivaroxaban is not inferior to warfarin with respect to the mean percentage change in ETP at six weeks: H1: μR - μW < 20% Where: μR = mean percentage change in ETP in the rivaroxaban group μW = mean percentage change in ETP in the warfarin group In order to conclude non-inferiority, we need to reject the null hypothesis. Using information on the ETP in patients on warfarin at a comparable INR range the standard deviation of % change in ETP was 36% six weeks after starting warfarin treatment.52 Using a onesided 2.5% significance level and 80% power it is calculated that 51 patients per group are required for the study. Based on our experience with the first 84 patients recruited, 2 patients have been lost to follow-up or withdrawn before the first trial visit post randomisation, and 5 patients have not provided paired ETP values. If we conservatively allow for 12% non evaluable patients, it is anticipated that a total of 116 patients will be required. The sample size has not been adjusted for non-compliance since, unlike in a superiority trial, it does not necessarily reduce the power for a non-inferiority design.75 Secondary outcomes: efficacy and safety The trial is not powered to detect differences between the two randomised groups for efficacy (recurrent thrombosis) or safety endpoints (i.e. SAEs and bleeding events). Therefore, for secondary outcomes the two groups will be compared using estimates and confidence intervals. 9.4 INTERIM MONITORING & ANALYSES The Independent Data Monitoring Committee (IDMC) is the only group who will see the confidential, accumulating data split by trial arm (see Section 14.3). Reports to the IDMC will be produced by the UCL CTU statisticians. The IDMC will meet within six months of the trial opening. The timing and frequency of subsequent meetings and any interim analyses will be stated in the IDMC ToR. 9.5 ANALYSIS PLAN (BRIEF) The analyses will be described in detail in the Statistical Analysis Plan located in the Statistics Master File. This section summarises the main issues. Summary statistics will be calculated for all baseline patient characteristics and study outcomes in each arm. Numbers and percentages will be used for categorical variables, whilst means/standard deviations or medians/inter-quartile ranges will be used for continuous variables, as appropriate. All analyses will be performed on an intention to treat basis. No subgroup analyses are planned. UCL CTU Page 54 RAPS Protocol Version 5.0 22 September 2014 Primary outcome Regression modelling will be used to estimate the difference in ETP between the two treatments (rivaroxaban - warfarin) at day 42 together with a two-sided 95% confidence interval, adjusting for the stratification variables. Baseline ETP will be included as a covariate. ETP will be log transformed for the statistical analysis. Estimates and 95% confidence intervals on the log scale will be back transformed to percentage changes for presentation, as the difference between two logarithms is the log of their ratio. If the upper end of the 95% confidence interval does not cross the noninferiority limit of 20%, then rivaroxaban will be regarded as non-inferior (see B and C in the figure below76). If the mean difference is negative and the 95% CI does not include a difference of zero then we can conclude that rivaroxaban is superior to warfarin (see A in the figure below76). Treatment Difference for Adverse Outcome (New Treatment minus Reference Treatment) Sensitivity analysis will be performed for the primary outcome measure using tobit regression which can include censored values, below the limit of detection of the assay77. In addition, a per protocol analysis will be performed for the primary outcome measure. Efficacy and safety secondary outcomes The differences between the two arms for the efficacy and safety secondary outcomes, efficacy (recurrent thrombosis alone as well as a composite of VTE and other thrombotic events) and safety (the rates of SAEs and all bleeding events), will be presented as estimates and confidence intervals. The trial is not powered to perform hypothesis tests for these outcomes. UCL CTU Page 55 RAPS Protocol Version 5.0 22 September 2014 Quality of Life Quality of life, as measured by EQ-5D-5L, will be measured at baseline and at six months. The scores at six months will be compared between treatment groups, adjusting for baseline values and stratifying variables. Estimates of treatment difference with 95% CI will be presented. Laboratory measures of compliance The anti-factor Xa assays in the patients on rivaroxaban (at Day 42) and the INRs and factor X amidolytic assays in patients on warfarin (at baseline and Day 42), done concurrently with the TGTs, will be correlated with log transformed ETP to assess whether there are any relationships. UCL CTU Page 56 RAPS Protocol Version 5.0 22 September 2014 10 ANCILLARY STUDIES Future translational research Patients are given the opportunity to consent for extra blood (20 mL extra) to be taken at baseline and the 42 day time point for storage at the UCL Haemostasis Research Unit for translational research purposes. This is an optional part of the trial and patients not wanting to enter this part of the trial are not excluded from the main trial. The transport, processing and storage of these samples are covered in Appendix C. UCL CTU Page 57 RAPS Protocol Version 5.0 22 September 2014 11 REGULATORY & ETHICAL ISSUES 11.1 COMPLIANCE 11.1.1 REGULATORY COMPLIANCE The trial will be conducted in accordance with the principles of the Declaration of Helsinki (version 2008). It will also be conducted in compliance with the approved protocol, the principles of GCP as laid down by the Commission Directive 2005/28/EC with the implementation in national legislation in the UK by Statutory Instrument 2004/1031 (The Medicines for Human Use (Clinical Trials) Regulations 2004) and subsequent amendments, the UK Data Protection Act (DPA number: Z5886415), and the National Health Service (NHS) Research Governance Framework for Health and Social Care (RGF). 