1. CKD 1.2
advertisement
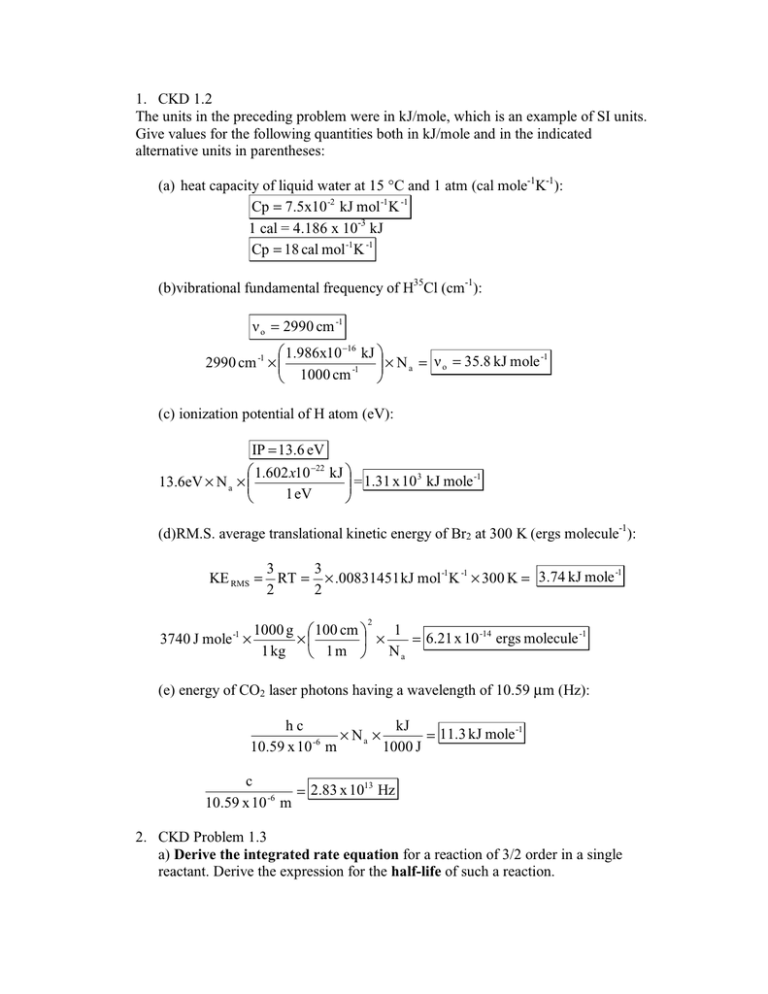
1. CKD 1.2
The units in the preceding problem were in kJ/mole, which is an example of SI units.
Give values for the following quantities both in kJ/mole and in the indicated
alternative units in parentheses:
(a) heat capacity of liquid water at 15 °C and 1 atm (cal mole-1K-1):
Cp = 7.5x10 -2 kJ mol -1K -1
1 cal = 4.186 x 10-3 kJ
Cp = 18 cal mol -1K -1
(b)vibrational fundamental frequency of H35Cl (cm-1):
ν o = 2990 cm -1
� 1.986x10 −16 kJ �
�� × N
a = ν o = 35.8 kJ mole -1
2990 cm ­1 × ��
­1
�
1000 cm
�
(c) ionization potential of H atom (eV):
IP = 13.6 eV
�
1.602 x10 −22 kJ �
�� =
1.31 x 10 3 kJ mole -1
13.6eV × N a × ��
1 eV
�
�
(d)RM.S. average translational kinetic energy of Br2 at 300 K (ergs molecule-1):
3
3
­1
KE RMS = RT = × .00831451 kJ mol -1K ­1 × 300 K = 3.74 kJ mole
2
2
2
1000 g � 100 cm �
1
-14
-1
�
= 6.21 x 10 ergs molecule
3740 J mole ×
� ×
1 kg �
1 m �
N a
-1
(e) energy of CO2 laser photons having a wavelength of 10.59 µm (Hz):
hc
kJ
-1
× Na ×
= 11.3 kJ mole
-6
1000 J
10.59 x 10 m
c
13
= 2.83 x 10 Hz
-6
10.59 x 10 m
2. CKD Problem 1.3
a) Derive the integrated rate equation for a reaction of 3/2 order in a single
reactant. Derive the expression for the half-life of such a reaction.
