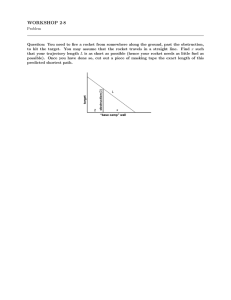
16.512, Rocket Propulsion Prof. Manuel Martinez-Sanchez Read Sutton’s, Chapter 12
advertisement

16.512, Rocket Propulsion Prof. Manuel Martinez-Sanchez Lecture 16: Solid Propellants: Design Goals and Constraints Solid Propellants Read Sutton’s, Chapter 12 Double Base (DB) Nitrocellulose + Nitroglycerine + Additives (for opacity, plasticity, …). Both NC and NG are explosives, dangerous sometimes JPN NC 51.5%, NG 43%, Diethyl phthalate 3.2%, Ethyl centralite 1%, H2SO4 1.2% + carbon black + candelilla wax Composite Modified Double base (CMBD) DB + Ammonium perchlorate (AP) or Aluminium (Al) Composite (C) AP (sometimes A Nitr) + Synthetic Rubber binder (fuel) + Al. Safer than DB Other Composite contain nitramine explosive (RDX, HMX), replacing some AP Type DB DB-AP-Al CTPB/AP/Al HTPB/AP/Al Isp (s) at 1000/14.7 psi 220-230 260-265 260-265 260-265 Tc (K) ρp Al % r (cm/s) @1000 psi η Fabrication 0 20-21 15-17 4-17 1.14 1.98 1.14 1.02 0.3 0.4 0.4 0.40 Extruded Extruded Cast Cast 3 (g/cm ) 2530 3866 3370-3490 3370-3490 1.60 1.79 1.76 1.85 The addition of Aluminum is not necessarily beneficial, as the following example shows: Problem 3. Adding Aluminum to the formulation of a solid rocket propellant increases the gas temperature, but incurs performance penalties related to the solid particles that are generated. Consider a simple model for the effect of adding a mass fraction xAl of Aluminium, of the form Tc = 1 + rx ; x= 1.85 x Al Tc o (1) where r ≅ 1.41 is a separately calculated coefficient, Tco is the flame temperature without aluminum (~2500K), and x is the solids fraction in the gas (the 1.85 factor accounts for the oxygen in the Al2O3 particles). 16.512, Rocket Propulsion Prof. Manuel Martinez-Sanchez Lecture 16 Page 1 of 3 Consider also a linearized model for the effect of the particulates, of the form ue = 1 − fx ueo (at fixed Tc ) (2) where f is as derived in class: γ −1 ⎧ γ ⎪⎪ 1 − cs ⎡1 + (1 − η) ln(1 − η) ⎤ ; η = 1 − ⎛ Pe ⎞ (small particles) ⎥ ⎜ ⎟ f = ⎨ 2 2c ⎢⎢ η ⎥⎦ pg ⎣ ⎝ Pc ⎠ ⎪ ⎪⎩ 1 − − − − − − − − − − − − − − − − (l arg e particles) (a) Show that the optimum loading is given by xO PT = (b) For r − 2f ; 3 rf ( x Al )OPT = xO PT 1.85 (3) Pe J = 0.01, γ g = 1.25, Mg = 18g / mol, cs = 1260 , calculate ( x A l ) for O PT Pc KgK both small and large particulates. Comment on results. Problem 3 – Solution Ignoring the exit pressure effect (or at matched conditions), g Isp = ve = γ −1 ⎡ ⎤ γ ⎛ ⎞ P ⎢ e 2Cp Tc 1 − ⎜ ⎟ ⎥ ⎢ ⎥ ⎝ Pc ⎠ ⎥ ⎢⎣ ⎦ which is proportional to Tc . The rest of the dependence (γ, cp ) are affected by particulates, but that is counted separately in the loss analysis. So we have (counting both effects) (a) ve ∼ (1 − fx ) 1 + rx To optimize, take the logarithmic derivative and equate to zero 16.512, Rocket Propulsion Prof. Manuel Martinez-Sanchez Lecture 16 Page 2 of 3 −f 1 r + =0 1 − fx 2 1 + rx 2f (1 + rx) = r (1 − fx) r - 2f = 3rfx r - 2f 3rf xOP T = and then (b) For small particulate f = ( x Al )OPT = xOPT 1.85 (1 − η) ln (1 − η) ⎤ ⎪⎫ , and using the given cs ⎡ 1 ⎪⎧ ⎢1 + ⎥⎬ ⎨1 − η 2 ⎪⎩ cpg ⎢⎣ ⎥⎦ ⎭⎪ values, η = 1 - (0.01)0.25/1.25 = 0.6012 f = 1 ⎧ 1260 ⎨1 − 2 ⎩ 2309 xO P T = ; Cpg = 1.25 8.314 = 2309 J / Kg / K 0.25 0.018 0.3988 ln(0.3988) ⎤ ⎫ ⎡ ⎢1 + ⎥ ⎬ = 0.3934 0.6012 ⎣ ⎦⎭ 1.41 − 2 × 0.3934 3 × 1.41 × 0.3934 xO P T = 0.3746 and then the Aluminum fraction should be ( X Al )O P T = 0.3746 = 0.2025 1.85 20.3% Al loading For the case of larger particle, the class derivation showed f=1, and so xOPT = 1.41 − 2 × 1 < 0 3 × 1.41 × 1 This nonsensical result simply means there is no good Al loading in this case. The losses due to the particles are stronger than the gains due to increased temperature, so no Aluminum should be added. Fortunately, the particles are small, not large. 16.512, Rocket Propulsion Prof. Manuel Martinez-Sanchez Lecture 16 Page 3 of 3