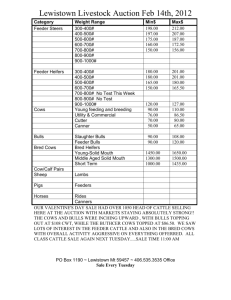
CHANGES IN TEMPORAL LEPTIN CONCENTRATIONS AND OTHER ANESTROUS, SUCKLED, BEEF COWS
advertisement

CHANGES IN TEMPORAL LEPTIN CONCENTRATIONS AND OTHER METABOLIC FACTORS IN PRIMIPAROUS, POSTPARTUM, ANESTROUS, SUCKLED, BEEF COWS EXPOSED TO BULLS by Jesse Riley Olsen A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Animal and Range Sciences MONTANA STATE UNIVERSITY Bozeman, Montana August, 2009 ©COPYRIGHT by Jesse Riley Olsen 2009 All Rights Reserved ii APPROVAL of a thesis submitted by Jesse Riley Olsen This thesis has been read by each member of the dissertation committee and has been found to be satisfactory regarding content, English usage, format, citation, bibliographic style, and consistency, and is ready for submission to the Division of Graduate Education. Dr. James G. Berardinelli Approved for the Department of Animal and Range Sciences Dr. Bret E. Olson Approved for the Division of Graduate Education Dr. Carl A. Fox iii STATEMENT OF PERMISSION TO USE In presenting this thesis in partial fulfillment of the requirements for a doctoral degree at Montana State University, I agree that the library shall make it available to borrowers under rules of the library. If I have indicated my intention to copyright this thesis by including a copyright notice page, copying is allowable only for scholarly purposes, consistent with “fair use” as prescribed in the U.S. Copyright Law. Requests for permission for extended quotation from or reproduction of this thesis in whole or in parts may be granted only by the copyright holder. Jesse Riley Olsen August, 2009 iv ACKNOWLEDGEMENTS I would like to thank my parents, Peter and Linda Olsen for their love and encouragement throughout my academic career at Montana State University. As well as, a special thanks to Dr. Duane Keisler for his collaboration with the leptin assay; my fellow graduate colleagues, Jarrod R. C. Wilkinson and Shaun A. Tauck for their insurmountable support, assistance, and input throughout this thesis; and undergraduate interns, Luke Runnion, Tim Gibbs, Katie Philips, Riley Wedlake, and Kyla Hendry. Also, I would like to express my sincerest thanks to my major professor, Dr. James G. Berardinelli, for his time, guidance, encouragement of new ideas, patience, constructive criticism, and friendship throughout the course of my graduate training. This study was supported by Award No. 2007-35203-17743, NRI Competitive Grants Program, CSREES, and the USDA, the Montana NSF EpsCOR program, the Montana Agricultural Experiment Station, and is a contributing project to Multistate Research Project, W1112, Reproductive Performance in Domestic Ruminants. I would also like to thank my graduate committee members, Drs. Milan Shipka and Dennis Hallford for their valuable time, assistance, and encouragement during the course of my studies and in the preparation of this thesis. v TABLE OF CONTENTS 1. INTRODUCTION .........................................................................................................1 2. LITERATURE REVIEW ..............................................................................................4 Physiology of the Postpartum Anestrous Cow ..............................................................4 Hypothalmic Neurohormone ...................................................................................4 Gonadotropin-Releasing Hormone (GnRH) ................................................4 Adenohypophyseal Gonadotropins ..........................................................................5 Luteinizing Hormone (LH) ..........................................................................5 Follicle Stimulating Hormone (FSH)...........................................................6 Ovarian Steroids.......................................................................................................7 Estrogen .......................................................................................................7 Progesterone .................................................................................................7 Role of the Hypothalamic Hypophyseal-Ovarian Axis ...........................................8 Hypothalmic Feedback Effect of Estrogen ................................................10 Factors Known to Influence the Postpartum Anestrous Period ...................................11 Nutrition .................................................................................................................12 Effect of Suckling ..................................................................................................15 Other Factors ..........................................................................................................18 Effect of Bull Biostimulation on the Postpartum Anestrous Period ............................19 Metabolic Regulation and Reproduction .....................................................................22 Metabolic Regulation and the Hypothalmic-Hypophyseal Ovarian Axis .............23 Cortisol.......................................................................................................23 Tri-iodothyronine (T3) and Thyroxine (T4) ..............................................24 Role of Leptin in Regulation of Energy Homeostasis and Reproduction ..............25 Leptin in Energy Homeostasis ...................................................................25 Leptin Regulation on Puberty and Gonadotropin Secretion ......................27 Direct Ovarian Effects of Leptin................................................................30 Ovarian Steroid Regulation on Leptin Secretion .......................................30 3. STATEMENT OF PROBLEM ....................................................................................32 4. EXPERIMENT 1: CONCENTRATIONS OF GLUCOSE, NEFA, THYROXINE, AND TRIIODOTHYRONINE IN PRIMIPAROUS, ANESTROUS, SUCKLED BEEF COWS EXPOSED TO BULLS ........................................................................34 Introduction ..................................................................................................................34 Materials and Methods .................................................................................................34 Animals and Treatments ........................................................................................35 Facilities .................................................................................................................35 Nutrition .................................................................................................................36 vi TABLE OF CONTENTS-CONTINUED Intensive Blood Sampling Procedures ...................................................................36 Metabolite and Hormone Assays ...........................................................................37 Statistical Analyses ................................................................................................38 Results ..........................................................................................................................38 Discussion ....................................................................................................................40 5. EXPERIMENT 2: BIOSTIMULATORY EFFECT OF BULLS ON TEMPORAL PATTERNS OF LEPTIN CONCENTRATIONS AND RESUMPTION OF LUTEAL ACTIVITY IN PRIMIPAROUS, POSTPARTUM, ANESTROUS, SUCKLED BEEF COWS ..........................................................................................................................43 Introduction ..................................................................................................................43 Materials and Methods .................................................................................................44 Animals and Treatments ........................................................................................44 Facilities .................................................................................................................45 Nutrition .................................................................................................................45 Blood Sampling for Progesterone, Leptin, and Resumption of Luteal Activity ....46 Statistical Analyses ................................................................................................48 Results ..........................................................................................................................48 Discussion ....................................................................................................................52 6. EXPERIMENT 3: EFFECT OF DURATION OF DAILY BULL EXPOSURE ON LEPTIN CONCENTRATIONS DURING RESUMPTION OF OVULATORY ACTIVITY IN PRIMIPAROUS, POSTPARTUM, ANESTROUS, SUCKLED BEEF COWS ..........................................................................................................................54 Introduction ..................................................................................................................54 Materials and Methods .................................................................................................55 Animals and Treatments ........................................................................................55 Facilities and Daily Bull Exposure ........................................................................56 Nutrition .................................................................................................................57 Blood Sampling, Leptin and Progesterone Concentrations, and Resumption of Ovarian Activity.....................................................................................................58 Statistical Analyses ................................................................................................59 Results ..........................................................................................................................60 Discussion ....................................................................................................................64 vii TABLE OF CONTENTS-CONTINUED GENERAL DISCUSSION ................................................................................................67 Hypothesis for a Metabolic Component or Pathway to the Biostimulatory Effect of Bulls .....................................................................................68 LITERATURE CITED ......................................................................................................72 viii LIST OF TABLES Table Page 1. Number of cows per treatment and least square means for days from calving to start of the experiment, cow BW at the start of treatment, calf birth weight (BW), BCS, calf sex ratio, and dystocia score for primiparous, suckled beef cows exposed to bulls (EB) or not exposed to steers (ES)…...……..…………..39 2. Least squared means of glucose, NEFA, thyroxine (T4), and triiobothyronine (T3) concentrations for primiparous, suckled beef cows exposed to bulls (EB) and steers (ES)………………………………...…………………………..40 3. Number of cows per treatment and least square means for calving date, cow BW, calf BW, BCS, calf sex ratio, and dystocia score for first calf suckled beef cows exposed (BE) or not exposed (NE) to bulls at the start of the experiment………………………………………………..……………...……..49 4. Number of cows per treatment and least squares means for intervals from calving to resumption of luteal activity (RLA) and proportions of cows that RLA during the experiment for primiparous, anestrous, suckled, beef cows exposed to bulls for 12-h daily (BE12), 6-h daily (BE6), or not exposed to bulls (NE) for 45 d starting 51.5 ± 2.3 d (± SE) after calving…...……………..61 ix LIST OF FIGURES Figure Page 1. Pattern of progesterone concentrations used to determine occurrence of resumption of ovarian cycling activity. Panel A represents a cow that resumed cycling activity. Panel B represents a cow that did not resume ovarian cycling activity...........................................................……..…………..47 2. Percentages of primiparous, suckled cows exposed (BE) or not exposed (NE) to bulls that resumed luteal activity (%RLA) at the start of the experiment and after the 30-d exposure period. Bars that lack common letters within each cycling classification differ, P < 0.05; cycling start of experiment, 2 = 1.02, d.f. = 1; cycling at start of breeding season,2 = 9.1, d.f. = 1.........49 3. Least squares means of leptin concentrations for 6-d intervals in cows exposed to bulls (BE) and cows not exposed to bulls (NE) that resumed ovulatory activity after start of experiment (D 0). Bars that lack common letters within and between each interval differ, P < 0.05. Interval by treatment interaction; P < 0.05............………………………………………………..………………..50 4. Percentages of primiparous, suckled cows exposed (BE) or not exposed (NE) to bulls that had not started to cycle by start of experiment (D0) and resumed luteal activity (%RLA) during intervals D 0 to 6, D 9 to 15, and D 18 to 24 of the experiment. Bars that lack common letters within and between each interval differ, P < 0.05; interval D 0 to 6, 2 = 1.99, d.f. = 1; interval D 9 to 15,2 = 8.53, d.f. = 1; interval D 18 to 24,2 = 8.53, d.f. = 1; and between intervals D 0 to 6 and D 9 to 15 for BE cows,2 = 6.2, d.f. = 1..................…..51 5. Facilities where cows were housed during experiment and schedule of daily bull exposure..........................................................................................………..57 6. Least squares means of leptin concentrations for 10-d intervals in cows exposed to bulls for 0 (NE), 6 (BE6), and12 (BE12) h daily. Bars that lack common letters within and between each interval differ, P < 0.05. Interval by treatment interaction, P = 0.06; treatment interaction, P < 0.0001................……………..62 7. Least squares means of leptin concentrations for 10-d intervals in cows exposed to bulls for 0 (NE), 6 (BE6), and12 (BE12) h daily. Non-cycling NE, BE6 and BE12 data are samples collected before the resumption of ovulatory activity. Bars that lack common letters within and between each interval differ, P < 0.05. Interval by treatment interaction, P = 0.08; treatment interaction, P < 0.0001…….................................................................63 x LIST OF FIGURES - CONTINUED Figure Page 8. Percentages of primiparous, suckled cows exposed to bulls for 0 (NE), 6 (BE6), and12 (BE12) h daily that resumed luteal activity (%RLA) during intervals D 0 to 10, D 11 to 20, D 21 to 30, and D 31 to 40 of the experiment. Bars that lack common letters within each interval differ, P < 0.05; interval D 0 to 10, 2 = 3.37, d.f. = 2; interval D 11 to 20,2 = 2.84, d.f. = 2; interval D 21 to 30,2 = 6.38, d.f. = 2; ; interval D 31 to 40,2 = 8.12, d.f. = 2 and the overall interaction, P < 0.05;2 = 382.7, d.f. = 11....................64 xi ABSTRACT Exposing cows to bulls or excretory products of bulls stimulates resumption of ovarian cycling activity in postpartum, suckled, anestrous cows. This biostimulatory effect may be mediated by pheromones produced by bulls that stimulate physiological changes in metabolic regulation of the hypothalamic-pituitary-ovarian (HPO) axis of cows. In Experiment 1, the hypotheses tested were that concentrations of glucose, NEFA, thyroxine (T4), tri-iodothyronine (T3), and T3:T4 ratios do not differ between cows exposed to bulls or steers. The biostimulatory effect of bulls was associated with lower mean concentrations of NEFA in postpartum cows. Experiment 2 was designed to determine if continuous (24-h daily) bull exposure alters temporal patterns of leptin concentrations in postpartum, anestrous cows. Cows exposed to bulls that resumed cycling activity after the start of the experiment tended to have higher leptin concentrations by the end of the 30-d exposure period than cows not exposed to bulls. However, it was not known if these changes were related to resumption of ovarian cycling activity in postpartum, anestrous cows. Experiment 3 tested the hypothesis that temporal leptin concentrations may depend upon duration of daily bull exposure. Cows had higher daily leptin concentrations and resumed ovarian cycling activity sooner as duration of daily bull exposure increased. In conclusion, as duration of daily bull exposure increases, the biostimulatory effect of bulls alters temporal leptin concentrations and this change may facilitate or support the function of the HPO axis and accelerate resumption of ovarian cycling activity in primiparous, postpartum, suckled, anestrous cows. 1 CHAPTER 1 INTRODUCTION The goal of cow-calf operations is to wean one calf per cow per year (Yavas and Walton, 2000). This can be referred to as reproductive efficiency. Postpartum anestrus, or the time after calving during which cows do not exhibit estrus and cannot become pregnant, decreases reproductive efficiency in beef cattle. Generally, after a gestation of approximately 280 d, cows are required to establish a pregnancy within 80 to 85 d after calving. This problem is more pronounced in first-calf suckled beef cows that require 15 to 25 d longer to resume ovarian cycling activity than multiparous cows (Short et al., 1994). Many management strategies have been developed to help reduce the interval of postpartum anestrus. Unfortunately, these strategies can be costly, labor intensive, unsustainable, and socially unacceptable. The use of the biostimulatory effect of bulls may be an effective management strategy to reduce postpartum anestrus that is cost effective, labor saving, sustainable, and socially acceptable to both producers and consumers. Although, implementation of the use of the biostimulatory effect of bulls in practical situations and the mechanism by which this effect is mediated is not well understood. The biostimulatory effect of bulls involves interactions between bulls and cows in which the physical presence of bulls stimulates the resumption of ovarian cyclic activity in multiparous and primiparous suckled beef cows (Zalesky et al., 1990; Custer et al., 1990). Berardinelli and Joshi (2005) reported that more cows exposed to bulls 55 d 2 postpartum resumed ovarian cycling activity within 20 d of exposure than cows exposed to bulls starting on d 15 or d 35. This result indicates that the ability of cows to respond to the biostimulatory effect of bulls increases as time after parturition increases. Bull exposure is known to alter cortisol concentrations and the characteristics of their pulsatile patterns (Tauck et al., 2007; Tauck, 2008). Furthermore, there is the possibility that the biostimulatory effect of bulls to accelerate resumption of ovulatory activity involves metabolic changes in cows that are induced by changes in cortisol concentrations, specifically changes involved with adipose metabolism (Landaeta-Hernandes et al.; 2004, Wilkinson et al., 2007). Experiment 1 of this thesis focuses on the influence of the biostimulatory effect of bulls on concentrations of thyroid hormones, thyroxine (T4) and tri-iodothyronine (T3), glucose, and nonesterified fatty acids (NEFA). Research conducted within the last ten years has shed light on the physiological mechanisms by which energy balance modulates reproductive functions. Leptin, an adipocyte-derived hormone or adipokine, is secreted by white adipose tissue and is an important factor in the control of energy balance, satiety, and food intake, and has been shown to have many levels of modulation within the hypothalamic-hypophyseal-gonadal axis (HPO; for review see, Tene-Sempere, 2007). In Experiment 2 and 3, we investigated the capacity of the biostimulatory effect of bulls to alter temporal leptin concentration patterns during postpartum anestrus and resumption of ovarian cycling activity. This review of literature will encompass: 1) an overview of the endocrinology of postpartum cows and factors that influence the postpartum interval to resumption of 3 ovarian cycling activity; 2) a review of the biostimulatory effect of bulls on the resumption of ovarian cycling activity of postpartum cows; and 3) an in depth review of literature related to metabolic regulation, specifically by leptin, of reproduction in mammals. 