CRIXIVAN®
(INDINAVIR SULFATE)
CAPSULES
DESCRIPTION
CRIXIVAN® (indinavir sulfate) is an inhibitor of the human immunodeficiency virus (HIV) protease.
CRIXIVAN Capsules are formulated as a sulfate salt and are available for oral administration in strengths
of 200 and 400 mg of indinavir (corresponding to 250 and 500 mg indinavir sulfate, respectively). Each
capsule also contains the inactive ingredients anhydrous lactose and magnesium stearate. The capsule
shell has the following inactive ingredients and dyes: gelatin and titanium dioxide.
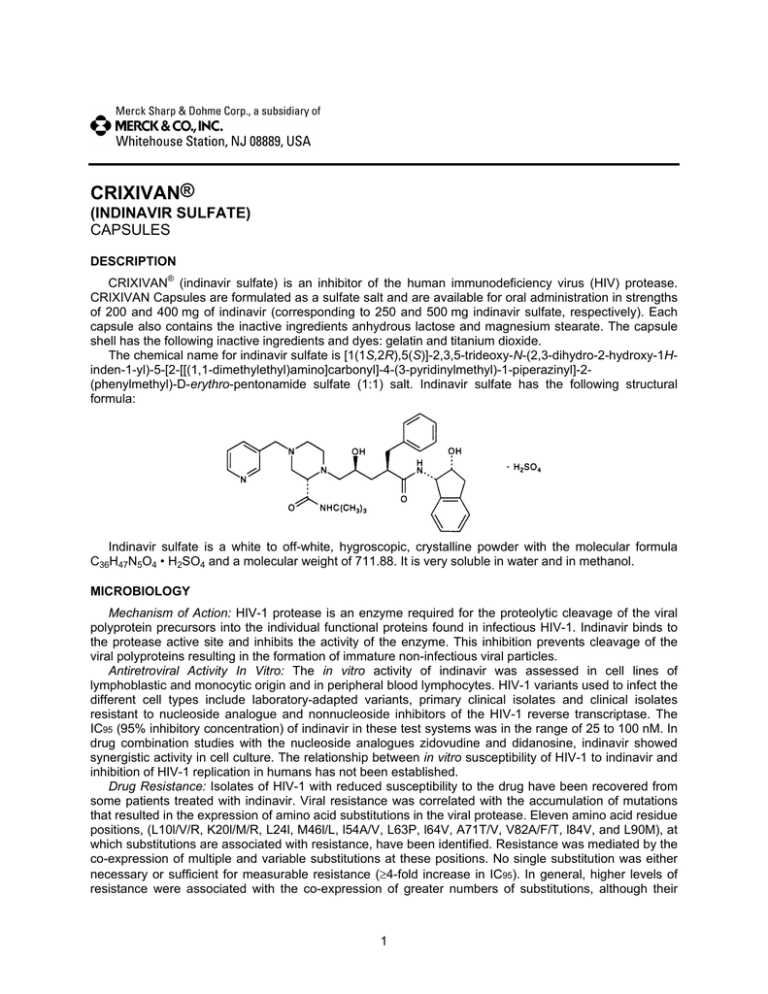
The chemical name for indinavir sulfate is [1(1S,2R),5(S)]-2,3,5-trideoxy-N-(2,3-dihydro-2-hydroxy-1Hinden-1-yl)-5-[2-[[(1,1-dimethylethyl)amino]carbonyl]-4-(3-pyridinylmethyl)-1-piperazinyl]-2(phenylmethyl)-D-erythro-pentonamide sulfate (1:1) salt. Indinavir sulfate has the following structural
formula:
Indinavir sulfate is a white to off-white, hygroscopic, crystalline powder with the molecular formula
C36H47N5O4 • H2SO4 and a molecular weight of 711.88. It is very soluble in water and in methanol.
MICROBIOLOGY
Mechanism of Action: HIV-1 protease is an enzyme required for the proteolytic cleavage of the viral
polyprotein precursors into the individual functional proteins found in infectious HIV-1. Indinavir binds to
the protease active site and inhibits the activity of the enzyme. This inhibition prevents cleavage of the
viral polyproteins resulting in the formation of immature non-infectious viral particles.
Antiretroviral Activity In Vitro: The in vitro activity of indinavir was assessed in cell lines of
lymphoblastic and monocytic origin and in peripheral blood lymphocytes. HIV-1 variants used to infect the
different cell types include laboratory-adapted variants, primary clinical isolates and clinical isolates
resistant to nucleoside analogue and nonnucleoside inhibitors of the HIV-1 reverse transcriptase. The
IC95 (95% inhibitory concentration) of indinavir in these test systems was in the range of 25 to 100 nM. In
drug combination studies with the nucleoside analogues zidovudine and didanosine, indinavir showed
synergistic activity in cell culture. The relationship between in vitro susceptibility of HIV-1 to indinavir and
inhibition of HIV-1 replication in humans has not been established.
Drug Resistance: Isolates of HIV-1 with reduced susceptibility to the drug have been recovered from
some patients treated with indinavir. Viral resistance was correlated with the accumulation of mutations
that resulted in the expression of amino acid substitutions in the viral protease. Eleven amino acid residue
positions, (L10l/V/R, K20l/M/R, L24l, M46l/L, l54A/V, L63P, l64V, A71T/V, V82A/F/T, l84V, and L90M), at
which substitutions are associated with resistance, have been identified. Resistance was mediated by the
co-expression of multiple and variable substitutions at these positions. No single substitution was either
necessary or sufficient for measurable resistance (4-fold increase in IC95). In general, higher levels of
resistance were associated with the co-expression of greater numbers of substitutions, although their
1
individual effects varied and were not additive. At least 3 amino acid substitutions must be present for
phenotypic resistance to indinavir to reach measurable levels. In addition, mutations in the p7/ p1 and p1/
p6 gag cleavage sites were observed in some indinavir resistant HIV-1 isolates.
In vitro phenotypic susceptibilities to indinavir were determined for 38 viral isolates from 13 patients
who experienced virologic rebounds during indinavir monotherapy. Pre-treatment isolates from five
patients exhibited indinavir IC95 values of 50-100 nM. At or following viral RNA rebound (after 12-76
weeks of therapy), IC95 values ranged from 25 to >3000 nM, and the viruses carried 2 to 10 mutations in
the protease gene relative to baseline.
Cross-Resistance to Other Antiviral Agents: Varying degrees of HIV-1 cross-resistance have been
observed between indinavir and other HIV-1 protease inhibitors. In studies with ritonavir, saquinavir, and
amprenavir, the extent and spectrum of cross-resistance varied with the specific mutational patterns
observed. In general, the degree of cross-resistance increased with the accumulation of resistanceassociated amino acid substitutions. Within a panel of 29 viral isolates from indinavir-treated patients that
exhibited measurable (4-fold) phenotypic resistance to indinavir, all were resistant to ritonavir. Of the
indinavir resistant HIV-1 isolates, 63% showed resistance to saquinavir and 81% to amprenavir.
CLINICAL PHARMACOLOGY
Pharmacokinetics
Absorption: Indinavir was rapidly absorbed in the fasted state with a time to peak plasma
concentration (Tmax) of 0.8 ± 0.3 hours (mean ± S.D.) (n=11). A greater than dose-proportional increase in
indinavir plasma concentrations was observed over the 200-1000 mg dose range. At a dosing regimen of
800 mg every 8 hours, steady-state area under the plasma concentration time curve (AUC) was 30,691
± 11,407 nM•hour (n=16), peak plasma concentration (Cmax) was 12,617 ± 4037 nM (n=16), and plasma
concentration eight hours post dose (trough) was 251 ± 178 nM (n=16).
Effect of Food on Oral Absorption: Administration of indinavir with a meal high in calories, fat, and
protein (784 kcal, 48.6 g fat, 31.3 g protein) resulted in a 77% ± 8% reduction in AUC and an 84% ± 7%
reduction in Cmax (n=10). Administration with lighter meals (e.g., a meal of dry toast with jelly, apple juice,
and coffee with skim milk and sugar or a meal of corn flakes, skim milk and sugar) resulted in little or no
change in AUC, Cmax or trough concentration.
Distribution: Indinavir was approximately 60% bound to human plasma proteins over a concentration
range of 81 nM to 16,300 nM.
Metabolism: Following a 400-mg dose of 14C-indinavir, 83 ± 1% (n=4) and 19 ± 3% (n=6) of the total
radioactivity was recovered in feces and urine, respectively; radioactivity due to parent drug in feces and
urine was 19.1% and 9.4%, respectively. Seven metabolites have been identified, one glucuronide
conjugate and six oxidative metabolites. In vitro studies indicate that cytochrome P-450 3A4 (CYP3A4) is
the major enzyme responsible for formation of the oxidative metabolites.
Elimination: Less than 20% of indinavir is excreted unchanged in the urine. Mean urinary excretion of
unchanged drug was 10.4 ± 4.9% (n=10) and 12.0 ± 4.9% (n=10) following a single 700-mg and 1000-mg
dose, respectively. Indinavir was rapidly eliminated with a half-life of 1.8 ± 0.4 hours (n=10). Significant
accumulation was not observed after multiple dosing at 800 mg every 8 hours.
