Harvard-MIT Division of Health Sciences and Technology
advertisement

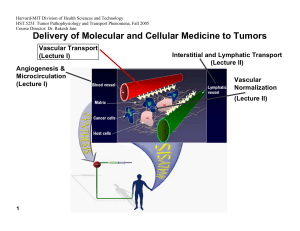
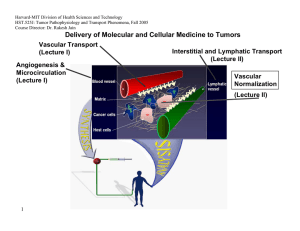
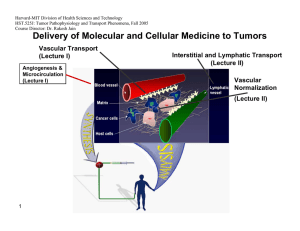
Harvard-MIT Division of Health Sciences and Technology HST.525J: Tumor Pathophysiology and Transport Phenomena, Fall 2005 Course Director: Dr. Rakesh Jain Delivery of Molecular and Cellular Medicine to Tumors Vascular Transport (Lecture I) Angiogenesis & Microcirculation (Lecture I) Interstitial and Lymphatic Transport (Lecture II) Vascular Normalization (Lecture Il) 1 DELIVERY OF MOLECULAR MEDICINE TO TUMORS Lecture II: Interstitial and Lymphatic Transport OVERVIEW R.K. Jain, “Transport of Macromolecules in the Tumor Interstitium: A Review,” Cancer Research, 3038- 47:3050 (1987). R.K. Jain, “1996 Landis Award Lecture: Delivery of Molecular and Cellular Medicine to Solid Tumors,” Microcirculation, 4:1-23 (1997). R.K. Jain, and B.T. Fenton, “Intra-Tumoral Lymphatic Vessels: A Case of Mistaken Identity or Malfunction?” JNCI, 94:417-421 (2002). R.K. Jain, and T.P. Padera, “Prevention and Treatment of Lymphatic Metastasis by Anti-Lymphangiogenic Therapy,” JNCI, 94:785-787 (2002). Jussila L, Alitalo K, “Vascular growth factors and lymphangiogenesis,” Physiology Reviews, 82:673-700 (2002). R.K. Jain and T. P. Padera, “ Lymphatics Make the Break,” Science. 299: 209-210 (2003) R.K. Jain, “Molecular Regulation of Vessel Maturation,” Nature Medicine, 9:685-693 (2003). R.K. Jain “Vascular and Interstitial Biology of Tumors,” In: Clinical Oncology, 3rd Edition (Editors: M. Abeleff, J. Armitage, J. Niederhuber, M. Kastan and G. McKenna), Elsevier, Philadelphia, PA, Chapter 9, pp. 153-172 (2004). 2005 Interstitial Transport S.R. Chary and R.K. Jain, “Direct Measurement of Interstitial Diffusion and Convection of Albumin in Normal and Neoplastic Tissues Using Fluorescence Photo-bleaching,” PNAS, 86:5385-5389 (1989). D.A. Berk, F. Yuan, M. Leunig and R.K. Jain, “Direct in vivo Measurement of Targeted Binding in a Human Tumor Xenograft, “ PNAS, 94:1785-1790 (1997). A. Pluen, P.A. Netti, R.K. Jain, and D.A. Berk, “Diffusion of Macromolecules in Agarose Gels: Comparison of Linear and Globular Configurations,” Biophysical Journal, 77: 542-552 (1999). P.A. Netti, D.A. Berk, M.A. Swartz, A.J. Grodzinsky and R.K. Jain, “Role of Extracellular Matrix Assembly in Interstitial Transport in Solid Tumors,” Cancer Research, 60: 2497-2503 (2000). A. Pluen, Y. Boucher, S. Ramanujan, T.D. McKee, T. Gohongi, E. di Tomaso, E.B. Brown, Y. Izumi, R.B. Campbell, D.A. Berk, R.K. Jain, "Role of Tumor-Host Interactions in Interstitial Diffusion of Macromolecules: Cranial vs. Subcutaneous tumors," PNAS, 98: 4628-4633 (2001). C. de L. Davies, D.A. Berk, A. Pluen, and R.K. Jain, “Comparison of IgG Diffusion and Extracellular Matrix Composition in Rhabdomyosarcomas Grown in Mice Versus In Vitro as Spheroids Reveals the Role of Host Stromal Cells,” BJC, 86:16391644 (2002) S. Ramanujan, A. Pluen, R.D. McKee, E.B. Brown, Y. Boucher, R.K. Jain, “Diffusion and Convection in Collagen Gels: Implications for Transport in the Tumor Interstitium,” Biophysical Journal. 