11.1.2 SITE COMPLIANCE The site will comply with the approved protocol, unless it is in the patients’ best interest to treat them otherwise, and with the above regulations and will comply with the principles of GCP as laid down by the ICH topic E6 (Note for Guidance on GCP), Commission Directive 2005/28/EC, the European Directive 2001/20/EC (where applicable) and applicable National regulations. An agreement will be in place between each site and UCL CTU, setting out respective roles and responsibilities (see Section 13). The site must inform UCL CTU as soon as they become aware of a possible protocol violation or serious breach of compliance, so that the CTU can report the breach if necessary within seven days as per the UK regulatory requirements. For the purposes of this regulation, a 'serious breach' is one that is likely to affect to a significant degree: The safety or physical or mental integrity of the patients in the trial, or The scientific value of the trial 11.1.3 DATA COLLECTION & RETENTION CRFs, clinical notes and administrative documentation should be kept in a secure location (for example, locked filing cabinets in a room with restricted access) and held for five years after the end of the trial. During this period, all data should be accessible to the competent or equivalent authorities and the Sponsor, with suitable notice. The data may be subject to an audit by the competent authorities. 11.2 ETHICAL CONDUCT OF THE STUDY 11.2.1 ETHICAL CONSIDERATIONS Patients will have to attend approximately three extra visits for the trial, but they will be reimbursed for the extra travelling expenses incurred for these visits. To assess the primary end point and to assess compliance, patients will have to give 20 mL of blood at baseline and at day 42 (ideally -12 days +14days). These are not standard tests and samples will be transported to the Haemostasis Research Unit at UCL, where the tests will be performed. Patients will also be given the opportunity to give a further 20 mL of blood for storage for future translational UCL CTU Page 58 RAPS Protocol Version 5.0 22 September 2014 research. This is optional and there is a separate patient information sheet and consent form for this. There are specific instructions for the management of bleeds and for elective surgical procedures for patient on rivaroxaban. The TMG have produced information sheets for both events which are available 24 hours of the day at each site. Patients are asked to carry patient cards, which have the number that the healthcare professional should call to gain this information. If a patient is allocated to rivaroxaban, after the study period of six months they may have to change back to warfarin, if this is considered to be best care at that time. These issues are covered in the Patient Information Sheet. 11.2.2 ETHICAL APPROVALS Before initiation of the trial at each clinical site, the protocol, all informed consent forms, and information materials to be given to the prospective participant will be submitted to the ethics committee for approval. Any further amendments will be submitted and approved by the ethics committee. The rights of the patient to refuse to participate in the trial without giving a reason must be respected. After the patient has entered into the trial, the clinician must remain free to give alternative treatment to that specified in the protocol, at any stage, if he/she feels it to be in the best interest of the patient. The reason for doing so, however, should be recorded; the patient will remain within the trial for the purpose of follow-up and will be included in the statistical analysis according to the treatment to which they were randomly allocated. Similarly, the patient must remain free to change their mind at any time about the protocol treatment and trial follow-up without giving a reason and without prejudicing his/her further treatment. 11.3 COMPETENT AUTHORITY APPROVALS This protocol will be submitted to the national competent authority (MHRA). This is a Clinical Trial of an Investigational Medicinal Product (IMP) as defined by the EU Directive 2001/20/EC. Therefore, a CTA is required in the UK. The EUdraCT number for the trial is 2012-002345-38. The progress of the trial and safety issues will be reported to the competent authority, regulatory agency or equivalent in accordance with local requirements and practices in a timely manner. Safety reports, including expedited reporting and SUSARS will be submitted to the competent authority in accordance with each authority’s requirements in a timely manner. 