Document 13478270
advertisement

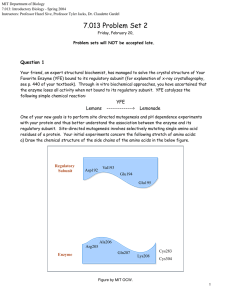
MIT Department of Biology 7.013: Introductory Biology - Spring 2004 Instructors: Professor Hazel Sive, Professor Tyler Jacks, Dr. Claudette Gardel 7.013 Problem Set 2 Friday, February 20, Problem sets will NOT be accepted late. Question 1 Your friend, an expert structural biochemist, has managed to solve the crystal structure of Your Favorite Enzyme (YFE) bound to its regulatory subunit (for explanation of x-ray crystallography, see p. 440 of your textbook). Through in vitro biochemical approaches, you have ascertained that the enzyme loses all activity when not bound to its regulatory subunit. YFE catalyzes the following simple chemical reaction: Lemons YFE --------------> Lemonade One of your new goals is to perform site directed mutagenesis and pH dependence experiments with your protein and thus better understand the association between the enzyme and its regulatory subunit. Site-directed mutagenesis involves selectively mutating single amino acid residues of a protein. Your initial experiments concern the following stretch of amino acids: a) Draw the chemical structure of the side chains of the amino acids in the below figure. Regulatory Subunit Asp192 CH2 Val193 Glu194 CH Glu195 CH3 CH3 CH2 COO <CHARGE> NH2 = C NH NH2 CH2 CH CH Enzyme CH2 CH2 CH2 O=C <VDW> O <CHARGE> COO O <POLAR> C NH2 CH3 CH2 Ala206 CH2 Arg205 Gln207 CH2 NH3 CH2 CH2 CH2 Lys208 <DISULFIDE BRIDGE> Cys283 CH2 S Cys304 CH2 S 1 Figure by MIT OCW. ii. For each interaction listed below, identify the strongest type of chemical bond or force expected between each amino acid pair. Choose from covalent, hydrogen, ionic, or van der Waals. a. Asp192:Arg205 ionic b. Val193:Ala206 van der Waals c. Glu194:Gln207 hydrogen d. Glu195:Lys208 ionic e. Cys283:Cys304 covalent c) Describe the expected results of the following mutations on the activity of the enzyme. Rationalize whether and why you will see a loss of activity of the enzyme by being specific as to how chemical bonds may be changed. [NOTE: Your explanation will constitute the majority of points awarded.] a. Arg205->Ala Loss – remove salt bridge b. Ala206->Leu No loss – conserved substitution – hydrophobic vs. hydrophobic (VDW) c. Gln207->Asn No loss- conserved substitution – polar vs. polar (H-bonds) d. Lys208->Arg No loss – conserved substitution – charged vs. charged (Salt-bridge) e. Cys283->Ala Loss – Disrupt disulfide bridge in enzyme 2 Question 2 The structure of the lipid bilayer does not allow for many polar molecules to pass into the cell. Using genetic approaches, your lab has identified pair of proteins hypothesized to be involved in transporting two different molecules from outside of the cell into the cytoplasm. Through common biochemical assays, Protein A has been identified as a monomer at physiological concentrations. You decide to analyze the amino acid sequence by constructing a hydropathy plot (which considers the relative hydrophobicity along the primary amino acid sequence—for example, tryptophan would be scored as relatively hydrophobic and serine would be scored as relatively hydrophilic). Your analysis yields the following result. Relative Hydrophobic Character 50 100 150 200 250 300 AA Residue # Relative Hydrophilic Character Scale: = 20 amino acids Figure by MIT OCW. a) Based upon the above hydropathy plot, and given that a transmembrane segment is about 20 amino acids long, might you expect Protein A to be embedded in the membrane? If so, show how it would interact with the lipid bilayer below: Extracellular Cytoplasm (The protein should be associated with the membrane and should only cross once.) Figure by MIT OCW. 3 You perform an identical analysis on Protein B and are puzzled by the results. A hydropathy plot does not yield any indication