R. G. Prinn 12.806/10.571: Atmospheric Physics &
advertisement

R. G. Prinn
12.806/10.571:
Atmospheric
Physics &
Chemistry,
March 7, 2006
COMPONENTS OF ATMOSPHERIC CHEMISTRY MODELS
DYNAMICAL EQUATIONS
MASS CONTINUITY EQUATION
Temperature
THERMODYNAMIC EQUATION
CHEMICAL
Rates
for
Heating
CONTINUITY EQUATIONS
∆
UV
r
Fo
ph
Flu
xe
s
.([i]V)
~
n
tio
ia
oc
iss
od es
ot rat
{
= Pi - Li -
EQUATIONS
For
unpredicted
green house
gases use
scenarios or
extrapolations
For source gases
use predictions,
extrapolations
or scenarios
∂t
Rates for Chemistry
{
∂ [i]
Concentrations
(O3, etc.)
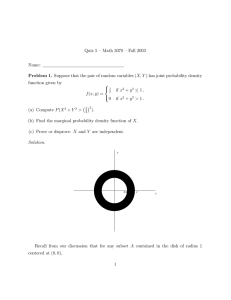
RADIATION
Figure by MIT OCW.
Winds , eddy diffusion coefficients
MOMENTUM EQUATIONS
Interactions Between Air Pollution and Climate
Stratosphere
UV
O2
NO2
O3
O2
UV
OH
NO
HNO3
O(1D)
OH
N2O
Lightning
H 2O
CO2
Hydrosphere
H2SO4
HO2
O2
CO
OH
CH4
CO2
OH
CFCs
BC
SO2
Biosphere & Human Activity
Greenhouse Gases
Primary & Secondary Pollutants
Absorbing Aerosols (BC)
Reactive Free Radical/Atom
Less Reactive Radicals
Reflective Aerosols
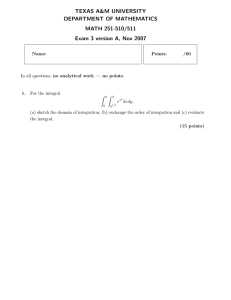
In the atmospheric column: Wind
vectors, humidity, clouds, temperature,
and chemical species
Horizontal exchange
between columns
Geography and orography
Ocean grid
Atmospheric Grid
Vertical exchange
between levels
At the surface: Ground temperature
water and energy, momentum and
CO2 fluxes
Bathymetry
Within the ocean column: Current vectors,
temperature and salinity
Figure by MIT OCW.
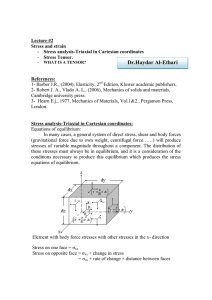
Combining Chemistry and Transport: The Continuity Equation
Physical picture:
(Eulerian)
i
Oz dxdy
i
Outputs
Oy dzdx
i
Ox dydz
Ixi dydz
Iyi dzdx
Inputs
Izi dxdy
Internal Net Production
(Pi - Li)dxdydz
Figure by MIT OCW.
