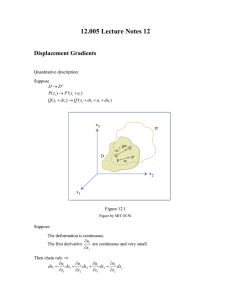
Recitation: 10/16/03

Recitation: 7
10/16/03
Solutions
Open Systems: Variables: S,V, N: dU = T dS − P dV +
�
µ i dn i i where
µ i
≡
∂U
�
∂n i
S,V,n j
For the Legendre Transforms, we have:
V
T S
G ≡ U − T S + P V
µ i
H
=
F
≡
≡
∂U
∂n i
U
U
�
+
−
P
S,V,n j
=
∂n
→
→
→ i
∂H
� dH = T dS + V dP + dF = − SdT − P dV +
�
µ i i �
µ i dn i dn i dG =
=
− sdT
∂F
�
+ V dP
=
+
�
µ i i
∂G
�
S,P,n j
∂n i
T ,V,n j
∂n i dn i
T,P,n j
The chemical potential, µ i has different definitions, depending on the potential. However, the def initions are equivalent, as can be seen in the previous equation.
Using the Euler Relations:
U = T S − P V +
�
H = T S +
F
G
=
=
−
� i
P
µ
V i n i
+ i
µ
� i n i
µ i i
�
µ i i n i n i
Using First Law, and Euler Relation for the Energy, one can get the GibbsDuhem Equation:
SdT − V dP +
� n i dµ i
= 0 i
The GibbsDuhem relation tells you that thermodynamic potentials in a system are not inde pendent. A change in one of the potentials has to be accompanied by a corresponding change in the rest.
At constant temperature and pressure, the GibbsDuhem relation implies that, by knowing the thermodynamic behavior of one of the components, i of the system, it is possible to determine the behavior of the rest. To do this we have to do a GibbsDuhem Integration.
For a composite system, at constant pressure and temperature, (at mechanical and thermal equilibrium), the condition of equilibrium is such that the system minimizes its Gibbs free energy by changing its composition, n i
. For each component for which there is no constraint in its transfer across the composite system’s internal boundaries, the equilibrium condition implies that:
µ
α i
α j
.
.
.
µ =
=
µ
µ
.
.
β i
β j
=
=
· · ·
· · ·
=
=
µ
µ
.
φ i
φ j
µ α k
= µ β k
= = µ φ k
For all φ phases and k components.
Note that this equilibrium condition is valid, as long as the internal variables that can change are not coupled. For example, it is common that solids change their molar volume as their com position changes, so, in this case, the condition of all the chemical potential of i being equal in all phases is not the correct one. force for the mass flow of i , � µ
So
When
− dn α i
µ α i
>
= dn β i
µ
β i
, and component i can pass across the α/β boundary, there will be a driving
. Component i i
= µ α i
− µ β i until the system reaches an equilibrium state. µ β i
= µ α i
.
flows from the high chemical potential region to the low chemical potential region. The mass flow of i is parallel to minus the gradient of the chemical potential of component i .
Partial Molar Quantities
For any extensive quantity, Y , it is possible to define a corresponding partial quantity Y i
:
∂Y
�
Y i
=
∂n i P,T ,n j
It is possible to define a change in the chemical potential µ i of component i : dµ i
( P, T, x i
)
P,T
= RT d ln ( a i
)
Where a i is an arbitrary activity function that just makes life easier when trying to describe the thermodynamics of the system. We can integrate the previous equation on both sides, obtaining:
µ i
( P, T, x i
)
P,T
= µ ∗ i
( P, T )
P,T
+ RT ln ( a i
) where µ ∗ i
( T, P ) is the reference state at the same P and T .
The standard state µ 0 i
( T, P = 1 atm.
) is at P = 1 atm.
.
In general,
µ ∗ i
− µ 0 i
=
� P
P 0
∂µ i
∂P
� P
=
¯ i
P 0
Since V i
∼ 0 for condensed matter, µ ∗ i
∼ µ 0 i for moderate pressures.
partial Pressures
When a gas is in equilibrium with a condensed phase, the activity of component i in the con densed phase is such that a i
= p i
P i where p i is the partial pressure of component i in the gas mixture and P i is the vapor pressure of when you have a gas of pure component i over a condensed phase composed only of i . i
In general, a i
= γ i x i where γ i is called the activity coefficient of i .