11.4 OTHER APPROVALS The protocol will be submitted to the relevant R&D department of each participating site by those delegated to do so. A copy of the local R&D approval (or other relevant approval as above) and of the PIS and Consent Form (CF) on local headed paper should be forwarded to the UCL CTU before any patients are entered. UCL CTU Page 59 RAPS Protocol Version 5.0 22 September 2014 12 INDEMNITY UCL holds insurance against claims from participants for injury caused by their participation in the clinical trial. Participants may be able to claim compensation if they can prove that UCL has been negligent. However, as this clinical trial is being carried out in a hospital, the hospital continues to have a duty of care to participants. UCL does not accept liability for any breach in the hospital’s duty of care, or any negligence on the part of hospital employees. This applies whether the hospital is a NHS Trust or otherwise. Participants may also be able to claim compensation for injury caused by participation in this clinical trial without the need to prove negligence on the part of UCL or another party. Participants who sustain injury and wish to make a claim for compensation should do so in writing in the first instance to the Chief Investigator, who will pass the claim to the Sponsor’s Insurers, via the Sponsor’s office. Hospitals selected to participate in this clinical trial shall provide clinical negligence insurance cover for harm caused by their employees and a copy of the relevant insurance policy or summary shall be provided to UCL, upon request. UCL CTU Page 60 RAPS Protocol Version 5.0 22 September 2014 13 FINANCE Funding for staff salaries and trial expenses is provided by Arthritis Research UK Clinical Studies funding stream (Grant reference number: 19708), which have been reduced as the UCL CTU is undertaking RAPS as a CTU development project. The supply of rivaroxaban is being provided by Bayer Healthcare AG free of charge. The company is also providing financial support for a specified amount of pharmacovigilance work. Research Nurse support at the sites is supported by the LCRN. UCL CTU Page 61 RAPS Protocol Version 5.0 22 September 2014 14 OVERSIGHT & TRIAL COMMITTEES There are a number of committees responsible for the oversight of the trial, which are detailed below. 14.1 TRIAL MANAGEMENT GROUP (TMG) A Trial Management Group (TMG) will be formed comprising the Chief Investigator, other lead investigators (clinical and non-clinical) and members of the UCL CTU. The TMG will be responsible for the day-to-day running and management of the trial. It will meet at least three times a year at least one of which will be in-person. The full details can be found in the TMG Terms of Reference (ToR). 14.2 TRIAL STEERING COMMITTEE (TSC) The Trial Steering Committee (TSC) has independent membership, including the Chair plus members from the TMG. The role of the TSC is to provide overall supervision for the trial and provide advice through its independent Chair. The ultimate decision for the continuation of the trial lies with the TSC. Further details of TSC functioning are presented in the TSC ToR. 14.3 INDEPENDENT DATA MONITORING COMMITTEE [IDMC] An Independent Data Monitoring Committee (IDMC) is the only group who will see the confidential, accumulating data for the trial split by trial arm. Reports to the IDMC will be produced by the UCL CTU statisticians. The IDMC will meet within six months of the trial opening. The timing and frequency of subsequent meetings and any interim analyses and will be stated in the IDMC ToR. The IDMC can recommend premature closure or reporting of the trial to the TSC. Further details of IDMC processes are provided in the IDMC ToR. 14.4 ROLE OF STUDY SPONSOR The sponsor is responsible for securing the arrangements to initiate, manage and finance a study. UCL is the trial sponsor and has delegated the duties as sponsor to UCL CTU with a memorandum of understanding (MoU). UCL CTU Page 62 RAPS Protocol Version 5.0 22 September 2014 15 PUBLICATION After completion of all data entry, the final analysis for the RAPS trial will be discussed by the TMG. Results of the analysis will be presented at an investigators’ meeting to provide an opportunity for them to discuss and comment on the results. Following the investigators’ meeting a paper will be written by the TMG. The TMG will determine the scientific meetings to present the work and the journal(s) to submit the publication(s). Drafts of any publications resulting from the analysis will be reviewed by the IDMC and TSC. Bayer plc will be given the proposed abstract for presentation at scientific meetings or proposed manuscript for publication(s) of the results at least 15 days prior to submission or at least 15 days prior to presentation to allow them the opportunity for comments. Bayer will be acknowledged for their support in any publications. Arthritis Research UK will be acknowledged for their financial support of the trial. The RAPS trial will have an ISRCTN; this will be included in any presentations and publications for provenance. UCL CTU Page 63 RAPS Protocol Version 5.0 22 September 2014 16 PROTOCOL AMENDMENTS Additions/amendments from protocol version 4.0 – 02 July 2013 The section, sub-section and where applicable paragraph and sentence are stated to give the location of the amendment. Substantial amendments: Summary of trial, Secondary outcome