Assume a reaction of the form:
A → B, with a rate expression,
- d[A]
= k[A]3 2
dt
[A]
−3 2
d[A] = - kt
A(t)
1 kt
=
1 2 �
2
[A] �
A o
[A(t)]−1
2
= [A o ]-1
(
[A(t)] = [A o ]−1
2
2
+
+ kt
kt
2
)
−2
half-life derivation:
At t1/2 ,[A t1 2 ] =
[A o ]
,
2
[A o ] −1 2
− [A o ]-1
2
2 − 1 kt 1 2
=
,
2
[A o ]1 2
t1
2
2
=+
kt 1
2
2
2( 2 − 1)
k[A o ]1 2
=
For reaction order n:
A → B, with a rate expression,
- d[A]
= k[A]n
dt
[A]
­n
d[A] = - kt
� [A]1− n
��
� n −1
A(t)
��
��� = kt
�
�
A o
(
[A(t)]1-n = [A o ]1−n + (n − 1)kt
(
[A(t)] = [A o ]1− n + (n − 1)kt
)
)
1 1− n
3. Show that the rate law for A + B → products (Eq. 1-46) reduces to the rate law for
2A → products (Eq. 1-41) when the initial concentrations [A]o and [B]o are
identical.
� [B] [A] t
1
ln �� o
[A]o − [B]o � [A]o [B] t
�
�� = k 2 t
�
(Eq. 1-46)
There are a number of different ways to solve this problem, starting with
Eq. 1-46. The following is only one way:
Start with the left hand side of Eq. 1-46; let [B]o→[A]o and do some re­
arranging:
� ln[B]t − ln[A]t ln[B]o − ln[A]o �
lim �
−
�
[B]o →[A]o
[B]o − [A]o �
�
[B]o − [A]o
{[B]o − [A]o = ([B]t + x) − ([A]t + x) = [B]t − [A]t }
�
ln[B] t − ln[A] t
= lim ��
[B]t →[A] t
�
[B] t − [A]t
=
d(ln[B]t )
−
d[B]t [B] =[A]
t
=
t
1
1
−
= k2t
[A] t [A]o
�
�
ln[B]o − ln[A]o
��
�� − [B]lim
o →[A]o
�
�
[B]o − [A]o
d(ln[B]o )
d[B]o [B]
�
��
�
o =[A]o
(getting closer to Eq.1 - 41)
We need to include a factor of 2, because[A]o = [B]o , which gets us Eq. 1 - 41 :
1
1
=
+ 2k 2 t
[A]t [A]o
4. CKD Problem 1.5
The kinetics of formation of ethyl acetate from acetic acid and ethyl alcohol as
homogeneously catalyzed by a constant amount of HCl has been studied by titrating
1-cc aliquots of the reaction mixture with 0.0612 N base at various times. The
following data have been obtained at 25 °C.
t, min
base, cc
0
24.37
Initial concentrations
44
22.20
62
21.35
[CH 3 COOH] = 1.000M
108
19.50
[C 2 H 5 OH] = 12.756M
117
19.26
[H 2 O] = 12.756M
148
18.29
313
14.14
[CH 3 COOC 2 H 5 ] = 0
384
13.40
Overall reaction :
442
13.09
k1
12.68
∞
CH COOH + C H OH ⇔ CH COOC H + H O
3
2
5
k ­1
3
2
5
2
The reaction has been found to be first order with respect to each of the four reactants.
Calculate the specific rate constants k1and k-1. What is the equilibrium constant Keq at
25 oC?