Raoult’s Law da
1 dx
1
� dγ
1 x
1 dx
1 � x
1
→ 1 dγ
1
=
= 0
γ
1 dx
1 x
1
� 1 d ln( γ
1
) dx
1 x �
1
→ 1
� x
1
= +1
→ 1
= 0
) x
1 d ln( γ
1
) dx
2 x
1
→ 1
= −
→ 1 d ln( γ
1
) dx
1
+
� x i dγ
1 dx
1 x
1
→ 1
� x
1
→ 1
= 0
= 1
Henry’s Law: x x da
2 dx
2 da
2 dx
2
2
2
�
� x
2
→ 0
= 0 , ∞}
�
= dγ
2 x
2 dx
2 x
2 x
2 dγ
2 dx
2
� d ln γ
2 dx
2
0 x � → 0
= 0 x
2
→ 0
= 0
→ 0
= γ ∞
2
+ x
2 dγ
2 dx
2
� x
2
→ 0
= γ ∞
2
Relationship between Henry’s and Raoult’s Laws:
The GibbsDuhem Equation tells you that: x
1 d ln γ
1
+ x
2 d ln γ
2
= 0
Differentiating the GibbsDuhem equation, with respect to x
2
, we have: x
1 d ln γ
1
� dx
2 x
2
→ 0
+ x
2 d ln dx
2
γ
2
� x
2
→ 0
= 0
If Henry’s Law is obeyed
� x
2 d ln γ
2 dx
2
� x
2
→ 0
= 0
�
, then Raoult’s Law has to be obeyed
� x
1 d ln γ
1 dx
2
� x
2
→ 0
= 0
�
.
The opposite is not true.
Raoult's Law
Intercept Rule: inf x
2
Henry's Law
Figure 1: Raoult’s Law and Henry’s Law
Y
M
Y
1
X
2
*
1 X
2
Figure 2: Intercepts Rule
Proof:
For a molar extensive property, Y
M
, in a binary system, we have:
Y
M
= x
1
¯
1
+ x
2
Y
2
2
Y
2
(1)
If we differentiate dY
M dx
2
, considering that x
1 dY dx
M
2
= −
¯
1
+ Y
2
+ x
2
= 1
+
� x
1
∂Y
1
∂x
2 and
+ dx x
2
1
∂x
=
2
−
¯
2
� dx
2
:
The last term (in brackets) is equal to zero because of the GibbsDuhem Equation, and we have:
¯
1
= Y
2
− dY
M dx
2
Substituting Eq. 2 into Eq. 1, we have:
Y
M
= x
1
�
¯
2
− dY
M
� dx
2
+ x
2
Y
2
Rearranging, we finally have:
Y
2
= Y
M
+ x
1 dY
M dx
2
For a Gibbs free energy, vs. composition curve, we have:
(2)
(3)
G
M
µ
1
µ
2
1 X
2
*
X
2
2
Figure 3: Intercept Rule: Molar Gibbs Energy and Chemical Potentials
Calculation of Solubility Limits:
For a system of components A and B , and assuming equilibrium between the liquid and the solid phases, the following condition must hold:
µ
µ
L
A
L
B
= µ
= µ
S
A
S
B
Assuming that component B in the solid phase does not dissolve any A ,
µ L
B
= µ L, 0
B
+ RT ln
� x L
B
�
= µ S
B
= µ S, 0
B
Therefore, x L
B
= exp
�
µ S, 0
B
− µ L, 0
B
RT
�
= exp
�
− Δ H m
RT
�
1 −
T
T m
��
Melting Point Depression
With the same example,
Δ Gm ( i ) = RT ln
� a S i a L i
�
The difference in the partial molar Gibbs free energy of i is just the difference in chemical potentials.
For B , we have:
Δ Gm ( B ) = RT ln
Δ Gm ( B )
RT
= ln
�
1 −
1 x
� a S
B
� a L
B
�
L
A
≈ x L
A
And x L
A
=
Δ H m
( B )
Δ T
R · ( T B
M
)
2
A lowers the melting point of B . Since A does not dissolve in solid B , at any given temperature below the melting point of B , there is a thermodynamic driving force for forming the liquid phase, which can dissolve A .
Ideal Solution a i
= x i for all i .
G m
= x
A
µ 0
A
+ x
B
µ 0
B
+ RT [ x
A ln ( x
A
) + x
B ln ( x
B
)]
Δ S mix
= − R [ x
A ln ( x
A
) + x
B ln ( x
B
)]
Ideal Entropy of Mixing is always positive.
→ Mixing is a highly irreversible process.
Δ H mix
= 0