5.60 Thermodynamics & Kinetics
advertisement

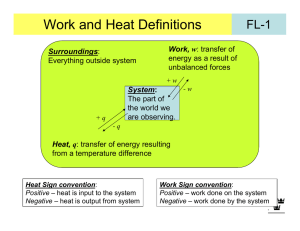
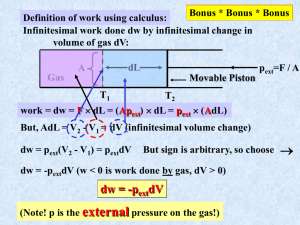
MIT OpenCourseWare http://ocw.mit.edu 5.60 Thermodynamics & Kinetics Spring 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 5.60 Spring 2008 Lecture #3 Isothermal Gas Expansion page 1 (∆T = 0) gas (p1, V1, T) = gas (p2, V2, T) Irreversibly (many ways possible) (1) Set pext = 0 p= 0 T T p= 0 p 1,V1 p 2,V2 v2 w (1) = − ∫ pext dV = 0 V1 (2) Set pext = p2 p2 T p2 T p 2,V2 p 1,V1 v2 w (2) = − ∫ p2dV = −p2 (V2 −V1 ) V1 p p1 p2 V1 -w(2) V2 Note, work is negative: system expands against surroundings 5.60 Spring 2008 (3) Lecture #3 page 2 Carry out change in two steps gas (p1, V1, T) = gas (p3, V3, T) = gas (p2, V2, T) p1 > p3 > p2 p2 p3 p3 T T T p 2,V2 p 3,V3 p 1,V1 v3 v2 V1 V3 w (3) = − ∫ p3dV − ∫ p2dV = − p3 (V3 −V1 ) −p2 (V2 −V3 ) p p1 More work delivered to surroundings in this case. p3 p2 V1 V3 V2 -w(3) (4) Reversible change p = pext throughout V p wrev = − ∫ 2 pdV V1 p1 p2 V1 - V2 rev For ideal gas: V wrev = − ∫ 2 V1 Maximum work delivered to surroundings for isothermal gas expansion is obtained using a reversible path V p nRT dV = −nRT ln 2 = nRT ln 2 V V1 p1 5.60 Spring 2008 Lecture #3 page 3 The Internal Energy U dU = d-q + d-w (First Law) dU = C pathdT − pext dV And U (T ,V ) ⇒ ⎛ ∂U ⎞ ⎛ ∂U ⎞ dU = ⎜ ⎟ dT + ⎜ ⎟ dV ∂ T ∂ V ⎝ ⎠V ⎝ ⎠T Some frequent constraints: dU = d-qrev + d-wrev = d-qrev – pdV Reversible ⇒ • Isolated ⇒ d-q = d-w = 0 • Adiabatic ⇒ d-q = 0 • Constant V ⇒ • (p = pext ) reversible ⇒ dU = d-w = -pdV w = 0 ⇒ dU = d-qV Constant V ∂U ⎞ ⎛ ∂U ⎞ dU = ⎛⎜ ⎟ dT + ⎜ ⎟ dV ⎝ ∂T ⎠V ⎝ ∂V ⎠T but also ⎛ ∂U ⎞ ⇒ d-qV = ⎜ ⎟ dT ⎝ ∂T ⎠V d-qV = CV dT So ⇒ ⎛ ∂U ⎞ ⎜ ⎟ = CV ⎝ ∂T ⎠V very important result!! ⎛ ∂U ⎞ ⎟ ⎝ ∂V ⎠T dU = CV dT + ⎜ dV what is this? 5.60 Spring 2008 Lecture #3 ⎛ ∂U ⎞ ⎟ ) ⎝ ∂V ⎠T Joule Free Expansion of a Gas gas (to get ⎜ vac gas (p1, T1, V1) = gas (p2, T2, V2) Since q = w = 0 Recall ⇒ Adiabatic q=0 Expansion into Vac. (pext=0) w=0 dU or ∆U = 0 Constant U ⎛ ∂U ⎞ ⎟ dV = 0 ⎝ ∂V ⎠T dU = CV dT + ⎜ ⎛ ∂U ⎞ ⎜ ⎟ ⎝ ∂V ⎠T dVU = −CV dTU ⎛ ∂U ⎞ ⎜ ⎟ = −CV ⎝ ∂V ⎠T Joule did this. • page 4 ⎛ ∂T ⎞ ⎜ ⎟ ⎝ ∂V ⎠U measure in Joule exp't! ⎛ ∆T ⎞ ⎜ ⎟ ⎝ ∆V ⎠U ⎛ ∆T ⎞ ⎛ ∂T ⎞ ∴ dU = CV dT − CV ηJ dV ⎟ =⎜ ⎟ ≡ ηJ ⎝ ∆V ⎠U ⎝ ∂V ⎠U Joule coefficient lim ⎜ ∆V →0 For Ideal gas ⇒ ηJ = 0 dU = CV dT U(T) exactly Always for ideal gas only depends on T The internal energy of an ideal gas depends only on temperature Consequences ⇒ ⇒ ∆U = 0 ∆U = ∫ CVdT For all isothermal expansions or compressions of ideal gases For any ideal gas change in state