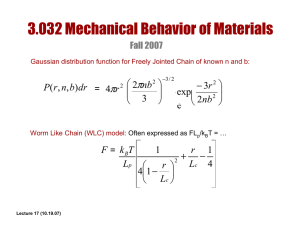
LECTURE 21: SINGLE CHAIN ELASTICITY OF BIOMACROMOLECULES:
advertisement

3.052 Nanomechanics of Materials and Biomaterials Thursday 05/03/07
Prof. C. Ortiz, MIT-DMSE
I
LECTURE 21: SINGLE CHAIN ELASTICITY OF
BIOMACROMOLECULES:
THE GIANT PROTEIN TITIN AND DNA
Outline :
REVIEW LECTURE #20 : EXTENSIBLE FJC AND WLC .......................................................................... 2
STRUCTURE OF MUSCLE AND TITIN .................................................................................................... 3
SINGLE MOLECULE ELASTICITY OF TITIN (AFM) ................................................................................ 4
SINGLE MOLECULE ELASTICITY OF DNA ............................................................................................ 5
Motivation........................................................................................................................... 5
Optcal Tweezers Data........................................................................................................ 6
Objectives:
To understand the elasticity of biopolymers and they differ from random coil entropic
elasticity
Readings: Course Reader Documents 40-43
Multimedia : Fibronectin and Titin unfolding simulation movies
.
1
3.052 Nanomechanics of Materials and Biomaterials Thursday 05/03/07
Prof. C. Ortiz, MIT-DMSE
REVIEW LECTURE 20 : EXTENSIBLE FJC AND WLC
0
-0.1
-0.1
a = 0.1 nm
a = 0.2 nm
a = 0.3 nm
a = 0.6 nm
a = 1.2 nm
a = 3.0 nm
-0.3
-0.4
-0.5
F
-0.2
n=100
-0.3
n=200
n=300
n=400
n=500
-0.4
-0.5
0
50
100
150
200
0
r (nm)
100
200
r (nm)
WLC
300
-0.1
Felastic
extensible
nonGaussian
FJC
-0.2
nonGaussian
FJC
-0.3
-0.4
-0.5
0
0
felastic (nN)
r
Felastic
0
felastic (nN)
-0.2
Felastic (nN)
0
Extensible FJC
≈
Felastic (nN)
-Effect of a and n on the inextensible FJC
100
200
300
400
r (nm)
-0.2
L total = L contour +
123
non-Gaussian
FJC
-0.4
=na
⎛ f ⎞
n⎜
⎟⎟
⎜k
segment ⎠
⎝
14243
extension beyond Lcontour
due to enthalpic stretching of
chain segments
-0.6
WLC
-0.8
-1
0
20
40
60
r (nm)
80
100
"Directed random walk"- segments are correlated,
polymer chains intermediate between a rigid rod and a
flexible coil (e.g. DNA)
- takes into account both local stiffness and long range
flexibility
-chain is an isotropic, homogeneous elastic rod whose
trajectory varies continuously and smoothly through
space as opposed to the jagged contours of FJC
p= persistence length, length over which statistical
segments remain directionally correlated in space
2
3.052 Nanomechanics of Materials and Biomaterials Thursday 05/03/07
Prof. C. Ortiz, MIT-DMSE
STRUCTURE OF MUSCLE AND TITIN
(*MARSZALEK, et. al Nature 402, 100 - 103 (1999))
Image removed due to copyright restrictions.
Figure by MIT OCW.
Figure by MIT OCW.
Sarcomere- fundamental contractile unit of muscle
-many proteins exhibit a modular motif (spectrin, fibronectic, seashell nacre, bone)
Titin:
- largest known protein
(1-3 mm in length)≈
25,000 amino acids
(a.a.)