4 CHAPTER 2 LITERATURE REVIEW Physiology of the Postpartum Anestrous Cow Endocrine control of the reproductive system includes the function of the hypothalamus, anterior pituitary gland and the ovaries. Gonadotropins are hormones secreted by the anterior pituitary gland that modulate processes of the ovary. Ovarian function includes: the development, maturation and release of the female gamete; and, the synthesis and secretion of protein and steroid hormones. Reproductive events in the female are regulated by a complex system of interactions between hormones secreted within the hypothalamic-pituitary-ovarian (HPO) axis. This section will review hormones of the hypothalamus, anterior pituitary, and ovary which play a major role in regulating reproductive function in the postpartum anestrous female bovine. Hypothalmic Neurohormone Gonadotropin-Releasing Hormone (GnRH). Gonadotropin-releasing hormone is a 10 amino acid neurohormone released from a subset of the hypothalamic neurosecretory neurons into the hypothalamo-hypophyseal portal circulation to the adenohypophysis. The primary function of GnRH is to stimulate secretion of gonadotropic hormones from the anterior pituitary gland. The release of the gonadotropins luteinizing hormone (LH) and follicle stimulating hormone (FSH) from the anterior pituitary (listed below), particularly when measured in the portal blood, are 5 characterized as pulsatile or otherwise reflecting a rapid increase followed by a decrease in circulating concentrations (Caldani et al., 1993; Padmanabhan et al., 2002a). However, pulsatile release is not spontaneous but induced by GnRH. Furthermore, gonadotropin secretion is regulated by the interaction of hypothalamic GnRH with gonadotropes and the feedback of gonadal steroids and peptides (Caldani et al., 1993). Additionally, other intra-adenohypophyseal factors modulate the secretion of LH and FSH (Padmanabhan et al., 2002a). Adenohypophyseal Gonadotropins Luteinizing Hormone (LH). The most consistent factor reported to precede resumption of ovarian cycling activity in anestrous female bovids is the onset and increase in LH pulsatility (Walters et al., 1982). The gonadotropin LH is a dimeric glycoprotein secreted by luteotropes of the anterior pituitary (for review see, Hafez and Hafez, 2000). During the luteal phase of the estrous cycle of cows, LH is secreted into the general circulation in a temporal pattern characterized as having low frequency and high amplitude pulses. During proestrus, the period proceeding estrus, the pulsatile patterns in LH changes to that of low amplitude and high frequency LH release. There have been many studies on LH pulsatility prior to ovulation and it is well established that this is the appropriate pattern of LH release that leads to the preovulatory surge of LH and causes ovulation (Hafez and Hafez, 2000). An increase in LH pulse frequency is observed before anestrous cows resume ovarian cycling activity (Walters et al., 1982; Humphery et al., 1983; Peters and 6 Lamming, 1990). Furthermore, the pulse frequency, amplitude (Rawlings et al., 1980; Garcia-Winder et al., 1984; Garcia-Winder et al., 1986; Savio et al., 1990; Wright et al., 1990), and average plasma concentration (Walters et al., 1982; Humphrey et al., 1983; Garcia-Winder et al., 1984; Garcia-Winder et al., 1986; Nett et al., 1988) of LH have been shown to increase with time after calving. Once LH pulse frequency, amplitude, and concentration change to patterns observed in proestrus, a surge of LH is released from the anterior pituitary which in turn causes ovulation and resumption of normal ovarian cycling activity in postpartum anestrous cows. Follicle Stimulating Hormone (FSH). Follicle stimulating hormone is also a dimeric gonadotropin glycoprotein like LH; however, it is synthesized and secreted by folliculotropes of the anterior pituitary and is responsible for the recruitment and development of secondary and tertiary follicles of the ovary (for review see, Hafez and Hafez, 2000). Follicle stimulating hormone and LH differ molecularly within the βsubunit, but α-subunit composition is the same for both FSH and LH. Follicular selection and dominance appear to be dependent on FSH (for review see, Hafez and Hafez, 2000). Concentrations of FSH have been shown to increase shortly after parturition (Moss et al., 1985; Crowe et al., 1998); however, these fluctuations do not result in ovulation. In a cow with normal ovarian cycling activity, FSH secretion is controlled by the negative feedback effects of follicular inhibin. As one follicle begins to become dominant, the granulosa cells within this dominant follicle release inhibin, inhibiting the release of FSH from pituitary folliculotropes causing smaller follicles within that cohort begin atresia. The dominant follicle then becomes 7 FSH independent-LH dependent and the appropriate temporal LH release pattern stimulates the final maturation and development of the largest or “dominant” follicle. In anestrous cows, maturation, development, and ovulation of follicles does not occur because of the inappropriate release of LH. Dominant follicles in this type of hormone milieu fail to synthesize and secrete estradiol-17β and do not grow beyond 7 to 9 mm in diameter (Gong et al., 1995; Gong et al., 1996). As a result, these follicles do not ovulate Ovarian Steroids Estrogen. Estradiol-17β, the dominant ovarian estrogen, is synthesized primarily by the granulosal cells of the ovarian antral follicles. Shortly after parturition, circulating concentrations of estradiol-17β are very low due to the ovaries being devoid of large follicles (Arije et al., 1974; Humphrey et al., 1983; Crowe et al., 1998). However, follicular waves that develop a dominant follicle (DF) begin to develop 5 to 11 d after parturition (Crowe et al., 1998). Thereafter, estradiol-17β concentrations remain relatively constant and low throughout postpartum anestrus (Carruthers et al., 1980; Chang et al., 1981), regardless of any effect suckling and milk production may have upon secretion (Carruthers and Hafs, 1980). Again, only the appropriate LH signal can stimulate a change in estradiol-17β concentrations, inducing a DF to become larger and secrete higher levels of estradiol-17β. This change can induce behavioral estrus and signal the preovulatory release of LH from the anterior pituitary. Progesterone. Progesterone is a steroid hormone produced by the theca interna cells of antral follicles and corpora lutea (CL; Hafez and Hafez, 2000). Following 8 parturition, progesterone concentrations decrease almost immediately and remain low throughout postpartum anestrus. Usually, the postpartum cow exhibits a small rise in progesterone concentration 3 to 7 days prior to the resumption of ovulatory activity (Arije et al., 1974; Stevenson and Britt, 1979; Rawlings et al., 1980; Humphrey et al., 1983; Werth et al., 1996). This increase in progesterone concentrations is followed by a decline to baseline concentrations before the first postpartum estrus and is often referred to as a “short cycle”. Short cycles are due to ovulation and formation of a CL (Castenson et al., 1976; Stevenson and Britt, 1979); however, the CL formed after this ovulation is short lived and produces small amounts of progesterone (Corah et al., 1974). Behavioral estrus does not generally accompany this ovulation probably because there is no preovulatory increase in estrogen. A priming effect by progesterone is believed to be responsible for the full expression of estrus in response to estrogen in the bovine (Kieborz-Loos et al., 2003). Mann and Lamming (2000) showed that the shortened lifespan of the CL is most likely caused by low concentrations of estradiol-17β before ovulation which, in turn, causes the retention of oxytocin receptors in the endometrium of the uterus; this allows for a premature increase in prostaglandin-F2α (PGF2α) and lysis of the CL. Role of the Hypothalamo-Hypophyseal-Ovarian Axis Considerable effort has gone into the study of the relationship between the postpartum anestrous interval to resumption of ovulatory activity and the physiological mechanisms responsible for its occurrence and subsidence (for reviews see, Peters and Lamming, 1986; Short et al., 1990; Williams, 1990; Yavas and Walton, 2000). Physiological controls of the postpartum period are located in the complex interactions of 9 neuroendocrine-endocrine relationships within the hypothalamo-hypophyseal-ovarian axis. Simply, the hypothalamus stimulates the pituitary which, in turn, stimulates the ovary, and again in turn, stimulates a feedback and exerts control over the hypothalamus and adenohypophysis. The following is an overview of the interaction between the hypothalamus, pituitary, and ovary during the postpartum, anestrous periods of the bovine. Normal ovarian function is dependent upon stimulation by gonadotropins secreted by the adenohypophysis, also called the anterior pituitary gland. The hypothalamus contains neurons that are known as neurosecretory neurons or peptidergic neurons because they synthesize peptides (neurohormones) that are secreted into the blood, instead of a synaptical space. Gonadotropin secretion is modulated by the secretion of GnRH. Once GnRH binds to its receptors on gonadotropes, it stimulates the synthesis and secretion of gonadotropins. More specifically, GnRH stimulates pituitary luteotropes and folliculotropes to secrete LH and FSH, respectively. Generally, GnRH neurons intrinsically secrete GnRH in small bursts or pulses; the intrinsic pattern of release is known as the GnRH “pulse generator”. A pulse of GnRH will stimulate a concomitant pulse in LH but not FSH (for review see, Hafez and Hafez, 2000). Therefore, factors that modulate the GnRH pulse generator will influence the secretion pattern of LH release as well. Luteinizing hormone pulsatility is an important factor in explaining the failure of DF to ovulate soon after parturition. Luteinizing hormone has direct stimulatory effect on the DF and in a cow exhibiting normal ovarian cycling activity, temporal release of 10 LH is characterized by low frequency and high amplitude pulses (Walters et al., 1982; Peters and Lamming, 1990). This temporal release pattern extends the life of the DF and causes it to grow beyond 7 to 9 mm into a pre-ovulation size of greater than 9 mm (Fortune et al., 1991; Savio et al., 1993; Gong et al., 1995; Rhodes et al., 1995; Gong et al., 1996). During postpartum anestrus, LH patterns are characterized by low frequency and high amplitude pulses (Rawlings et al., 1980; Humphery et al., 1983; Garcia-Winder et al., 1984; Garcia-Winder et al., 1986; Savio et al., 1990; Wright et al., 1990). The temporal release pattern of LH is the primary endocrine cause of postpartum anestrus. Therefore, to understand the physiological mechanisms involved with regulating resumption of ovarian cycling activity in cows, one must understand those mechanisms that limit pulsatile LH release. Hypothalmic Feedback Effect of Estrogen. Secretion of gonadotropin releasing hormone (GnRH), a critical neurohormone produced by the hypothalamus that stimulates the release of both LH and FSH, is greatly influenced by production of gonadal steroids (Fernandes et al., 1978; Jagger et al., 1987). Progesterone is high during the luteal phase of the estrous cycle, however as progesterone begins to decrease during the early proestrous phase of the estrous cycle, low estradiol concentrations cause neurosecretory neurons to release GnRH with an increased frequency, causing release of LH from the anterior pituitary in low amplitude, high frequency manner (Rawlings et al., 1980; Humphery et al., 1983; Garcia-Winder et al., 1984; Garcia-Winder et al., 1986; Savio et al., 1990; Wright et al., 1990, Evans et al., 1994), described as the positive feedback mechanism of estradiol. The release of LH in this temporal manner stimulates the 11 continued growth and development of the DF in the ovary, causing the synthesis and secretion of estadiol-17β. Once estrogen concentrations reach a threshold, luteotropes are stimulated for the episodic or preovulatory release of LH. This episodic release of LH assures development and ovulation of the dominant follicle. Concomitantly, the sexual behavior center of the brain detects the rise in estrogen concentration and stimulates behavioral estrus. During postpartum anestrus, circulating estrogen concentrations do not change dramatically (Carruthers et al., 1980; Chang et al., 1981). Neurosecretory neurons responsible for the tonic release of GnRH are quite sensitive to low constant levels of estradiol-17β (Acosta et al., 1983) causing a decrease in the pulsatile release of LH from the anterior pituitary. This is known as the negative feedback effect of estrogen. As time after calving increases, sensitivity of neurosecretory neurons to estrogen declines, allowing GnRH pulses to occur more frequently. This increased GnRH pulse frequency stimulates the appropriate low amplitude, high frequency LH secretion required for ovulation and resumption of ovarian cycling activity following parturition. Factors Known to Influence the Period of Postpartum Anestrus The primary factors influencing the length of time from calving to the resumption of ovarian cycling activity are nutrition, suckling stimuli, and parity. Other factors that affect this interval are breed, environmental stress, dystocia, and social factors (for review see, Short et al., 1990; Yavas and Walton, 2000). 12 Nutrition Basal metabolism, activity, growth and basic energy stores have priority over reproduction (Grimard et al., 1997; Guedon et al., 1999), and a negative energy balance would reduce availability of glucose for use in processes such as resumption of ovarian cycling activity and maintenance of a pregnancy (Yavas and Walton, 2000). Such a negative metabolic state would increase the mobilization of energy reserves stored as adipose tissue. Additionally, energy balance greatly influences the length of postpartum anestrus in the bovine, whereby low energy intake before and after calving increases the time interval from calving to resumption of ovarian cycling activity (Dunn et al., 1969; Wiltbank, 1970; Falk et al., 1975; Dunn et al., 1985). Furthermore, if cows remain on a low plane of nutrition after calving, they may fail to resume ovarian cycling activity throughout the breeding season (Wiltbank, 1970). On the other hand, the interval to resumption of ovarian cycling activity can be shortened if cows are fed a higher plane of nutrition after calving (Bellows and Short, 1978; Ducker et al., 1985; Henricks and Rone, 1986). As a consequence, cows fed a high plane of nutrition have a greater chance of becoming pregnant before the end of the breeding season than cows fed a low plane of nutrition (Bellows and Short, 1978; Henricks and Rone, 1986; DeRouen et al., 1993). A moderate to high plane of nutrition is important for the resumption of ovarian cycling activity after calving (Dunn et al., 1969; Wiltbank, 1970; Falk et al., 1975; Dunn et al., 1985). However, there are conflicting reports regarding the prepartum and postpartum nutrition regimens affecting postpartum ovarian cycling activity. Houghton 13 et al. (1990) reported that cows fed a low energy diet before calving and switched to a high energy diet after calving had shorter postpartum intervals to estrus and more cows cycling than cows fed the low energy diet prepartum and low energy diet postpartum. Contrary to these results, Dunn and Kaltenbach (1980) concluded that prepartum nutrition was more important than postpartum nutrition in reducing the postpartum interval. These results beg to question as to how nutrition might affect follicular development. Henricks and Rone (1986) reported that cows fed a high energy diet had more medium- and small-sized follicles and higher estradiol-17β concentrations than cows fed a low energy diet. Also, it has been observed that increasing the energy intake beginning either two wk before parturition (Lammoglia et al., 1996), at parturition (Beam and Butler, 1997; DeFries et al., 1998), or at four wk after calving (Khireddine et al., 1998) increased the number of antral follicles in the ovaries. Further evidence has shown that feeding a higher energy ration at parturition prolongs the life of the corpus luteum (Williams, 1989). Progesterone, the hormone of pregnancy, prepares the uterus for embryonic implantation and sustains pregnancy after implantation. Gombe and Hansel (1973) and Beal et al. (1978) found that progesterone concentrations were lower for first-calf cows fed a restricted-energy diet. The low concentrations of progesterone in these cows could have been due in part to the low rate of follicular development caused by poor nutrition documented by Henricks and Rone (1986) and Perry et al. (1991). Also, energyrestricted cows had smaller corpora lutea with lower progesterone contents on d 10 of the 14 third postpartum cycle (Gombe and Hansel, 1973). This indicates that energy intake can be directly related to the ability of the cow to conceive and maintain pregnancy (Dunn et al., 1969). Percent body fat at the time of and after parturition can also influence the length of postpartum anestrus in cows (Bartle et al., 1984; Richards et al., 1986). Cows of good body condition at time of parturition resume ovarian cycling activity and conceive sooner compared to thin cows that have longer intervals from calving to resumption of ovarian cycling activity and therefore a greater difficulty conceiving before the end of the breeding season (DeRouen et al., 1994; Spitzer et al., 1995; Vizcarra et al., 1998). Body condition score positively correlates with follicular development early postpartum (Ryan et al., 1994), pituitary LH concentrations at 30 d after calving (Connor et al., 1990), and influences circulating concentrations of insulin-like growth factor-1 (IGF-1), LH pulse frequency, and postpartum interval to resumption of ovulatory activity after weaning (Bishop et al., 1994). However, Wright et al. (1990) reported that average LH concentrations in systemic circulation were not affected by body condition. Severe nutrient restriction has been shown to have a negative effect on LH secretion in sexually-mature and prepubertal mammals, including cattle (Day et al., 1986a; McCann and Hansel, 1986) and sheep (Foster et al., 1989; Keisler and Lucy, 1996). Restricted and control-fed heifers respond to administration of exogenous GnRH with an LH surge-like release; however, the release of LH was lower in restricted than in control heifers (Day et al., 1986a). These results indicate that under-nutrition suppresses pulsatile LH secretion by impairing hypothalamic GnRH release. Thus, the diminished 15 pulsatile release of LH observed during low energy balance has been generally accepted as the result of a reduced frequency of GnRH pulses (Williams, 1999). However, under nutrition may have a modulatory effect directly at the level of the luteotropes in the anterior pituitary. Simultaneous blood sampling for LH from the adenohypophyseal portal system and the jugular vein has demonstrated that there is a greater reduction in frequency of LH pulses in feed-restricted female lambs than the reduction in frequency of GnRH pulses (I’Anson et al., 2000). This suggests low amplitude pulses of GnRH did not