measures Markers of in vivo coagulation activation (point iv) have been added to the outcome measures. Summary of trial, Number of participants to be studied The number of participants to be studied has been reduced from 156 to 116. Summary of trial, Duration The planned recruitment period has been increased from 10 to 18 months, and the overall planned duration of the study has been increased from 24 to 44 months. Trial assessment schedule The requirement to measure the patient’s abdominal circumference at screening and Visit 3 has been removed. Glossary, Clinical outcome events The reference to the Independent Adjudication Committee has been removed. Classification of thrombotic events will be done by the PI at the trial site. Section 1.5 Rationale and objectives of the study, Objectives, Secondary aims Additional markers of coagulation activation (point iv) have been added to the secondary outcome measures. Section 3.2 Patient exclusion criteria An additional exclusion criterion has been added to exclude patients on dronedarone. Section 3.3 Number of patients The number of participants to be studied has been reduced from 156 to 116. Section 3.5 Screening procedures and pre-randomisation investigations, Screening The requirement to measure the patient’s abdominal circumference at screening has been removed. Section 5.3.6 Accountability and unused drugs/devices Additional wording has been added to state that unused rivaroxaban will either be destroyed or taken into general hospital stock. Section 5.10.2 Medications not permitted Additional wording has been added regarding the co-administration of dronedarone for patients on rivaroxaban. Section 5.10.3 Medications to be used with caution Additional wording has been added regarding prescribing rivaroxaban in patients with renal impairment who are concomitantly receiving other medicinal products that increase rivaroxaban UCL CTU Page 64 RAPS Protocol Version 5.0 22 September 2014 concentrations and that rivaroxaban should be used with caution in patients who require dronedarone. Section 6.1 Trial Assessment Schedule, Visit 3 The requirement to measure the patient’s abdominal circumference at Visit 3 has been removed. Section 6.2 Procedure for assessing efficacy A paragraph has been inserted describing the secondary laboratory in vivo markers of coagulation activation measures of efficacy. Section 6.8 Loss to follow-up Additional wording has been added regarding the rate of non evaluable patients in the first 84 patients. Section 9.2 Outcome measures, Secondary outcome measures, a) Efficacy outcomes Additional markers of coagulation activation have been added to the outcome measures. Section 9.3 Sample size, Primary outcome The sample size has been recalculated, the power has been reduced from 90% to 80% and the total sample size been reduced from 156 to 116. UCL CTU Page 65 RAPS Protocol Version 5.0 22 September 2014 17 REFERENCES 1. Hughes GRV. The antiphospholipid syndrome: ten years on. Lancet 1993; 342: 341-44. 2. Khamashta MA (Ed). Hughes syndrome: antiphospholipid syndrome. 2nd edition. Springer-Verlag London, 2006. 3. Austin S and Cohen H. Antiphospholipid syndrome. Medicine, 2009; 34: 472-5. 4. Bushnell CD, Goldstein LB. Diagnostic testing for coagulopathies in patients with ischaemic stroke. Stroke 2000; 31; 3067-78. 5. Roldan V, Lecumberri R, Munoz-Torrero JFS Vicente V, Rocha E, Brenner B, Monreal M; RIETE Investigators. Thrombophilia testing in patients with venous thromboembolism. Findings from the REITE registry. Thromb Res 2009; 124: 174-77. 6. Mok CC, Tang SS, To CH, Petri M. Incidence and risk factors of thromboembolism in systemic lupus erythematosus: a comparison of three ethnic groups. Arthritis Rheum 2005; 52: 2774-82. 7. Ruiz-Irastorza G, Egurbide MV, Ugalde J, Aguirre C. High impact of antiphospholipid syndrome on irreversible organ damage and survival of patients with systemic lupus erythematosus. Arch Intern Med 2004 ; 164: 77-82. 8. Tektonidou MG, Laskari K, Panagiotakos DB, Moutsopoulos HM. Risk factors for thrombosis and primary thrombosis prevention in patients with systemic lupus erythematosus with or without antiphospholipid antibodies. Arthritis Rheum 2009; 61: 29-36. 9. Crowther MA, Ginsberg JS, Julian J, Denburg J, Hirsh J, Douketis J, Laskin C, Fortin P, Anderson D, Kearon C, Clarke A, Geerts W, Forgie M, Green D, Costantini L, Yacura W, Wilson S, Gent M, Kovacs MJ. A comparison of two intensities of warfarin for the prevention of recurrent thrombosis in patients with the antiphospholipid syndrome. N Engl J Med 2003; 12: 1133-38. 10. Finazzi G, Marchioli R, Brancaccio V, Schinco P, Wisloff F, Musial J, Baudo F, Berrettini M, Testa S, D'Angelo A, Tognoni G, Barbui T. A randmised clinical trial of high-intensity warfarin vs. conventional antithrombotic therapy for the prevention of recurrent thrombosis in patients with the antiphospholipid syndrome (WAPS). J Thromb Haemost 2005; 3: 848-853; 11. Keeling D, et al. British Committee for Standards in Haematology (BCSH) - Guidelines for General Haematology, Haemostasis and Thrombosis. Guidelines on oral anticoagulation with warfarin – fourth edition. Br J Haematol 2011; 154: 311–324. 12. Ruiz-Irastorza G, Crowther M, Branch W, Khamashta M. Antiphospholipid syndrome. Lancet 2010; 376: 1498-509. 