83: 1650-1660 (2002). E. Brown, T. McKee, E. di Tomaso, B. Seed, Y. Boucher, R.K. Jain, “Dynamic Imaging of Collagen and its Modulation in Tumors in vivo using Second Harmonic Generation,”Nature Medicine. 9: 796-801 (2003). G. Alexandrakis, E. B. Brown, R. T. Tong, T. D. McKee, R. B. Campbell, Y. Boucher and R. K. Jain, “Two-photon fluorescence correlation microscopy reveals the two-phase nature of transport in tumors,” Nature Medicine, 10:203-207 (2004). E. B. Brown, Y. Boucher, S. Nasser, and R. K. Jain,”Measurement of Macromolecular Diffusion Coefficients in Human Tumors”, Microvascular Research, 67 231-236 (2004). 3 2005 Lymphatic Transport A. Leu, D.A. Berk, F. Yuan and R.K. Jain, “Flow Velocity in the Superficial Lymphatic Network of the Mouse Tail,” American Journal of Physiology, 267:H1507-H1513 (1994). M.A. Swartz, D.A. Berk and R.K. Jain, “Transport in Lymphatic Capillaries: I. Macroscopic Measurements Using Residence Time Distribution Theory,” American Journal of Physiology, 270:H324-H329 (1996). D.A. Berk, M.A. Swartz, A.J. Leu, and R.K. Jain, “Transport in Lymphatic Capillaries: II. Microscopic Velocity Measurement with Fluorescence Recovery After Photobleaching,” American Journal of Physiology, 270:H330-H337 (1996). M. Jeltsch, A. Kaipainen, M. Swartz, D. Fukumura, R.K. Jain, and K. Alitalo, “Hyperplasia of Lymphatic Vessels in VEGFC Transgenic Mice,” Science, 276:1423-1425 (1997). S.A. Slavin, A.D. van den Abbeele, A. Losken, M. Swartz, and R.K. Jain, “Return of Lymphatic Function Following Flap Transfer for Acute Lymphedema,” Annals of Surgery, 229:421-427 (1999). M.A. Swartz, A. Kaipainen, P. Netti, C. Brekken, Y. Boucher, A.J.Grodzinsky and R.K.Jain, “Mechanics of InterstitialLymphatic Fluid Transport: Theoretical Foundation and Experimental Validation,” Journal of Biomechanics, 32: 1297-1303 (1999). A.J. Leu, D.A. Berk, A. Lymboussaki, K. Alitalo and R.K. Jain, “Absence of Functional Lymphatics within a Murine Sarcoma: a Molecular and Functional Evaluation,” Cancer Research, 60: 4324-4327 (2000). M.A. Swartz, A. Kaipainen, P. Netti, C. Brekken, Y. Boucher, A.J.Grodzinsky and R.K.Jain, “Mechanics of InterstitialLymphatic Fluid Transport: Theoretical Foundation and Experimental Validation,” Journal of Biomechanics, 32: 1297-1303 (1999). A.J. Leu, D.A. Berk, A. Lymboussaki, K. Alitalo and R.K. Jain, “Absence of Functional Lymphatics within a Murine Sarcoma: a Molecular and Functional Evaluation,” Cancer Research, 60: 4324-4327 (2000). A. Kadambi, C.M. Carreira, C.-O. Yun, T.P. Padera, D.E.J.G.J. Dolmans, P. Carmeliet, D. Fukumura and R.K. Jain. “Vascular endothelial Growth Factor (VEGF)-C Differentially Affects Tumor Vascular Function and Leukocyte Recruitment: Role of VEGF-Receptor 2 and Host VEGF-A,” Cancer Research, 61: 2404-2408 (2001). 4 2005 C. Mouta Carreira, S.M. Nasse, E. diTomaso, T.P. Padera, Y. Boucher, S.I. Tomarev, R.K. Jain, “LYVE-1 is not Restricted to the Lymph Vessels: Expression in Normal Liver Blood Sinusoids and Down-regulation in Human Liver Cancer and Cirrhosis,” Cancer Research, 61:8079-8084 (2001). T.P. Padera, B.R. Stoll, P.T.C. So, and R.K. Jain, “High-Speed Intravital Multiphoton Laser Scanning Microscopy of Microvasculature, Lymphatics, and Leukocyte-Endothelial Interactions,” Molecular Imaging, 1:9-15 (2002) T.P. Padera, E. di Tomaso, A. Kadambi, C. Mouta