always result in concomitant LH pulses and that gonadotropes tend to have a decreased sensitivity to GnRH in long-term, feed-restricted lambs. Although, short-term fasting had effects on indicators of metabolic status, such as insulin, IGF-I, and nonesterified fatty acids (NEFA) (McCann and Hansel, 1986; Kadokawa and Yamada, 1999), there was no affect on circulating LH concentrations in mature cows. However, in prepubertal heifers, 48 to 72 h of total feed restriction reduces LH pulsatility (Amstalden et al., 2000; Maciel et al., 2003a). Taken together, energy balance, maturity of the central reproductive axis, and interactions with the negative feedback of estradiol may be major factors in influencing the postpartum anestrous period in cows. Good body condition and medium to high energy rations fed pre- or postpartum are necessary for cows to resume ovarian cycling activity, conceive, and maintain pregnancy after calving. Effect of Suckling If nutrition is not a limiting factor, suckling stimuli are the primary factor contributing to prolonged postpartum anestrus in beef and dairy cows (Short et al., 1990; Williams, 1990; Stagg et al., 1998). Cows suckled once daily and non-suckled cows have 16 shorter postpartum anestrous intervals to resumption of ovarian cycling activity than cows suckled twice daily, cows suckled normally, and cows suckled intensively (Smith and Vincent, 1972; Laster et al., 1973; Carter et al., 1980; Odde et al., 1980; La Voie et al., 1981; Randel, 1981; Reeves and Gaskins, 1981; Ramirez-Godinez et al., 1982; Garcia-Winder et al., 1984; Houghton et al., 1990). Removal of the calf at birth and weaning calves early causes a significant reduction in the length of postpartum anestrus compared to cows suckling calves (Bellows et al., 1974; Walter et al., 1982; Williams, 1990; Short et al., 1990); whereas, intensive suckling can prolong postpartum anestrus in cows (Wettemann et al., 1986; McNeilly, 1988). Suckling stimuli have the potential to modulate the neuroendocrine-endocrine physiological events that control the resumption of ovarian cycling activity. Cows who have been weaned from their calves have higher LH concentrations than suckled cows (Walters et al., 1982; Carter et al., 1980). Non-suckled cows and restricted-suckled cows exhibit a sustained increase in LH concentrations within 20 d after calving, while cows subjected to intensive suckling exhibit no sustained increase until 48 d postpartum (Garcia-Winder et al., 1984). Also, LH mean concentration, peak frequency (Carruthers and Hafs, 1980; Whisant et al., 1986), and amplitude (Carruthers and Hafs, 1980; Chang et al., 1981; Whisnant et al., 1986) increase significantly within 48 h post-weaning compared to non-weaned, suckled cows. Additionally, suckled cows have lower frequency and amplitude of LH release than milked cows (Carruthers and Hafs, 1980). Therefore, suckling may affect the hypothalamus and GnRH neurosecretory system 17 reducing the tonic release of LH causing the postpartum anestrous state of ovarian quiescence. Inhibition of LH release by suckling may involve more than mammarysomatosensory pathways. The physical presence of the calf may also be needed to elicit this effect. Williams et al. (1993) found that LH pulse frequency increased within 9 to 13 days after weaning when compared to that of suckled cows. Also, these investigators found that denervation of the mammary gland failed to increase the LH pulse frequency. Therefore, the physical presence of a calf appeared to be a component of the inhibitory effect of the suckling stimulus and suggested that cows recognize calves through olfactory and visual cues. This is known as the cow-calf bond (Williams and Griffith, 1995; Lamb et al., 1997). In summary, it is possible to model the mechanism by which suckling stimuli fits into the endocrinology of the postpartum anestrous cow. Following parturition, suckling stimuli is intense due to the close relationship to and the physical presence of the calf. Suckling stimuli causes the GnRH pulse generator of the hypothalamus to be more sensitive to the negative feedback effects of estrogen; resulting in high amplitude, low frequency patterns of GnRH and causes high amplitude, low frequency pulses of LH. This type of LH pattern signals the ovary to remain anovular. As time increases postpartum and the calf grows older, it becomes more nutritionally and socially independent, reducing suckling stimuli. There is then a decrease in the sensitivity of the GnRH pulse generator to the negative feedback effect of estrogen, causing a change in 18 the release of LH in a high frequency, low amplitude manner. This type of pulsatile pattern in LH signals to the ovary to resume ovarian cycling activity. Other Factors The following is a summary of other factors contributing to postpartum anestrus reviewed by Short et al. (1990). Multiparous cows have shorter anestrous intervals than do primiparous cows. The reason for this is believed to be a result of a combination of increased incidence of dystocia and greater nutrient demands on primiparous cows due to energy requirements for growth, lactation, and production of a calf. Dystocia in both primiparous and multiparous cows increases the length of postpartum anestrus. The greater degree of difficulty during parturition causes more damage to the reproductive tract and increases amount of physical stress. Stress increases energy demand which, in turn, causes prolonged periods of anestrus after parturition. Stress can come in many forms; studies have shown that stressing the cow with extreme heat or cold will prolong postpartum anestrus. Breed or breed-type also plays a role in the length of postpartum anestrus. Continental cattle breeds have higher growth and milking genetic potentials, and longer postpartum anestrous intervals than do British breeds. Increased milking and growth potentials cause an increase in nutrient demands, which may result in longer intervals from calving to resumption of ovarian cycling activity. 19 Effect of Bull Biostimulation on the Postpartum Anestrous Period Exposing cows to the physical presence of bulls with intent to hasten the resumption of ovarian cycling activity was first documented in early breeding experiments (Nersesjan, 1962; Sipilov, 1964; Ebert et al., 1972; Macmillan et. al., 1979; Zalesky et al., 1984; Berardinelli et. al., 1987; Scott and Montgomery, 1987; Custer et. al., 1990; Naasz and Miller, 1990; Stumpf et. al., 1992; Cupp et. al., 1993; Hornbuckle et. al., 1995; Fernandez et al., 1996; Peres-Hernandez et al., 2002; Anderson et al., 2002). This biostimulatory effect of bulls is described as the influence of bulls on the reproductive function of cows that is mutually beneficial for successful reproduction. Bull biostimulation interacts with other factors known to influence the length of postpartum anestrus (Stumpf et al., 1992; Hornbuckle et al., 1995). Stumpf et al. (1992) showed cows exposed to bulls and fed a low plane of nutrition had postpartum anestrous intervals that were 14 d shorter than cows fed a low plane of nutrition and not exposed to bulls. In the same study, cows fed a high plane of nutrition that were exposed to bulls had postpartum anestrous intervals to resumption of ovarian cycling activity 6 d shorter than non-exposed cows also fed on a high plane of nutrition. These data indicate that the biostimulatory effect of bulls shortens postpartum anestrus to a greater extent under nutrient-restricted conditions in cows. This suggests the possibility that an increase in sensitivity to the biostimulatory effect of bulls is associated with a decrease in energy balance. The mechanism by which the bull stimulates the hypothalamo-hypophsealovarian control axis is not well understood. Data from previous research showed that an 20 increase in LH pulse frequency occurred directly before postpartum anestrous cows resumed ovarian cycling activity (Walters et al., 1982; Peters and Lamming, 1990), and this change in LH secretion patterns is believed to be required for the resumption of ovarian cycling acitivity after calving. Cows exposed continuously to bulls and cows exposed to bulls intermittently had higher mean LH concentrations and greater frequency of LH pulses than cows that were not exposed to bulls (Fernandez et al., 1996). Additionally, Baruah and Kanchev (1993) administered bull urine oronasally to cows and found that mean LH and FSH concentrations increased in the blood within 80 min after urine application. However, Fernandez et al., (1996) and Baruah and Kanchev (1993) reported that exposing cows to bulls intermittently or administering bull urine oronasally to cows did not decrease the length of postpartum anestrus. These data indicate that the quantity of stimulation was not sufficient to cause a biostimulatory effect. Taken together, these studies support the hypothesis that the mechanism whereby the biostimulatory effect of bulls acts involves the hypothalamo-hypophseal-ovarian axis and allows one to speculate that a pheromone(s) may be involved with this effect. Berardinelli et al. (2004) explored the hypothesis that a social bond may be formed between the bull and cow, causing the biostimulatory effect of bulls. This notion was tested by comparing the length of postpartum anestrus of cows exposed to familiar bulls, then switched to exposure to unfamiliar bulls, and cows exposed to familiar ovariectomized (OVX) cows and switched to exposure of unfamiliar OVX cows. Cows exposed to familiar and unfamiliar bulls had shorter intervals from calving to the resumption of ovarian cycling activity than cows exposed to OVX cows. However, 21 familiarity had no effect on the length of postpartum anestrus. Therefore, social interaction and bonding between bulls and cows may not be an important factor that mediates the biostimulatory effect of bulls. Instead, it appears that the physical presence of bulls acts independent of social interaction and is probably related to a pheromonal factor. Berardinelli and Joshi, (2005) tested this idea by comparing intervals to resumption of ovarian cycling activity in cows that were: 1) exposed to continuous presence of bulls, 2) exposed to excretory products of bulls for 12 h daily, 3) exposed to their own excretory products for 12 h daily, or 4) not exposed to bulls, excretory products of bulls or cows beginning 35 d after calving. Cows exposed to bulls or excretory products of bulls or their own excretory products had shorter intervals to resumption of cycling activity than cows not exposed to bulls, excretory products of bulls or excretory products of cows. These data indicate that the biostimulatory effect of bulls is mediated through exposure to excretory products of bulls. Previously, Burns and Spitzer (1992) showed that length of postpartum anestrus for cows exposed to testosterone-treated cows and cows exposed to bulls did not differ but were shorter than that for control cows. The data from these experiments indicate that the biostimulatory effect of bulls could be mediated through a pheromone(s) present in bull excretory products, and that production and excretion of this pheromone could be androgen-dependent. There is evidence that the biostimulatory effect of bulls involves activation of the hypothalamo-hypophyseal-adrenal axis as well. Tauck et al. (2007) found that cows exposed to bulls for 30 d had greater cortisol concentrations and shorter postpartum intervals to resumption of ovulatory activity than cows not exposed to bulls. Recently, 22 research in the same laboratory evaluated the effect of acute bull exposure on characteristics of temporal cortisol and LH concentration patterns. They found that cortisol pulse frequency was lower, cortisol pulse duration was longer, and LH pulse frequency was higher in postpartum anestrous cows exposed continuously to bulls within 10 d of the start of the exposure period and after a 2 d intensive sampling acclimatization period (Tauck, 2008). Furthermore, Landaeta-Hernandez et al. (2004) found that nonesterified fatty acids (NEFA) were 33% higher in suckled cows exposed to bulls than in non-exposed suckled cows. It is possible that these results could be related to an increase in cortisol concentrations associated with peri-ovulatory events in resumption of ovarian cycling activity in postpartum cows (Humphreys et al., 1983). A well known catabolic effect of cortisol is the liberation of NEFA and amino acids from skeletal muscle and adipose tissue to be used as substrates for hepatic gluconeogenensis (for review see, Hadley and Levine, 2007). These findings indicate that the biostimulatory effect of bulls activates or alters the hypothalamic-hypophyseal-adrenal axis and the hypothalamichypophyseal-ovarian axis prior to the resumption of ovulatory activity in postpartum, anestrous, beef cows. Furthermore these results indicate the potential for adrenal involvement in the mechanism of bull exposure to accelerate the resumption of ovulatory activity in postpartum, anovular cows via alterations in metabolic processes. Metabolic Regulation and Reproduction Energy balance or metabolic status of an organism is defined by the availability of energy and nutrients to the tissues. An organism’s intrinsic perception of this status is a 23 critical component in the modulation of various biological functions. In mammals, it is very well known that reproduction, the ability to produce and rear a viable offspring, is dependent upon availability of nutrients to support growth, onset of puberty and sexual maturity, copulation, pregnancy, lactation, and rearing. Energy balance is of even greater importance in the female, wherein pregnancy and lactation are a considerable energetic drain. However, the physiological basis for the coupling of regulation of energy balance and reproductive function has been only briefly explained in relatively recent research. Reproduction depends upon the development and function of proper neuroendocrine control. The HPO axis has three levels of regulation: the secretion of GnRH from neurosecretory neurons in the hypothalamus, LH and FSH from the anterior pituitary, and hormonal products or sex steroids, from the gonads. These factors all participate in the aforementioned positive and negative feed-forward and feed-back loops in the regulation of proper reproductive function. This section will review some of the many central and peripheral metabolic modulatory factors, both stimulatory and inhibitory, that participate in the regulation of reproductive function at all levels of the HPO axis. Metabolic Regulation and the Hypothalmic-Hypophyseal-Ovarian Axis Cortisol. Cortisol , as well as other adrenal glucocorticoids, is associated with stress and can affect carbohydrate, lipid, and protein metabolism. Actions of glucocorticoids on the liver are anabolic and increase the synthesis of a variety of gluconeogenic enzymes within hepatocytes. On the other hand, glucocortisoids enhance 24 catabolic actions in skeletal muscle and adipose tissue. Inhibition of glucose uptake in peripheral tissues by glucocorticoids causes a void of metabolic substrate for ATP production, resulting in the proteolysis of muscle proteins and lipolysis of fat. Once mobilized, free fatty acids and amino acids become substrates available for use in hepatic gluconeogenesis (for review see, Hadley and Levine, 2007). Hormone milieu in total at the time of stress may influence the effect of cortisol on function of the HPO axis. Additionally, the effects of cortisol on the HPO axis seem to be attenuated in the presence of gonads and their products (Tilbrook et al., 1999; Daley et al., 2000; Stackpole et al., 2006). Cortisol has been shown to be involved with the physiological mechanism by which postpartum anestrous interval is shortened due to the biostimulatory effect of bulls. Tauck et al. (2007) found that cows exposed to bulls had greater cortisol concentrations and shorter postpartum intervals than cows not exposed to bulls. Recently, research in the same laboratory further evaluated the effect of acute bull exposure on characteristics of temporal cortisol and LH concentration patterns. They found cortisol pulse frequency was lower and pulse duration was longer while coinciding with a higher LH pulse frequency in postpartum anestrous cows continuously exposed to bulls (Tauck, 2008). These findings indicate that the biostimulatory effect of bulls activates or alters the HPA axis and the HPO axis prior to the resumption of ovulatory activity in postpartum, anestrous, beef cows. This suggests the potential for adrenal involvement in the mechanism of bull exposure to accelerate the resumption of ovulatory activity in postpartum, anovular cows. Tri-iodothyronine (T3) and Thyroxine (T4). Thyroid hormones, T3 and T4, are 25 required for normal growth and development, stimulate thermogenesis and oxygen consumption, are altered by energy balance, and are believed to play a permissive role in the function of many other hormones (for review see, Hadley and Levine, 2007). Thyroid hormone synthesis and secretion is regulated by thyrotropin (thyroid stimulating hormone; TSH) secretion from the anterior pituitary (Hafez and Hafez, 2000). Thyrotropin shares the same α-subuinit as the adenohypophyseal gonadotropes; luteinizing hormone (LH) and follicle stimulating hormone (FSH) (Hadley and Levine, 2007). Additionally, TSH secretion is controlled by hypothalamic thyrotropin-releasing hormone (TRH). Therefore, the thyroid hormones T3 and T4 are modulators of metabolic processes and are closely related to modulators of reproductive function within the hypothalamic-pituitary axis. Role of Leptin in Regulation of Energy Homeostasis and Reproduction Leptin in Energy Homeostasis. Energy balance is defined by the equilibrium between food intake and energy expenditure, with energy homeostasis being the most tightly regulated functions of the organism (Casanueva and Dieguez, 1999). It wasn’t until the mid-1990s that our knowledge of the endocrine control mechanism for the control of energy stores was elucidated, exclusively due to the discovery of the adipocyte derived hormone leptin (Zhang et al., 1994; Friedman and Halaas, 1998; Rosenbaum and Leibel, 1998; Casanueva and Dieguez, 1999; Ahima et al., 2000;). The 167-amino acid hormone synthesized and secreted by adipocytes has been implicated in communicating nutritional status to the brain and is involved in regulation of food intake, metabolism, 26 and reproduction (Houseknecht et al., 1998). It has been widely stated that serum leptin concentrations inform the brain about the level of adipocity or the availability of metabolic fuels (Schneider, 2004). The adipokine is expressed in placenta (Hoggard et al., 1997a), stomach (Bado et al., 1998), skeletal muscle (Wang et al., 1998), brain (Morash et al.,1999; Wiesner et al., 1999), adenohypophysis (Morash et al., 1999) and leptin mRNA is detected in the cerebral cortex, cerebellum, hypothalamus, and pineal gland (Morash et al., 1999). There is a great deal of evidence supporting the idea that leptin increases the intracellular availability and oxidation of free-fatty acids (FFA). Treatment with leptin decreases de novo FFA synthesis, increases intracellular FFA esterification to triglycerides, increases triglyceride breakdown and FFA oxidation, and increases FFA exportation to nonadipocytes in rats (William et al., 2002). Ultimately, this results in a greater efflux of FFAs from adipocytes thereby reducing FFA oxidation within adipocytes and increasing availability to nonadipocytes (William et al., 2002). If leptin positively influences reproductive function by increasing fuel oxidation and availability, then it is possible that leptin would no longer improve reproductive function as metabolic fuels became