13. Gardiner C, Longair, I, Pescott M,A, Erwin H, Hills J, Machin SJ, Cohen H. Self-monitoring of oral anticoagulation: does it work outside trial conditions? J Clin Pathol 2009; 62: 168071. 14. Tripodi A, Chantarangkul V, Clerici M, Negri B, Galli M, Mannucci PM. Laboratory control of anticoagulant treatment by the INR system in patients with the antiphospholipid syndrome and lupus anticoagulant. Br J Haematol 2001; 115 (3): 672-678. UCL CTU Page 66 RAPS Protocol Version 5.0 22 September 2014 15. Ruiz-Irastorza G, Hunt BJ, Khamashta M. A systematic review of secondary thromboprophylaxis in patients with antiphospholipid antibodies. Arthritis and Rheumatism 2007; 57: 1487-95. 16. Pengo V, Tripodi A, Reber G, Rand JH, Ortel TL, Galli M, DE Groot PG. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 2009; 7: 1737-40. 17. Pradaxa 110mg hard capsules: summary of product characteristics (SPC), EU. Boehringer Ingelheim International GmBH, 18 March 2008. Available from: www.emc.medicines.org.uk 18. Xarelto 10mg film-coated tablets. Summary of product characteristics (SPC), EU. Bayer HealthCare AG. Date of first authorisation/renewal of authorisation: 30/09/08. Date of revision 12/2011. Available from: www.emc.medicines.org.uk 19. Erikkson BI, Dahl OE, Rosencher N et al; RE-MODEL Study Group. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the Re-MODEL randomized trial. J Thromb Haemost 2007; 5: 2178-85. 20. Eriksson BI, Dahl OE, Rosencher N et al; RE-NOVATE Study group. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet 2007; 370: 949-56. 21. Eriksson BI, Borris LC, Dahl OE et al; RECORD 2 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008; 358: 2765-75. 22. Kakkar AK, Brenner B, Dahl OE et al; RECORD 2 Investigators. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind randomised controlled trial. Lancet 2008; 372: 31-9. 23. Lassen MR, Ageno W, Borris LC et al; RECORD 3 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008; 358: 2776-86. 24.Turpie AG, Lassen MR, Davidson BL et al; RECORD 4 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee replacement (RECORD 4): a randomised trial. Lancet 2009; 373: 1673-80. 25. Cohen H and Gates C. Anticoagulation with dabigatran or rivaroxaban. Drugs and Therapeutics Bulletin, 2009; 47: 116-20. (also see H Cohen and C Gates, Rapid response, BMJ online 19 November 2009: New oral agents vs subcutaneous alternatives. Has the DTB drawn the correct conclusion?) 26. NICE technology appraisal guidance 157. Dabigatran etexilate for the prevention of venous thromboembolism after hip or knee replacement surgery in adults. September 2008. www.nice.org.uk/TA157 27. NICE technology appraisal guidance 170. Rivaroxaban for the prevention of venous thromboembolism after total hip or total knee replacement in adults. April 2009. www.nice.org.uk/TA170 28. Schulman S, Kearon C, Kakkar et al for the Re-COVER Study Group. Dabigatran versus warfarin for UCL CTU Page 67 RAPS Protocol Version 5.0 22 September 2014 the treatment of acute venous thromboembolism. N Engl Med 2009; 361: 2341-52. 29. EINSTEIN Investigators: Bauersachs R, Berkowitz SD, Brenner B et al. Oral rivaroxaban for symptomatic venous thromboembolism (and supplementary appendix). N Engl J Med. 2010; 363: 2499-510. 30. Connolly SJ, Ezekowitz MD, Yusuf S et al; Re-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361: 1139-51. 31. Patel MR, Mahaffey KW, Gar J, Pan G, Singe DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccin JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KAA, Califf RM, and the ROCKET AF Steering Committee for the ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation (and supplementary appendix). N Engl J Med 2011; 365: 883-91. 32. Khamashta MA, Cuadrado MJ, Mujic F et al. The management of thrombosis in the antiphospholipid-antibody syndrome. N Engl J Med 1995; 332: 993-7. 33. The EINSTEIN–PE Investigators. Oral Rivaroxaban for the Treatment of Symptomatic Pulmonary Embolism. N Engl J Med 2012; 366: 1287-1297. 34. Schulman S, Kakkar A, Schellong S, Goldhaber S, Henry E, Mismetti P, Vedel Christiansen A, Schnee J, Kearon C. A Randomized Trial of Dabigatran Versus Warfarin in the Treatment of Acute Venous Thromboembolism (RE-COVER II). Blood (ASH Annual Meeting Abstracts) 2011; 118: Abstract 205. 35. Wallentin L, Yusuf S, Ezekowitz MD, Alings M, Flather M, Franzosi MG, Pais P, Dans A, Eikelboom J, Oldgren J, Pogue J, Reilly PA, Yang S, Connolly SJ; RE-LY investigators. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet 2010; 376: 975-83. 36. Cariou R, Tobelem G, Soria C, Caen J. Inhibition of Protein C Activation by Endothelial Cells in the Presence of Lupus Anticoagulant. N Engl J Med 1986; 314: 1193-94. 37. Rossi E, Gatti L, Guarneri D, Finotto E, Lombardi A, Preda L. Functional protein S in women with lupus anticoagulant inhibitor. Thrombosis Research 1992; 65: 253-262. 38. Liestol S, Sandset PM, Mowinckel M-C, Wisloff F. Activated protein C resistance determined with a thrombin generation-based test is associated with thrombotic events in patients with lupus anticoagulants. J Thromb Haemost 2007; 5: 2204-10. 39. Cohen H and Machin SJ. Antithrombotic treatment failures in antiphospholipid syndrome: the new anticoagulants? Lupus 2010; 19: 486 40. Besser M, Baglin C, Luddington R, van Hylckama Vlieg A, Baglin T. High rate of unprovoked recurrent venous thrombosis is associated with high thrombin generation potential in a prospective cohort study. J Thromb Haemost 2008; 6: 1720-48. 