Carreira, E.B. Brown, Y. Boucher, N.C. Choi, D. Mathisen, J. Wain, E.J. Mark, L.L. Munn, R.K. Jain, “Lymphatic Metastasis in the Absence of Functional Intratumor Lymphatics,” Science, 296:1883-1886 (2002) T.P. Padera, E. di Tomaso, A. Kadambi, C. Mouta Carreira, E.B. Brown, Y. Boucher, N.C. Choi, D. Mathisen, J. Wain, E.J. Mark, L.L. Munn, R.K. Jain, “Lymphatic Metastasis in the Absence of Functional Intratumor Lymphatics,” Science, 296:1883-1886 (2002) T. Padera, B. Stoll, J. Tooredman, D. Capen, E. di Tomaso, and R. K. Jain, “Cancer cells compress intratumor vessels,” Nature, 427: 695 (2004). J. Hagendoorn, T.P. Padera, S. Kashiwagi, N. Isaka, F. Noda, M.I. Lin, P.L.Huang, W.C. Sessa, D. Fukumura, and R.K. Jain. “Endothelial nitric oxide synthase regulates microlymphatic flow via collecting lymphatics,” Circulation Research, 95: 204-9 (2004). N. Isaka, T. P. Padera, J. Hagendoorn, D. Fukumura, and R. K. Jain, “Peritumor lymphatics induced by vascular endothelial growth factor-C exhibit abnormal function,” Cancer Research, 64: 4400-4404 (2004). 5 2005 Interstitial Transport Figure by MIT OCW. 6 2005 Measuring Convection, Diffusion and Binding 7 2005 Fluorescence Recovery After Photobleaching I0 I/I0 0 8 time 5 2005 Typical Values of Interstitial Convective Velocities & Directions 9 Reference: Chary & Jain, PNAS (1989) 2005 Interstitial Diffusion of IgG in LS174T Xenograft Dmean = 1.3 x10-7 50 Count 40 30 D0 20 10 0 10-8 10-7.5 10-7 10-6.5 10-6 2 D (cm /s) 1 0.8 0.6 D/D0 0.4 0.2 0 10 Fab' Albumin IgG Diffusing molecule Reference: Berk et al. PNAS (1997) 2005 Time Constant for Diffusion 11 Distance IgG Fab 100 µm ~ 0.5 hours ~ 0.2 hours 1 mm ~ 2–3 days ~ 0.5–1 days 1 cm ~ 7 months ~ 2 months Plasma Half-life ~ days ~ hours 2005 Specific Antibody 1 sec 10 sec 100 sec Non-Specific Antibody 12 Reference: Berk et al. PNAS (1997) 2005 Effect of Molecular Configuration on Diffusion 10 -5 10 -6 2 s -1 in 0.1M PBS Diffusion coefficient <r > p 10 -7 10 -8 10 -9 10 <rp>: experimental pore radius "Spherical" molecules Molecular size DNA -10 1 10 100 Experimental Hydrodynamic Radius, R 1000 H , nm 13 Reference: Pluen et al. Biophysical Journal (1999) Interstitial diffusion varies with site of growth Dorsal window Diffusion coefficient (cm2s-1) 2005 Cranial window 10-5 10-6 10-7 10-8 10-9 10-10 0.1 14 1 10 100 Hydrodynamic radius (nm) Courtesy of National Academy of Sciences, U. S. A. Used with permission. Source: Tomaso, E., E. B. Brown, Y. Izumi, R. B. Campbell, D. A. Berk, R. K. Jain. "Role of tumor-host interactions in interstitial diffusion of macromolecules: cranial vs. subc Pluen A, Boucher Y, Ramanujan S, McKee TD, Gohongi T, di utaneous tumors." Proc Natl Acad Sci 98 (2001): 4628-4633. (c) National Academy of Sciences, U.S.A. 2005 Tumor-Host Interaction Influences Drug Transport Image removed for copyright reasons. Multicell spheroid human squamous cell carcinoma. See: Science 240, no. 4849 (April 8, 1988): cover page. 15 Figure by MIT OCW. After Jain. 2005 Role of Collagen in Interstitial Transport 15 10 Collagen content (mg/g) 5 0 MCa-IV LS174T HSTS U87 Tumor Increase in D (%) 16 120 100 80 60 40 20 0 -20 Treatment: saline collagenase (N=3) (N=4) Reference: Netti et al. Cancer Research (2000) 2005 Collagen