Special Populations
Hepatic Insufficiency: Patients with mild to moderate hepatic insufficiency and clinical evidence of
cirrhosis had evidence of decreased metabolism of indinavir resulting in approximately 60% higher mean
AUC following a single 400-mg dose (n=12). The half-life of indinavir increased to 2.8 ± 0.5 hours.
Indinavir pharmacokinetics have not been studied in patients with severe hepatic insufficiency (see
DOSAGE AND ADMINISTRATION, Hepatic Insufficiency).
Renal Insufficiency: The pharmacokinetics of indinavir have not been studied in patients with renal
insufficiency.
Gender: The effect of gender on the pharmacokinetics of indinavir was evaluated in 10 HIV
seropositive women who received CRIXIVAN 800 mg every 8 hours with zidovudine 200 mg every 8
hours and lamivudine 150 mg twice a day for one week. Indinavir pharmacokinetic parameters in these
women were compared to those in HIV seropositive men (pooled historical control data). Differences in
indinavir exposure, peak concentrations, and trough concentrations between males and females are
shown in Table 1 below:
2
Table 1
PK Parameter
% change in PK parameter for females
relative to males
90% Confidence Interval
AUC0-8h (nM•hr)
13%
(32%, 12%)
Cmax (nM)
13%
C8h (nM)
22%
Indicates a decrease in the PK parameter; indicates an increase in the PK parameter.
(32%, 10%)
(47%, 15%)
The clinical significance of these gender differences in the pharmacokinetics of indinavir is not known.
Race: Pharmacokinetics of indinavir appear to be comparable in Caucasians and Blacks based on
pharmacokinetic studies including 42 Caucasians (26 HIV-positive) and 16 Blacks (4 HIV-positive).
Pediatric: The optimal dosing regimen for use of indinavir in pediatric patients has not been
established. In HIV-infected pediatric patients (age 4-15 years), a dosage regimen of indinavir capsules,
500 mg/m2 every 8 hours, produced AUC0-8hr of 38,742 ± 24,098 nM•hour (n=34), Cmax of
17,181 ± 9809 nM (n=34), and trough concentrations of 134 ± 91 nM (n=28). The pharmacokinetic
profiles of indinavir in pediatric patients were not comparable to profiles previously observed in
HIV-infected adults receiving the recommended dose of 800 mg every 8 hours. The AUC and Cmax values
were slightly higher and the trough concentrations were considerably lower in pediatric patients.
Approximately 50% of the pediatric patients had trough values below 100 nM; whereas, approximately
10% of adult patients had trough levels below 100 nM. The relationship between specific trough values
and inhibition of HIV replication has not been established.
Pregnant Patients: The optimal dosing regimen for use of indinavir in pregnant patients has not been
established. A CRIXIVAN dose of 800 mg every 8 hours (with zidovudine 200 mg every 8 hours and
lamivudine 150 mg twice a day) has been studied in 16 HIV-infected pregnant patients at 14 to 28 weeks
of gestation at enrollment (study PACTG 358). The mean indinavir plasma AUC0-8hr at weeks 30-32 of
gestation (n=11) was 9231 nM•hr, which is 74% (95% CI: 50%, 86%) lower than that observed 6 weeks
postpartum. Six of these 11 (55%) patients had mean indinavir plasma concentrations 8 hours post-dose
(Cmin) below assay threshold of reliable quantification. The pharmacokinetics of indinavir in these 11
patients at 6 weeks postpartum were generally similar to those observed in non-pregnant patients in
another study (see PRECAUTIONS, Pregnancy).
Drug Interactions: (also see CONTRAINDICATIONS, WARNINGS, PRECAUTIONS, Drug Interactions)
Indinavir is an inhibitor of the cytochrome P450 isoform CYP3A4. Coadministration of CRIXIVAN and
drugs primarily metabolized by CYP3A4 may result in increased plasma concentrations of the other drug,
which could increase or prolong its therapeutic and adverse effects (see CONTRAINDICATIONS and
WARNINGS). Based on in vitro data in human liver microsomes, indinavir does not inhibit CYP1A2,
CYP2C9, CYP2E1 and CYP2B6. However, indinavir may be a weak inhibitor of CYP2D6.
Indinavir is metabolized by CYP3A4. Drugs that induce CYP3A4 activity would be expected to
increase the clearance of indinavir, resulting in lowered plasma concentrations of indinavir.
Coadministration of CRIXIVAN and other drugs that inhibit CYP3A4 may decrease the clearance of
indinavir and may result in increased plasma concentrations of indinavir.
Drug interaction studies were performed with CRIXIVAN and other drugs likely to be coadministered
and some drugs commonly used as probes for pharmacokinetic interactions. The effects of
coadministration of CRIXIVAN on the AUC, Cmax and Cmin are summarized in Table 2 (effect of other
drugs on indinavir) and Table 3 (effect of indinavir on other drugs). For information regarding clinical
recommendations, see Table 9 in PRECAUTIONS.
Table 2: Drug Interactions: Pharmacokinetic Parameters for Indinavir in the Presence of the Coadministered Drug (See PRECAUTIONS,
Table 9 for Recommended Alterations in Dose or Regimen)
Ratio (with/without coadministered drug) of Indinavir
Dose of Coadministered Dose of CRIXIVAN
Pharmacokinetic Parameters
Coadministered drug
drug (mg)
(mg)
n
(90% CI); No Effect = 1.00
Cmax
AUC
Cmin
Cimetidine
600 twice daily, 6 days
400 single dose
12
1.07
0.98
0.82
(0.77, 1.49)
(0.81, 1.19)
(0.69, 0.99)
3
Clarithromycin
500 q12h, 7 days
800 three times
daily, 7 days
10
1.08
(0.85, 1.38)
1.19
(1.00, 1.42)
1.57
(1.16, 2.12)
Delavirdine
400 three times daily
28
Delavirdine
400 three times daily
400 three times
daily, 7 days
600 three times
daily, 7 days
1000 three times
daily, 10 days
0.64*
(0.48, 0.86)
No significant
change
No significant
change*
1.53*
(1.07, 2.20)
2.18*
(1.16, 4.12)
3.98*
(2.04, 7.78)
No significant
change*
No significant
change*
0.71*
(0.57, 0.89)
0.87
(0.72, 1.05)
0.65
(0.53, 0.79)
0.95
(0.88, 1.03)
0.78*
(0.69, 0.88)
0.69*
(0.61, 0.78)
0.67*
(0.61, 0.74)
0.63*
(0.54, 0.74)
0.54*
(0.46, 0.63)
0.76
(0.59, 0.98)
0.73
(0.60, 0.87)
0.99
(0.87, 1.13)
0.99*
(0.91, 1.06)
0.80*
(0.74, 0.87)
0.61*
(0.49, 0.76)
0.48*
(0.43, 0.53)
0.43*
(0.37, 0.50)
0.90
(0.72, 1.12)
0.90
(0.71, 1.15)
0.89
(0.75, 1.06)
1.49*
(1.28, 1.74)
1.29*
(1.11, 1.51)
Efavirenz
†
600 once daily, 10 days
Fluconazole
†
400 once daily, 8 days
Grapefruit Juice
8 oz.
Isoniazid
Itraconazole
300 once daily in the
morning, 8 days
200 twice daily, 7 days
Ketoconazole
400 once daily, 7 days
400 once daily, 7 days
Methadone
20-60 once daily in the
morning, 8 days
200 single dose
Quinidine
Rifabutin
Ritonavir
150 once daily in the
morning, 10 days
300 once daily in the
morning, 10 days
600 once daily in the
morning, 8 days
100 twice daily, 14 days
Ritonavir
200 twice daily, 14 days
Sildenafil
25 single dose
Rifabutin
Rifampin
St. John’s wort
(Hypericum perforatum,
standardized to 0.3 %
hypericin)
300 three times daily
with meals, 14 days
Stavudine (d4T)
40 twice daily, 7 days
†
Trimethoprim/
Sulfamethoxazole
†
Zidovudine
Zidovudine/Lamivudine
800 Trimethoprim/
160 Sulfamethoxazole
q12h, 7 days
200 three times daily, 7
days
200/150 three times
daily, 7 days
28
20
After morning
dose
After afternoon
dose
After evening
dose
1000 three times
daily, 7 days
400 single dose
11
10
800 three times
daily, 7 days
600 three times
daily, 7 days
600 three times
daily, 7 days
11
12
12
0.42*
0.44*
0.73*
(0.37, 0.47)
(0.41, 0.48)
(0.62, 0.85)
See text below for discussion of interaction.
12
400 three times
daily, 7 days
800 three times
daily, 8 days
400 single dose
10
10
800 three times
daily, 10 days
800 three times
daily, 10 days
800 three times
daily, 7 days
800 twice daily,
14 days
800 twice daily,
14 days
800 three times
daily
800 three times
daily
‡
0.96
1.07
0.93
(0.79, 1.18)
(0.89, 1.28)
(0.73, 1.19)
0.80
0.68
0.60
(0.72, 0.89)
(0.60, 0.76)
(0.51, 0.72)
0.75
0.66
0.61
(0.61, 0.91)
(0.56, 0.77)
(0.50, 0.75)
0.13
0.08
Not Done
(0.08, 0.22)
(0.06, 0.11)
See text below for discussion of interaction.
