WAVE-PARTICLE DUALITY of MATTER ! x ≥
5.61 Fall 2007 Lecture #6 page 1
WAVE-PARTICLE DUALITY of MATTER
Consequences (II)
Heisenberg Uncertainty Principle Δ x Δ p x
≥
!
2
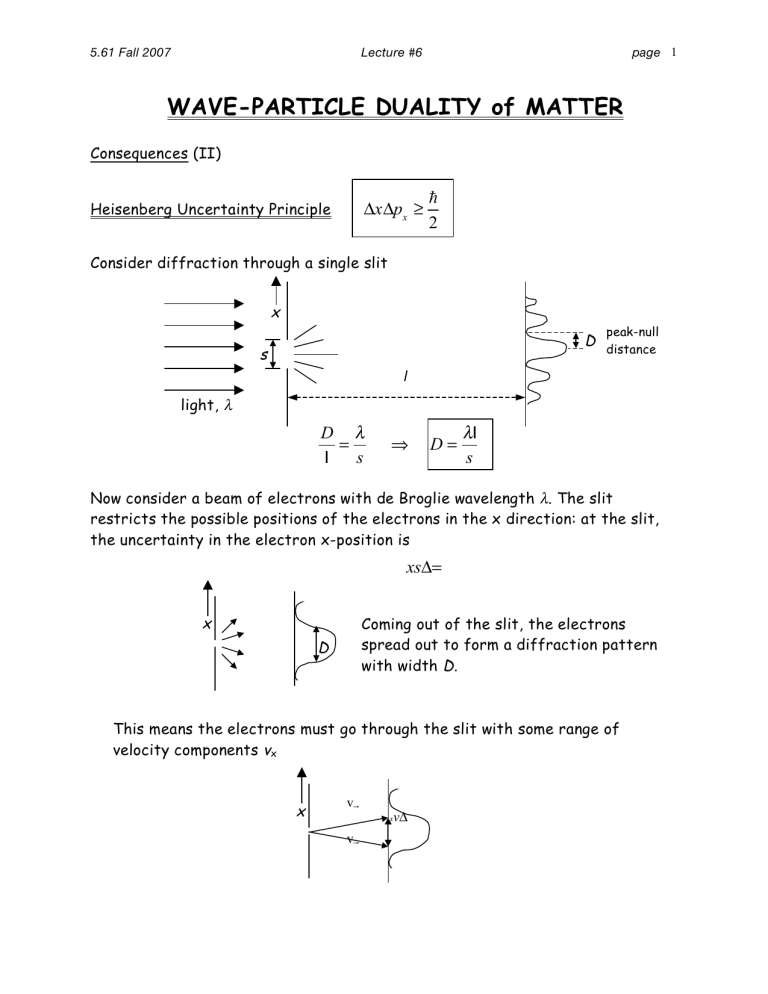
Consider diffraction through a single slit x s
D peak-null distance l light, λ
D l
=
λ s
⇒ D =
λ l s
Now consider a beam of electrons with de Broglie wavelength λ . The slit restricts the possible positions of the electrons in the x direction: at the slit, the uncertainty in the electron x-position is xs Δ = x
D
Coming out of the slit, the electrons spread out to form a diffraction pattern with width D.
This means the electrons must go through the slit with some range of velocity components v x x v !
v !
x v Δ
5.61 Fall 2007 Lecture #6 page 2
Δ v v x
Δ x Δ v
≈ x
D
=
λ
=
λ
!
s Δ x
= λ v or Δ x Δ p x
= λ p
But from deBroglie λ = h p
∴ Δ x Δ p x
= h
So the position and momentum of a particle cannot both be determined with arbitrary position! Knowing one quantity with high precision means that the other must necessarily be imprecise!
The conventional statement of the Heisenberg Uncertainty Principle is
Δ x Δ p x
≥
!
2
(depends on how “uncertainty” is defined: 1/e half-width, FWHM, etc.)
Uncertainty can always be larger than !
2 , but not smaller.
Note that this sort of uncertainty is standard in classical wave mechanics. If you focus a light beam or a water wavelet to a small spot size, at the focus there is a wide range of propagation directions. What is new is the idea that particles inherently show wavelike behavior, with similar consequences.
Implications for atomic structure
Apply Uncertainty Principle to e- in H atom m e
= 9.11 x 10 -31 kg Δ x ≈ 10 -10 m (1 Å )
5.61 Fall 2007 Lecture #6 page 3
Δ p x
≥
!