41. Eichinger S, Gregor H, Kollars M, Kyrle PA. Prediction of recurrent venous thromboembolism by endogenous thrombin potential and D-dimer. Clinical Chemistry 2008; 54: 2042-48. 42. Hemker HC, Beguin NS. Phenotyping the clotting system. J Thromb Haemost 2000; 84: 747-51. UCL CTU Page 68 RAPS Protocol Version 5.0 22 September 2014 43. Hemker HC, Al Dieri R, De Smedt E, Béguin S. Thrombin generation, a function test of the haemostatic-thrombotic system. Thromb Haemost. 2006; 96(5): 553-61. 44. Bauer KA, Rosenberg RD. The pathophysiology of the prethrombotic state in humans: insights gained from studies using markers of hemostatic system activation. Blood 1987;70:343-50. 45. Boisclair MD, Ireland H, Lane DA. Assessment of hypercoagulable states by measurement of activation fragments and peptides. Blood Rev 1990;4:25-40. 46. Wells PS, Anderson DR, Rodger M, Forgie M, Kearon C, Dreyer J, Kovacs G, Mitchell M, Lewandowski B, Kovacs MJ. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003; 25; 349: 1227-35. 47. Eichinger S, Hron G, Kollars M, Kyrle PA. Prediction of recurrent venous thromboembolism by endogenous thrombin potential and D-dimer. Clin Chem 2008;54:2042-8. 48. Palareti G, Cosmi B, Legnani C, Tosetto A, Brusi C, Iorio A, et al. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med 2006;355:1780-9. 49. Poli D, Antonucci E, Ciuti G, Abbate R, Prisco D. Combination of D-dimer, F1+2 and residual vein obstruction as predictors of VTE recurrence in patients with first VTE episode after OAT withdrawal. J Thromb Haemost 2008;6:708-10. 50. Tripodi A, Cattaneo M, Molteni A, Cesana BM, Mannucci PM. Changes of prothrombin fragment 1+2 (F 1+2) as a function of increasing intensity of oral anticoagulation--considerations on the suitability of F 1+2 to monitor oral anticoagulant treatment. J Thromb Haemost 1998; 79: 571-3. 51. Millenson MM, Bauer KA, Kistler JP, Barzegar S, Tulin L, Rosenberg RD. Monitoring "miniintensity" anticoagulation with warfarin: comparison of the prothrombin time using a sensitive thromboplastin with prothrombin fragment F1+2 levels. Blood 1992;79:2034-8. 52. Brodin E, Seljeflot, Arnesen H, Hurlen M, Applebom H, Hansen JB. Endogenous thrombin potential (ETP) in plasma from patients with AMI during antithrombotic treatment. Thrombosis Research 2009; 123: 573-79. 53. Gerotziafas GT, Dupont C, Spyropoulos AC, Hatmi M, Samama MM. Differential inhibition of thrombin generation by vitamin K antagonists alone and associated with low-molecular-weight heparin. Blood Coagulation, Fibrinolysis and Cellular Haemostasis 2009; 102: 42-8. 54. Gerotziafas GT, Elalamy I, Depasse F, Perzborn E, Samama MM. In vitro inhibition of thrombin generation, after tissue factor activation, by the oral, direct factor Xa inhibitor rivaroxaban. J Thromb Haemost 2007; 5: 886-8. 55. Gerotziafas GT, Petropoulou AD, Verdy E, Samama MM, Elalamy I. Effect of the anti-factor Xa and anti-factor IIa activities of low-molecular weight heparins upon the phases of thrombin generation. J Thromb Haemost 2007; 5: 955-62. 56. Green L, Lawrie AS, Patel S, Hossain F, Chitolie A, Mackie IJ, Haddad FS, Machin SJ. The impact of elective knee/hip replacement surgery and thromboprophylaxis with rivaroxaban or dalteparin on thrombin generation. Br J Haematol 2010; 151: 469-76. UCL CTU Page 69 RAPS Protocol Version 5.0 22 September 2014 57. Eerenberg ES, Kamphuisen PW, Sijpken MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebocontrolled, crossover study in healthy subjects. Circulation 2011; 124: 1573-9. 58. Samama MM, Martinoli J-L, Guinet C, Plu-Bureau G, Depasse F, Perzborn E. Assessment of laboratory assays to measure rivaroxaban – an oral, direct factor Xa inhibitor. J Thromb Haemost 2010; 103: 815–825 59. Rosborough, T.K., et al. (2010) Factor X and factor II activity levels do not always agree in warfarin-treated lupus anticoagulant patients. Blood Coagul Fibrinolysis 2010; 21: 242-244. 60. Rosendaal FR, Cannegieter SC, et all. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993; 69: 236-239. 61. McNally T, Purdy G, Mackie IJ, Machin SJ, Isenberg DA. The use of an anti-beta 2-glycoprotein-1 assay for discrimination between anticardiolipin antibodies associated with infection and increased risk of thrombosis. Br J Haematol 1995; 91(2): 471-3. 62. Loizou S, McCrea JD, Rudge AC, Reynolds R, Boyle CC, Harris EN. Measurement of anti-cardiolipin antibodies by an enzyme-linked immunosorbent assay (ELISA): standardization and quantitation of results. Clin Exp Immunol 1985; 62(3): 738-45. 63. Harris EN, Gharavi AE, Patel SP, Hughes GR. Evaluation of the anti-cardiolipin antibody test: report of an international workshop held 4 April 1986. Clin Exp Immunol 1987; 68(1): 215-22. 64. McNally T, Mackie IJ, Machin SJ, Isenberg DA. Increased levels of beta 2 glycoprotein-I antigen and beta 2 glycoprotein-I binding antibodies are associated with a history of thromboembolic complications in patients with SLE and primary antiphospholipid syndrome. Br J Rheumatol 1995; 34(11): 1031-6. 65. Keeling D, Mackie I, Moore GW, Greer IA, Greaves M; British Committee for Standards in Haematology. Guidelines on the investigation and management of antiphospholipid syndrome. Br J Haematol. 2012 Apr;157(1):47-58. 