of membrane localization. Thankfully, your pal, an expert biochemist, has characterized Protein B as a homohexamer with strong alpha helical character. Quaternary structural analysis predicts the following arrangement of molecules. Figure by MIT OCW. b) Would you expect this protein could serve the role of a channel for the transport of polar molecules? What is your reasoning after hearing of the above structural data. Because of the hexamer structure of the complex, it is possible that the protein spans the membrane.The alpha helical nature would allow for the protein to be “amphipathic”. c) Why is the alpha helical nature of the protein crucial to your assessment of its potential membranous or cytoplasmic localization? Draw a possible orientation of a Protein B hexamer with respect to a lipid bilayer. Alpha helical rotations encompass 3.6 aa residues. Therefore, polar residues could point in toward the aqueous channel and hydrophobic residues could point into the bilayer. You cannot rule out a membranous localization for this protein based on the hydropathy plot cannot rule out a membranous localization for this protein based on the hydropathy plot.) Figure by MIT OCW. 4 Question 3 H a) Draw the tetrahedral cage-like crystalline structure of water in its solid form with unshared electron pairs, etc. Draw at least one water molecule and its nearest neighbors. H O H H O H O H O H H O H Figure by MIT OCW. b) What type of bonds was made between one water molecule and another in the figure above? Hydrogen bonds c) What property of molecular water allows for the structure you have drawn? unpaired electrons attract electropositive hydrogens from other water molecules – up to 4 H-bonds can be made per water molecule. This is the case for ice. The polar character of water allows hydrogen bonds to form. Consider the following thermodynamic data (DG=DH -TDS.) H20 (solid) ‡ H20 (liquid) DH = + 5.9 kJ/mol DG=0, at 0°C d) Would you predict that the reaction is favorable at room temperature? __Yes____ e) Using your knowledge of the solid and liquid forms of water, explain why the DH of this reaction is positive? Considering your explanation, why does ice melt? Considering your explanation, why does ice melt? Going from solid to liquid water requires the breaking of hydrogen bonds, which requires the input of energy. Ambient heat is responsible for the melting of ice at room temperature. Also entropy is increased in water as opposed to ice so both a higher temperature and positive entropy value is subtracted from the DH value resulting in a spontaneous reaction at room temperature.. f) Consider the following observation about tertiary structure of proteins – hydrophobic residues tend to be clustered and often buried deep within the protein. Explain this observation using what you know about the favorable nature of hydrogen bonds. (It is energetically unfavorable for hydrophobic residues to be exposed to water. Water forms ordered structures around hydrophobic regions. It is favorable for hydrophobic residues to cluster so that water is removed from their environment and is free to form hydrogen bonds. This also increases the entropy or disorder, which is favorable for a free energy change.) 5 Question 4 You have recently picked up a research project that was initially undertaken by a former student in your lab. The project involves the biochemical characterization of a protein with a known enzymatic activity. You pull out the student’s notebook and see the following graph: 8,000 Reaction Velocity (S-1) 7,000 Km = 30nm Vmax = 8,000 S-1 6,000 5,000 4,000 3,000 Km = 30nm Vmax = 4,000 S-1 2,000 1,000 0 50 100 150 200 250 [S] (nM) Figure by MIT OCW. a) Describe what is going on in this experiment. Estimate a vmax and Km and write these on the graph above. A fixed level of