2 Δ x
⇒ Δ v x
≥
2 m
!
Δ x
≈
1
2 x 10 − 6 m s
Basically, if we know the e- is in the atom, then we can’t know its velocity at all!
Bohr had assumed the electron was a particle with a known position and velocity.
To complete the picture of atomic structure, the wavelike properties of the electron had to be included.
So how do we properly represent where the particle is??
Schrödinger (1933 Nobel Prize)
A particle in a “stable” or time-independent state can be represented mathematically as a wave, by a “wavefunction” ψ ( x) (in 1-D) which is a solution to the differential equation
−
!
2
2 m
∂ 2
∂ x
ψ
2
+
( )
ψ
( x
)
= E ψ
( )
Time-independent
Schrödinger equation potential energy total energy
We cannot prove the Schrödinger equation. But we can motivate why it might be reasonable.
A v
φ
1
( x , t
)
= A sin
( kx − ω t
) is a right-traveling wave. k =
2 π
ω
ω = 2 πν λν = v
Similarly, a left-traveling wave can be represented as
φ
2
( x , t
)
= A sin
( kx + ω t
)
.
Both are solutions to the wave equation
5.61 Fall 2007 Lecture #6 page 4
∂ 2 φ
∂ x
( x , t
)
=
1
∂ 2 φ
( x , t
)
2 v 2 ∂ t 2
Further, the sum also a solution.
Ψ
( x , t
)
= φ
1
( x , t
)
+ φ
2
( x , t
) of left and right traveling waves is
Ψ
( x , t
)
= A ⎡⎣ sin
( kx − ω t
)
+ sin
( kx + ω t
)
⎤⎦ = 2 A sin
( ) cos
(
ω t
)
This is a stationary wave or standing wave. Its peaks and nulls remain stationary.
At various times during a full cycle ( 2 π / ω ):
Ψ
( x ,0
)
= 2 A sin kx
Ψ
⎝⎜
⎛ x ,
1 2
8
⋅
ω
π ⎞
⎠⎟
= 2 A ⎜
⎝
⎛
2
⎞
2 ⎠
⎟ sin
( ) t = 0 t = π /4 ω
Ψ
⎛
⎝⎜ x ,
1 2
4
⋅
ω
π ⎞
⎠⎟
Ψ
⎝⎜
⎛ x ,
3 2
8
⋅
ω
π ⎞
⎠⎟
Ψ
⎛
⎝⎜ x ,
1 2
2
⋅
π ⎞
ω ⎠⎟
= 0 t = π /2 ω
= 2 A
⎝
⎜
⎛
− 2
2
⎟
⎞
⎠ sin
( ) t = 3 π /4 ω t = π / ω
= 2 A
( ) sin
( ) nodes
As in a vibrating violin string, the node positions are independent of time. Only the amplitude of the fixed waveform oscillates with time.
More generally, we can write wave equation solutions in the form
Ψ
( x , t
)
= ψ
( ) cos
(
ω t
)
In the particular case above, ψ
( x
)
= 2 A sin
( )
.
5.61 Fall 2007 Lecture #6
For the general case,
∂ 2 Ψ
∂ x
( x , t
2
)
=
1 v 2
∂ 2 Ψ
∂ t
( x , t
2
)
=
− v
ω 2
2
Ψ
( x , t
)
= − k 2 Ψ
( x , t
) (
ω k = v
)
Plugging in Ψ
( x , t
)
= ψ
( ) cos
(
ω t
) de Broglie relation
⇒
∂ 2 ψ
( )
= − k
∂ x 2
λ = h p
⇒
2 ψ
( )
= −
⎛ 2 π ⎞ 2
⎝⎜ λ ⎠⎟
∂ 2 ψ
( x
)
∂ x 2
= −
⎝⎜
⎛
ψ
( ) p ⎞ 2
!
⎠⎟
ψ
( )
∴
− !
2
∂ 2 ψ
( )
∂ x 2
= p 2 ψ
( )
But p 2 = 2 m
(
K.E.
)
= 2 m ⎡⎣ E −
( )
⎤⎦ (assuming t-independent potential)
−
!
2
∂ 2 ψ
2 m ∂ x 2
( )
+
( )
ψ
( x
)
= E ψ
( ) time-independent Schrödinger equation in one dimension page 5
We now have the outline of:
• a physical picture involving wave and particle duality of light and matter !
• a quantitative theory allowing calculations of stable states and their properties !