-modular structure,
linear array of folded
immunoglobulin
domains covalently
attached in series
("beads on a string")
-subunit 7-stranded βbarrel
-highly extensible, "giant
rubberband"
(Lfolded domain = 3 nm
( ~90 a.a.), Lunfolded
domain = 30 nm)
-plays a major role in
the passive elasticity of
muscle; serves as an
anchoring spring to
keep myosin aligned on
actin tracks, resist large
sarcomere lengths,
allows for overstretching
of muscles without
permanent damage to
the sarcomere
3
3.052 Nanomechanics of Materials and Biomaterials Thursday 05/03/07
Prof. C. Ortiz, MIT-DMSE
SINGLE MOLECULE ELASTICITY OF TITIN-AFM (Rief, et al. CHEMPHYSCHEM 2002, 3, 255-261)
Distance = r chain end
to end distance
Figure by MIT OCW.
-domain "breaks" - rupture of some critical noncovalent interactions needed to keep stability of folded structure
-domain "extends" - even though there is some noncovalent rupture, entropic elasticity dominates
# of unfolded domains = # of peaks - 1 (last peak) - any short range nonspecific substrate peak
Lcontour (entire folded protein) = D at first unfolding peak
Lcontour (unfolded module) = D at 2nd unfolding peak -3 (folded domain lengths) - distance between peaks
- Sawtooth force profile : sequential unfolding (weakest to strongest) of domains where each peak corresponds to the unfolding
(mechanical denaturation) nanomechanical properties of an individual module or domain (many domains in series lead to huge
extensibility)
4
3.052 Nanomechanics of Materials and Biomaterials Thursday 05/03/07
Prof. C. Ortiz, MIT-DMSE
SINGLE MOLECULE ELASTICITY OF DNA - MOTIVATION
solenoid
chromatin
fibre
{
Individual histone octamer
nucleosomes
DNA
Figure by MIT OCW.
histone octamer
Figure by MIT OCW.
In an average human cell 2 meters of DNA (hydrated!) has to fit into a 10 μm diameter while still maintaining accessibility
to proteins and enzymes.
- The compaction of DNA is achieved by winding it around small proteins called histones
- Histones are composed of many positively charged amino acids that form ionic bonds to the negatively charged groups
on DNA (polyelectrolyte).
-Elasticity of DNA is critically important to this process-has to be just the right stiffness (too stiff- will be too hard to bend
around histones).
5
3.052 Nanomechanics of Materials and Biomaterials Thursday 05/03/07
Prof. C. Ortiz, MIT-DMSE
SINGLE MOLECULE ELASTICITY OF DNA - OPTCAL TWEEZERS
(Bustamante, et al. Science 1999, 271, 795)
overstretching transition
I. low stretched behaves like WLC (p ≈
50 nm under physiological conditions,
much larger than most polymers ~
1nm, hence much smaller forces,
need optical tweezers)
Force (pN)
II. intermediate stretches -some
extensibility as apparent by finite
slope beyond Lcontour (B-form)
III. At 65 pN ~ 0.06 nN, reversible
strain-induced conformational
transition; chain "yields" and stretches
out almost 2× its native B-form
contour length at relatively constant
force (plateau in force region)
-All of hydrogen bonding and binding
between 2 strands is still in tact, tilting
of base pairs, tightened helix,
reduction in diameter
"overstretching transition"
IV. entropic elasticity of S-form
Distance (μm)
V. can't see here - if you go to high
enough stretches, separation between
strains (mechanical "melting")
6
3.052 Nanomechanics of Materials and Biomaterials Thursday 05/03/07
Prof. C. Ortiz, MIT-DMSE
AFM SINGLE MOLECULE FORCE SPECTROSCOPYOF DNA
(Rief, et al. Nature Structural Biology 6, 346, 1999)
Biological Relevance of Overstretching Transition? Ability to switch between different structures is critical to the
processes of transcription, replication, condensaton, e.g. the base pairs are much more exposed in S-DNA than normal
DNA, the transition may be biologically significant for accessing information contained in the DNA code
V. At 150 pN another transition is found- force induced melting in which the double strands are split apart into single
strands, which in many cases is reversible
Graphs of force vs. extension distance removed due to copyright restrictions.
7