9, 16
‡
See text below for discussion of interaction.
6
See text below for discussion of interaction.
14
10
12
10, 16
8
800 three times
daily, 7 days
400 four times
daily, 7 days
11
1000 three times
daily, 7 days
800 three times
daily, 7 days
12
Not Available
12
¶
§
0.19
§
(0.06, 0.33)
0.95
(0.80, 1.11)
1.12
(0.87, 1.46)
0.95
(0.80, 1.12)
0.98
(0.81, 1.18)
1.13
(0.83, 1.53)
0.83
(0.72, 0.95)
1.06
(0.91, 1.25)
1.05
(0.83, 1.33)
1.05
(0.86, 1.28)
1.04
(0.67, 1.61)
1.02
(0.77, 1.35)
0.98
(0.56, 1.73)
6, 9
†
(3TC)
All interaction studies conducted in healthy, HIV-negative adult subjects, unless otherwise indicated.
* Relative to indinavir 800 mg three times daily alone.
†
Study conducted in HIV-positive subjects.
‡
Comparison to historical data on 16 subjects receiving indinavir alone.
§
95% CI.
¶
Parallel group design; n for indinavir + coadministered drug, n for indinavir alone.
4
0.46
(0.34, 0.58)
Table 3: Drug Interactions: Pharmacokinetic Parameters for Coadministered Drug in the Presence of Indinavir (See PRECAUTIONS, Table 9
for Recommended Alterations in Dose or Regimen)
Dose of
Ratio (with/without CRIXIVAN) of Coadministered Drug
Coadministered drug Dose of CRIXIVAN
Pharmacokinetic Parameters
Coadministered drug
(mg)
(mg)
n
(90% CI); No Effect = 1.00
Cmax
AUC
Cmin
Clarithromycin
500 twice daily, 7
days
800 three times
daily, 7 days
12
1.19
(1.02, 1.39)
1.47
(1.30, 1.65)
Efavirenz
200 once daily, 14
days
800 three times
daily, 14 days
20
No significant
change
No significant
change
35 mcg, 8 days
800 three times
daily, 8 days
18
1.02
(0.96, 1.09)
1.22
(1.15, 1.30)
1.37
(1.24, 1.51)
300 once daily in the
morning, 8 days
20-60 once daily in
the morning, 8 days
1 mcg, 8 days
800 three times
daily, 8 days
800 three times
daily, 8 days
800 three times
daily, 8 days
11
1.34
(1.12, 1.60)
0.93
(0.84, 1.03)
1.05
(0.95, 1.16)
1.12
(1.03, 1.22)
0.96
(0.86, 1.06)
1.26
(1.20, 1.31)
1.00
(0.92, 1.08)
1.06
(0.94, 1.19)
1.44
(1.32, 1.57)
150 once daily in the
morning, 10 days
800 three times
daily, 10 days
14
1.29
(1.05, 1.59)
1.54
(1.33, 1.79)
1.99
(1.71, 2.31)
n=13
300 once daily in the
morning, 10 days
800 three times
daily, 10 days
10
2.34
(1.64, 3.35)
2.73
(1.99, 3.77)
100 twice daily, 14
days
800 twice daily,
14 days
10, 4
1.61
(1.13, 2.29)
1.72
(1.20, 2.48)
3.44
(2.65, 4.46)
n=9
1.62
(0.93, 2.85)
200 twice daily, 14
days
800 twice daily,
14 days
9, 5
‡
1.19
(0.85, 1.66)
1.96
(1.39, 2.76)
600 single dose
800 three times
daily, 2 days
6
4.7
(2.7, 8.1)
6.0
(4.0, 9.1)
800 three times
daily, 2 days
6
6.5
(4.7, 9.1)
7.2
(4.3, 11.9)
800 three times
daily, 2 days
6
4.0
(2.7, 5.9)
4.6
(3.2, 6.7)
Ethinyl Estradiol
*
(ORTHO-NOVUM 1/35)
Isoniazid
Methadone
†
Norethindrone
*
(ORTHO-NOVUM 1/35)
Rifabutin
150 mg once daily in the
morning, 11 days +
indinavir compared to
300 mg once daily in the
morning, 11 days alone
Ritonavir
12
18
‡
1.97
(1.58, 2.46)
n=11
--
4.71
(2.66, 8.33)
n=9, 4
Saquinavir
Hard gel formulation
Soft gel formulation
Soft gel formulation
Sildenafil
Stavudine
800 single dose
1200 single dose
25 single dose
¶
40 twice daily, 7 days
Theophylline
250 single dose
(on Days 1 and 7)
800 three times
daily
800 three times
daily, 7 days
800 three times
daily, 6 days
(Days 2 to 7)
2.9
§
(1.7, 4.7)
5.5
§
(2.2, 14.1)
5.5
§
(3.7, 8.3)
See text below for discussion of interaction.
6
13
‡
12, 4
0.86
(0.73, 1.03)
0.88
(0.76, 1.03)
1.21
(1.09, 1.33)
1.14
(1.04, 1.24)
Not Done
1.13
(0.86, 1.49)
n=7, 3
Trimethoprim/
Sulfamethoxazole
Trimethoprim
800 Trimethoprim/
160 Sulfamethoxazole
q12h, 7 days
400 q6h, 7 days
12
1.18
(1.05, 1.32)
1.18
(1.05, 1.33)
1.18
(1.00, 1.39)
800 Trimethoprim/
160 Sulfamethoxazole
q12h, 7 days
10 single dose
400 q6h, 7 days
12
1.01
(0.95, 1.08)
1.05
(1.01, 1.09)
1.05
(0.97, 1.14)
18
200 three times daily,
7 days
800 three times
daily
1000 three times
daily, 7 days
200/150 three times
daily, 7 days
800 three times
daily, 7 days
6, 7
Trimethoprim/
Sulfamethoxazole
Sulfamethoxazole
Vardenafil
¶
Zidovudine
Zidovudine/Lamivudine
Zidovudine
See text below for discussion of interaction.
12
0.89
(0.73, 1.09)
1.17
(1.07, 1.29)
1.51
(0.71, 3.20)
n=4
1.23
(0.74, 2.03)
1.39
(1.02, 1.89)
1.08
(0.77, 1.50)
n=5, 5
¶
‡
5
Zidovudine/Lamivudine
¶
‡
800 three times
200/150 three times
0.73
6, 7
daily, 7 days
daily, 7 days
(0.52, 1.02)
All interaction studies conducted in healthy, HIV-negative adult subjects, unless otherwise indicated.
* Registered trademark of Ortho Pharmaceutical Corporation.
†
Study conducted in subjects on methadone maintenance.
‡
Parallel group design; n for coadministered drug + indinavir, n for coadministered drug alone.
§
C6hr
¶
Study conducted in HIV-positive subjects.
Lamivudine
0.91
(0.66, 1.26)
0.88
(0.59, 1.33)
Delavirdine: Delavirdine inhibits the metabolism of indinavir such that coadministration of 400-mg or
600-mg indinavir three times daily with 400-mg delavirdine three times daily alters indinavir AUC, Cmax
and Cmin (see Table 2). Indinavir had no effect on delavirdine pharmacokinetics (see DOSAGE AND
ADMINISTRATION, Concomitant Therapy, Delavirdine), based on a comparison to historical delavirdine
pharmacokinetic data.
Methadone: Administration of indinavir (800 mg every 8 hours) with methadone (20 mg to 60 mg daily)
for one week in subjects on methadone maintenance resulted in no change in methadone AUC. Based
on a comparison to historical data, there was little or no change in indinavir AUC.
Ritonavir: Compared to historical data in patients who received indinavir 800 mg every 8 hours alone,
twice-daily coadministration to volunteers of indinavir 800 mg and ritonavir with food for two weeks
resulted in a 2.7-fold increase of indinavir AUC24h, a 1.6-fold increase in indinavir Cmax, and an 11-fold
increase in indinavir Cmin for a 100-mg ritonavir dose and a 3.6-fold increase of indinavir AUC24h, a 1.8fold increase in indinavir Cmax, and a 24-fold increase in indinavir Cmin for a 200-mg ritonavir dose. In the
same study, twice-daily coadministration of indinavir (800 mg) and ritonavir (100 or 200 mg) resulted in
ritonavir AUC24h increases versus the same doses of ritonavir alone (see Table 3).
Sildenafil: The results of one published study in HIV-infected men (n=6) indicated that coadministration
of indinavir (800 mg every 8 hours chronically) with a single 25-mg dose of sildenafil resulted in an 11%
increase in average AUC0-8hr of indinavir and a 48% increase in average indinavir peak concentration
(Cmax) compared to 800 mg every 8 hours alone. Average sildenafil AUC was increased by 340%
following coadministration of sildenafil and indinavir compared to historical data following administration
of sildenafil alone (see CONTRAINDICATIONS, WARNINGS, Drug Interactions and PRECAUTIONS,
Drug Interactions).
Vardenafil: Indinavir (800 mg every 8 hours) coadministered with a single 10-mg dose of vardenafil
resulted in a 16-fold increase in vardenafil AUC, a 7-fold increase in vardenafil Cmax, and a 2-fold increase
in vardenafil half-life (see WARNINGS, Drug Interactions and PRECAUTIONS, Drug Interactions).