To save myself some time, the reaction above will be represented as the following:
A = CH 3 COOH
A + B → C + D , where
B = C 2 H 5 OH
C = CH 3 COO
2
H5
D = H 2O
Define a progress variable, x:
Reactant/product
A
B
Initial concentration
1.00
12.756
Progress
-x
-x
Concentration at time, t
1.00-x
12.756-x
dx
= k 1 (1.00 − x)(12.756 − x) − k −1 (12.756 + x)(x)
dt
dx
at t = ∞,
= 0, or,
dt
k
[C] ∞ [D]∞
(12.756 + x ∞ )(x ∞ )
K eq = 1 =
=
k −1 [A]∞ [B]∞ (1.00 − x ∞ )(12.756 − x ∞ )
C
0
+x
x
D
12.756
+x
12.756+x
�
1.000
� �
�
x ∞ = [A]
o − [A]
∞ = 1.000M
− ��12.68
−
� 24.37
−
� � = 0.715,
0.0612
�
��
�
�
therefore,
K eq = 2.81
Make a substitution, k -1 =
(
k1
, in the rate equation above;
2.81
dx
= k 1 0.644x 2 − 18.30x + 12.756
dt
Integrated to give,
)
�1.288x - 35.68 �
k 1 t = 0.575 ln �
− 0.210
� 1.288x - 0.92 ��
Using the time values given in the table above, and plugging in x-values, where
�
�
1.000
�
�
x(t) =
1 −
�� vol base (t) −
� 24.37
−
� × 0.0612
��
0.0612
�
�
�
�
time (min) x
k 1t
0
volume
0
24.37
22.2
21.35
19.5
19.26
18.29
14.14
13.4
13.09
12.68
0
44 0.132796 0.011884
62 0.184816 0.017164
108 0.298036 0.030761
117 0.312724 0.032796
148 0.372088
0.04187
313 0.626068 0.119279
384 0.671356 0.160597
442 0.690328 0.194094
0.71542
y = 2.84354E-04x - 2.88281E-04
R2 = 9.99679E-01
0.05
k1t
0.04
0.03
0.02
0.01
0
0
25
50
75
100
125
150
time (min)
The slope of the graph is k1 in units of M-1min-1:
k 1 = 2.84x10 −4 M -1min −1 , and
k -1 = 1.01x10 −4 M -1 min −1
5. CKD Problem 1.6
1
Nitrogen pentoxide decomposes according to the reaction N 2 O 5 → 2NO 2 + O 2
2
with a rate constant k. The measured rates between 273 K and 338 K are included in
the chart below.
Make an Arrhenius plot of the data, and determine Eact and A for the first-order
decomposition of nitrogen pentoxide.
The slope and y-intercept of the Arrhenius plot are related to Eact and A in the
following manner:
E
�
k(T) =
A exp�� act
RT ��
�
E
lnk = lnA − act
RT
E
slope = − act and
R
y - intercept = ln A
T/ K
273
298
308
318
328
338
1/T
0.003663
0.003356
0.003247
0.003145
0.003049
0.002959
k (sec^-1) ln k
7.87E-07
-14.055
3.46E-05 -10.2717
1.35E-04 -8.91024
4.98E-04 -7.60491
1.50E-03 -6.50229
4.87E-03 -5.32466
Arrhenius Plot: N2O5 decomposition
ln k
0.0029
0.0031
0.0033
-5
-6
-7
-8
-9
-10
-11
-12
-13
-14
-15
0.0035
0.0037
y = -12376x + 31.273
1/T (1/K)
E act
= −12376 K
R
E act = 12376 K × 8.3144 J K -1 mole −1
−
E act = 103 kJ mole -1
ln A = 31.273
A = 3.82x1013 s -1
6. According to the information provided on some milk cartons, homogenized milk will
keep for 1/3 day at 80°F, for ½ day at 70°F, for 1 day at 60°F, for 2 days at 50°F, for 10
days at 40°F, and for 24 days at 32°F. Calculate the activation energy for the process
that spoils milk.
We are not told how far the spoiled milk is after the given times, but it will not make a
difference to the activation energy as long as we can assume the spoilage is the same at
each given time (the spoilage would affect the Arrhenius coefficient, but nobody asked us
about that). The given times are the 1/e time for spoilage, so that the rate is the simply
the reciprocal of the indicated time; e.g., rate at 80°F is 3 days-1. Remember to convert
from °F to K.
T/ F
80
70
60
50
40
32
T/ K
299.6667
294.1111
288.5556
283
277.4444
273
1/T
time / days k
ln k
0.003337 0.33333333
3 1.098612
0.0034
0.5
2 0.693147
0.003466
1
1
0
0.003534
2
0.5 -0.69315
0.003604
10
0.1 -2.30259
0.003663
24 0.041667 -3.17805
Spoiled Milk
0.0033
1.5
0.0034
0.0035
0.0037
y = -13462x + 46.393
0.5
ln k
0.0036
-0.5
-1.5
-2.5
-3.5
-1
1/T (K )
The slope of this graph will give us Eact.
-1
E act = −(R × −13462) = 112 kJ mole



