1: 2: 3: 4: Laplace’s equation, Uniqueness
advertisement

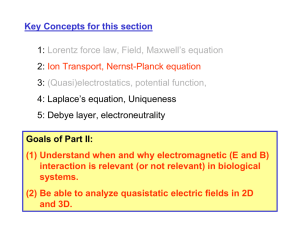
Key Concepts for this section 1: Lorentz force law, Field, Maxwell’s equation 2: Ion Transport, Nernst-Planck equation 3: (Quasi)electrostatics, potential function, 4: Laplace’s equation, Uniqueness 5: Debye layer, electroneutrality Goals of Part II: (1) Understand when and why electromagnetic (E and B) interaction is relevant (or not relevant) in biological systems. (2) Be able to analyze quasistatic electric fields in 2D and 3D. EM interactions in media - polarization (linear medium) G Eext = 0 G G E pol ∝ Eext -+ -+ -+ -+ - -+ -+ -+ -+ - -+ -+ -+ -+ - G Eext G E pol + + + G Eext G G G 1 G Emedia = Eext − E pol = Eext εr εr : relative permittivity (dielectric constant ) of the medium (=>1) ε of various media Medium εr Water (pure) ~80 0.9% NaCl solution ~60 Ethanol 24 Methanol 34 Acetic acid 15~16 Gases ~1 Glass 3~4 Plastics and rubbers 2~9 Maxwell’s equation in a medium (Magnetic field) Image source: MIT 8.02 class notes. Courtesy of Dr. Sen-ben Liao, Dr. Peter Dourmashkin, and Professor John W. Belcher. Used with permission. G G Bmag ∝ Bext G G G G Bmedia = Bext + Bmag = μr Bext ( μr ≥ 1) G G G G G ∂E ∂E ∇ × B = μ 0 μ r J + μ 0 μ r ε 0ε r = μ J + με ∂t ∂t μr : relative magnetic permeability of the medium μο : free space permeability (4π×10-7 H/m) μ of various media μr for water : very close to 1 μr (Ni)~600, μr (Fe)~5000 Mobility of various ions in water Species Mobility Ui (cm2/v/s) Diffusion coefficient Di (cm2/s) Cations in H2O (25oC) H+ K+ Na+ Li+ 36.30×10-4 7.62×10-4 5.19×10-4 4.01×10-4 9.33×10-5 1.96×10-5 1.33×10-5 1.03×10-5 Anions in H2O (25oC) OHSO42ClNO3- 20.52×10-4 8.27×10-4 7.91×10-4 7.40×10-4 5.27×10-5 1.06×10-5 2.03×10-5 1.90×10-5 Electrons in Si at 25oC Holes in Si at 25oC 1500 600 38.55 15.42 Comparative Number densities and Conductivities Material DI water 0.1M NaCl Copper Si (intrinsic) Si (doped) Nd=1016 Quartz ni (#/cm3) ~1017 6×1019 ~1022 n=p~1010 ne=1016 Np=104 σ (m-1Ω-1) 4 ×10-6 1.07 5.8 ×107 3.36 ×10-4 2.4 10-18 In silicon (semiconductor), n×p~1020 (constant) In aqueous solutions, [H+][OH-] = 10-14 = Kw (pH= -log10[H+]) Electrolytes (biological systems) are conductors. electrode E V=Vext Charge Relaxation in electrolyte + + + ++ ++ +++ + τ + + ~10-9 sec + ρ=0 + + <ρ>=0 within the medium Fixed charges + + + + τ ~10-9 sec - - --- + - - + + + + + <ρ>=0 within the medium • (Quasi) Electroneutrality Approximation – It is an approximation. • Valid only after the relaxation time constant τ, and outside of the Debye length (κ-1, to be discussed) – Not valid when there is a discontinuity • Valid only within a single medium (σ=constant) • Boundary of the medium could carry (surface) free charge – No inter-charge interaction in liquid media Electroneutrality Buffer counterions ~ Debye length (κ-1) + -- - - Protein A + + + + + Protein B Capillary Electrophoresis (1980s) Electroosmotic flow (UV; laser fluorescence and mass spec; radioisotope) Capillary inlet Data acquisition Capillary outlet 10 - 200 µm Detector Electrolyte buffer Electrolyte buffer + Reservoir 105 V/m + Reservoir HV Generic diagram of a capillary electrophoresis system. Figure by MIT OCW. Micro Total Analysis System (microTAS): Parallelism • 96~356 samples analyzed in a single chip simultaneously • fluorescence detection of DNA at the center of the chip (rotating optical head) Figure 1 removed due to copyright restrictions. Yining Shi et al., Analytical Chemistry, 71, 5354 (1999)