scarce. This is the case in rats restricted to 70% of their ad libitum food intake wherein leptin failed to improve reproductive function, however leptin fully reversed the effects of fasting in rats restricted to only 80% of ad libitum intake (Cheung et al., 1997). Furthermore, treatment with leptin can shorten the duration of lactational diestrus in rats exposed to acute food deprivation but not in rats exposed to prolonged or chronic food restriction (Woodside et al., 1998 and 2000). This suggests that leptin can 27 facilitate reproductive function but only under adequate metabolic fuel availability. Effects of leptin in control of food intake appear to be within the hypothalamus, where leptin in known to modulate orexigenic neurons as well as anorexigenic neurons (for review see, Sahu, 2004). In addition, these neuronal pathways are subject to other modulators of food intake that are either synergistic or antagonistic towards the effects of leptin on energy homeostasis (Zigman and Elmquist, 2003). Thus, knowing leptin’s potential as a pleiotropic modulator of a vast array of endocrine functions involved with the hypothalamus (Wauters et al., 2000), it is considered a “genuine” neuroendocrine integrator, communicating control of biological functions to the organism’s state of energy balance (Tene-Sempere, 2007). Leptin Regulation on Puberty and Gonadotropin Secretion. In agreement that metabolic control of reproduction is much more restrictive in the female due to the energetic drain of pregnancy and lactation, it has been suggested that leptin plays a prominent role in control of female reproductive function (Tene-Sempere, 2007). Homozygous mutation for the gene that encodes leptin, ob gene, results in obesity, infertility, and atrophy of reproductive organs in mice (Barash et al., 1996). However, these manifestations can be reversed via treatment with exogenous leptin (for review see, Schneider, 2004). Leptin treatment induced weight loss and development of reproductive organs, and restored reproduction in male (Mounzih et al., 1997) and female (Barash et al., 1996) ob/ob mice. In normal prepubertal mice, leptin treatment induced earlier onset of estrous cycles (Ahima et al., 1997; Chehab et al., 1997). Circulating concentrations of leptin are correlated positively with adiposity and 28 increases in body weight in normal mice (Frederich et al., 1995), humans (Ahren et al., 1997), sheep (Delavaud et al., 2000), cattle (Ehrhardt et al., 2000), and developing heifers (Garcia et al., 2002a). Exogenous treatment with leptin reduces feed intake and the effects of negative energy balance on reproduction, including the onset of puberty, length of the estrous cycle, length of lactational diestrus, gonadotropin and gonadal steroid levels, and pulsatile LH secretion in mice, rats, hamsters, and non-human primates (for review see, Schneider, 2004). Additionally, treatments of leptin to within physiological ranges reverse the effects of food deprivation on the estradiol–induced LH and prolactin surges in rats (Watanobe et al., 1999). Initially, direct actions by leptin on GnRH neurons were suggested after the discovery of leptin receptor (LR) expression within immortalized GnRH-secreting cell line, GT1-7 (Magni et al., 1999). However, under physiological conditions it has been accepted that GnRH receptors do not express the LR in rats (Watanobe et al., 2002). Thus, it has been suggested that the influence of leptin on the GnRH and LH surges occur through intermediate neurons projecting from LR-containing neurons to ultimately synapse upon GnRH neurons (for review see, Schneider, 2004). These pathways have been postulated to include neuropeptide-Y (NPY), pro-opiomelanocortin (POMC), and cocaine- and amphetamine-regulated transcript (CART) secreting neurons, all of which are well known to influence GnRH neuron function (Schneider, 2004). Messenger RNA for the LR has been detected in NPY neurons (Mercer et al., 1996; Finn et al., 1998; Williams et al., 1999). Expression of NPY in the hypothalamus is increased in ob/ob mice and treatment with leptin diminishes hypothalamic NPY in this mutant mouse 29 (Stephens et al., 1995). Interestingly, feed restriction increases NPY mRNA in sheep (McShane et al., 1993), and intra-cerebral-ventricular (ICV) infusion of NPY decreases circulating concentrations of LH in sheep (McShane et al., 1992) and cows (Gazal et al., 1998). In addition, ICV infusion of leptin prevented NPY-induced feed intake in rats (Sahu, 1998). However, in the ovariectomized, estradiol implanted cow, pretreatment with leptin did not prevent the NPY-mediated decrease in plasma concentrations of LH (Garcia et al., 2002b). Short-term fasting reduces circulating concentrations of leptin in rodents and ruminants (Amstalden et al., 2000; Nagatani et al., 2000). In rats, prepuberal heifers (Amstalden et al., 2000), castrated lambs (Nagatani et al., 2000), and ovariectomized gilts (Whisnant and Harrel, 2001) the fasting-mediated reduction in mean concentrations of leptin is associated with decreased pulsatility of LH. Moreover, administration of leptin to castrated, estradiol-implanted lambs (Nagatani et al., 2000), and peripubertal heifers (Maciel et al., 2003a) during fasting prevents the reduction in frequency of LH pulses. In ovariectomized, estradiol-implanted cows under acute nutrient restriction, leptin increases mean concentrations of LH (Zieba et al., 2002). However, when higher doses (10 times greater) are used, leptin loses its ability to stimulate LH. Similarly, long-term undernourished, ovariectomized ewes, but not normal-fed ewes, exhibited increased secretion of LH after ICV infusions of recombinant human leptin (Henry et al., 1999; Henry et al., 2001). More recent research has elucidated much of the neuroendocrine networks for the signaling of leptin to GnRH neurons. One example of this is the discovery that leptin 30 directly targets kisspeptin neurons within the hypothalamus in mice (Smith et al., 2006). Kisspeptin neurons are one of the most potent stimulators of the GnRH/LH axis known thus far (Tena-Sempere, 2006). Furthermore, gene expression of kisspeptin is decreased during undernutrition while administration of kisspeptin attenuates negative effects of undernutrition on the onset of puberty. These results indicate the potential that changes in endogenous leptin concentrations participate in metabolic regulation in reproduction. However, it is commonly assumed that the majority of modulation by leptin occurs directly upon the HPO axis. It is important to note that leptin greatly upregulates energy expenditure, thermogenesis, and fuel oxidation (Schneider, 2004), therefore, has the potential to influence reproduction simply by means of regulating metabolic fuel availability. Direct Ovarian Effects of Leptin. Many studies of different species have documented the ability of leptin to modulate ovarian steroidogenesis, most of which suggest an inhibitory action by leptin (Spicer and Francisco, 1991; Zachow and Magoffin, 1997; Agarwal et al., 1999; Ghizzoni et al., 2001; Kendall et al., 2004). This contradicts apparent stimulatory and permissive effects upon the hypothalamus and pituitary. In addition to leptin receptor (Ob-R) mRNA expression within granulosal and thecal cells of the human ovary (Karlsson et al., 1997) and long (Ob-Rb) and short (ObRa) forms of leptin receptor in the rat ovary, the adipokine, leptin, itself appears to be expressed in the ovaries and in concentrations that fluctuate with ovarian cyclic activity (Loffler et al., 2001; Ryan et al., 2002; Archanco et al., 2003; Ryan et al., 2003). Though many studies have found contradictory results as to positive or negative effects of ovarian 31 leptin on follicular development (Duggal et al., 2000; Craig et al., 2004; Olatinwo et al., 2005), Burkan et al. (2005) interestingly reported that leptin induced LH-independent ovulation in GnRH deficient mice. Ovarian Steroid Regulation on Leptin Secretion. Expression and secretion of leptin, leptin receptor, and biological actions of leptin may be influenced by sex steroids. Leptin mRNA decreases in adipose tissue after ovariectomy in rats and estradiol stimulates mRNA expression and secretion of leptin in adipocytes in culture (Machinal et al., 1999). Central infusion of leptin increases mean concentrations of LH and FSH in ovariectomized rats treated with estradiol (Walczewska et al., 1999). However, ovariectomized rats without estradiol treatment did not respond to central infusions of leptin and high doses of estradiol attenuated the leptin-induced increase in the release of LH (Walczewska et al., 1999). Furthermore, estradiol may influence expression of leptin receptor. Expression of the long form of leptin receptor (Ob-Rb) in the hypothalamus of rats decreases following estradiol treatment (Bennett et al., 1998). 32 CHAPTER 3 STATEMENT OF THE PROBLEM As pointed out in the review of literature, the biostimulatory effect of bulls appears to be mediated by pheromones that influence reproductive behavior and function of cows. Stumpf et al. (1992) found that cows of lower body condition had a greater reduction in postpartum anestrous interval when exposed to bulls than cows of higher body condition exposed to bulls. Furthermore, Tauck (2008) described the activation and involvement of the hypothalamic-pituitary-adrenal axis with the biostimulatory effect of bulls on primiparous, postpartum, anestrous, suckled beef cows. One interpretation of these observations may be that bull exposure alters metabolic regulation and energy homeostasis, ultimately influencing the HPO axis and accelerating resumption of ovarian cycling activity in primiparous, postpartum, anestrous, suckled cows. As stated in the review of literature, energy balance is of particular importance to the postpartum, lactating, and still growing primiparous cow. Many metabolic hormones and metabolites serve as indicators of energy balance and have the ability to modulate functions of reproduction by influencing the HPO axis. One of which, leptin, is a peptide hormone secreted by adipose tissue and is accepted as an indicator of adiposity and satiety. Leptin is known to influence feed intake, onset of puberty, as well as influencing the gonadotropic hormone secretion patterns. The pulsatile pattern of luteinizing hormone, a gonadotropin secreted from the anterior pituitary, is what must be altered in order for the postpartum anestrous cow to resume ovarian cycling activity. 33 The goals of this research were: 1) to determine if thyroid hormones (T3 and T4), glucose, or NEFA are involved with the physiological response of cows to the biostimulatory effect of bulls, 2) determine the role of leptin in the biostimulatory effect of bulls on the function of the HPO axis and resumption of ovarian cycling activity, and 3) to determine if duration of daily bull exposure is a factor involved with the appropriate manner whereby bull-pheromonal stimuli alters temporal leptin concentrations and accelerates resumption of ovarian cycling activity in primiparous, postpartum, anestrous, suckled, beef cows. 34 CHAPTER 4 EXPERIMENT 1: CONCENTRATIONS OF GLUCOSE, NEFA, THYROXINE, AND TRI-IODOTHYRONINE IN PRIMIPAROUS, ANESTROUS, SUCKLED BEEF COWS EXPOSED TO BULLS Introduction Resumption of luteal function in primiparous, anovular, suckled beef cows after calving is accelerated if cows are exposed to bulls (Custer et al., 1990) or excretory products of bulls (Berardinelli and Joshi, 2005). Olsen et al. (2006) found that cortisol concentrations increase in cows exposed to bulls. Cortisol is intimately involved with metabolic regulation of energy utilization (Hadley and Levine, 2007). There is the possibility that the biostimulatory effect of bulls to accelerate resumption of ovulatory activity involves metabolic changes in cows that are induced by changes in cortisol concentrations. The objective of this study was to determine if exposing cows to bulls alters systemic glucose, NEFA, and thyroid hormones concentrations in primiparous, postpartum, anestrous, suckled beef cows. The specific hypothesis tested in this experiment were that systemic concentrations of glucose, NEFA, thyroxine (T4), triiodothyronine (T3), and T3:T4 ratios do not differ in postpartum, anestrous, suckled beef cows exposed daily for 5 h to bulls or steers for 9 d. Materials and Methods This experiment was conducted at the Montana State University Bozeman Agricultural Research and Teaching Facility. Animal care, handling, and protocols used 35 in this experiment were approved by the Montana State University Agriculural Animal Care and Use Committee. Animals and Treatments Sixteen spring-calving 2-yr-old Angus X Hereford primiparous, suckled beef cows, 2 mature Angus X Hereford bulls, and 2 yearling Angus X Hereford steers were used in this experiment. Cows and calves were maintained in a single pasture and had no contact with bulls or their excretory products during pregnancy and from calving until the start of the experiment. Average calving date for these cows was Feb. 4, 2006. At the start of the experiment (D 0) cows averaged 67 ± 3.5 d postpartum. One week before the start of the experiment cows were stratified by body weight, BCS, calf birth weight, calving date, sex of calf and dystocia score and assigned randomly to two treatments: exposed to bulls (EB, n = 8) or steers (ES, n = 8) 5 h daily for 9 d (D 0 to 8). Facilities Cows were housed within pens in separate lot areas at the Bozeman Livestock Teaching and Research Center. Pens within the north lot were used for maintaining EB cows while pens within the south lot were used for maintaining ES cows. During the daily sample collection and exposure period cows were moved into individual stalls within open-air sheds adjacent to pens that housed cows in each treatment. Sheds were similar in structure, area and light density. Light density within sheds was tested using a Minolta Autometer Pro Photometer and tarps were used to manipulate the light density so 36 that cows in each sampling area were exposed to the same amount of light. During daily sample collections and exposure periods cows were halter-restrained within aligned (sideby-side) stalls, and bulls or steers were contained in front of cows. Bulls and steers were unrestrained and allowed to eat, roam, come into contact with the frontal aspect of cows and interact with cows individually within each stanchion. Excretory products of bulls and steers were not removed from the exposure area throughout the experimental period. Nutrition Cows had free access to good quality, chopped mixed-grass alfalfa hay, and any pasture grasses that were available before the start of the experiment. Once cows and calves were moved into pens they were given free access to the same hay, 0.5 kghd-1d-1 cracked barley, water, and a trace mineral-salt supplement. The TDN of the diet exceeded the NRC requirement for lactating beef cow with a mature weight of 545 kg by approximately 18% (NRC, 1996). Bulls were fed 0.5 kg of cracked barely and good quality, chopped mixed-grass alfalfa hay. Steers were fed a finishing ration that consisted of 70% concentrate and 30% roughage throughout the experiment. Intensive Blood Sampling Procedures Two days before the start of the experiment, each cow was fitted with an indwelling jugular catheter. Blood samples (~7 mL) were collected daily from each cow at 15-min intervals (starting at Time 0 and ending at 360 min) for 6 h (1000 to 1600 h) beginning 1 h before the 5-h exposure period each day during the 9 d period (D 0 to 8). Serum was harvested for each sample and stored at −20°C. Blood samples were 37 collected by the same technician for cows in each treatment throughout the experiment. A total of 143 (3.9%) samples were not collected during this experiment due to noncooperative animals, coagulation within catheters, or catheter replacement. Metabolite and Hormone Assays It was determined from a preliminary analysis that randomly selected samples throughout the intensive bleeding period were representative of the daily mean concentrations. Glucose concentrations in serum for Times 15 and 225 min on D 2 and 8 were determined using a commercially available, hand-held glucose/ketone monitor (Precision Xtra™, MediSense/Abbott Laboratories, Bedford, MA). In order to compensate for assay drift, high and low serum pools were repeated 5 times every 20 samples. Mean high and low pool concentrations were 135.8 and 56.5 mg/dL with an inter-assay CV of 4.5 and 6.3%. Concentrations of NEFA at Times 15 and 300 min on all days were quantified with a commercially available enzymatic-colorimetric assay (HR Series NEFA – HR [2], Wako Diagnostics, Richmond, VA). Inter- and intra-assay CV were 5.8 and 4.6% for a serum pool that contained 0.415 mmol/L; and, 14 and 5.6%, respectively, for a serum pool that contained 0.100 mmol/L. Thyroxine (T4) and triiodothyronine (T3) concentrations at Time 225 min on each day were assayed using solid-phase RIA kits (Siemens Medical Solutions Diagnostics, Los Angeles, CA, USA). The intra-assay CV for serum pools that contained 155 and 105 ng/mL of T4 were 5.8 and 4.9%, respectively. The intra-assay CV for serum pools that contained 10.3 and 4.5 ng/mL of T3 were 11.2 and 24.5%, respectively. 38 Statistical Analyses Glucose and NEFA concentrations(reasoning for times, presentation) were analyzed by separate ANOVA for a completely random design using PROC GLM of SAS (SAS Inst. Inc., Cary, NC). The model included treatment, time, day, animal within treatment, and all possible interactions. Animal within treatment variance component was used to test the effect of treatment. Thyroxine, T3, and T3:T4 ratio concentrations were analyzed by separate ANOVA for a completely random design using PROC GLM of SAS (SAS Inst. Inc., Cary, NC). The model included treatment, day, animal within treatment, and all possible interactions. Animal within treatment variance component was used to test the effect of treatment. Means were separated by Bonferroni multiple comparison tests. Results Stratification factors, days form calving to start of the experiment, cow BW at start of the experiment, calf BW, BCS of cows, calf sex ratio, and dystocia score did not differ (P > 0.30) between EB and ES cows (Table 1). 