enzyme is initially present with varying concentrations of substrate. The line is made by measurements from a series of experiments. Vmax is the maximal velocity at “maximal substrate” Km is the concentration of substrate at half-maximal reaction velocity.). b) Why does the curve approach a maximum? The curve levels off because the amount of enzyme is small relative to substrate and it became saturated. c) On the same graph, draw the corresponding curve if twice as much enzyme was added initially in the experiment. From your new data, estimate a vmax and Km and write these next to your new data. 6 d) On the coordinates below, draw a simple reaction profile for an energetically favorable one-step reaction with a high activation energy. Label the reactants and products. Relative Energy Uncatalyzed reaction Reactants Enzymecatalyzed reaction Products Reaction Coordinate Figure by MIT OCW. e) Now draw the profile for the catalyzed reaction on the same graph with a dashed line. Finally, comment on the changes that the addition of enzyme imposes on the following: Choose from “Increases” or “Decreases“ or “No Change”. A. Free energy of products None B. Overall free energy change (DG) None C. Activation Energy of forward reaction Decreases D. Activation Energy of reverse reaction Decreases E. Rate of forward reaction Increases F. Rate of reverse reaction Increases 7 Question 5 a) Nucleotides are the building blocks for DNA polymers. Draw the below dNTPs and indicate clearly the 5’ end and the 3’-hydroxyl. Deoxyadenosine 5’-triphosphate (dATP) O O P O O <5' end> O O - P O O P H O O CH CH2 C H H N O H C C C OH H N N H C C N C C N H H <3' OH> Figure by MIT OCW. Deoxycytosine 5’-triphosphate (dCTP) O O P O <5' end> O O - P O O O P O C H <3' OH> C CH CH2 H H O C N O H C C OH H H H N C H N C O Figure by MIT OCW. Melting of double stranded DNA to single strands is an important technique in molecular biology. Your favorite computer program predicts the below melting temperatures for the corresponding oligonucleotide sequences you plan to use for a technique called PCR (polymerase chain reaction). In this process we will learn about later in the semester, the oligonucleotides will anneal to complementary DNA. Oligonucleotide sequence TGTAACCATTATCATATTCATGAC AAGAGCGCTTGGTGCTTGCATT Melting temperature (Tm) 50.2° C 62.1° C b) Explain a possible reason for the disparity in melting temperature. The GC content is the reason for the difference in melting temperature c) What is the chemical basis for this disparity? G-C base pairs are capable of making more hydrogen bond contacts and thus form stronger bonds. Therefore, the more GC-rich, the higher the melting temperature. To understand this visually, consider the sites which could form hydrogen bonds in the nucleotides you have drawn above. There are three such sites in dCTP and only two in dATP. 8 STRUCTURES OF AMINO ACIDS at pH 7.0 O O H C CH3 ALANINE (ala) H O O H N C H + C C CH2 NH3 + O N C H H H O- O C CH2CH2 S CH3 H H C C CH3 NH OH + 3 THREONINE (thr) C CH3 NH3 + CH3 H H O- H H C CH2 O- C C H C CH2 OH NH3 + SERINE (ser) PROLINE (pro) H H H H O O NH3 + TRYPTOPHAN (trp) O - O H N C NH3+ NH3 + H C CH2 CH2 H N CH2 H + H C CH2CH2CH2CH2 LYSINE (lys) O PHENYLALANINE (phe) O OC H C CH2 LEUCINE (leu) C CH2 O C H C NH3 + METHIONINE (met) GLYCINE (gly) H H C H NH3 + O C NH3 + O H H O- O C H C C CH2CH3 ISOLEUCINE (ile) HISTIDINE (his) NH2 C NH3 CH3 + H H C O O O C O GLUTAMINE (gln) O C H O- ASPARTIC ACID (asp) O NH3 + GLUTAMIC ACID (glu) CYSTEINE (cys) NH3 + OC CH2CH2 O- O C CH2 C NH2 C H C H ASPARAGINE (asn) O NH3 + NH3 + NH3 + O C CH2CH2 C O C CH2 C NH2 + C C CH2 SH H NH2 O- O C H C ARGININE (arg) O O C CH2CH2CH2 N O- O C H NH3 + NH3 + O O C C H O O H H C C H O O C CH2 OH NH3 + H TYROSINE (tyr) H H C C NH3 H + CH3 CH3 VALINE (val) 9