INDICATIONS AND USAGE
CRIXIVAN in combination with antiretroviral agents is indicated for the treatment of HIV infection.
This indication is based on two clinical trials of approximately 1 year duration that demonstrated: 1) a
reduction in the risk of AIDS-defining illnesses or death; 2) a prolonged suppression of HIV RNA.
Description of Studies
In all clinical studies, with the exception of ACTG 320, the AMPLICOR HIV MONITOR assay was
used to determine the level of circulating HIV RNA in serum. This is an experimental use of the assay.
HIV RNA results should not be directly compared to results from other trials using different HIV RNA
assays or using other sample sources.
Study ACTG 320 was a multicenter, randomized, double-blind clinical endpoint trial to compare the
effect of CRIXIVAN in combination with zidovudine and lamivudine with that of zidovudine plus
lamivudine on the progression to an AIDS-defining illness (ADI) or death. Patients were protease inhibitor
and lamivudine naive and zidovudine experienced, with CD4 cell counts of 200 cells/mm3. The study
enrolled 1156 HIV-infected patients (17% female, 28% Black, 18% Hispanic, mean age 39 years). The
mean baseline CD4 cell count was 87 cells/mm3. The mean baseline HIV RNA was 4.95 log10 copies/mL
(89,035 copies/mL). The study was terminated after a planned interim analysis, resulting in a median
follow-up of 38 weeks and a maximum follow-up of 52 weeks. Results are shown in Table 4 and Figures
1 & 2.
6
Table 4: ACTG 320
Number (%) of Patients with AIDS-defining Illness or Death
IDV+ZDV+L
ZDV+L
(n=577)
(n=579)
Endpoint
HIV
Progression
Death
or
35 (6.1)
63 (10.9)
Death*
10 (1.7)
19 (3.3)
* The number of deaths is inadequate to assess the impact of Indinavir on survival.
IDV = Indinavir, ZDV = Zidovudine, L = Lamivudine
7
Study 028, a double-blind, multicenter, randomized, clinical endpoint trial conducted in Brazil,
compared the effects of CRIXIVAN plus zidovudine with those of CRIXIVAN alone or zidovudine alone on
the progression to an ADI or death, and on surrogate marker responses. All patients were antiretroviral
naive with CD4 cell counts of 50 to 250 cells/mm3. The study enrolled 996 HIV-1 seropositive patients
[28% female, 11% Black, 1% Asian/Other, median age 33 years, mean baseline CD4 cell count of
152 cells/mm3, mean serum viral RNA of 4.44 log10 copies/mL (27,824 copies/mL)]. Treatment regimens
containing zidovudine were modified in a blinded manner with the optional addition of lamivudine (median
time: week 40). The median length of follow-up was 56 weeks with a maximum of 97 weeks. The study
was terminated after a planned interim analysis, resulting in a median follow-up of 56 weeks and a
maximum follow-up of 97 weeks. Results are shown in Table 5 and Figures 3 and 4.
Table 5: Protocol 028
Endpoint
HIV Progression or Death
Number (%) of Patients with AIDS-defining Illness or Death
IDV+ZDV
IDV
ZDV
(n=332)
(n=332)
(n=332)
21 (6.3)
27 (8.1)
Death*
8 (2.4)
5 (1.5)
* The number of deaths is inadequate to assess the impact of Indinavir on survival.
8
62 (18.7)
11 (3.3)
Study 035 was a multicenter, randomized trial in 97 HIV-1 seropositive patients who were zidovudineexperienced (median exposure 30 months), protease-inhibitor- and lamivudine-naive, with mean baseline
CD4 count 175 cells/mm3 and mean baseline serum viral RNA 4.62 log10 copies/mL (41,230 copies/mL).
Comparisons included CRIXIVAN plus zidovudine plus lamivudine vs. CRIXIVAN alone vs. zidovudine
plus lamivudine. After at least 24 weeks of randomized, double-blind therapy, patients were switched to
open-label CRIXIVAN plus lamivudine plus zidovudine. Mean changes in log10 viral RNA in serum, the
proportions of patients with viral RNA below 500 copies/mL in serum, and mean changes in CD4 cell
counts, during 24 weeks of randomized, double-blinded therapy are summarized in Figures 5, 6, and 7,
respectively. A limited number of patients remained on randomized, double-blind treatment for longer
periods; based on this extended treatment experience, it appears that a greater number of subjects
randomized to CRIXIVAN plus zidovudine plus lamivudine demonstrated HIV RNA levels below
500 copies/mL during one year of therapy as compared to those in other treatment groups.
9
Genotypic Resistance in Clinical Studies
Study 006 (10/15/93-10/12/94) was a dose-ranging study in which patients were initially treated with
CRIXIVAN at a dose of <2.4 g/day followed by 2.4 g/day. Study 019 (6/23/94-4/10/95) was a randomized
comparison of CRIXIVAN 600 mg every 6 hours, CRIXIVAN plus zidovudine, and zidovudine alone.
Table 6 shows the incidence of genotypic resistance at 24 weeks in these studies.
10
Table 6: Genotypic Resistance at 24 Weeks
Resistance
to IDV
n/N*
Resistance
to ZDV
n/N*
—
—
31/37 (84%)
9/21 (43%)
—
1/17 (6%)
IDV/ZDV
4/22 (18%)
1/22 (5%)
ZDV
1/18 (6%)
11/17 (65%)
Treatment Group
IDV
<2.4 g/day
2.4 g/day
*N - includes patients with non-amplifiable virus at 24 weeks who
had amplifiable virus at week 0.
CONTRAINDICATIONS
CRIXIVAN is contraindicated in patients with clinically significant hypersensitivity to any of its
components.
Inhibition of CYP3A4 by CRIXIVAN can result in elevated plasma concentrations of the following
drugs, potentially causing serious or life-threatening reactions:
Table 7: Drug Interactions With Crixivan: Contraindicated Drugs
Drug Class
Alpha 1-adrenoreceptor antagonist
Antiarrhythmics
Ergot derivatives
GI motility agents
HMG-CoA Reductase Inhibitors
Neuroleptics
PDE5 Inhibitors
Sedative/hypnotics
*
Registered trademark of Pfizer, Inc.
Drugs Within Class That Are Contraindicated With CRIXIVAN
alfuzosin
amiodarone
dihydroergotamine, ergonovine, ergotamine, methylergonovine
cisapride
lovastatin, simvastatin
pimozide
Revatio* (sildenafil) [for treatment of pulmonary arterial hypertension]
oral midazolam, triazolam, alprazolam
WARNINGS
ALERT: Find out about medicines that should NOT be taken with CRIXIVAN. This statement is
included on the product’s bottle label.
Nephrolithiasis/Urolithiasis
Nephrolithiasis/urolithiasis has occurred with CRIXIVAN therapy. The cumulative frequency of
nephrolithiasis is substantially higher in pediatric patients (29%) than in adult patients (12.4%; range
across individual trials: 4.7% to 34.4%). The cumulative frequency of nephrolithiasis events increases
with increasing exposure to CRIXIVAN; however, the risk over time remains relatively constant. In some
cases, nephrolithiasis/urolithiasis has been associated with renal insufficiency or acute renal failure,
pyelonephritis with or without bacteremia. If signs or symptoms of nephrolithiasis/urolithiasis occur,
(including flank pain, with or without hematuria or microscopic hematuria), temporary interruption (e.g., 13 days) or discontinuation of therapy may be considered. Adequate hydration is recommended in all
patients treated with CRIXIVAN. (See ADVERSE REACTIONS and DOSAGE AND
ADMINISTRATION, Nephrolithiasis/Urolithiasis.)
Hemolytic Anemia
Acute hemolytic anemia, including cases resulting in death, has been reported in patients treated with
CRIXIVAN. Once a diagnosis is apparent, appropriate measures for the treatment of hemolytic anemia
should be instituted, including discontinuation of CRIXIVAN.
Hepatitis
Hepatitis including cases resulting in hepatic failure and death has been reported in patients treated
with CRIXIVAN. Because the majority of these patients had confounding medical conditions and/or were
11
receiving concomitant therapy(ies), a causal relationship between CRIXIVAN and these events has not
been established.
Hyperglycemia
New onset diabetes mellitus, exacerbation of pre-existing diabetes mellitus and hyperglycemia have
been reported during post-marketing surveillance in HIV-infected patients receiving protease inhibitor
therapy. Some patients required either initiation or dose adjustments of insulin or oral hypoglycemic
agents for treatment of these events. In some cases, diabetic ketoacidosis has occurred. In those
patients who discontinued protease inhibitor therapy, hyperglycemia persisted in some cases. Because
these events have been reported voluntarily during clinical practice, estimates of frequency cannot be
made and a causal relationship between protease inhibitor therapy and these events has not been
established.
Risk of Serious Adverse Reactions Due to Drug Interactions
Initiation of CRIXIVAN, a CYP3A inhibitor, in patients receiving medications metabolized by CYP3A or
initiation of medications metabolized by CYP3A in patients already receiving CRIXIVAN, may increase
plasma concentrations of medications metabolized by CYP3A. Initiation of medications that inhibit or
induce CYP3A may increase or decrease concentrations of CRIXIVAN, respectively. These interactions
may lead to:
Clinically significant adverse reactions, potentially leading to severe, life-threatening, or fatal
events from greater exposures of concomitant medications.