66. Cohen H and Baglin T. Plasma, plasma products and indications for their use. In: ABC of Transfusion Medicine British Medical Journal Books (Wiley-Blackwell) 2009 4th edition: p 40-7. 67. Green L. MD Thesis: the impact of total hip and knee replacement surgery and chemical prophylaxis on thrombin generation. University of London 2011. 68. Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4(2): 295-306.51. 69. National Patient Safety Agency (NPSA). http://npsa.nhs.uk/ 70. Gatt A, van Veen JJ, Bowyer A et al. Wide variation in thrombin generation in patients with atrial fibrillation and therapeutic International Normalised Ratio is not due to inflammation. Br J Haematol 2008; 142: 946–52. 71. Lawrie AS, Hills J, Longair I, Green L, Gardiner C, Machin SJ, Cohen H. The clinical significance of UCL CTU Page 70 RAPS Protocol Version 5.0 22 September 2014 differences between point-of-care and laboratory INR methods in over-anticoagulated patients. Thromb Res. 2012;130:110-4. 72. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihaemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3: 69-74. 73. EQ 5D 5L Quality of Life questionnaire. www.euroqol.org 74. Dargaud Y, Luddington R et al. Standardisation of thrombin generation test – which reference plasma for TGT?: An international multicentre study. Thrombosis Research 2010; 125: 353-356. 75. Julious S. Tutorial in biostatistics. Sample sizes for clinical trials with Normal data. Statist.Med. 2004; 23: 1921-1986. 76. Piaggio G, Elbourne D et al. Reporting of noninferiority and equivalence randomized trials. An extension of the consort statement. JAMA 2006; 295: 1152-1160. 77. StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP. Acknowledgements: UCL CTU acknowledges the kind support and the provision of the MRC Clinical Trials Unit Protocol Template that has been used as a basis for that of the UCL CTU. UCL CTU Page 71 RAPS Protocol Version 5.0 22 September 2014 18 APPENDICES APPENDIX A - FORMULA Cockcroft and Gault FORMULA (C&G) in μmol/L Estimated CrCl (mL/min) = (140 – age) x weight*(kg) [x 1.23 if male] or [x 1.04 if female] serum creatinine (µmol/L) CrCl is creatinine clearance (mL/min) corrected for body weight *Calculate weight as follows (steps 1 and 2) 1. Estimate ideal body weight (IBW) kg Males: IBW = 50 kg + 2.3 kg for each inch over 5 feet. Females: IBW = 45.5 kg + 2.3 kg for each inch over 5 feet. 2. Compare IBW to patient’s total body weight (TBW) kg (as measured using weighing scales). a. If TBW is 120% or less of the IBW then use TBW in the C&G formula b. If TBW is more than 120% of the IBW, then calculate the adjusted body weight (ABW) kg, and use ABW in the C&G formula Adjusted body weight (ABW) = IBW + 0.4 x (TBW-IBW) REFERENCES 1. 2. Cockcroft DW and Gault H. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16: 31-41. Basic Clinical Pharmacokinetics 4th edition; 2004. Michael Winter. Editor: DB Troy. Lippincott Williams& Wilkins, Philadelphia UCL CTU Page 72 RAPS Protocol Version 5.0 22 September 2014 APPENDIX B – EXPECTED TOXICITIES WARFARIN Expected warfarin toxicities taken from the warfarin SPC Mercury Pharma Group Limited (date of last revision 18 Sep 2012), obtained from the Medicines Compendium (www.medicines.org.uk). MedDRA system organ class Adverse Reaction Infections and infestations Fever Immune system disorders Hypersensitivity Nervous system disorders Cerebral haemorrhage; Cerebral subdural haematoma Vascular disorders Haemorrhage Respiratory, thoracic and Haemothorax, epistaxis mediastinal disorders Gastrointestinal disorders Gastrointestinal haemorrhage, rectal haemorrhage, haematemesis; pancreatitis; diarrhoea; nausea; vomiting; melaena Hepatobiliary disorders Jaundice; hepatic dysfunction Skin and subcutaneous disorders Rash; alopecia; purpura; 'purple toes' syndrome; erythematous swollen skin patches leading to ecchymosis, infarction and skin necrosis Renal and Urinary disorders Haematuria Investigations Unexplained drop in haematocrit; haemoglobin decreased UCL CTU Page 73 RAPS Protocol Version 5.0 22 September 2014 RIVAROXABAN Expected rivaroxaban toxicities taken from the 15mg and 20mg rivaroxaban (Xarelto) SPC Bayer plc (Date of last revision November 2013), obtained from the Medicines Compendium (www.medicines.org.uk), for the following therapeutic indications: Prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation with one or more risk factors, such as congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, prior stroke or transient ischaemic attack. Treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrent DVT and PE in adults. Common Uncommon Blood and lymphatic system disorders Anaemia (incl. respective laboratory parameter) Rare Not known* Thrombocythaemia (incl. platelet count increased)A Immune system disorders Allergic reactions, dermatitis allergic Nervous system disorders Dizziness, headache Cerebral and intracranial haemorrhage, syncope Eye Disorders Eye haemorrhage (incl. conjunctival haemorrhage) Cardiac disorders Tachycardia Vascular disorders Hypotension, haematoma Respiratory, thoracic and mediastinal disorders Epistaxis, haemoptysis Gastrointestinal disorders Gingival bleeding, gastrointestinal tract haemorrhage (incl. rectal haemorrhage), gastrointestinal and abdominal pains, dyspepsia, nausea, constipationA, diarrhoea, vomitingA Dry mouth Hepatobiliary disorders Hepatic function