39 Table 1. Number of cows per treatment and least square means for days from calving to start of the experiment, cow BW at the start of treatment, calf birth weight (BW), BCS, calf sex ratio, and dystocia score for primiparous, suckled beef cows exposed to bulls (EB) or exposed to steers (ES) Treatment SEMa P value 67 3.5 0.94 526 531 36 0.76 Calf BW (kg) 31 32 3.5 0.61 BCSc 5.3 5.3 0.23 0.92 e 0.62 0.34 Variable EB ES 8 8 Day postpartum 67 Cow BW (kg) n b Calf sex ratio d Dystocia Score f 0.50 0.63 0.25 1.1 1.0 0.16 a SEM = Standard error of the mean. Days from calving to start of the experiment. c BCS; 1 = emaciated, 9 = obese. b Calf sex ratio = ratio of male to female calves, 1 = male and 0 = female. Tested with ϰ2 analysis. d ϰ2 value. e f Dystocia Score: 0 = No assistance to 5 = Caesarean section. There was no interaction among or between treatments, days, and times for glucose concentrations. Glucose concentrations did not differ between Times 15 and 225 min, or D 2 and 8 or between EB and ES cows (Table 2). There was no interaction among or between treatments, days, and times for NEFA concentrations. Concentrations of NEFA did not differ between Times 15 and 300 min or among days (0 to 8). However, NEFA concentrations were greater (P < 0.05) in ES cows (0.262 ± 0.024 mmol/L) than in EB cows (0.197 ± 0.020 mmol/L) over the course of the experiment (Table 2). There was no interaction between treatments and days for concentrations of 40 T4 and T3, or T3:T4 ratios. Concentrations of T4 and T3, and T3:T4 ratios did not differ among days or between EB and ES cows (Table 2). Table 2. Least squared means of glucose, NEFA, thyroxine (T4), and triiobothyronine (T3) concentrations for primiparous, suckled beef cows exposed to bulls (EB) and steers (ES) Treatment SEMa P value 82.4 6.2 0.79 0.197 0.262 0.099 0.06 T4, ng/mLc 17.7 14.0 7.9 0.15 T3, ng/mLc 9.5 8.8 3.5 0.41 T3:T4 ratio 0.68 0.79 0.36 0.25 Variable EB ES 8 8 Glucose, mg/dLb 81.5 NEFA, mmol/L n a SEM = Pooled standard error of the mean. Glucose concentrations for samples obtained at Time 15 and 225 min on Days 2 and 8. c T4 and T3 concentration for samples obtained at Time 225 min on each day of the experiment. b Discussion In the present experiment we evaluated concentrations of glucose on D 2 and 8 at Times 15 and 225 min, NEFA concentrations every day at Times 15 and 300 min, and T4, T3 concentrations and T3:T4 ratios every day at Time 225 min. Blood samples were collected every 15 min for 6 h daily for 9 d. Concentrations of glucose, NEFA, T4, and T3 for a full collection period were evaluated in 1 or 2 cows and on 1 or 2 days chosen randomly. We found that the patterns of glucose, NEFA, T4, and T3 concentrations did exhibit pulsatile rhythms, in fact they exhibited a random pattern of change throughout the sampling period. Thus, we randomly chose D 2 and 8 and Times 15 and 225 min to 41 evaluate changes in glucose concentrations: Times 15 and 300 min for all days for NEFA concentrations and Time 225 on all days for T4 and T3. Exposing anestrous, suckled beef cows to bulls for 5 h daily over a period of 9 d did not influence glucose, T4, T3 concentrations and T3:T4 ratios. However, exposing anestrous cows to bulls decreased concentrations of NEFA compared to concentrations of NEFA in cows exposed to steers. This result of the present study for NEFA concentrations in suckled cows exposed to bulls is quite different from the result reported by Landaeta-Hernandez et al. (2004). They found that concentrations of NEFA were 33% higher in suckled cows exposed to bulls than in non-exposed suckled cows. The reason for this difference is not clear; however, the data reported by Landaeta-Hernandez et al. (2004) may have been confounded by the fact that more bull exposed cows had resumed ovulatory activity by the end of the experiment than non-exposed cows. This may have been due to an increase in cortisol concentrations that are associated with periovulatory events of resumption of ovulatory activity in postpartum cows (Humphreys et al., 1983). A well-known catabolic affect of cortisol is the liberation of NEFA and amino acids from skeletal muscle and adipose tissue to be used as substrates for gluconeogenesis in the liver (for review see, Hadley and Levine, 2007). Whereas, in the present study all samples were collected in anestrous cows that had not resumed ovulatory activity and this difference may be related to NEFA concentrations in anestrous cows compared to those of cows during the peri-ovulatory period. The primary objective of this experiment was to determine if exposing anestrous, suckled beef cows to bulls alters glucose, NEFA, and thyroid hormones that may directly 42 or indirectly affect carbohydrate and lipid metabolism. This idea was based on the following observations that alterations in carbohydrate and lipid metabolism can either delay or accelerate the resumption of ovulatory activity in postpartum, anestrous, suckled beef cows (for reviews see, Randel, 1990; Short et al., 1990). Exposing postpartum cows to bulls increased systemic cortisol concentrations relative to cows not exposed to bulls (Tauck et al., 2007). Cortisol is a hormone known to influence carbohydrate and lipid metabolism (for review see, Hadley and Levine, 2007). Recently, Landaeta-Hernandez et al. (2004) reported that systemic concentrations of NEFA were greater in postpartum cows exposed to bulls than in cows not exposed to bulls. One could hypothesize that the biostimulatory effect of bulls to reduce the interval of postpartum may be mediated by inducing changes in cortisol concentrations which in turn may directly affect metabolic processes of postpartum, anestrous, suckled beef cows. This in turn could alter the hypothalamic release patterns of GnRH stimulating follicular development and ovulation. Thus, the affect of bull exposure on anestrous cows appears to be related to a decrease in catabolism of adipose tissue and may be involved in the mechanism whereby bulls accelerate the resumption of ovulatory activity. 43 CHAPTER 5 EXPERIMENT 2: BIOSTIMULATORY EFFECT OF BULLS ON TEMPORAL PATTERNS OF LEPTIN CONCENTRATIONS AND RESUMPTION OF LUTEAL ACTIVITY IN PRIMIPAROUS, POSTPARTUM, ANESTROUS, BEEF COWS. Introduction Resumption of luteal function in primiparous, anovular, suckled beef cows after calving is accelerated if cows are exposed to bulls (Custer et al., 1990) or excretory products of bulls (Berardinelli and Joshi, 2005). Tauck et al. (2007) found that cortisol concentrations increase in cows exposed to bulls. Cortisol is intimately involved with metabolic regulation of energy utilization (Hadley and Levine, 2007). Furthermore, there is the possibility that the biostimulatory effect of bulls to accelerate resumption of ovulatory activity involves metabolic changes in cows that are induced by changes in cortisol concentrations, specifically changes involved with adipose metabolism (Landaeta-Hernandes et al., 2004, Wilkinson et al., 2007; Tauck, 2008). Stumpf et al. (1992) reported that cows of low body condition have a greater reduction in postpartum interval to resumption of luteal activity when exposed to bulls than cows of high body condition. The physiological mechanisms by which energy balance modulates reproductive functions have recently been elucidated within the last decade. Leptin, an adipocyte-derived hormone or adipokine, is secreted by white adipose tissue and is an important factor on the control of energy balance, satiety, food intake and has been shown to have many levels of modulation upon the hypothalamic-hypophyseal-gonadal axis (for review see Tene-Sempere, 2007). The objectives of this study were to determine 44 if 1) short-term bull exposure (30 d) increased the number of cows cycling before the start of the breeding season; and 2) bull exposure alters temporal leptin concentration patterns during postpartum anestrus and resumption of luteal activity. The specific hypothesis tested in this experiment was that the proportion of cows that resume luteal activity and systemic concentrations of leptin do not differ in postpartum, anestrous, suckled beef cows continuously exposed or not exposed to bulls. Materials and Methods Animals and Treatments Fifty-three spring-calving two-yr-old, primiparous, suckled, Angus Hereford crossbred beef cows and four mature, epididymectomized Angus x Hereford bulls were used in this experiment conducted at the Montana State University Bozeman Agricultural Research and Teaching Facility. Animal care, handling, and protocols used in this experiment were approved by the Montana State University Agricultural Animal Care and Use Committee. Cows and calves were maintained in a single pasture and had no contact with bulls or their excretory products during pregnancy and from calving until the start of the experiment. Average calving date for these cows was Feb. 16, 2003. At the start of the experiment (D 0) cows averaged 63 d (± 14 d; ± SE) postpartum; D 30 of the experiment was day of start of estrous synchronization (ES). One week before the start of treatment cows were stratified by calving date, cow BW, calf birth weight, calf sex, dystocia score, and BCS. Once cows were stratified they were assigned randomly to one of two 45 treatments; exposure to mature bulls (BE; n = 26) or no bull exposure (NE; n = 27) from the start of the experiment (D 0) until the start of ES (D 30). Facilities Two lots were used for this experiment, designated north and south by their geographic location. Each lot contained four pens (41 m x 18 m) that were identical in east-west configuration, bunk space, aspect, slope, and connection to open-shed shelters. Lots were approximately 0.35 km apart, and the arrangement was such that the prevailing wind blew from south to north. Animals housed in one lot were not able to see or smell animals housed in the other lot; however, there was a possibility that sounds made by animals in one lot could be heard by animals in the other lot. These lots and arrangement have proven to be effective in previous experiments involving bull-cow interactions (Fernandez et al., 1993; 1996). Pens in the south lot had not held bulls for more than six years. Pens in the north lot had not held bulls for ten months. Nutrition Cows had free access to good quality, chopped mixed-grass alfalfa hay, and any pasture grasses that were available before the start of the experiment. Once cows and calves were moved into pens they were given free access to the same hay, 0.5 kg/hd/d cracked barley, water, and a trace mineral-salt supplement. The TDN of the diet exceeded the NRC requirement for lactating beef cows with a mature weight of 545 kg by approximately 18% (NRC, 1996). Bulls were fed the same ration as cows. 46 Blood Sampling for Leptin, Progesterone, and Resumption of Luteal Acitivity Blood samples were collected from each cow by jugular venepuncture at 3-d intervals from the start of the experiment to the start of the breeding season. Serum concentrations of leptin were determined in triplicate and were quantified by using a competitive liquid-liquid phase, double-antibody leptin RIA procedure described previously (Delavaud et al., 2000). The intra and inter assay coefficients of variation were 2.01% and 3.22%, respectively. Serum was assayed for progesterone concentration using solid-phase RIA kit (Siemens Medical Solutions Diagnostics, Los Angeles, CA, USA). Intra- and interassay CV’s for a serum pool that contained 0.8 ng/mL were 4.8 and 11%, respectively; and for a pool that contained 7.0 ng/mL were 2.0 and 5.4%, respectively. Progesterone concentrations patterns were used to assess the resumption of ovarian cycling activity. A rise in progesterone concentration of > 0.5 ng/ml in three consecutive samples that exceeded 1 ng/mL provided evidence that cows resumed ovarian cycling activity during the experimental period. Progesterone (ng/mL) 47 7 6.5 6 5.5 5 4.5 4 3.5 3 2.5 2 1.5 1 0.5 0 0 3 6 9 12 15 18 21 24 27 30 24 30 Progesterone (ng/mL) Day of treatment 7 6.5 6 5.5 5 4.5 4 3.5 3 2.5 2 1.5 1 0.5 0 0 3 6 9 12 15 18 21 27 Day of treatment Figure 1. Pattern of progesterone concentrations used to determine occurrence of resumption of ovarian cycling activity. Panel A represents a cow that resumed cycling activity. Panel B represents a cow that did not resume ovarian cycling activity. 48 Graphic representation of the pattern of progesterone concentrations used to determine the occurrence of resumption of ovarian cycling activity is illustrated in Figure 1. Statistical Analyses Comparisons of calving date, calf birth weight, calf sex ratio, dystocia score, and body condition score were analyzed by analysis of variance for a completely random design using PROC GLM of SAS (SAS, Cary, NC). The model included treatment. Means were separated by PDIFF procedure of SAS. Leptin concentrations were analyzed by PROC MIXED repeated measures analysis of SAS. The model included TRT, Animal as experimental unit, Day as the repeated measure, and the TRT by Day interaction and means were separated using Bonferroni Multiple Comparison tests. Results Average calving date, cow BW, calf birth weight, calf sex ratio, dystocia score, and BCS did not differ between BE and NE cows (Table 3). Proportions of BE (50%) and NE (44%) cows cycling at the start of the experiment did not differ (P = 0.68; Figure 2). However, a greater proportion (P < 0.05) of cows exposed to bulls (BE; 100%) were cycling by the end of the 30-d exposure period than cows not exposed to bulls (NE; 70.4%; Figure 2). 49 Table 3. Number of cows per treatment and least square means for calving date, cow BW, calf BW, BCS, calf sex ratio, and dystocia score for first calf suckled beef cows exposed (BE) or not exposed (NE) to bulls at the start of the experiment Treatment Variable BE NE SEMa P value n 26 27 Calving dateb 47 46 14.37 0.93 Cow BW (kg) 518 509 41.72 0.40 Calf BW (kg) 37 37 3.89 0.66 BCS 4.7 4.8 1.11 0.98 Calf sex ratioc 0.53 0.59 0.49 0.96 Dystocia scored 1.08 1.15 0.39 a SEM = Standard error for means. b Day of year. c Calf sex ratio = ratio of male to female calves. d Dystocia Score: 0 = No assistance to 5 = Caesarean section. 0.91 BE NE y 100% 90% % RLA 80% x 70% 60% 50% x x 40% 30% 20% Start of experiment End of experiment Figure 2. Percentages of primiparous, suckled cows exposed (BE) or not exposed (NE) to bulls that resumed luteal activity (%RLA) at the start of the experiment and after the 30-d exposure period. Bars that lack common letters within each cycling classification differ, P < 0.05; cycling start of experiment, 2 = 1.02, d.f. = 1; cycling at start of breeding season,2 = 9.1, d.f. = 1. 50 Temporal patterns of leptin concentrations did not differ between BE and NE cows. However, there was a treatment by day interaction (P < 0.05; Figure 3) for leptin concentrations in BE and NE cows that began to cycle after D 0. Leptin concentrations increased on D 15 and remained stable until D 21, then increased again by D 27. Whereas, concentrations of leptin in NE cows increased on D 18, decreased on D 21, then increased by D 27. In order to better understand this treatment by day interaction, the experiment period was grouped into three intervals; D 0 to 6, D 9 to 15, and D 18 to 24. Here, leptin concentrations during the 18 to 24 d period were greater (P < 0.05; Figure 3) in BE cows than in NE cows that resumed luteal activity after the start of the experiment. Leptin concentrations during 0 to 6 d and 9 to 15 d did not differ (P > 0.10; Figure 3). NE 10 Leptin, ng/mL 9.5 9 BE SEM; P < 0.05 y x, y x, y 8.5 x x x 9 to 15 d 18 to 24 d 8 7.5 7 0 to 6 d Interval Figure 3. Least squares means of leptin concentrations for 6-d intervals in cows exposed to bulls (BE) and cows not exposed to bulls (NE) that resumed ovulatory activity after start of experiment (D 0). Bars that lack common letters within and between each interval differ, P < 0.05. Interval by treatment interaction; P < 0.05. 51 In cows that had not resumed luteal activity before the start of the experiment (D0), proportions of BE (62%) and NE (33%) cows that had resumed luteal activity during the D 0 to 6 interval did not differ (P > 0.10; Figure 4). However, a greater proportion (P < 0.05) of cows exposed to bulls (BE; 100%) resumed luteal activity by the intervals D 9 to 15 and D 18 to 24 than cows not exposed to bulls (NE; 46.7%; Figure 4). BE 100% NE y y 90% % RLA 80% 70% x 60% x 50% 40% x x 30% 20% 0 to 6 9 to 15 18 to 24 Interval Figure 4. Percentages of primiparous, suckled cows exposed (BE) or not exposed (NE) to bulls that had not started to cycle by start of experiment (D0) and resumed luteal activity (%RLA) during intervals D 0 to 6, D 9 to 15, and D 18 to 24 of the experiment. Bars that lack common letters within and between each interval differ, P < 0.05; interval D 0 to 6, 2 = 1.99, d.f. = 1; interval D 9 to 15,2 = 8.53, d.f. = 1; interval D 18 to 24,2 = 8.53, d.f. = 1; and between intervals D 0 to 6 and D 9 to 15 for BE cows,2 = 6.2, d.f. = 1. 52 Discussion Long-term bull exposure (> 60 d) reduces the postpartum interval to resumption of ovarian cycling activity in first-calf suckled beef cows (Custer et al., 1990, Fernandez et al., 1993). In this experiment cows exposed to mature bulls for 30 d increased the proportion of cows that started to cycle by 24% relative to cows that were not exposed to bulls. Macmillan et al. (1979) reported a greater proportion of cows exposed to bulls than cows not exposed to bulls for 21 d before the breeding season showed estrus during the 60 d breeding season. Similarly, Scott and Montgomery (1987) reported that more cows exposed to bulls for 20 d before the breeding season began cycling than cows not exposed to bulls. These results indicate that short-term bull exposure has the same effect as long-term exposure. Short-term bull exposure may be an effective strategy to increase the proportion of first-calf suckled cows that are cycling at the beginning of the breeding season. Additionally, we asked, “Does bull exposure for 30 d influence temporal leptin concentration patterns during postpartum anestrus and the resumption of ovarian cycling activity?” In the previous experiment of this thesis we found that there is a decrease in NEFA concentrations associated with the biostimulatory effect of bulls. These two experiments are complimentary wherein we find that there is a difference between treatments of bull-exposed and non-exposed in leptin concentrations by day, wherein leptin concentrations are higher in cows exposed to bulls for 18to 24 d than cows not exposed. Furthermore, Tauck et al. (2007) and Tauck (2008) described adrenal activation and involvement associated with exposure to bulls whereby cows exposed to bulls had 53 higher cortisol concentrations; and increased duration, decreased frequency of cortisol pulses, respectively. Cortisol is well known to be involved with lipid metabolism as well. This suggests that there may be a metabolic component to the biostimulatory effect of bulls in hastening the resumption of ovarian cycling activity, particularly in the area of lipid mobilization and metabolism. We conclude that short-term bull exposure of primiparous suckled beef cows altered leptin concentrations such that temporal leptin concentrations were higher in cows after 18 d of bull exposure. 