Clinically significant adverse reactions from greater exposures of CRIXIVAN.
Loss of therapeutic effect of CRIXIVAN and possible development of resistance.
See Table 9 for steps to prevent or manage these possible and known significant drug interactions,
including dosing recommendations. Consider the potential for drug interactions prior to and during
CRIXIVAN therapy; review concomitant medications during CRIXIVAN therapy; and monitor for the
adverse reactions associated with the concomitant medications.
Concomitant use of CRIXIVAN with lovastatin or simvastatin is contraindicated due to an increased
risk of myopathy including rhabdomyolysis. Caution should be exercised if CRIXIVAN is used
concurrently with atorvastatin or rosuvastatin. Titrate the atorvastatin and rosuvastatin doses carefully
and use the lowest necessary dose with CRIXIVAN. (See PRECAUTIONS, Drug Interactions.)
Midazolam is extensively metabolized by CYP3A4. Co-administration with CRIXIVAN with or without
ritonavir may cause a large increase in the concentration of this benzodiazepine. No drug interaction
study has been performed for the co-administration of CRIXIVAN with benzodiazepines. Based on data
from other CYP3A4 inhibitors, plasma concentrations of midazolam are expected to be significantly
higher when midazolam is given orally. Therefore CRIXIVAN should not be co-administered with orally
administered midazolam (see CONTRAINDICATIONS), whereas caution should be used with coadministration of CRIXIVAN and parenteral midazolam. Data from concomitant use of parenteral
midazolam with other protease inhibitors suggest a possible 3-4 fold increase in midazolam plasma
levels. If CRIXIVAN with or without ritonavir is co-administered with parenteral midazolam, it should be
done in a setting which ensures close clinical monitoring and appropriate medical management in case of
respiratory depression and/or prolonged sedation. Dosage reduction for midazolam should be
considered, especially if more than a single dose of midazolam is administered.
Particular caution should be used when prescribing sildenafil, tadalafil, or vardenafil in patients
receiving indinavir. Coadministration of CRIXIVAN with these medications is expected to substantially
increase plasma concentrations of sildenafil, tadalafil, and vardenafil and may result in an increase in
adverse events, including hypotension, visual changes, and priapism, which have been associated with
sildenafil, tadalafil, and vardenafil (see CONTRAINDICATIONS and PRECAUTIONS, Drug Interactions
and Information for Patients, and the manufacturer’s complete prescribing information for sildenafil,
tadalafil, or vardenafil).
Concomitant use of CRIXIVAN and St. John’s wort (Hypericum perforatum) or products containing St.
John’s wort is not recommended. Coadministration of CRIXIVAN and St. John’s wort has been shown to
substantially decrease indinavir concentrations (see CLINICAL PHARMACOLOGY, Drug Interactions)
and may lead to loss of virologic response and possible resistance to CRIXIVAN or to the class of
protease inhibitors.
12
PRECAUTIONS
General
Indirect hyperbilirubinemia has occurred frequently during treatment with CRIXIVAN and has
infrequently been associated with increases in serum transaminases (see also ADVERSE REACTIONS,
Clinical Trials and Post-Marketing Experience). It is not known whether CRIXIVAN will exacerbate the
physiologic hyperbilirubinemia seen in neonates. (See Pregnancy.)
Tubulointerstitial Nephritis
Reports of tubulointerstitial nephritis with medullary calcification and cortical atrophy have been
observed in patients with asymptomatic severe leukocyturia (>100 cells/ high power field). Patients with
asymptomatic severe leukocyturia should be followed closely and monitored frequently with urinalyses.
Further diagnostic evaluation may be warranted, and discontinuation of CRIXIVAN should be considered
in all patients with severe leukocyturia.
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral
therapy, including CRIXIVAN. During the initial phase of combination antiretroviral treatment, patients
whose immune system responds may develop an inflammatory response to indolent or residual
opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii
pneumonia [PCP], or tuberculosis), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves’ disease, polymyositis, and Guillain-Barré syndrome) have
also been reported to occur in the setting of immune reconstitution; however, the time to onset is more
variable, and can occur many months after initiation of treatment.
Coexisting Conditions
Patients with hemophilia: There have been reports of spontaneous bleeding in patients with
hemophilia A and B treated with protease inhibitors. In some patients, additional factor VIII was required.
In many of the reported cases, treatment with protease inhibitors was continued or restarted. A causal
relationship between protease inhibitor therapy and these episodes has not been established. (See
ADVERSE REACTIONS, Post-Marketing Experience.)
Patients with hepatic insufficiency due to cirrhosis: In these patients, the dosage of CRIXIVAN should
be lowered because of decreased metabolism of CRIXIVAN (see DOSAGE AND ADMINISTRATION).
Patients with renal insufficiency: Patients with renal insufficiency have not been studied.
Fat Redistribution
Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement
(buffalo hump), peripheral wasting, facial wasting, breast enlargement, and “cushingoid appearance”
have been observed in patients receiving antiretroviral therapy. The mechanism and long-term
consequences of these events are currently unknown. A causal relationship has not been established.
Information for Patients
A statement to patients and health care providers is included on the product’s bottle label. ALERT:
Find out about medicines that should NOT be taken with CRIXIVAN. A Patient Package Insert (PPI)
for CRIXIVAN is available for patient information.
CRIXIVAN is not a cure for HIV-1 infection and patients may continue to experience illnesses
associated with HIV-1 infection, including opportunistic infections. Patients should remain under the care
of a physician when using CRIXIVAN.
Patients should be advised to avoid doing things that can spread HIV-1 infection to others.
Do not share needles or other injection equipment.
Do not share personal items that can have blood or body fluids on them, like toothbrushes
and razor blades.
Do not have any kind of sex without protection. Always practice safe sex by using a latex or
polyurethane condom to lower the chance of sexual contact with semen, vaginal secretions, or
blood.
Do not breastfeed. We do not know if CRIXIVAN can be passed to your baby in your breast milk
and whether it could harm your baby. Also, mothers with HIV-1 should not breastfeed because
HIV-1 can be passed to the baby in the breast milk.
13
Patients should be advised to remain under the care of a physician when using CRIXIVAN and should
not modify or discontinue treatment without first consulting the physician. Therefore, if a dose is missed,
patients should take the next dose at the regularly scheduled time and should not double this dose.
Therapy with CRIXIVAN should be initiated and maintained at the recommended dosage.
CRIXIVAN may interact with some drugs; therefore, patients should be advised to report to their
doctor the use of any other prescription, non-prescription medication or herbal products, particularly St.
John’s wort.
For optimal absorption, CRIXIVAN should be administered without food but with water 1 hour before
or 2 hours after a meal. Alternatively, CRIXIVAN may be administered with other liquids such as skim
milk, juice, coffee, or tea, or with a light meal, e.g., dry toast with jelly, juice, and coffee with skim milk and
sugar; or corn flakes, skim milk and sugar (see CLINICAL PHARMACOLOGY, Effect of Food on Oral
Absorption and DOSAGE AND ADMINISTRATION). Ingestion of CRIXIVAN with a meal high in calories,
fat, and protein reduces the absorption of indinavir.
Patients receiving a phosphodiesterase type 5 (PDE5) inhibitor (sildenafil, tadalafil, or vardenafil)
should be advised that they may be at an increased risk of PDE5 inhibitor-associated adverse events
including hypotension, visual changes, and priapism, and should promptly report any symptoms to their
doctors (see CONTRAINDICATIONS and WARNINGS, Drug Interactions).
Patients should be informed that redistribution or accumulation of body fat may occur in patients
receiving antiretroviral therapy and that the cause and long-term health effects of these conditions are not
known at this time.
CRIXIVAN Capsules are sensitive to moisture. Patients should be informed that CRIXIVAN should be
stored and used in the original container and the desiccant should remain in the bottle.
Drug Interactions
Indinavir is an inhibitor of the cytochrome P450 isoform CYP3A4. Coadministration of CRIXIVAN and
drugs primarily metabolized by CYP3A4 may result in increased plasma concentrations of the other drug,
which could increase or prolong its therapeutic and adverse effects (see CONTRAINDICATIONS and
WARNINGS).
Indinavir is metabolized by CYP3A4. Drugs that induce CYP3A4 activity would be expected to
increase the clearance of indinavir, resulting in lowered plasma concentrations of indinavir.
Coadministration of CRIXIVAN and other drugs that inhibit CYP3A4 may decrease the clearance of
indinavir and may result in increased plasma concentrations of indinavir.
Table 8: Drugs That Should Not Be Coadministered with CRIXIVAN
Drug Class: Drug Name
Alpha 1-adrenoreceptor antagonist:
alfuzosin
Antiarrhythmics:
amiodarone
Antimycobacterial:
rifampin
GI motility agents:
cisapride
Clinical Comment
Potentially increased alfuzosin concentrations can result in
hypotension.
CONTRAINDICATED due to potential for serious and/or lifethreatening reactions such as cardiac arrhythmias.