Notation:
Inputs = Iix dydz + Iiy dzdx + Iiz dxdy
Outputs = Oix dydz + Oiy dzdx + Oiz dxdy
⎡
∂Iiy ⎤
⎡
⎤
⎡
∂Ii
∂Ii ⎤
= ⎢ Iix + x dx ⎥ dydz + ⎢ Iiy +
dy ⎥ dzdx + ⎢ Iiz + z dz ⎥ dxdy
∂x ⎦
∂y ⎦⎥
∂z ⎦
⎣
⎣
⎣⎢
i
i
i
I x = [i ] u, I y = [i ] v, I z = [i ] w (input fluxes)
dx
dy
dz
, v= , w=
(wind velocities)
dt
dt
dt
Pi , Li = rates of chemical production, loss
u=
[i] = concentration of i
Local rate of change of [i] given by:
∂ [i ]
Inputs - Outputs + Internal net production
∂t
dxdydz
∂
∂
∂
= Pi − Li − ([i ] u ) − ([i ] v ) − ([i ] w )
∂x
∂y
∂z
G
= Pi − Li − ∇ ⋅ [i ] V
=
(
)
(1)
Continuity equation for i
For total molecular concentration [ M ] , PM − L M 0 so:
G
∂ [M]
= −∇ ⋅ [ M ] V
∂t
(
)
(2)
Continuity equation for M
Defining mixing ratio = [i ] [ M ] = Xi :
∂ [i ]
∂ [M]
[M] −
[i ]
∂X i ∂
∂t
= ([i ] [ M ]) = ∂t
2
∂t
∂t
[M]
G
G
Pi − Li − ∇ ⋅ X i [ M ] V [ M ] + ∇ ⋅ [ M ] V X i [ M ]
(Using equations (1) and (2))
=
2
[M]
G
G
G
Pi − Li − X i∇ ⋅ [ M ] V − [ M ] V ⋅∇X i [ M ] + ∇ ⋅ [ M ] V X i [ M ]
==
2
[M]
G
Pi − Li − [ M ] V ⋅∇X i [ M ]
=
2
[M]
P − Li G
(3)
= i
− V ⋅∇X i
[M]
(
(
(
))
(
(
)
(
)
)
(
)
)
Continuity Equation for i (mixing ratio form)
Theorem: If there is no gradient in the mixing ratio of i ( ∇Xi = 0 ) then there can be no
local changes in i due to transport.
Rate of change of Xi traveling with the air given by ( Lagrangian view ) :
(a)
dXi d
= ⎡⎣ X i ( x, y, z, t ) ⎤⎦
dt
dt
∂X i dx ∂X i dy ∂X i dz ∂X i
=
+
+
+
∂t
∂x dt ∂y dt ∂z dt
∂Xi
∂X
∂X
∂X
u+ i v+ i w+ i
=
∂x
∂y
∂z
∂t
G
G
P − Li
= V ⋅∇X i + i
− V ⋅∇X i
[M]
P − Li
= i
[M]
(chain rule)
(using equation (3))
(4)
Theorem: If there is no “net chemical production” ( Pi − Li = 0 ), then the mixing ratio of i
is conserved moving with the air.
(b)
(s*, t*)
s*
dX i dt
ds
dt ds
0
X it* = X i0 + ∫
s*
= X i0 + ∫
0
(s, t)
Pis − Lis
ds
[M] us
(0, 0)
(using equation (4)) (5)
Theorem: The change in mixing ratio in an air mass from its initial value is a line integral
of the “net chemical production” over the trajectory of the air mass.
A steady state exists when the local rate of change is zero:
∂ [i ]
=0
∂t
∂ [M]
=0
∂t
∂X i
=0
∂t
G
i.e. Pi − Li = ∇ ⋅ [i ] V ⎫
⎪⎪
⎬ ( 6)
G
⎪
i.e. ∇ ⋅ [ M ] V = 0 ⎪
⎭
(6)
Pi − Li G
i.e.
= V ⋅∇X i (7)
[M]
(
(
)
)
One-Dimensional (Horizontal) Model
Source Region Downwind Region
[Constant wind speed u , Pi = 0, Li =
[Constant Xi]
0
z=0
Equation (7) with v = w = 0 gives:
X
Pi − Li = 0 − [ M ] i
τi
dX i
dx
d ln X i
1
i.e.
=−
dx
uτ i
= [M] u
⎛ x ⎞
i.e. X i ( x ) = X i ( 0 ) exp ⎜ −
⎟
⎝ uτi ⎠
(8)
[i] [M]X i
]
=
τi
τi
x
[chemical (e-folding) distance, h = uτi ]
[advection time = x u ]
i.e.
Xi(x)
Xi(0)
(a) Inert Case
(x<<h, τi >> xu (
(b) Reactive Case
(x>h, τi < xu (
X
Figure by MIT OCW.