54 CHAPTER 6 EXPERIMENT 3: EFFECT OF DURATION OF DAILY BULL EXPOSURE ON LEPTIN CONCENTRATIONS DURING RESUMPTION OF OVULATORY ACTIVITY IN PRIMIPAROUS, POSTPARTUM, ANESTROUS, BEEF COWS Introduction The biostimulatory effect of bulls involves interactions between bulls and cows in which the physical presence of bulls stimulates the resumption of luteal activity in multiparous and primiparous suckled beef cows (Custer et al., 1990). Bull exposure is known to alter cortisol concentrations and the characteristics of their pulsatile patterns (Tauck et al. 2007). Furthermore, there is the possibility that the biostimulatory effect of bulls to accelerate resumption of luteal activity involves metabolic changes in cows that are induced by changes in cortisol concentrations, specifically changes involved with adipose metabolism (Landaeta-Hernandes et al., 2004, Wilkinson et al., 2007). Stumpf et al., 1992 reported that cows of low body condition have a greater reduction in postpartum interval when exposed to bulls than cows of high body condition. In the previous experiments of this thesis, the biostimulatory effect of bulls was shown to decrease NEFA and increase leptin concentrations in cows after short-term (5h/d for 9d and 24h/d for 30d; respectively) bull exposure. Taken together these results indicate a metabolic component to the biostimulatory effect of bulls, specifically at the level of the adipocytes in cows. However, little is known as to whether this component is influenced more by the biostimulatory effect of bulls or simply by the resumption of luteal activity in the cow. This begs the questions: “Is the alteration or increase associated with bull exposure 55 in temporal leptin concentrations an all-or-nothing response or is it dose-dependent and is this change in temporal leptin concentrations influenced by cycling status”?. Leptin, an adipocyte-derived hormone or adipokine, is secreted by white adipose tissue and is an important factor on the control of energy balance, satiety, food intake and has been shown to have many levels of modulation upon the hypothalamic-hypophyseal-gonadal axis (for review see Tene-Sempere, 2007). In this study, our objective was to investigate the effect of daily duration of bull exposure to alter the temporal leptin concentration patterns during postpartum anestrus and the resumption of luteal activity. The specific hypothesis tested in this experiment were that systemic concentrations of leptin do not differ in postpartum, anestrous, suckled beef cows exposed to bulls for 12, 6, or 0 h daily. Materials and Methods This experiment was conducted at the Montana State University Bozeman Area Research and Teaching Facility. Animal care, handling, and protocols used in this experiment were approved by the Montana State University Institutional Agricultural Animal Care and Use Committee. Animals and Treatment Thirty-nine, spring-calving, two-yr-old Angus X Hereford primiparous, suckled beef cows and four mature epididymectomized Angus X Hereford bulls were used in this experiment. Cows and calves were maintained in a single pasture and had no contact with bulls or their excretory products from the previous breeding season until the start of the experiment (D 0). Average calving date for these cows was Feb. 18, 2008. Before 56 the start of the experiment, cycling status of each cow was rated by two ultrasound examinations of each ovary for the presence or absence of a corpus luteum. The first and second ultrasonic examinations were conducted 10 and 2 d, respectively, before the start of the experiment. Cows that did not exhibit the presence of a corpus luteum on either ovary in both ultrasound examinations were used in this experiment. The interval from calving to D 0 averaged 51.5 ± 2.3 d. Two d before the start of the experiment cows were stratified by calving date, calf birth weight, dystocia score, cow body weight, cow BCS, and sex of calf and assigned randomly to be exposed to bulls for 12 h daily (BE12; n = 15), 6 h daily (BE6; n =14) or not exposed to bulls (NE; n = 10) for 45 d. Facilities and Daily Bull Exposure. Cows were housed within pens in separate lot areas. Pens within the south lot were used to maintain BE12 and BE6 cows while pens within the north lot were used to maintain NE cows. A common holding pen, approximately 0.35 km from the lot that housed NE cows and 30 m from the lot that housed BE12 and BE6 cows, was used to house bulls before and after daily exposure periods. During daily exposure periods two bulls were moved from the common holding pen into the pen that housed BE12 cows and two bulls were moved into the pen that housed BE6 cows. Cows in each treatment were exposed to bulls at 0700 h each day for 45 d (D 0 to 44). At 1900 and1300 h bulls were removed from pens that housed BE12 and BE6 cows, respectively, and housed in the common holding pen until the following day. Cows in each treatment could not see or 57 smell bulls before or after daily exposure periods. Figure 5 below illustrates pen arrangements and method of daily bull exposure. Figure 5. Facilities where cows were housed during experiment and schedule of daily bull exposure. Nutrition. Cows had free access to good quality, chopped mixed-grass alfalfa hay, and any pasture grasses that were available before the start of the experiment. Once cows and calves were moved into pens they were given free access to the same hay, 0.5 kghd-1d1 cracked corn, water, and a trace mineral-salt supplement. Average body weight of cows was 467.5 ± 37.7 kg. The TDN of the diet was 110% of the recommended energy requirement for lactating beef cows with a mature weight of 533 kg (NRC, 1996). Bulls were fed the same diet as cows. 58 Blood Sampling, Leptin and Progesterone Concentrations, and Resumption of Luteal Activity. Blood samples were collected from each cow by jugular venepuncture every other day from D 0 to the end of the experiment (D 44). Serum concentrations of leptin were determined in triplicate and were quantified by using a competitive liquid-liquid phase, double-antibody leptin RIA procedure described previously (Delavaud et al., 2000). The intra and inter assay coefficients of variation were 2.01% and 3.22%, respectively. Serum was assayed for progesterone concentration in duplicate using solid-phase RIA kits (Siemens Medical Solutions Diagnostics, Los Angeles, CA, USA) validated for bovine serum in our laboratory (Custer et al., 1990). Intra- and interassay CV for a serum pool that contained 2.2 ng/mL of progesterone were 10.2 and 15.4%, respectively, and 8.9 and 11.8%, respectively, for a pool that contained 5.75 ng/mL. Progesterone concentrations in these samples were used to determine the interval from calving to resumption of luteal activity (RLA), interval from the start of the experiment to RLA, and the proportion of cows that RLA during the experiment. An increase of progesterone concentration, above the average progesterone baseline of individual cows in three consecutive samples that exceeded 1 ng/mL was used to determine the occurrence of RLA. Intervals from calving and D 0 to RLA were determined by the number of days from the treatment to the lowest inflection point before a rise in three consecutive samples that exceeded 1 ng/mL. Progesterone concentrations and RLA were confirmed by transrectal ultrasonographic examination of ovaries of each cow using a Titan ultrasound with a 7.5 to 10 MHz rectal transducer every other day throughout the 45-d exposure period used in this experiment 59 (SonoSite Inc., Bothell, WA, USA). The presence of a corpus luteum in the same anatomical position of an ovary in 4 successive scans was used as evidence to confirm RLA. Cows that failed to exhibit a rise in progesterone over three consecutive samples and did not have a corpus luteum in their ovaries were assigned an interval from calving or the start of treatment to the end of the experiment. Statistical Analyses. Intervals from D 0 to RLA were evaluated by ANOVA for a completely randomized design using PROC GLM of SAS (SAS, Cary, NC). The model included treatment (TRT) and means were separated using Bonferroni Multiple Comparison tests. Linear regression analyses were used to determine the relationship between intensity of bull exposure (hours/d) and intervals (days) from calving and the start of the experiment to RLA using the PROC REG procedure of SAS. Data for intervals from D 0 to RLA showed heterogeneous variances among treatments using Bartlett’s Box F-test. Therefore, data from the start of the experiment to RLA that were used in ANOVA were transformed using the Empirical Method by intervals to the power of 10.3. Least squares means and standard errors of means for intervals and D 0 to RLA, reported herein, were transformed to original values after analysis. Differences in proportions of cows that resumed OA during the experiment were analyzed by chi-square using the PROC FREQ procedure of SAS. Leptin concentrations were analyzed by PROC MIXED repeated measures analysis of SAS. The model included TRT, Animal as experimental unit, Day as the repeated measure, and the TRT by Interval interaction and means were separated using 60 Bonferroni Multiple Comparison tests. Linear regression analyses between leptin concentrations and hours of daily bull exposure were conducted using PROC REG procedure of SAS. Results Interval from D 0 to RLA was shorter (P < 0.05) for BE12 cows than for NE cows, 30.6 d and 43.0 d, respectively. However, interval from D 0 to RLA did not differ (P > 0.10) between BE6 cows, 35.0 d, and either BE12 or NE cows. The proportion of BE12 and BE6 cows that RLA during the experiment did not differ (P > 0.10), 60 and 64.3%, respectively. The proportion of cows that RLA for either BE6 cows or BE12 cows were greater than for NE cows, 10% (Table 2). 61 Table 4. Number of cows per treatment and least squares means for intervals from calving to resumption of luteal activity (RLA) and proportions of cows that RLA during the experiment for primiparous, anestrous, suckled, beef cows exposed to bulls for 12-h daily (BE12), 6-h daily (BE6), or not exposed to bulls (NE) for 45 d starting 51.5 ± 2.3 d (± SE) after calving. Variable n Interval from D0 to RLA, db NE 10 43.0y Treatment a BE6 14 35.0x,y BE12 15 30.6x SEM P -Value 10.6 <0.05 Proportion that 10.0y 64.3x 60.0x 8.0c <0.05 RLA, % a Means and proportions that lack common superscript differ (P < 0.05). b Cows that failed to RLA were assigned an interval from calving to the end of the experiment. c 2 X value. There was a treatment by day interaction (P < 0.05) for leptin concentrations. Temporal patterns of leptin concentrations differed among NE, BE6, and BE12 cows. However, leptin concentrations tended to be greater (P = 0.09) in cows that RLA than cows that did not RLA, indicating this interaction may be due to cycling status. In order to better understand this treatment by day interaction, the experiment period was grouped into four intervals; D 0 to 10, D 11 to 20, D 21 to 30, and D 31 to 40. Figure 6 illustrates patterns of leptin concentrations by treatment, wherein leptin exhibits a similar pattern in all levels of daily bull exposure. However, it appears that as the duration of bull exposure increases, treatments exhibit leptin patterns at slightly higher levels, respectively. 62 NE 9 BE6 BE12 z SEM z z Leptin, ng/mL 8 y 7 6 y y y x, y y y x, y x 5 0 to 10 d 11 to 20 d 21 to 30 d 31 to 40 d Interval Figure 6. Least squares means of leptin concentrations for 10-d intervals in cows exposed to bulls for 0 (NE), 6 (BE6), and12 (BE12) h daily. Bars that lack common letters within and between each interval differ, P < 0.05. Interval by treatment interaction, P = 0.06; treatment interaction, P < 0.0001. Bull exposed groups, both BE6 and BE12, had higher (P < 0.05) leptin concentrations over the D 0 to 10 interval than NE cows. Leptin concentrations seemed to increase through the first 20 d of the experiment and then level out. Furthermore, leptin concentrations from D 11 to 20, D 21 to 30, and D 31 to 40 were greater (P < 0.05) for BE12 cows than BE6 and NE cows. Again, there is the possibility that these effects could be attributed to the proportion cycling within each treatment. However, when samples taken after animals had resumed luteal activity were removed from the analysis, there was a similar significant treatment by day interaction (P < 0.05) as well (Figure 7). Again to better explain this interaction the experiment period was grouped into four intervals; D 0 to 10, D 11 to 20, D 21 to 30, and D 31 to 40. 63 non-cycling NE 9 non-cycling BE6 z non-cycling BE12 z SEM Leptin, ng/mL 8 y, z y y 7 6 y x, y y y, z x, y y, z x 5 0 to 10 d 11 to 20 d 21 to 30 d 31 to 40 d Interval Figure 7. Least squares means of leptin concentrations for 10-d intervals in cows exposed to bulls for 0 (NE), 6 (BE6), and12 (BE12) h daily. Non-cycling NE, BE6 and BE12 data are samples collected before the resumption of ovulatory activity. Bars that lack common letters within and between each interval differ, P < 0.05. Interval by treatment interaction, P = 0.08; treatment interaction, P < 0.0001. Similarly, concentrations of leptin increased faster within the first 20 d than through the rest of the experiment and were greater (P < 0.05) for bull exposed groups, both BE6 and BE12, than for NE cows during the D 0 to 10 interval. Additionally, leptin concentrations during intervals D 11 to 20 and D 21 to 30 were greater for BE12 cows than both BE6 and NE cows. However, in this analysis of samples from cows before they resumed luteal activity, leptin concentrations from D 21to 30 were greater (P < 0.05) in BE12 than both BE6 and NE cows. However, it appears that during the D 31 to 40 interval treatments did not differ (P > 0.10). There was a tendency for leptin concentrations to be greater (P = 0.09) in cows that had resumed luteal activity during the experiment than cows that did not resume OA, 8.0 and 6.9 ng/mL, respectively. 64 Furthermore, there was a tendency for a linear relationship (b1 = 0.123 ng/h; P = 0.09) between mean leptin concentrations and hours of daily bull exposure. NE BE6 BE12 70% y y 60% y %RLA 50% 40% y 30% x 20% x 10% 0% x x x x 0 to 10 d x x 11 to 20 d 21 to 30 d 31 to 40 d Interval Figure 8. Percentages of primiparous, suckled cows exposed to bulls for 0 (NE), 6 (BE6), and12 (BE12) h daily that resumed luteal activity (%RLA) during intervals D 0 to 10, D 11 to 20, D 21 to 30, and D 31 to 40 of the experiment. Bars that lack common letters within each interval differ, P < 0.05; interval D 0 to 10, 2 = 3.37, d.f. = 2; interval D 11 to 20,2 = 2.84, d.f. = 2; interval D 21 to 30,2 = 6.38, d.f. = 2; ; interval D 31 to 40,2 = 8.12, d.f. = 2 and the overall interaction, P < 0.05;2 = 382.7, d.f. = 11. Discussion The purpose of this study was to evaluate changes in leptin concentrations in primiparous, postpartum, anovular, suckled, beef cows that were exposed to bulls for durations of 0, 6, and 12 hours daily. Even though, mean leptin concentrations did not 65 differ among treatments, we found that there was a treatment by day interaction for leptin concentrations. This data indicates that the temporal patterns of leptin differed among cows exposed to bulls for 0, 6, and 12 hours daily. Interestingly, across all treatments, leptin concentrations tended to be greater in cows that had resumed luteal activity during the experimental period (D 0 to D 44) than in cows that had not resumed luteal activity by the end of the experiment. Specifically, cows that had resumed luteal activity during the experimental period had a mean leptin concentration of 8.0 ng/mL, whereas cows that did not resume luteal activity by the end of the experiment (D 44) had a mean leptin concentration of 6.9 ng/mL. This suggests that the treatment by day interaction seen with leptin concentrations is perhaps more directly related to the cycling status of the animal and indirectly influenced by the biostimulatory effect of bulls to hasten the resumption of luteal activity in the postpartum anestrous cow. If cycling status is more responsible for mean leptin concentrations, one could expect that the proportion of cows cycling would correlate to mean leptin concentrations. However, we found a tendency for a positive linear relationship between the level of bull exposure (0, 6, and 12 hours daily) and mean leptin concentrations, wherein the proportion of cows exposed to bulls for 6 and 12 hours daily did not differ, 64.3 and 60.0% respectively; but were significantly greater than the 10% cycling of cows not exposed to bulls. Though it is only a tendency, this relationship suggests the possibility that the biostimulatory effect of bulls may have a direct influence on leptin concentrations. In order to better understand the treatment by day interaction, the 66 experiment period was grouped into four periods; D 0 to 10, D 11 to 20, D 21 to 30, and D 31 to 40. In Fig. 6, mean leptin concentrations of each period are represented for cows exposed to bulls for 0, 6, and 12 hours daily. This illustrates a graded curve by duration of bull exposure on leptin concentrations, wherein leptin exhibits a similar pattern in all treatments. However, it seems that as the duration cows are exposed to a bull increases, treatments indicate leptin patterns at slightly higher levels, respectively. Again, there is the possibility that this could be attributed to the proportion cycling within each treatment. However, when samples taken after animals had resumed ovulatory activity were removed from the analysis, there was a similar tendency for a treatment by interval interaction as well (Fig. 7). These interactions are further supported by the tendency for a linear relationship between mean leptin concentrations and duration of daily bull exposure. In conclusion, daily bull exposure reduced the interval to resumption of luteal activity and altered the temporal pattern of leptin concentrations in the postpartum, anovular, suckled, beef cows. This suggests that the biostimulatory effect of bulls may have a metabolic component. The results of this study suggest that leptin concentrations are increased by both the biostimulatory effect of bulls and the resumption of luteal activity in postpartum, suckled cows. Further investigations are necessary to explain how the presences of bulls influence leptin; and to understand the role of changes in leptin concentration patterns during the resumption of luteal activity in the postpartum, primiparous, anestrous, suckled, beef cow. 67 CHAPTER 9 GENERAL DISCUSSION In Experiment 1 of this thesis, we reported non-esterified fatty acids (NEFA) were significantly lower in cows that were exposed to bulls than cows not exposed. If leptin concentrations are high, this would be a signal of satiety to the brain and would be concurrent with a decrease in the mobilization of NEFA (Tena-Sempere, 2007). T hese results are complementary of the findings of Experiments 2 and 3 wherein we find that bull exposure not only results in an increase temporal leptin concentrations, but it does so in a dose-dependent manner. However, Landaeta-Hernandez et al., (2004) found that NEFA concentrations were 33% higher in suckled cows exposed to bulls than in nonexposed cows. Perhaps this difference is due to the greater proportion in resumption of luteal activity of cows that were exposed to bulls than cows not exposed to bulls as reported by Landaeta-Hernandez et al., (2004). The conclusions of Experiments 1, 2, and 3 of this thesis all suggest that there is a metabolic component involved with the biostimulatory effect of bulls. Specifically, the physical presence of bulls seems to decrease the mobilization of free-fatty acids (or NEFA) and over time increase temporal leptin concentrations. One interpretation of these results could be that within this metabolic component of the biostimulatory effect there is a physiological change representative of an increasing energy state for the cow. If so, it is possible that cows are “tricked” into perceiving a higher energy state than if they were not exposed to bulls. The physiological advantage to this perception is the 68 potential to change LH pulsatility and hasten the resumption of luteal activity in the postpartum anestrous cow through action of leptin on neural inputs and within the hypothalamus; as described by Schneider, (2004). In both Experiments 2 and 3, temporal leptin concentrations were increased shortly after the start of bull exposure. Continuous (24h/d) bull exposure in Experiment 2 tended to increase temporal leptin concentrations by 18 to 20 d after the start of bull exposure wherein 100% of cows exposed to bulls and 46% of non-exposed cows had resumed luteal activity by 9 to 15 d from the start of bull exposure. Furthermore, in Experiment 3 cows exposed to bulls for 12h/d (BE12) and 6h/d (BE6) had higher temporal leptin concentrations than non-exposed (NE) cows within the first 10 d of bull exposure and had mean intervals from the start of the experiment to the resumption of luteal activity of 31, 35, and 43d; respectively. This indicates a definite relationship between the biostimulatory effect of bulls to hasten the resumption of luteal activity between the anestrous cow and changes in temporal leptin concentrations. There is the possibility that the observed changes in leptin concentrations could be related simply to cycling status, specifically the presence of progesterone in the blood. However, from the analysis of non-cycling cows in Experiment 3 it appears that there is a dose-dependent response to bull exposure in leptin concentrations in the absence of luteal activity. Hypothesis for a Metabolic Component or Pathway to the Bull Biostimulatory Effect of Bulls Reproductive efficiency