Restricts HSV Distribution 50 µm Fibrillar collagen HSV particles (150 nm) 17 Reference: McKee, Grandi, Mok et al., submitted (2005) 2005 Collagenase Co-Injection Improves Virus Distribution 1 mm Virus Alone 1 mm 18 Collagenase Co-injection Reference: McKee, Grandi, Mok et al., submitted (2005) 2005 Collagenase Improves Efficacy of MGH2 Oncolytic Viral Therapy Courtesy of the American Association for Cancer Research. Used with permission. From McKee, T. D., P. Grandi, W. Mok, G. Alexandrakis, N. Insin, J. P. Zimmer, M. G. Bawendi, Y. Boucher, X. O. Breakefield, and R. K. Jain. "Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector." Cancer Research 66 (2006): 2509-2513. 19 2005 Relaxin fibroblasts Decreases collagen synthesis 20 Decreased collagen production Increases MMPs Decreases TIMPs Increased collagen degradation 2005 Relaxin treatment modifies collagen structure Day 0 Day 2 Day 5 Day 8 Day 9 Day 12 75 µm relaxin 21 placebo Reference: Brown, McKee et al., Nature Medicine (2003) 2005 Relaxin treatment enhances macromolecular transport 2 -1 Diffusion coefficients, D, cm s 4 3.5 10 -7 3.0 10 -7 2.5 10 -7 2.0 10 -7 1.5 10 -7 1.0 10 -7 5.0 10 -8 3.5 3 2.5 2 1.5 1 0.5 7.0 10 -9 6.0 10 -9 5.0 10 -9 4.0 10 -9 3.0 10 -9 2.0 10 -9 1.0 10 -9 0 w/o Relaxin 1 22 IgG w Relaxin 1.5 w/o Relaxin 2 w Relaxin 2.5 Dextran 2,000,000 MW Reference: Brown, McKee et al. Nature Medicine (2003) 2005 Summary • FRAP can provide direct measurements of diffusion, convection and binding in vivo • Convection ~ 0.1 - µm/sec • Diffusion ~ 1/3 of water • Non-specific binding ~ 1/4 - 1/3 • Interfering with collagen synthesis and/or assembly may enhance interstitial transport. • Interstitial diffusion depends on the host-tumor interaction. • Fluorescence correlation microscopy can reveal the two-phase nature of transport in tumors. 23 2005 25 Courtesy of Lance Munn. Used with permission. 2005 Intravital Lymphangiography 26 Reference: Leu et al. Am J Physiol. (1994) Hagendoorn et al. (2004) 2005 27 2005 Lack of Functional Lymphatics in Tumors Normal tail Tumor bearing Courtesy of the American Association for Cancer Research. Used with permission. From Leu, A. J., D. A. Berk, A. Lymboussaki, K. Alitalo, and R. K. Jain. "Absence of functional lymphatics within a murine sarcoma: a molecular and functional evaluation." Cancer Research 60 (2000): 4324-4327. 28 2005 VEGF-C Induces Lymphatic Hyperplasia Control – Lymph Vessels VEGF-C Induced Hyperplasia 29 Reference: Jeltsch et al. Science, (1997) 2005 Effect of VEGF-C on Lymphatic Metastases Property P-value* Cell Line B16F10-MT B16F10-VEGF-C Lymphatic Metastasis 6/21 15/19 Local Invasion 3/21 12/18 5/22 2/19 Lung Metastasis† 0.002 0.001 N.S. T-241-MT Lymphatic Metastasis 2/14 Local Invasion 4/14 † Lung Metastasis 5/14 0.046 N.S. N.S. T-241-VEGF-C 8/14 5/14 6/14 Numerator is the number of mice that exhibited the property, denominator is the total number of mice. *: P-value based on Fisher’s Exact Test. N.S.: not significant †: Hematogenous metastasis was analyzed microscopically in 10-20 sections of lung per animal. 