May lead to loss of virologic response and possible resistance
to CRIXIVAN or to the class of protease inhibitors or other
coadministered antiretroviral agents.
CONTRAINDICATED due to potential for serious and/or lifethreatening reactions such as acute ergot toxicity
characterized by peripheral vasospasm and ischemia of the
extremities and other tissues.
CONTRAINDICATED due to potential for serious and/or lifethreatening reactions such as cardiac arrhythmias.
Herbal products:
St. John’s wort (Hypericum perforatum)
May lead to loss of virologic response and possible resistance
to CRIXIVAN or to the class of protease inhibitors.
HMG-CoA Reductase inhibitors:
lovastatin, simvastatin
CONTRAINDICATED due to an increased risk for serious
reactions such as myopathy including rhabdomyolysis.
Neuroleptic:
pimozide
PDE5 inhibitor:
Revatio* (sildenafil) [for treatment of pulmonary
arterial hypertension]
CONTRAINDICATED due to potential for serious and/or lifethreatening reactions such as cardiac arrhythmias.
A safe and effective dose has not been established when used
with CRIXIVAN. There is increased potential for sildenafilassociated adverse events (which include visual disturbances,
hypotension, prolonged erection, and syncope).
Ergot derivatives:
dihydroergotamine, ergonovine, ergotamine,
methylergonovine
14
*
Protease inhibitor:
atazanavir
Both CRIXIVAN and atazanavir are associated with indirect
(unconjugated) hyperbilirubinemia. Combinations of these
drugs have not been studied and coadministration of
CRIXIVAN and atazanavir is not recommended.
Sedative/hypnotics:
Oral midazolam, triazolam, alprazolam
CONTRAINDICATED due to potential for serious and/or lifethreatening reactions such as prolonged or increased sedation
or respiratory depression.
Registered trademark of Pfizer, Inc.
15
Table 9: Established and Other Potentially Significant Drug Interactions: Alteration in Dose or Regimen May Be Recommended Based on
Drug Interaction Studies or Predicted Interaction (See also CLINICAL PHARMACOLOGY for magnitude of interaction, WARNINGS and
DOSAGE AND ADMINISTRATION.)
Drug Name
Delavirdine
Didanosine
Efavirenz
Nelfinavir
Nevirapine
Ritonavir
Saquinavir
Antiarrhythmics:
bepridil, lidocaine
(systemic) and
quinidine
Anticonvulsants:
carbamazepine,
phenobarbital,
phenytoin
Antidepressant:
Trazodone
Anti-gout:
Colchicine
Effect
Clinical Comment
HIV Antiviral Agents
Dose reduction of CRIXIVAN to 600 mg every 8 hours should be
indinavir concentration
considered when taking delavirdine 400 mg three times a day.
Indinavir and didanosine formulations containing buffer should
be administered at least one hour apart on an empty stomach.
The optimal dose of indinavir, when given in combination with
indinavir concentration
efavirenz, is not known. Increasing the indinavir dose to 1000
mg every 8 hours does not compensate for the increased
indinavir metabolism due to efavirenz.
The appropriate doses for this combination, with respect to
indinavir concentration
efficacy and safety, have not been established.
Indinavir concentrations may be decreased in the presence of
indinavir concentration
nevirapine. The appropriate doses for this combination, with
respect to efficacy and safety, have not been established.
The appropriate doses for this combination, with respect to
indinavir concentration
efficacy and safety, have not been established. Preliminary
ritonavir concentration
clinical data suggest that the incidence of nephrolithiasis is
higher in patients receiving indinavir in combination with ritonavir
than those receiving CRIXIVAN 800 mg q8h.
The appropriate doses for this combination, with respect to
saquinavir concentration
efficacy and safety, have not been established.
Other Agents
Caution is warranted and therapeutic concentration monitoring
antiarrhythmic agents
is recommended for antiarrhythmics when coadministered with
concentration
CRIXIVAN.
indinavir concentration
trazodone concentration
colchicine concentration
Use with caution. CRIXIVAN may not be effective due to
decreased indinavir concentrations in patients taking these
agents concomitantly.
Concomitant use of trazodone and CRIXIVAN may increase
plasma concentrations of trazodone. Adverse events of nausea,
dizziness, hypotension and syncope have been observed
following coadministration of trazodone and ritonavir. If
trazodone is used with a CYP3A4 inhibitor such as CRIXIVAN,
the combination should be used with caution and a lower dose
of trazodone should be considered.
Patients with renal or hepatic impairment should not be given
colchicine with CRIXIVAN.
Treatment of gout flares:
Co-administration of colchicine in patients on CRIXIVAN: 0.6 mg
(1 tablet) x 1 dose, followed by 0.3 mg (half tablet) 1 hour later.
Dose to be repeated no earlier than 3 days.
Prophylaxis of gout flares:
Co-administration of colchicine in patients on CRIXIVAN:
If the original colchicine regimen was 0.6 mg twice a day, the
regimen should be adjusted to 0.3 mg once a day.
If the original colchicine regimen was 0.6 mg once a day, the
regimen should be adjusted to 0.3 mg once every other day.
Antipsychotics:
Quetiapine
quetiapine
Treatment of familial Mediterranean fever (FMF):
Co-administration of colchicine in patients on CRIXIVAN:
Maximum daily dose of 0.6 mg (may be given as 0.3 mg twice a
day).
Initiation of CRIXIVAN in patients taking quetiapine:
Consider alternative antiretroviral therapy to avoid increases in
quetiapine drug exposures. If coadministration is necessary,
reduce the quetiapine dose to 1/6 of the current dose and
monitor for quetiapine-associated adverse reactions. Refer to
the quetiapine prescribing information for recommendations on
adverse reaction monitoring.
Initiation of quetiapine in patients taking CRIXIVAN:
Refer to the quetiapine prescribing information for initial dosing
and titration of quetiapine.
Calcium Channel
dihydropyridine calcium
Caution is warranted and clinical monitoring of patients is
16
Blockers,
Dihydropyridine: e.g.,
felodipine, nifedipine,
nicardipine
Clarithromycin
Endothelin receptor
antagonist:
Bosentan
HMG-CoA Reductase
Inhibitors: atorvastatin,
rosuvastatin
Immunosuppressants:
cyclosporine,
tacrolimus, sirolimus
Inhaled beta agonist:
Salmeterol
Inhaled/nasal steroid:
Fluticasone
channel blockers concentration
recommended.
clarithromycin concentration
indinavir concentration
bosentan concentration
The appropriate doses for this combination, with respect to
efficacy and safety, have not been established.
Co-administration of bosentan in patients on CRIXIVAN or coadministration of CRIXIVAN in patients on bosentan: Start at or
adjust bosentan to 62.5 mg once daily or every other day based
upon individual tolerability.
The atorvastatin and rosuvastatin doses should be carefully
titrated; use the lowest dose necessary with careful monitoring
during treatment with CRIXIVAN.
atorvastatin concentration
rosuvastatin concentration
immunosuppressant agents
concentration
salmeterol
fluticasone concentration
Itraconazole
indinavir concentration
Ketoconazole
indinavir concentration
Midazolam (parenteral
administration)
midazolam concentration
Rifabutin
indinavir concentration
rifabutin concentration
Sildenafil
sildenafil concentration
(only the use of sildenafil at
doses used for treatment of
erectile dysfunction has
been studied with
CRIXIVAN)
Tadalafil
tadalafil concentration
Plasma concentrations may be increased by CRIXIVAN.
Concurrent administration of salmeterol with CRIXIVAN is not
recommended. The combination may result in increased risk of
cardiovascular adverse events associated with salmeterol,
including QT prolongation, palpitations and sinus tachycardia.
Concomitant use of fluticasone propionate and CRIXIVAN may
increase plasma concentrations of fluticasone propionate. Use
with caution. Consider alternatives to fluticasone propionate,
particularly for long-term use.
Fluticasone use is not recommended in situations where
CRIXIVAN is coadministered with a potent CYP3A4 inhibitor
such as ritonavir unless the potential benefit to the patient
outweighs the risk of systemic corticosteroid side effects.
Dose reduction of CRIXIVAN to 600 mg every 8 hours is
recommended when administering itraconazole concurrently.
Dose reduction of CRIXIVAN to 600 mg every 8 hours should be
considered.
Concomitant use of parenteral midazolam with CRIXIVAN may
increase plasma concentrations of midazolam. Coadministration
should be done in a setting which ensures close clinical
monitoring and appropriate medical management in case of
respiratory depression and/or prolonged sedation. Dosage
reduction for midazolam should be considered, especially if
more than a single dose of midazolam is administered.
Coadministration of oral midazolam with CRIXIVAN is
CONTRAINDICATED (see Table 8).
Dose reduction of rifabutin to half the standard dose and a dose
increase of CRIXIVAN to 1000 mg every 8 hours are
recommended when rifabutin and CRIXIVAN are
coadministered.
May result in an increase in PDE5 inhibitor-associated adverse
events, including hypotension, syncope, visual disturbances,
and priapism.
Use of sildenafil for pulmonary arterial hypertension (PAH):
Use of Revatio* (sildenafil) is contraindicated when used for the
treatment of pulmonary arterial hypertension (PAH) [see
CONTRAINDICATIONS].