increases as the interval of postpartum anestrous decreases. Exposing postpartum, anestrous cows to bulls accelerates the resumption of 69 ovulatory activity and may be used as a strategy to increase reproductive efficiency. The mechanism by which bulls decrease the postpartum, anestrous interval may be related to changes in metabolic regulation of adipose tissue. This is the first report wherein the presence of bull alters a metabolic processes related to adipose metabolism in postpartum, anestrous cows. The results suggest that leptin concentrations are increased by both the biostimulatory effect of bulls and the resumption of ovulatory activity. Further investigations are necessary to explain how the daily presence of bulls influences the secretion of leptin, and to further understand the patterns of leptin concentrations during the resumption of ovulatory activity in the postpartum, primiparous, anestrous, suckled, beef cow. The following diagram (adapted from Schneider, 2004; Tena-Sempere, 2007; and Tauck, 2008) reviews the hypothetical relationship between bull biostimulation, metabolic hormones, and the hypothalamic-pituitary-ovarian (HPO) axis. 70 The above diagram depicts the many metabolic endocrine pathways that can influence the HPO, LH pulsatility, and ultimately cause the resumption of ovarian cycling activity in postpartum, anestrous cows. Again from the literature review, the positive or negative feedback by each of these hormones can change and is dependent upon the energy state and the specific hormone milieu. However, it is clear that a change in metabolic fuels, energy balance, and metabolic hormones can greatly influence the function of the HPO and/or metabolic hormone sensitivity in effectors of GnRH neurons. As aforementioned, energy homeostasis is a very tightly regulated system with many redundant pathways and levels of modulation upon the HPO. However, it would be advantageous for reproduction if it was possible that bull exposure stimulates the HPO axis by “tricking” the cow’s perception of this system into that of a higher energy state. Is it then possible that noted changes in adrenal secretion of cortisol (Tauck, 2008) in cows under the biostimulatory effect sets into motion a permissive or positive influence upon the effect by other metabolic hormones (Schneider, 2004 and Tena-Sempere, 2007) on the HPO to ultimately alter LH pulsatility? Further investigations are necessary to understand the potential role of changes in leptin concentration patterns in hastening the resumption of luteal activity in the postpartum, primiparous, anestrous, suckled, beef cow. In future research specific to the influence of bull exposure on leptin concentrations, questions that must be asked are 1) “Is the change in temporal leptin concentrations due to an increase in secretion and synthesis or to a decrease in its degredation”?; 2), “Does an increase in leptin during bull exposure directly alter LH secretion patterns for the resumption of ovarian cycling 71 activity”?; and 3), “Does bull exposure change the expression or sensitivity of the Ob-R (leptin receptor) within the HPO”? Further research could also investigate the effect of bull exposure on other metabolic hormones such as insulin, ghrelin, IGF-1, etc. 72 LITERATURE CITED Acosta, B., G.K. Tarnavsky, T.E. Platt, D.L. Hamernik, J.L. Brown, H.M. Schoenemann, and J.J. Reeves. 1983. Nursing enhances the negative effect of estrogen on LH release in the cow. J. Anim. Sci. 57:1530-1536. Agarwal SK, Vogel K, Weitsman SR, Magofin DA. 1999. Leptin antagonizes the insulinlike growth factor augmentation of steroidogenesis in granulosa and thecal cells of the human ovary. J. Clin. Endocrinol Metab. 84: 1072-1076 Ahima RS, Dushay J, Flier SN, Prabakaran D and Flier JS. 1997. Leptin accelerates the onset of puberty in normal female mice Journal of Clinical Investigation 99 391395 Ahima RS, Saper CB, Flier JS, Elmquist JK. 2000. Leptin regulation of neuroendocrine systems. Front Neuroendocronol. 21: 263-307. Ahren B, Larsson H, Wilhelmsson C, Nasman B and Olsson T. 1997. Regulation of Amstalden M, Garcia MR, Williams SW, Stanko RL, Nizielski SE, Morrison CD, Keisler DH and Williams GL. 2000. Leptin gene expression, circulating leptin, and luteinizing hormone pulsatility are acutely responsive to short-term fasting in prepubertal heifers: relationships to circulating insulin and insulin-like growth factor I. Biology of Reproduction 63 127-133 Anderson, K. A., J. G. Berardinelli, P.S. Joshi, and B. Robinson. 2002. Effects of exposure to bull or excretory products of bulls on the breeding performance of first-calf restricted suckled beef cows using a modified CO-synch protocol. Proc. West. Sec. Amer. Soc. Anim. Sci. 53:457-460. Archanco M, Muruzabal FJ, Llopiz D, Garayoa M, Gomez-Ambrosi J, Fruhbeck G, Burrell MA. 2003. Leptin expression in the rat ovary depends on estrous cycle. J. Histochem. Cytochem. 51: 1269-1277. Arije, G. R., J. N. Wiltbank, and M. L. Hopwood. 1974. Hormone levels in pre- and post-parturient beef cows. J. Anim. Sci. 39:338-347. Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, Moizo L, Lehy T, Guerre-Mille M, Marchand-Brustel Y and Lewin MJM. 1998. The stomach is a source of leptin Nature 394 790-793 Barash IA, Cheung CC, Weigle DS, Ren H, Kabigting EB, Kuijper JL, Clifton DK and Steiner RA. 1996. Leptin is a metabolic signal to the reproductive system Endocrinology 137 3144-3147 73 Barkan D, Hurgin V, Dekel N, Amsterdam A, Rubinstein M. 2005. Leptin induces ovulation in GnRH-deficient mice. FASEBJ. 19:133-135. Bartle, S. J., J. R. Males, and R. L. Preston. 1984. Effect of energy intake on the postpartum interval in beef cows and the adequacy of the cow’s milk production for calf growth. J. Anim. Sci. 58:1068. Baruah, K. K. and L. N. Kanchev. 1993. Hormonal response to olfactory stimulation with bull urine in postpartum dairy cows. Proc. VII World Conf. Anim. Prod. 3:356-361. Beal, W.E., R.E. Short, R.B. Staigmiller, R.A. Bellows, C.C. Kaltenbach, and T. G. Dunn. 1978. Influence of dietary energy intake on bovine pituitary and luteal function. J. Anim. Sci. 46:181-188. Beam, S.W., and W.R. Butler.1997. Energy balance and ovarian follicle development prior to the first ovulation postpartum in dairy cows receiving three levels of dietary fat. Biol. Reprod. 56:133-142. Bellows, R.A. and R.E. Short. 1978. Effects of precalving feed level on birth weight, calving difficulty and subsequent fertility. J. Anim. Sci. 46:1522-1528. Bennett PA, Lindell K, Karlsson C, Robinson ICAF, Carlsson LMS and Carlsson B. 1998. Differential expression and regulation of leptin receptor isoforms in the rat brain: effects of fasting and oestrogen Neuroendocrinology 67 29-36 Berardinelli, J. G., M S. Roberson, and R. Adair. 1987. Shortening the anestrous period. Montana Ag Research 4:25-28. Berardinelli, J. G., P. S. Joshi, and S. A. Tauck. 2004. Breeding performance of firstcalf suckled beef cows exposed to familiar or unfamiliar bulls using a modified CO-Synch protocol. Postpartum interval in first-calf suckled beef cows exposed to familiar or unfamiliar bulls. Proc. West. Sec. Amer. Soc. Anim. Sci. 55:291293. Berardinelli, J. G., P. S. Joshi., and S. A Tauck. 2005. Postpartum resumption of ovarian cycling activity in first-calf suckled beef cows exposed to familiar or unfamiliar bulls. Anim. Reprod. Sci. 90:201-209. Bishop D.K., R.P. Wettemann, and L.J. Spicer. 1994. Body energy reserves influence the onset of luteal activity after early weaning of beef cows. J. Anim. Sci. 72:27032708. Burns, P.D. and J.C. Spitzer. 1992. Influence of biostimulation on reproduction in postpartum beef cows. J. Anim. Sci. 70:358-362. 74 Caldani M, Caraty A, Pelletier J, Thiery JC and Tillet Y. 1993. LH pulsatile release and its control. In Reproduction in Mammals and Man pp 80-96 Eds C Thibault, MC Levasseur and RHF Hunter. Ellipses, Paris Carruthers, T. D. and H. D. Hafs. 1980a. Suckling and four-times daily milking: influence on ovulation, estrus and serum luteinizing hormone, glucocorticoids and prolactin in postpartum holsteins. J. Anim. Sci. 50:919-925. Carruthers, T.D., E.M. Convey, J.S. Kesner, H.D. Hafs, and K.W. Cheng. 1980b. The hypothalamic-pituitary gonadotrophic axis of suckled and nonsuckled dairy cows postpartum. J. Anim. Sci. 51:949-957. Carter, M. L., D. J. Dierschke, J. J. Rytledge, and E. R. Hauser. 1980. Effect of gonadotropin-releasing hormone and calf removal on pituitary-ovarian function and reproductive performance in postpartum beef cows. J. Anim. Sci. 51:903. Casanueva FF, Dieguez C. 1999. Neuroendocrine regulation and actions of leptin. 20: 317-363 Castenson, P. E., A.M. Sorensen Jr., C. R. Cobos, and J. L. Fleeger. 1976. Source of -OHP preceding estrus in heifers. J. Anim. Sci.43:277. Chang, C. H., T. Gimenez, and D. M. Henricks. 1981. Modulation of reproductive by suckling and exogenous gonadal hormones in young beef cows post-partum. J. Reprod. Fertil. 63: 31-38. Chehab FF, Mounzih K, Lu R and Lim ME. 1997. Early onset of reproductive function in normal female mice treated with leptin Science 275 88-90 Cheung CC, Clifton DK and Steiner RA. 1997. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus Endocrinology 138 4489-4492 Cheung CC, Thornton JE, Kuijper JL, Weigle DS, Clifton DK, Steiner RA. 1997. Leptin is a metabolic gate for the onset of puberty in the female rat. Endocrinology 138 855-858 Connor, H.C., P.L. Houghton, R.P. Lemenager, P.V. Malven, J.R. Parfet, and G.E. Moss. 1990. Effect of dietary energy, body condition and calf removal on pituitary gonadotropins, gonadotropin-releasing hormone (GnRH) and hypothalamic opioids in beef cows. Domest. Anim. Endocrinol. 7:403-411. Corah, L. R., A. P. Quealy, T. G. Dunn, and C. C. Kaltenbach. 1974. Prepartum and postpartum levels of progesterone and estradiol in beef heifers fed two levels of energy. J. Anim. Sci.39:380-385. 75 Craig J, Zhu H, Dyce PW, Petrik J, Li J. 2004. Leptin enhances oocyte nuclear and cytoplasmatic maturation via the mitogen-activated protein kinase pathway. Endocrinology. 145: 5355-5363 Crowe, M. A., V. Padmanabhan, M. Mihm, I. Z. Beitins, and J. F. Roche. 1998. Resumption of follicular waves in beef cows is not associated with periparturient changes in follicle-stimulating hormone heterogeneity despite major changes in steroid and luteinizing hormone concentrations. Biol. Reprod. 58:1445-1450. Cupp, A.S., M.S. Roberson, T.T. Stumpf, M.W. Wolfe, L.A. Werth, N. Kojima, R.J. Kittok, J.E. Kinder.1993. Yearling bulls shorten the duration of postpartum anestrus in beef cows to the same extent as do mature bulls. J. Anim. Sci. 71:306309. Custer, E. E., J. G. Berardinelli, R. E. Short, M. Wehrman, and R. Adair. 1990. Postpartum interval to estrus and patterns of LH and progesterone in first-calf suckled beef cows exposed to mature males. J. Anim. Sci. 668:1370-1377. Daley, C. A., H. Sakurai, B. M. Adams, and T. E. Adams. 2000. Effect of stress-like concentrations of cortisol on feedback potency of oestradiol in orchidectomized sheep. Anim. Reprod. Sci. 59:167-178. Day ML, Imakawa K, Zalesky DD, Kittok RJ and Kinder JE. 1986. Effects of restriction of dietary energy intake during the prepubertal period on secretion of luteinizing hormone and responsiveness of the pituitary to luteinizing hormonereleasing hormone in heifers Journal of Animal Science 62 1641-1649 DeFries, C.A., D.A. Neuendorff, and R.D. Randel. 1998. Fat supplementation influences postpartum reproductive performance in Brahman cows. J. Anim. Sci. 76:864870. Delavaud C, Bocquier F, Chilliard Y, Keisler DH, Gertler A and Kann G. 2000. Plasma leptin in ruminants: effect of nutritional status and body fatness on plasma leptin concentration assessed by a specific RIA in sheep Journal of Endocrinology 165 519-526 DeRouen, S.M. , D.E. Franke, D.G. Morrison, W.E. Wyatt, D.F. Coombs, T.W. White, P.E. Humes, and B.B. Greene. 1993. Prepartum body condition and dietary energy on pre- and postpartum performance of beef heifers. J. Anim. Sci. 71 (Suppl. 1): 236. DeRouen, S.M., D.E. Franke, D.G. Morrison, W.E. Wyatt, D.F. Coombs, T.W. White, P.E. Humes and B.B. Greene. 1994. Prepartum body condition and weight influences on reproductive performance of first-calf beef cows. J. Anim. Sci. 72: 1119-1125. 76 Ducker, M.J., R.A. Haggett, W.J. Fisher, S.V. Morant, and G.A. Bloomfield. 1985. Nutrition and reproductive performance of dairy cattle. I. The effect of level of feeding in late pregnancy and around the time of insemination on the reproductive performance of first lactation dairy heifers. Anim. Prod. 41:1-12. Duggal PS, van der Hoek, KH, Milner CR, Ryan NK, Armstrong DT, Magoffin DA, Norman RJ. (2000). The in vivo and in vitro effects of exogenous leptin on ovulation in the rat. Endocrinology. 141: 1971-1976. Dunn, R.T., Jr., M.F. Smith, H.A. Garverick, and C.W. Foley. 1985. Effect of 72 hr calf removal and / or gonadotropin releasing hormone on luteinizing hormone release and ovarian activity in postpartum beef cows. Theriogenology 23:767. Dunn, T.G. and C.C. Kaltenbach. 1980. Nutrition and the postpartum interval of the ewe, sow, and cows. J. Anim. Sci. 51 (Suppl. 2): 29. Dunn, T.G., J.E. Ingalls, D.R. Zimmerman, and J.N. Wiltbank.1969. Reproductive performance of 2-year-old Hereford and Angus heifers as influenced by pre- and post-calving energy intake. J. Anim. Sci. 29:719-726. Ebert, J. J., P. Conteras, and P. Saelzer. 1972. Influence of teaser bull on puerperium and fertility in dairy cows. VII Int. Cong. Anim. Reprod. Artif. Insem. Munich. 3:1743-1748. Ehrhardt RA, Slepetis RM, Siegal-Willott J, van Amburgh ME, Bell AW and Boisclair YR. 2000. Development of a specific radioimmunoassay to measure physiological changes of circulating leptin in cattle and sheep Journal of Endocrinology 166 519-528 Falk, D.G., R.E. Christian, R.C. Bull, and R.G. Sasser. 1975. Prepartum energy effects on cattle reproduction. J. Anim. Sci. 41:267. Fernandes, L. C., W. W. Thatcher, C. J. Wilcox, and E. P. Call. 1978. LH release in response to GnRH during the postpartum period of dairy cows. J. Anim. Sci. 46:443. Fernandez, D. L., J. G. Berardinelli, R. E. Short, and R. Adair. 1996. Acute and chronic changes in luteinizing hormone secretion and postpartum interval to estrus in first-calf suckled beef cows exposed continuously or intermittently to mature bulls. J. Anim. Sci. 74:1098-1103. Fernandez, D.L, J.G. Berardinelli, R.E. Short and R .Adair.1993. The time required for the presence of bulls to alter the interval from parturition to resumption of ovarian activity and reproductive performance in first-calf suckled beef cows. Theriogenology 39:411-419. 77 Finn PD, Cunningham MJ, Pau KF, Spies HG, Clifton DK and Steiner RA. 1998. The stimulatory effect of leptin on the neuroendocrine reproductive axis of the monkey Endocrinology 139 4652-4662 Fortune, J. E., J. Sirois, A. M. Turzillo, and M. Lavoir. 1991. Follicle selection in domestic ruminants. J. Reprod. Fertil. Suppl. 43:187-198. Frederich RC, Haman A, Anderson S, Lollmann B, Lowell BB and Flier JS. 1995. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action Nature Medicine 1 1311-1314 Friedman JM and Halaas JL. 1998. Leptin and the regulation of body weight in mammals Nature 395 763-770 Garcia MR, Amstalden M, Keisler DH, Raver N, Gertler A and Williams GL. 2002b. Leptin attenuates the central effects of neuropeptide-Y on somatotropin but not gonadotropin secretion in cows Journal of Animal Science 80 (Supplement 1) 352 Abstract 1411 Garcia MR, Amstalden M, Williams SW, Stanko RL, Morrison CD, Keisler DH, Nizielski SE and Williams GL. 2002a. Serum leptin and its adipose gene expression during pubertal development, the estrous cycle, and different seasons in cattle Journal of Animal Science 80 2158-2167 Garcia-Winder, M., K. Imakawa, M. L. Day, D. D. Zalesky, R.J. Kittok, and J. E. Kinder. 1984. Effect of suckling and ovariectomy on the control of luteinizing hormone secretion during the postpartum period of cows. Biol. Reprod. 31:771-778. Garcia-Winder, M., K. Imakawa, M. L. Day, D. D. Zalesky, R.J. Kittok, and J. E. Kinder. 1986. Effects of suckling and low doses of estradiol on luteinizing hormone secretion during the postpartum period in beef cows. Domest. Anim. Endocrinol. 3:79-85. Gazal OS, Leshin LS, Stanko RL, Thomas MG, Keisler DH, Anderson LL and Williams GL. 1998. Gonadotropin-releasing hormone secretion into third-ventricle cerebrospinal fluid of cattle: correspondence with the tonic and surge release of luteinizing hormone and its tonic inhibition by suckling and neuropeptide Y Biology of Reproduction 59 676-683 Ghizzoni L, Barreca A, Mastorakos G, Furlini M, Vottero A, Ferrari B, Chrousos GP, Bernasconi S. 2001. Leptin inhibits steroid biosynthesis by human granulosalutein cells. Horm. Metab. Res. 33: 323-328. Gombe, S., and W. Hansel. 1973. Plasma luteinizing hormone (LH) and progesterone levels in heifers on restricted energy intakes. J. Anim. Sci. 37:728-733. 78 Gong, J. G., B. K. Campbell, T. A. Bramley, C. G. Gutierrez, A.R. Peters, and R. Webb. 1996. Suppression in the secretion of follicle-stimulating hormone and luteinizing hormone, and ovarian follicular development in heifers continuously infused with a gonadotrophin-releasing hormone agonist. Biol. Reprod. 55:68-74. Gong, J. G., T. A. Bramley, C. G. Gutierrez, A.R. Peters, and R. Webb. 1995. Effects of a chronic treatment with a gonadotrophin-releasing hormone agonist on peripheral concentrations of FSH and LH, and ovarian function in heifers. J. Reprod. Fertil. 105:263-270. Grimard B., P. Humblot, J.P. Mialot, N. Jeanguyot, D. Sauvant, and M. Thibier. 1997. Absence of response to estrus induction and synchronization treatment is related to lipid mobilization in suckled beef cows. Reprod. Nutr. Dev. 37:129-140. Guedon L., J. Saumande, and B. Desbals. 1999. Relationships between calf birth weight, prepartum concentrations of plasma energy metabolites and resumption of ovulation postpartum in Limousine suckled beef cows. Theriogenology 52:779789. Hadley, M.E., and Levine, J.E. 2006. Adrenal steroid hormones. In Endocrinology.. Sixth ed. Pages 337-365, Pearson Prentice Hall, New Jersey, USA. Hafez, E. S. and E., B. Hafez. 2000. Reproduction in farm animals. Seventh ed. Page 55 in chapter 4. Lippincott Williams and Wilkins, Baltimore, MD. Henricks, D.M., and J.D. Rone. 1986. A note on the effect of nutrition on ovulation and ovarian follicular populations in the individually fed post-partum beef heifer. Anim. Prod. 557-560. Henry BA, Goding JW, Alexander WS, Tilbrook AJ, Canny BJ, Dunshea F, Rao A, Mansell A and Clarke IJ. 1999. Central administration of leptin to ovariectomized ewes inhibits food intake without affecting the secretion of hormones from the pituitary gland: evidence for a dissociation of effects on appetite and neuroendocrine function Endocrinology 140 1175-182 Henry BA, Goding JW, Tilbrook AJ, Dunshea FR and Clarke IJ. 2001. Intracerebroventricular infusion of leptin elevates the secretion of luteinizing hormone without affecting food intake in long-term food-restricted sheep, but increases growth hormone irrespective of bodyweight Journal of Endocrinology 168 67-77 Hoggard N, Hunter L, Duncan JS, Williams LM, Thayhurn P and Mercer JG. 1997. Leptin and leptin receptor mRNA and protein expression in the murine fetus and placenta Proceedings of the National Academy of Sciences USA 94 11073-11078 79 Hornbuckle, II, T, R.S. Ott, M.W. Ohl, G.M. Zinn, P.G. Weston, and J.E. Hixon. 1995. Effects of bull exposure on the cyclic activity of beef cows. Theriogenology 43:411-418. Houghton, P.L., R.P. Lemenager, L.A. Horstman, K.S. Hendrix, and G.E. Moss. 1990. Effects of body composition, pre- and postpartum energy level and early weaning on reproductive performance of beef cows and preweaning calf gain. J. Anim. Sci. 68:1438-1446. Houseknecht KL and Portocarrero CP. 1998. Leptin and its receptors: regulators of whole-body energy homeostasis Domestic Animal Endocrinology 15 457-475. Humphrey, W.D., C. C. Kaltenbach, T. G. Dunn, D. R. Koritnik, and G. D. Niswender. 