30 Reference: Padera et al., Science, (2002) 2005 Lymphatic Function Studies • Interstitial fluid pressure measurements • Epifluorescence lymphangiography • Multi-photon laser scanning microscopy • Ferritin lymphangiography Immunohistochemistry • Lectin, CD31, LYVE-1, Prox 1 31 2005 IFP is not changed by VEGF-C overexpression 25 * 20 * 15 10 5 0 Normal tissue T-241-MT T-241-VC+ p < 0.05 * compared to normal tissue 32 Reference: Padera et al., Science, (2002) 2005 Diameter (microns) VEGF-C induced hyperplasia of peri-tumor lymphatics 60 50 40 30 20 10 0 MT VEGF-C Green: Lymphatic vessels Red: Blood vessels 33 Reference: Padera et al., Science, (2002) 2005 HCCs in Patients Lack Lymphatics Lymphatic vessels Peritumor Tumor •42 biopsies of liver tumor (HCC + metastases) examined •No staining for Prox 1 + LYVE-1 was observed in the tumor area Courtesy of the American Association for Cancer Research. Used with permission. From C, Mouta Carreira, S. M. Nasser, E. di Tomaso, T. P. Padera, Y. Boucher, S. I. Tomarev, and R. K. Jain. "LYVE-1 is not restricted to the lymph vessels: expression in normal liver blood sinusoids and down-regulation in human liver cancer and cirrhosis." Cancer Research 61 (2001): 8079-8084. 34 2005 Human Lung Tumors Lymphatic vessels Tumor edge Lymphatic vessels 875 microns 200 microns IFP = 9.5 ± 1.5 mmHg n = 27 35 Reference: Padera et al., Science (2002) 2005 Fluid pressure can compress impermeable objects Fluid pressure can NOT compress permeable vessels, such as blood and lymphatic vessels Solid stress can compress permeable vessels, such as blood and lymphatic vessels 36 Courtesy of Lance Munn. Used with permission. 2005 DT treatment opens lymphatic vessels 37 Reference: Padera, Stoll et al. Nature, (2004) Peritumor lymphatics induced by VEGF-C exhibit abnormal function 2005 Normal tissue B16F10-MT B16F10-VEGF-C T Lymphatics T Retrograde lymphatics * 38 10 10 5 5 0 0 Control MT VEGF-C * Control MT VEGF-C Courtesy of the American Association for Cancer Research. Used with permission. From Isaka, N., T. P. Padera, J. Hagendoorn, D. Fukumura, and R. K. Jain. "Peritumor lymphatics induced by vascular endothelial growth factor-C exhibit abnormal function." Cancer Research 64 (2004): 4400-4404. 2005 Image removed for copyright reasons. See: Jain, R.K., and B.T. Fenton. "Intra-Tumoral Lymphatic Vessels: A Case of Mistaken Identity or Malfunction?" JNCI 94 (2002): 417-421. 39 2005 Summary • Despite the presence of VEGF-C and its receptor VEGFR3, functional lymphatics cannot be detected in tumors. • VEGF-C overexpression increases diameter and number of peritumor lymphatics. • Overexpression of VEGF-C and -D increases vascular angiogenesis. • Levels of VEGF-C and -D correlate with increased lymphatic and hematogeneous metastasis. • Blocking VEGF-C/-D can prevent lymphatic metastasis. 40 2005 Overall Summary Mechanisms of Heterogeneous Distribution of Macromolecules in Tumors • Heterogeneous blood flow • Elevated interstitial pressure • Fluid oozing from the periphery • Slow interstitial diffusion • Retardation by binding • Heterogeneous expression of antigens 41 2005 Strategies for Improved Delivery • Make the vasculature the target for therapy • Modify physiological parameters – Increase perfusion – Modulate interstitial pressure – Interfere with collagen synthesis (e.g., relaxin) – Use multi-step strategies – “Normalize” the abnormal tumor vasculature 42