abnormal Jaundice Skin and subcutaneous tissue disorders Pruritus (incl. uncommon cases of generalised pruritus), rash, ecchymosis, cutaneous and subcutaneous haemorrhage Urticaria Musculoskeletal and connective tissue disorders Pain in extremityA Haemarthrosis Muscle haemorrhage Compartment syndrome secondary to a bleeding Renal and urinary disorders UCL CTU Page 74 RAPS Protocol Version 5.0 22 September 2014 Urogenital tract haemorrhage (incl. haematuria and menorrhagiaB) renal impairment (incl. blood creatinine increased, blood urea increased)A Renal failure/acute renal failure secondary to a bleeding sufficient to cause hypoperfusion General disorders and administration site conditions FeverA, peripheral oedema, decreased general strength and energy (incl. fatigue and asthenia) Feeling unwell (incl. malaise) Localized oedemaA Increased bilirubin, increased blood alkaline phosphataseA, increased LDHA, increased lipaseA, increased amylaseA, increased GGTA Bilirubin conjugated increased (with or without concomitant increase of ALT) Investigations Increase in transaminases Injury, poisoning and procedural complications Postprocedural haemorrhage (incl. postoperative anaemia, and wound haemorrhage), contusion, wound secretionA Vascular pseudoaneurysmC Frequencies are defined as: very common (≥ 1/10) common (≥ 1/100 to < 1/10) uncommon (≥ 1/1,000 to < 1/100) rare (≥ 1/10,000 to < 1/1,000) very rare (< 1/10,000) not known (cannot be estimated from the available data) A: observed in prevention of venous thromboembolism (VTE) in adult patients undergoing elective hip or knee replacement surgery B: observed in treatment of DVT, PE and prevention of recurrence as very common in women < 55 years C: observed as uncommon in prevention of cardiovascular death and MI in patients after an ACS (following percutaneous coronary intervention) UCL CTU Page 75 RAPS Protocol Version 5.0 22 September 2014 APPENDIX C – BLOOD SAMPLES 1. Introduction Blood samples for trial assessments A 20 mL blood sample for the TGT, anti-Xa, INR, amidolytic factor X, and aPL should be collected in 0.105M sodium citrate tubes. These should then be processed preferably within one hour to separate the plasma for storage, ready for testing. Blood samples for translational research (optional) A further 20 mL blood sample is collected from patients giving consent to the storage of their blood for translational research. These samples will undergo the same processing and storage as the trial samples. The samples will be stored for the duration of the RAPS trial plus a further maximum of five years, after which they will be destroyed. 2. Equipment Trial samples: Citrate Tubes - Tubes containing 0.105M tri-sodium citrate, to give a ratio of 9:1, blood: anticoagulant (preferably: Vacutainers, Becton Dickinson). The same type of tube must be used by the site throughout the study. 3. Blood collection The blood collection tubes should be labelled with the labels provided by CTU and the following information entered on the label. The sample collection form should be completed to document that the sample was taken, transported and stored. Note: For patients on the rivaroxaban arm, blood samples should preferably be collected 2-4 hour after taking the rivaroxaban tablet (when the maximum concentrations (Cmax) appear in the blood). Label data fields Trial number: Arm: Warfarin/Rivaroxaban Site: Patient initials: Collection date: Sample type: trial assessment/translational research 4. Sample transportation Transportation of samples from UCLH Blood samples from UCLH, will be transported to the Haemostasis Research Unit by hand, to the following address. These samples need to be processed preferably within one hour of collection as specified in section 5. UCL Haemostasis Research Unit Department of Haematology University College London 1st Floor, 51 Chenies Mews London WC1E 6HX UCL CTU Page 76 RAPS Protocol Version 5.0 22 September 2014 Transportation of samples from other sites Blood samples from other sites will be processed at the site preferably within one hour of collection as specified in section 5 and stored as specified in section 6. The Haemostasis Research Unit will arrange for the bulk collection of these samples and their transportation on dry ice using a UCL approved courier. 5. Centrifugation and separation of plasma – trial samples Centrifuge the citrate tube at 2000g (approximately 3800 rpm in a typical laboratory bench-top centrifuge), at room temperature (18-20°C), for 15 minutes, preferably within one hour of collection. Carefully remove the supernatant plasma, avoiding disturbance of the buffy coat, with a polypropylene transfer pipette, and deliver into polypropylene tubes, which are then capped. Repeat the centrifugation step above, to remove any residual platelets. Ensure that the tubes are labelled throughout the separation process. 6. Storage of plasma – trial samples The aliquots of plasma must be frozen rapidly and stored at a minimum temperature of –40oC but preferably –70°C, where they should be maintained until required. When placed in the freezer, the samples must be allowed to freeze rapidly, preferably in a metal rack, they should not be placed in polystyrene racks as this can delay freezing. For any questions regarding the above, please direct your questions to the RAPS Trial Manager. UCL CTU Page 77