1983. Characterization of hormonal patterns in the beef cow during postpartum anestrus. J. Anim. Sci. 56:445-453. I’Anson H, Manning JM, Herbosa CG, Pelt J, Friedman CR, Wood RI, Bucholtz DC and Foster DL 2000 Central inhibition of gonadotropin-releasing hormone secretion in the growth-restricted hypogonadotropic female sheep Endocrinology 141 520-527 Jagger, J. P., A. R. Peters, and G. E. Lamming. 1987. Hormone responses to low-dose GnRH treatment in post-partum beef cows. J. Reprod. Fert. 80:263. Kadokawa H and Yamada Y (1999) Enhancing effect of acute fasting on ethanol suppression of pulsatile luteinizing hormone release via an estrogen-dependent mechanism in holstein heifers Theriogenology 51 673-680. Karlsson C, Lindell K, Svenson E, Bergh C, Lind P, Billig H, Carlsson LM, Carlsson B. 1997. Expression of functional leptin receptors in the human ovary. J Clin Endocrinol Metab. 82: 4144-4148. Kendall NR, Gutierrez CG, Scaramuzzi RJ, Baird DT, Webb R, Campbell. 2004. Direct in vivo effects of leptin on ovarian steroidogenesis in sheep. Reproduction. 128: 757-765. Khireddine, B., B. Grimard, A.A. Ponter, C. Ponsart, H. Boudjenah, J.P. Mialot, D. Sauvant, and P. Humblot. 1998. Influence of flushing on LH secretion, follicular growth and the response to estrus synchronization treatment in suckled beef cows. Theriogenology 49:1409-1423. Kieborz-Loos, K. R., H. A. Garverick, D. H. Keisler, S. A. Hamilton, B. E. Salfen, R. S. Youngquist, and M. F. Smith. 2003. Oxytocin-induced secretion of prostaglandin F2α in postpartum beef cows: Effects of progesterone and estradiol17-β treatment. J. Anim. Sci. 81:1830-1836. 80 Kikuchi N, Andoh K, Abe Y, Yamada K, Mizunuma H, Ibuki Y. 2001. Inhibitory action of leptin on early follicular growth differs in immature and adult female mice. Biol Reprod. 65:66-71. La Voie, V., D. K. Han, D. B. Foster, and E. L. Moody. 1981. Suckling effect on estrus and blood plasma progesterone in postpartum beef cows. J. anim. Sci. 52:802. Lamb, G. C., J. M. Lynch, D. M. Grieger, J. E. Minton, and J. S. Stevenson. 1997. Adlibitum suckling by unrelated calf in the presence or absence of a cow’s own calf prolongs postpartum anovulation. J. Anim. Sci. 75:2762-2769. Lammoglia, M.A., S.T. Willard, J.R. Oldham, and R.D. Randel. 1996. Effects of dietary fat and season on steroid hormone profiles before parturition and on hormonal, cholesterol, triglycerides, follicular patterns, and postpartum reproduction in Brahman cows. J. Anim. Sci. 74:2253-2262. Landaeta-Hernandez, A. J., M. Giangreco, P. Melendez, J. Bartolome, F. Bennet, D. O. Rae, and L. F. Archbald. 2004. Effect of biostimulation on uterine involution, early ovarian activity and first postpartum estrous cycle in beef cows. Therigenology 61:1521-1532. Landaeta-Hernandez, A. J., P. Melendez, J. Bartolome, D. O. Rae, and L. F. Archbald. 2006. Effect of biostimulation on the expression of estrus in postpartum Angus cows. Theriogenology 66:710-716. Laster, D. B., H. A. Glimp, and K. E. Gregory. 1973. Effects of early weaning on postpartum reproduction of cows. J. Anim. Sci. 36:734. Loffler S, Aust G, Kohler U, Spanel-Borrowski K. 2001. Evidence of leptin expression in normal and polycystic human ovaries. Mol Hum Reprod. 7: 1143-1149. Machinal F, Dieudonne MN, Leveveu MC, Pecquery R and Giudicelli Y. 1999. In vivo and in vitro ob gene expression and leptin secretion in rat adipocytes: evidence for a regional specific regulation by sex steroid hormones Endocrinology 140 15671574 Maciel MN, Zieba DA, Amstalden M, Keisler DH, Neves J and Williams GL. 2003. Recombinant oleptin prevents fasting-mediated reductions in pulsatile LH release and stimulates GH secretion in peripubertal heifers Proceedings of the MidWest Section of the American Society of Animal Science 66 Abstract 263 Macmillan, K. L., A. J. Allison, and G.A. Struthers. 1979. Some effects of running bulls with suckling cows or heifers during the premating period. New Zealand J. Exp. Agr. 7:121. 81 Magni P, Vettor R, Pagano C, Calcagno A, Beretta E, Messi E, Zanisi M, Martini L and Motta M. 1999. Expression of a leptin receptor in immortalized gonadotropinreleasing hormone-secreting neurons Endocrinology 140 1581-1585 Mann,G. E. and G. E. Lamming. 2000. The role of sub-optimal preovulatory oestradiol secretion in the aetiology of premature luteolysis during the short oestrous cycle in the cow. Anim. Reprod. Sci. 64:171-180. Marchlewska-Koj, A., and M. Zacharczuk-Kakietek. 1990. Acute increase in plasma corticosterone level in female mice evoked by pheromones. Physiol. Behav. 48:577-580. McCann JP and Hansel W. 1986. Relationships between insulin and glucose metabolism and pituitary-ovarian functions in fasted heifers Biology of Reproduction 34 630641 McNeilly, A.S. 1988. Suckling and the control of gonadotropin secretion. Page 2323 in The Physiology of Reproduction. E. Knobil and J Neill, ed., Raven Press, New York. McShane TM, May T, Miner JL and Keisler DH. 1992. Central actions of neuropeptideY may provide a neuromodulatory link between nutrition and reproduction Biology of Reproduction 46 1151-1157 McShane TM, Petersen SL, McCrone S and Keisler DH. 1993. Influence of food restriction on neuropeptide-Y, proopiomelanocortin, and luteinizing hormonereleasing hormone gene expression in sheep hypothalami Biology of Reproduction 49 831-839 Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Morgan PJ and Trayhurn P. 1996. Coexpression of leptin receptor and preproneuropeptide Y mRNA in arcuate nucleus of mouse hypothalamus Journal of Neuroendocrinology 8 733-735 Mora, O. A., and J. E. Sanchez-Criado. 2004. Involvement of the corticoadrenal hormones in the pheromonal restoration activity in aging rats. Life Sciences 74:3285-3290. Morash B, Li A, Murphy PR, Wilkinson M and Ur E. 1999. Leptin gene expression in the brain and pituitary gland Endocrinology 140 5995-5998 Moss, G. E., J. R. Parfet, C. A. Marvin, R. D. Allrich, and M. A. Diekman. 1985. Pituitary concentrations of gonadotropins and receptors for GnRH in suckled beef cows at various intervals after calving. J. Anim. Sci. 60:285-293. 82 Mounzih K, Lu R and Chehab FF. 1997. Leptin treatment rescues the sterility of genetically obese ob/ob males Endocrinology 138 1190-1193 Naasz, C.D., and H.L. Miller. 1990. Effects of bull exposure on postpartum interval and reproductive performance in beef cows. Can. J. Anim. Sci.70:537-542. Nagatani S, Zeng Y, Keisler DH, Foster DL and Jaffe CA. 2000. Leptin regulates pulsatile luteinizing hormone and growth hormone secretion in the sheep Endocrinology 141 3965-3975 Nersesjan, S. S. 1962. The use of vasectomized bulls as biological stimulators in controlling infertility in cows. Anim. Breed. Abstr. 30:48. Nett, T. M., D. Cermak, T. Braden, J. Manns, and G. Niswender. 1988. Pituitary receptors for GnRH and estradiol, and pituitary content of gonadotropins in beef cows. II. Changes during the postpartum period. Domest. Anim. Endocrinol. 5:81-89. NRC. 1996. 7th revised (ed.) Nutrient Requirements of Beef Cattle. Natl. Acad. Press, Washington, DC. Page 227. Odde, K. G., H. S. Ward, G. H. Kirakofe, R. M. Mckee, and R. J. Kittok. 1980. Short estrous cycles and associated serum progesterone levels in beef cows. Theriogenology 14:105. Olatinwo MO, Bhat GK, Stah, CD, Mann DR. 2005. Impact of gonadotropin administration on folliculogenesis in prepubertal ob/ob mice. Mol Cell Endocrinol. 245:121-127 Padmanabhan V, Karsch FJ and Lee JS. 2002. Hypothalamic, pituitary and gonadal regulation of FSH Reproduction 59 (Supplement) 67-82 Perez-Hernandez, P., M. Garcia-Winder, and J. Gallegos-Sanchez. 2002. Bull exposure and an increased within-day suckling interval reduced postpartum anoestrous in dual purpose cows. Anim. Repro. Sci. 74:111-119. Perry, R.C., L.R. Corah, R.C. Cochran, W.E. Beal, J.S. Stevenson, J.E. Minton, D.D. Simms, and J.R. Brethour. 1991. Influence of dietary energy on follicular development, serum gonadotropins and first postpartum ovulation in suckled beef cows. J. Anim. Sci. 69:3762-3773. Peters, A. R. and G.E. Lamming. 1990. Lactational anestrus in farm animals. In Milligan S.R. (ed.), Oxford Reviews of Reproductive Biology, Vol. 12, Pages 245-288. New York: Oxford University Press. 83 Ramirez-Godinez, J. A., G. H. Kirakofe, R. R. Schalles, and G. D. Niswender. 1982. Endocrine patterns in the postpartum beef cows associated with weaning: a comparison of the short and subsequent normal cycles. J. Anim. Sci. 55:153-158. Randel, R. D. 1981. Effects of once-daily suckling on postpartum interval and cow-calf performance of first-calf Brahman X Hereford heifers. J. Anim. Sci. 53:755 -757. Rawling, N. C., L. Weir, B. Todd, J. Manns, and J.H. Hyland. 1980. Some endocrine changes associated with the postpartum period of the suckling beef cow. J. Reprod. Fertil. 60:301-308. Reeves, J. J. and C. T. Gaskins. 1981. Effect of once-a-day nursing on rebreeding efficiency of beef cows. J. Anim. Sci. 53:889. Rhodes, F. M., L. A. Fitzpatrick, K. W. Entwistle, and J. E. Kinder. 1995. Hormone concentrations in caudal vena cava during the first ovarian follicular wave of the oestrous cycle in heifers. J. Reprod. Fertil. 104:33-39. Richards, M. W., J. C. Spitzer, and M.B. Warner. 1986. Effect of varying levels of postpartum nutrition and body condition at calving on subsequent reproductive performance in beef cattle. J. Anim. Sci. 62:300. Rosenbaum M, Leibel RL. (1998). Leptin; a molecule integrating somatic energy stores, energy expenditure and fertility. Trends Endocrinol Metab. 9: 117-124 Ryan NK, Woodhouse CM, van der Hoek KH, Gilchrist RB, Armstrong DT, Norman RJ. (2002). Expression of leptin and its receptor in the murine ovary: possible role in the regulation of oocyte maturation. Biol Reprod. 66:1548-1554. Ryan, D.P., R.A. Spoon, M.K. Griffith, and G.L. Williams. 1994. Ovarian follicular recruitment, granulose cell steroidogenic potential and growth hormone/insulinlike growth factor-I relationships in suckled beef cows consuming high lipid diets: effects of graded differences in body condition maintained during the puerperium. Domest. Anim. Endocrinol. 11:161-174. Ryan, van der Hoek KH, Robertson SA, Norman RJ. 2003. Leptin and leptin receptor expression in the rat ovary. Endocrinology. 144:5006-5013. Sahu A. 1998. Leptin decreases food intake induced by melanin-concentrating hormone (MCH), galanin (GAL) and neuropeptide Y (NPY) in the rat Endocrinology 139 4739-4742 Sahu A. 2004. A hypothalamic role in energy balance with special emphasis on leptin. Endocrinology. 145: 2613-2620. SAS Institute Inc. 2004. SAS Version 9.0. SAS Institute Inc., Cary, NC, USA. 84 Savio, J. D., M. P. Boland, N. Hynes, and J. F. Roche. 1990. Resumption of follicular activity in the early postpartum period of dairy cows. J. Reprod. Fertil. 88:569579. Savio, J. D., W. W. Thatcher, L. Badinga, R. L. Sota, and D. Wolfenson. 1993. Regulation of dominant follicle turnover during the oestrous cycle in cows. J. Reprod. Fertil. 97:197-203. Scott, I. C. and G. W. Montgomery. 1987. Introduction of bulls induces return of cyclic ovarian functioning post-partum beef cows. New Zealand J. Agr. Res. 30:189194. Short, R. E., R. A. Bellows, R. B. Staigmiller, D. C. Adams, and J. G. Berardinelli. 1994. Effects of suckling on postpartum reproduction. In: M. J. Fields, And R.S. Sand RS (ed.), Factors Affecting Calf Crop, Pages 179-187. CRC Press, Boca Rotan, FL. Short, R. E., R. D. Randel, R. B. Staigmiller, and R. A. Bellows. 1990. Physiological mechanisms controlling anestrus and infertility in postpartum beef cattle. J. Anim. Sci. 68:799-816. Sipilov, V. S. 1966. The use of males teasers in cattle breeding. Anim. Breed. Abstr. 35:244. Smith JT, Acohido BV, Clifton DK, Seiner RA. 2006. KiSS-1 neurons are direct targets fro leptin in the ob/ob mouse. J. Neuroendocrinol. 18: 298-303. Smith, L. E. and C. K. Vincent. 1972. Effects of early weaning and exogenous hormone treatment on bovine postpartum reproduction. J. Anim. Sci. 35:1228. Spicer LJ, Fransisco CC. 1991. The adipose obese gene producte, leptin: evidence of a direct inhibitory role in ovarian function. Endocrinology. 138: 3374-3379. Spitzer, J.C., D.G. Morrison, R.P. Wettemann, and L.C. Faulkner. 1995. Reproductive responses and calf birth and weaning weights as affected by body condition at parturition and postpartum weight gain in primiparous beef cows. J. Anim. Sci. 73:1251-1257. Stackpole, C. A., I. J. Clarke, K. M. Breen, A. I. Turner, F. J. Karsch, and A. J. Tilbrook. 2006. Sex difference in the suppressive effect of cortisol on pulsatile secretion of luteinizing hormone in sheep. Endocrinology 147: 5921-5931. Stagg, K., L. J. Spicer, J. M. Sreenan, J. F. Roche, and M. G. Diskin. 1998. Effect of calf isolation on follicular wave dynamics, gonadotropin and metabolic hormone changes, and interval to first ovulation in beef cows fed either of two energy levels postpartum. Biol. Reprod. 59:777-783. 85 Stephens TW, Basinski M, Bristow PK, Bue-Valleskey JM, Burgett SG, Craft L, Hale J, Hoffman J, Hsiung HM, Kriauciunas A, MacKellar W, Rosteck PR Jr., Schoner B, Smith D, Tinsley FC, Zhang XY and Heiman M. 1995. The role of neuropeptide Y in the antiobesity action of the obese gene product Nature 377 530-532 Stevenson, J. S. and J. H. Britt. 1979. Relationship among luteinizing hormone, estradiol, progesterone, glucocorticoids,milk yield,body weight and postpartum ovarian activity in Holstein cows. J. Anim. Sci. 48:570-577. Stumpf, T. T., M. W. Wolfe, P. L. Wolfe, M. L. Day, R. J. Kittok, and J. E. Kinder. 1992. Weight changes prepartum and presence of bulls postpartum interact to affect duration of postpartum anestrus in cows. J. Anim. Sci. 70:3133-3137. Tauck, S. A. 2008. The biostimulatory effect of bulls on the hypothalamic-pituitaryadrenal and –ovarian axis and on temporal aspects of resumption of ovarian cycling activity in primiparous, postpartum, anestrous, suckled, beef cows. PhD Dissertation, Montana State University, Bozeman, MT Tauck, S. A., J. R. Olsen, J. G. Berardinelli. 2007. Adrenal involvement in the biostimulatory effect of bulls. Reprod. Biol. Endo. 5: 33 Tilbrook, A.J., B.J. Canny, M.D. Serapiglia, T.J. Ambrose, and I.J. Clarke. 1999. Suppression of the secretion of luteinizing hormone due to isolation/restraint stress in gonadectomised rams and ewes is influenced by sex steroids. J. Endocrinol. 160:469-481. Vizcarra, J.A., R.P. Wettemann, J.C. Spitzer, and D.G. Morrison. 1998. Body condition at parturition and postpartum weight gain influence luteal activity and concentrations of glucose, insulin, and nonesterified fatty acids in plasma of primiparous beef cows. J. Anim. Sci. 76:927-936. Walczewska A, Yu WH, Karanth S and McCann SM (1999) Estrogen and leptin have differential effects on FSH and LH release in female rats Proceedings of the Society for Experimental Biology and Medicine 222 170-177 Walters, D. L., C. C. Kaltenbach, T. G. Dunn, and R.E. Short. 1982. Pituitary and ovarian function in postpartum beef cows. I. Effect of suckling on serum and follicular fluid hormones and follicular gonadotropin receptors. Biol. Reprod. 26:640-646. Wang J, Liu R, Hawkins M, Barzilai N and Rossetti L (1998) A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature 393 684-688 86 Watanobe H (2002) Leptin directly acts within the hypothalamus to stimulate gonadotropin-releasing hormone secretion in vivo in rats. Journal of Physiology 545 255-268 Wauters M, Considine RV, van Gaal LF. 2000. Human leptin: From an adipocyte hormone to an endocrine mediator. Eur J. Endocrinol. 143: 292-311 Werth, L. A., J. C. Whittier, S. M. Azzam, G. H. Deutscher, and J. E. Kinder. 1996. Relationship between circulating progesterone and conception at the first postpartum estrus in young primiparous beef cows. J. Anim. Sci. 74:616-619. Wettemann, R.P., G.M. Hill, M.E. Boyd, J.C. Spitzer, D.W. Forrest, and W.E. Beal. 1986. Reproductive performance of postpartum beef cows after short-term calf separation and dietary energy and protein supplementation. Theriogenology 26:411. Whisnant CS and Harrell RJ. 2002. Effect of short-term feed restriction and refeeding on serum concentrations of leptin, luteinizing hormone and insulin in ovariectomized gilts. Domestic Animal Endocrinology 22 73-80 Whisnant, C.S., T.E. Kiser, F.N. Thompson, and C.R. Barb. 1986. Opioid inhibition of luteinizing hormone secretion during the postpartum period in suckled beef cows. J. Anim. Sci. 63:1445-1448. Wiesner G, Vaz M, Collier G, Seals D, Kaye D, Jennings G, Lambert G, Wilkinson D and Esler M (1999) Leptin is released from the human brain: influence of adiposity and gender Journal of Clinical Endocrinology and Metabolism 84 22702274 William Jr WN, Ceddia RB, Curi R. 2002. Leptin controls the fate of fatty acids in isolated rat white adipocytes. J. Endocrinol. 175: 735-744 Williams LM, Adam CL, Mercer JG, Moar KM, Slater D, Hunter L, Findlay PA and Hoggard N. 1999. Leptin receptor and neuropeptide Y gene expression in the sheep brain Journal of Endocrinology 11 165-169 Williams, G. L. 1990. Suckling as a regulator of postpartum rebreeding in cattle: A review. J. Anim. Sci. 68:831-852. Williams, G. L. and M. K. Griffith. 1995. Sensory and behavioral control of gonadotrophin secretion during suckling-mediated anovulation in cows. J. Reprod. Fertil. Suppl. 49:463-475. Williams, G.L. 1989. Modulation of luteal activity in postpartum beef cows through changes in dietary lipid. J. Anim. Sci. 67:785-793. 87 Williams, G.L., W.R. McVey Jr., and J.F. Hunter. 1993. Mammary somatosensory pathways are not required for suckling-mediated inhibition of luteinizing hormone secretion and delay of ovulation in cows. Biol. Reprod. 49:1329-1337. Wiltbank, J.N. 1970. Research needs in beef cattle reproduction. J. Anim. Sci. 31:755762. Woodside B, Abizaid A, Jafferali S. 1998. Acute food deprivation lengthens lactational infertilitiy in rats and this effect is reduced by systemic leptin administration. Am. J. Physiol. 274: R16553-8 Wright, I. A., S. M. Rhind, T. K. Whyte, A. J. Smith, S. R. McMillen, and R. Prado. 1990. Circulating concentrations of LH and FSH and pituitary responsiveness to GnRH in intact and ovariectomized suckled beef cows in two levels of body condition. Anim. Prod. 51:93-101. Wright, I.A., S.M. Rhind, T.K. Whyte, and A.J. Smith. 1992. A note on the effects of pattern intake and body condition on the duration of the post-partum anoestrous period and LH profiles in beef cows. Anim. Prod.54:143-146. Yavas, Y., and J.S. Walton. 2000. Postpartum acyclicity in suckled beef cows: A Review. Theriogenology 54:25-55. Zachow RJ, Magoffin DA. 1997. Direct intraovarian effects of leptin: impairment of the synergistic action of insulin-like growth factor-I on follicle-stimulating hormonedependent estradiol-17β production by rat ovarian granulosa cells. Endocrinology. 138: 847-850. Zalesky, D. D., M. L. Day, M. Garcia-Winder, K. Imakawa, R. J.Kittok, M. J. D’Occhio, and J. E. Kinder. 1984. Influence of exposure to bulls on resumption of estrous cycles following parturition in beef cows. J. Anim. Sci. 59:1135-1139. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L and Friedman JM (1994) Positional cloning of the mouse obese gene and its human homologue Nature 372; 425-432 Zieba DA, Amstalden M, Maciel MN, Keisler DH, Raver N, Gertler A and Williams GL (2003a) Divergent effects of leptin on luteinizing hormone and insulin secretion are dose dependent Experimental Biology and Medicine 228 325-330 Zigman JM, Elmquist JK. 2003. From anorexia to obesity – the Yin and Yang of body weight control. Endocrinology. 144: 3749-3756