Use of sildenafil for erectile dysfunction:
Sildenafil dose should not exceed a maximum of 25 mg in a 48hour period in patients receiving concomitant CRIXIVAN
therapy. Use with increased monitoring for adverse events.
May result in an increase in PDE5 inhibitor-associated adverse
events, including hypotension, visual disturbances, and
priapism.
Use of tadalafil for pulmonary arterial hypertension (PAH):
The following dose adjustments are recommended for use of
Adcirca† (tadalafil) with CRIXIVAN:
Co-administration of Adcirca in patients on CRIXIVAN or coadministration of CRIXIVAN in patients on Adcirca:
Start at or adjust Adcirca to 20 mg once daily. Increase to 40 mg
once daily based upon individual tolerability.
Use of tadalafil for erectile dysfunction:
Tadalafil dose should not exceed a maximum of 10 mg in a 72-
17
Vardenafil
vardenafil concentration
Venlafaxine
indinavir concentration
hour period in patients receiving concomitant CRIXIVAN
therapy. Use with increased monitoring for adverse events.
Vardenafil dose should not exceed a maximum of 2.5 mg in a
24-hour period in patients receiving concomitant indinavir
therapy.
In a study of 9 healthy volunteers, venlafaxine administered
under steady-state conditions at 150 mg/day resulted in a 28%
decrease in the AUC of a single 800 mg oral dose of indinavir
and a 36% decrease in indinavir Cmax. Indinavir did not affect the
pharmacokinetics of venlafaxine and ODV. The clinical
significance of this finding is unknown.
Note: = increase; = decrease
*
Registered trademark of Pfizer, Inc.
†
Registered trademark of Eli Lilly and Company.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies were conducted in mice and rats. In mice, no increased incidence of any
tumor type was observed. The highest dose tested in rats was 640 mg/kg/day; at this dose a statistically
significant increased incidence of thyroid adenomas was seen only in male rats. At that dose, daily
systemic exposure in rats was approximately 1.3 times higher than daily systemic exposure in humans.
No evidence of mutagenicity or genotoxicity was observed in in vitro microbial mutagenesis (Ames) tests,
in vitro alkaline elution assays for DNA breakage, in vitro and in vivo chromosomal aberration studies,
and in vitro mammalian cell mutagenesis assays. No treatment-related effects on mating, fertility, or
embryo survival were seen in female rats and no treatment-related effects on mating performance were
seen in male rats at doses providing systemic exposure comparable to or slightly higher than that with the
clinical dose. In addition, no treatment-related effects were observed in fecundity or fertility of untreated
females mated to treated males.
Pregnancy
Pregnancy Category C: Developmental toxicity studies were performed in rabbits (at doses up to
240 mg/kg/day), dogs (at doses up to 80 mg/kg/day), and rats (at doses up to 640 mg/kg/day). The
highest doses in these studies produced systemic exposures in these species comparable to or slightly
greater than human exposure. No treatment-related external, visceral, or skeletal changes were observed
in rabbits or dogs. No treatment-related external or visceral changes were observed in rats. Treatmentrelated increases over controls in the incidence of supernumerary ribs (at exposures at or below those in
humans) and of cervical ribs (at exposures comparable to or slightly greater than those in humans) were
seen in rats. In all three species, no treatment-related effects on embryonic/fetal survival or fetal weights
were observed.
In rabbits, at a maternal dose of 240 mg/kg/day, no drug was detected in fetal plasma 1 hour after
dosing. Fetal plasma drug levels 2 hours after dosing were approximately 3% of maternal plasma drug
levels. In dogs, at a maternal dose of 80 mg/kg/day, fetal plasma drug levels were approximately 50% of
maternal plasma drug levels both 1 and 2 hours after dosing. In rats, at maternal doses of 40 and
640 mg/kg/day, fetal plasma drug levels were approximately 10 to 15% and 10 to 20% of maternal
plasma drug levels 1 and 2 hours after dosing, respectively.
Indinavir was administered to Rhesus monkeys during the third trimester of pregnancy (at doses up to
160 mg/kg twice daily) and to neonatal Rhesus monkeys (at doses up to 160 mg/kg twice daily). When
administered to neonates, indinavir caused an exacerbation of the transient physiologic
hyperbilirubinemia seen in this species after birth; serum bilirubin values were approximately fourfold
above controls at 160 mg/kg twice daily. A similar exacerbation did not occur in neonates after in utero
exposure to indinavir during the third trimester of pregnancy. In Rhesus monkeys, fetal plasma drug
levels were approximately 1 to 2% of maternal plasma drug levels approximately 1 hour after maternal
dosing at 40, 80, or 160 mg/kg twice daily.
Hyperbilirubinemia has occurred during treatment with CRIXIVAN (see PRECAUTIONS and
ADVERSE REACTIONS). It is unknown whether CRIXIVAN administered to the mother in the perinatal
period will exacerbate physiologic hyperbilirubinemia in neonates.
There are no adequate and well-controlled studies in pregnant patients. CRIXIVAN should be used
during pregnancy only if the potential benefit justifies the potential risk to the fetus.
A CRIXIVAN dose of 800 mg every 8 hours (with zidovudine 200 mg every 8 hours and lamivudine
150 mg twice a day) has been studied in 16 HIV-infected pregnant patients at 14 to 28 weeks of gestation
18
at enrollment (study PACTG 358). Given the substantially lower antepartum exposures observed and the
limited data in this patient population, indinavir use is not recommended in HIV-infected pregnant patients
(see CLINICAL PHARMACOLOGY, Pregnant Patients).
Antiretroviral Pregnancy Registry
To monitor maternal-fetal outcomes of pregnant patients exposed to CRIXIVAN, an Antiretroviral
Pregnancy Registry has been established. Physicians are encouraged to register patients by calling 1800-258-4263.
Nursing Mothers
Studies in lactating rats have demonstrated that indinavir is excreted in milk. Although it is not known
whether CRIXIVAN is excreted in human milk, there exists the potential for adverse effects from indinavir
in nursing infants. Mothers should be instructed to discontinue nursing if they are receiving CRIXIVAN.
This is consistent with the recommendation by the U.S. Public Health Service Centers for Disease
Control and Prevention that HIV-infected mothers not breast-feed their infants to avoid risking postnatal
transmission of HIV.
Pediatric Use
The optimal dosing regimen for use of indinavir in pediatric patients has not been established. A dose
of 500 mg/m2 every eight hours has been studied in uncontrolled studies of 70 children, 3 to 18 years of
age. The pharmacokinetic profiles of indinavir at this dose were not comparable to profiles previously
observed in adults receiving the recommended dose (see CLINICAL PHARMACOLOGY, Pediatric).
Although viral suppression was observed in some of the 32 children who were followed on this regimen
through 24 weeks, a substantially higher rate of nephrolithiasis was reported when compared to adult
historical data (see WARNINGS, Nephrolithiasis/Urolithiasis). Physicians considering the use of indinavir
in pediatric patients without other protease inhibitor options should be aware of the limited data available
in this population and the increased risk of nephrolithiasis.
Geriatric Use
Clinical studies of CRIXIVAN did not include sufficient numbers of subjects aged 65 and over to
determine whether they respond differently from younger subjects. In general, dose selection for an
elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal or cardiac
function and of concomitant disease or other drug therapy.
ADVERSE REACTIONS
Clinical Trials in Adults
Nephrolithiasis/urolithiasis, including flank pain with or without hematuria (including microscopic
hematuria), has been reported in approximately 12.4% (301/2429; range across individual trials: 4.7% to
34.4%) of patients receiving CRIXIVAN at the recommended dose in clinical trials with a median follow-up
of 47 weeks (range: 1 day to 242 weeks; 2238 patient-years follow-up). The cumulative frequency of
nephrolithiasis events increases with duration of exposure to CRIXIVAN; however, the risk over time
remains relatively constant. Of the patients treated with CRIXIVAN who developed
nephrolithiasis/urolithiasis in clinical trials during the double-blind phase, 2.8% (7/246) were reported to
develop hydronephrosis and 4.5% (11/246) underwent stent placement. Following the acute episode,
4.9% (12/246) of patients discontinued therapy. (See WARNINGS and DOSAGE AND
ADMINISTRATION, Nephrolithiasis/Urolithiasis.)
Asymptomatic hyperbilirubinemia (total bilirubin 2.5 mg/dL), reported predominantly as elevated
indirect bilirubin, has occurred in approximately 14% of patients treated with CRIXIVAN. In <1% this was
associated with elevations in ALT or AST.
Hyperbilirubinemia and nephrolithiasis/urolithiasis occurred more frequently at doses exceeding
2.4 g/day compared to doses 2.4 g/day.
Clinical adverse experiences reported in 2% of patients treated with CRIXIVAN alone, CRIXIVAN in
combination with zidovudine or zidovudine plus lamivudine, zidovudine alone, or zidovudine plus
lamivudine are presented in Table 10.
