3.044 MATERIALS PROCESSING Radiation: ∂T −k
advertisement

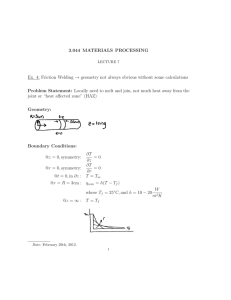
3.044 MATERIALS PROCESSING LECTURE 8 Radiation: −k 4 ∂T 4 = −εσ Tsurf − Tsource ∂x L 3 M = εσ Tsurf k ∂T = α ∇2 T ∂t 4 4 − Tsource ) q = εσ(Tsurf ⇒ Very few analytical solutions, some charts Date: March 5th, 2012. 1 2 LECTURE 8 CVD: Chemical Vapor Deposition At steady state the thermocouple outputs a temperature reading TTC TC is heated by gas (convection) out in 4 4 −h(T − Tf )A = εσ(Tsurf − Tsource )A εσ 4 4 − Tsource ) TTC = Tf − (Tsurf h TTC = Tf Tf ≈ 1000◦ C, Twall ≈ 500◦ C, W ε = 0.1, h = 100 2 mK ◦ TT C ≈ 830 C, ΔT ≈ 200◦ C Conclusions: 1. Objects that “see” cold surroundings are colder than you think 2. If an object “sees” a hot source it can be unexpectedly hot 3. “In vacuum” indicates no convection → radiation must be important Topics Covered So Far: Heat Beat Mix heat transfer solid mechanics diffusion fluid mechanics phase transition 3.044 MATERIALS PROCESSING ⇒ 3 Next step is to discuss heat transfer combined with diffusion and phase transformations Solidification: Heat transfer plus phase transition, single component solidification What are the B.C at the solid/liquid interface? 1. T = Tm 2. heat balance: q q out in ∂T ∂T −ks −k = l ∂x s ∂s l 3. heat of fusion Look closely: ∂T qin = −kl ∂x x=s,l 4 LECTURE 8 ∂T qout = −ks ∂x x=s,s kJ Fusion: − Hf kg In time Δt the interface moved Δs ⇒ Volume transformed = AΔs ∂T ∂T Δs A + ks A − Hf ρ A=0 −kl ∂x ∂x Δt in where out Heat of Fusion Δs ∂s = = interface velocity Δt ∂t − kl ∂T −ks ∂T ∂x s ∂x l = Hf ρ k ∂T ∂T = (within factor of two) − Hf ρ ∂x s ∂x l MIT OpenCourseWare http://ocw.mit.edu 3.044 Materials Processing Spring 2013 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms.