26 October 2015
Practice Group(s):
Biosimilars/Follow-on
Biologics
BPCIA Statute: Has the Music Stopped or Will the
Patent Dance Continue?
By Maria E. Doukas, Jennifer M. Dienes, Margaux L. Nair, and Christopher J. Betti, Ph.D.
IP Litigation
Food, Drugs, Medical
Devices and
Cosmetics (FDA)
In July, a divided Federal Circuit issued a ruling in the Amgen Inc. et al. v. Sandoz Inc., Case
No. 2015-1499 appeal and held: (1) the Biologics Price Competition and Innovation Act’s
(“BPCIA’s”) “patent dance” provisions are optional, and (2) a biosimilar applicant may only
give effective notice of commercial marketing to meet the 180-day notice provision after the
FDA licenses its product. Based on the FDA licensure requirement prior to a biosimilar
applicant giving its 180-day notice, the Federal Circuit held Sandoz was precluded from
marketing its product, Zarxio, prior to September 2, 2015. See BPCIA Statute: Patent Dance
is Optional, But Opting Out has Consequences (describing the Federal Circuit’s ruling).
Following this ruling, on August 20, 2015, both Amgen Inc. and Amgen Manufacturing
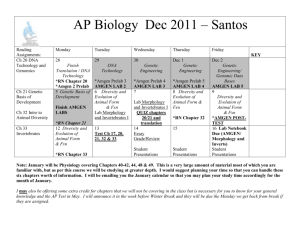
Limited (“Amgen”) and Sandoz Inc. (“Sandoz”) filed a petition for rehearing en banc. Each
party mainly tracked the arguments it had made in its appeal brief to the Federal Circuit.
Amgen argued the BPCIA’s “patent dance” provisions are mandatory, and Sandoz argued
FDA licensure is not required for the 180-day notice requirement.
On August 26, 2015, Amgen followed its petition for rehearing en banc with an emergency
motion for an injunction pending en banc consideration and review. In this emergency
motion, Amgen requested the Federal Circuit “preserve the status quo” and continue
preventing Sandoz from launching Zarxio even after the September 2, 2015 date until the
rehearing en banc issue is resolved. Amgen Inc., et al. v. Sandoz Inc., Case No. 2015-1499,
at 3 (Aug. 26, 2015). Sandoz followed up with its opposition to this emergency motion for an
injunction on August 31, 2015. A few days later, the Federal Circuit ruled in favor of Sandoz
and issued an Order denying Amgen’s emergency motion for an injunction.
Finally, on October 16, 2015, the Federal Circuit ruled and issued a short Order merely
stating the petitions for panel rehearing, and the petitions for rehearing en banc were denied.
At this point, it is unclear whether either Amgen or Sandoz will file a petition for certiorari to
the Supreme Court. However, for now, the July Federal Circuit holding regarding the
optional “patent dance” provisions and the 180-day exclusivity period remain. As more
BPCIA biosimilars “patent dances” are already beginning; it will be interesting to see how
district courts will rule in light of the Federal Circuit holding in Amgen v. Sandoz.
K&L Gates will continue to monitor this litigation and future litigations to see what
developments may arise.
BPCIA Statute: Has the Music Stopped or Will the Patent Dance
Continue?
Authors:
Maria E. Doukas
maria.doukas@klgates.com
+1.312.807.4223
Jennifer M. Dienes
jennifer.dienes@klgates.com
+1.312.807.4219
Margaux L. Nair
margaux.nair@klgates.com
+1.312.807.4280
Christopher J. Betti, Ph.D.
christopher Betti@klgates.com
+1.312.807.4313
Anchorage Austin Beijing Berlin Boston Brisbane Brussels Charleston Charlotte Chicago Dallas Doha Dubai Fort Worth Frankfurt
Harrisburg Hong Kong Houston London Los Angeles Melbourne Miami Milan Moscow Newark New York Orange County Palo Alto Paris
Perth Pittsburgh Portland Raleigh Research Triangle Park San Francisco São Paulo Seattle Seoul Shanghai Singapore Spokane
Sydney Taipei Tokyo Warsaw Washington, D.C. Wilmington
K&L Gates comprises more than 2,000 lawyers globally who practice in fully integrated offices located on five
continents. The firm represents leading multinational corporations, growth and middle-market companies, capital
markets participants and entrepreneurs in every major industry group as well as public sector entities, educational
institutions, philanthropic organizations and individuals. For more information about K&L Gates or its locations,
practices and registrations, visit www.klgates.com.
This publication is for informational purposes and does not contain or convey legal advice. The information herein should not be used or relied upon in
regard to any particular facts or circumstances without first consulting a lawyer.
© 2015 K&L Gates LLP. All Rights Reserved.
2