19
Table 10: Clinical Adverse Experiences Reported in 2% of Patients
Study 028
Considered Drug-Related and of Moderate or Severe
Intensity
Study ACTG 320
of Unknown Drug Relationship
and of Severe or Life-threatening
Intensity
Percent
(n=332)
CRIXIVAN plus
Zidovudine plus
Lamivudine
Percent
(n=571)
Zidovudine
plus
Lamivudine
Percent
(n=575)
16.0
4.2
1.5
2.7
12.0
3.6
2.1
1.8
1.9
2.4
3.8
0
0.7
4.5
3.0
0
11.7
3.3
8.4
2.7
2.7
2.1
1.5
1.5
31.9
3.0
17.8
5.4
5.4
1.5
2.7
2.1
19.6
2.4
9.0
1.8
3.0
1.2
0.9
0.3
2.8
0.9
1.4
0.4
0.5
0
0
0
1.4
1.2
1.4
0
0.2
0
0
0
Hemic and Lymphatic System
Anemia
0.6
1.2
2.1
2.4
3.5
Musculoskeletal System
Back pain
8.4
4.5
1.5
0.9
0.7
Nervous System/Psychiatric
Headache
Dizziness
Somnolence
5.4
3.0
2.4
9.6
3.9
3.3
6.0
0.9
3.3
2.4
0.5
0
2.8
0.7
0
Skin and Skin Appendage
Pruritus
Rash
4.2
1.2
2.4
0.6
1.8
2.4
0.5
1.1
0
0.5
1.5
0
0.3
0.6
0.6
0.3
1.6
1.8
1.0
1.0
Urogenital System
Nephrolithiasis/urolithiasis*
Dysuria
8.7
1.5
7.8
2.4
2.1
0.3
2.6
0.4
0.3
0.2
Special Senses
Taste perversion
2.7
8.4
1.2
0.2
0
Zidovudine
Percent
(n=332)
CRIXIVAN
plus
Zidovudine
Percent
(n=332)
Body as a Whole
Abdominal pain
Asthenia/fatigue
Fever
Malaise
16.6
2.1
1.5
2.1
Digestive System
Nausea
Diarrhea
Vomiting
Acid regurgitation
Anorexia
Appetite increase
Dyspepsia
Jaundice
CRIXIVAN
Adverse Experience
Respiratory System
Cough
Difficulty breathing/
dyspnea/shortness of breath
*Including renal colic, and flank pain with and without hematuria
In Phase I and II controlled trials, the following adverse events were reported significantly more
frequently by those randomized to the arms containing CRIXIVAN than by those randomized to
nucleoside analogues: rash, upper respiratory infection, dry skin, pharyngitis, taste perversion.
Selected laboratory abnormalities of severe or life-threatening intensity reported in patients treated
with CRIXIVAN alone, CRIXIVAN in combination with zidovudine or zidovudine plus lamivudine,
zidovudine alone, or zidovudine plus lamivudine are presented in Table 11.
20
Table 11: Selected Laboratory Abnormalities of Severe or Life-threatening Intensity Reported in Studies 028 and ACTG 320
Percent
(n=329)
Study 028
CRIXIVAN
plus
Zidovudine
Percent
(n=320)
0.6
0.9
3.3
2.4
3.5
0.9
0.9
1.8
0.2
0.9
2.4
2.2
6.7
5.1
14.6
4.9
4.1
3.0
2.6
2.6
3.7
2.8
2.7
3.3
2.8
11.9
9.7
0.6
6.1
1.4
2.1
1.9
1.8
0.9
0.3
0.9
0.9
0.6
1.6
1.9
0
0
0.6
0.2
0
CRIXIVAN
Hematology
Decreased hemoglobin
<7.0 g/dL
Decreased platelet count
<50 THS/mm3
Decreased neutrophils
<0.75 THS/mm3
Blood chemistry
Increased ALT
>500% ULN*
Increased AST
>500% ULN
Total serum bilirubin
>250% ULN
Increased serum amylase
>200% ULN
Increased glucose
>250 mg/dL
Increased creatinine
>300% ULN
Zidovudine
Percent
(n=330)
Study ACTG 320
Zidovudine
CRIXIVAN plus
plus
Zidovudine plus
Lamivudine
Lamivudine
Percent
Percent
(n=575)
(n=571)
*Upper limit of the normal range.
Post-Marketing Experience
Body As A Whole: redistribution/accumulation of body fat (see PRECAUTIONS, Fat Redistribution).
Cardiovascular System: cardiovascular disorders including myocardial infarction and angina pectoris;
cerebrovascular disorder.
Digestive System: liver function abnormalities; hepatitis including reports of hepatic failure (see
WARNINGS); pancreatitis; jaundice; abdominal distention; dyspepsia.
Hematologic: increased spontaneous bleeding in patients with hemophilia (see PRECAUTIONS);
acute hemolytic anemia (see WARNINGS).
Endocrine/Metabolic: new onset diabetes mellitus, exacerbation of pre-existing diabetes mellitus,
hyperglycemia (see WARNINGS).
Hypersensitivity: anaphylactoid reactions; urticaria; vasculitis.
Musculoskeletal System: arthralgia, periarthritis.
Nervous System/Psychiatric: oral paresthesia; depression.
Skin and Skin Appendage: rash including erythema multiforme and Stevens-Johnson syndrome;
hyperpigmentation; alopecia; ingrown toenails and/or paronychia; pruritus.
Urogenital System: nephrolithiasis/urolithiasis, in some cases resulting in renal insufficiency or acute
renal failure, pyelonephritis with or without bacteremia (see WARNINGS); interstitial nephritis sometimes
with indinavir crystal deposits; in some patients, the interstitial nephritis did not resolve following
discontinuation of CRIXIVAN; renal insufficiency; renal failure; leukocyturia (see PRECAUTIONS),
crystalluria; dysuria.
Laboratory Abnormalities
Increased serum triglycerides; increased serum cholesterol.
OVERDOSAGE
There have been more than 60 reports of acute or chronic human overdosage (up to 23 times the
recommended total daily dose of 2400 mg) with CRIXIVAN. The most commonly reported symptoms
were renal (e.g., nephrolithiasis/urolithiasis, flank pain, hematuria) and gastrointestinal (e.g., nausea,
vomiting, diarrhea).
It is not known whether CRIXIVAN is dialyzable by peritoneal or hemodialysis.
21
DOSAGE AND ADMINISTRATION
The recommended dosage of CRIXIVAN is 800 mg (usually two 400-mg capsules) orally every
8 hours.
CRIXIVAN must be taken at intervals of 8 hours. For optimal absorption, CRIXIVAN should be
administered without food but with water 1 hour before or 2 hours after a meal. Alternatively, CRIXIVAN
may be administered with other liquids such as skim milk, juice, coffee, or tea, or with a light meal, e.g.,
dry toast with jelly, juice, and coffee with skim milk and sugar; or corn flakes, skim milk and sugar. (See
CLINICAL PHARMACOLOGY, Effect of Food on Oral Absorption.)
To ensure adequate hydration, it is recommended that adults drink at least 1.5 liters (approximately
48 ounces) of liquids during the course of 24 hours.
Concomitant Therapy (See CLINICAL PHARMACOLOGY, Drug Interactions, and/or PRECAUTIONS,
Drug Interactions.)
Delavirdine
Dose reduction of CRIXIVAN to 600 mg every 8 hours should be considered when administering
delavirdine 400 mg three times a day.
Didanosine
If indinavir and didanosine are administered concomitantly, they should be administered at least one
hour apart on an empty stomach (consult the manufacturer's product circular for didanosine).
Itraconazole
Dose reduction of CRIXIVAN to 600 mg every 8 hours is recommended when administering
itraconazole 200 mg twice daily concurrently.
Ketoconazole
Dose reduction of CRIXIVAN to 600 mg every 8 hours is recommended when administering
ketoconazole concurrently.
Rifabutin
Dose reduction of rifabutin to half the standard dose (consult the manufacturer's product circular for
rifabutin) and a dose increase of CRIXIVAN to 1000 mg every 8 hours are recommended when rifabutin
and CRIXIVAN are coadministered.
Hepatic Insufficiency
The dosage of CRIXIVAN should be reduced to 600 mg every 8 hours in patients with mild-tomoderate hepatic insufficiency due to cirrhosis.
Nephrolithiasis/Urolithiasis
In addition to adequate hydration, medical management in patients who experience
nephrolithiasis/urolithiasis may include temporary interruption (e.g., 1 to 3 days) or discontinuation of
therapy.
HOW SUPPLIED
CRIXIVAN Capsules are supplied as follows:
No. 3756 — 200 mg capsules: semi-translucent white capsules coded "CRIXIVAN™ 200 mg" in blue.
Available as:
NDC 0006-0571-43 unit-of-use bottles of 360 (with desiccant).
No. 3758 — 400 mg capsules: semi-translucent white capsules coded "CRIXIVAN™ 400 mg" in
green. Available as:
NDC 0006-0573-62 unit-of-use bottles of 180 (with desiccant)
Storage
Bottles: Store in a tightly-closed container at room temperature, 15-30°C (59-86°F). Protect from
moisture.
CRIXIVAN Capsules are sensitive to moisture. CRIXIVAN should be dispensed and stored in the
original container. The desiccant should remain in the original bottle.
22
For patent information: www.merck.com/product/patent/home.html
Copyright © 1996, 1997, 1998, 1999, 2004 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
All rights reserved.
Revised 03/2015
uspi-mk0639-c-1503r017
23


