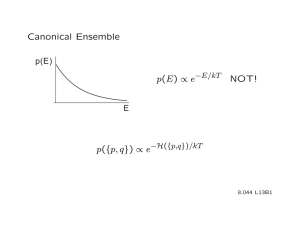
Document 13445448
advertisement
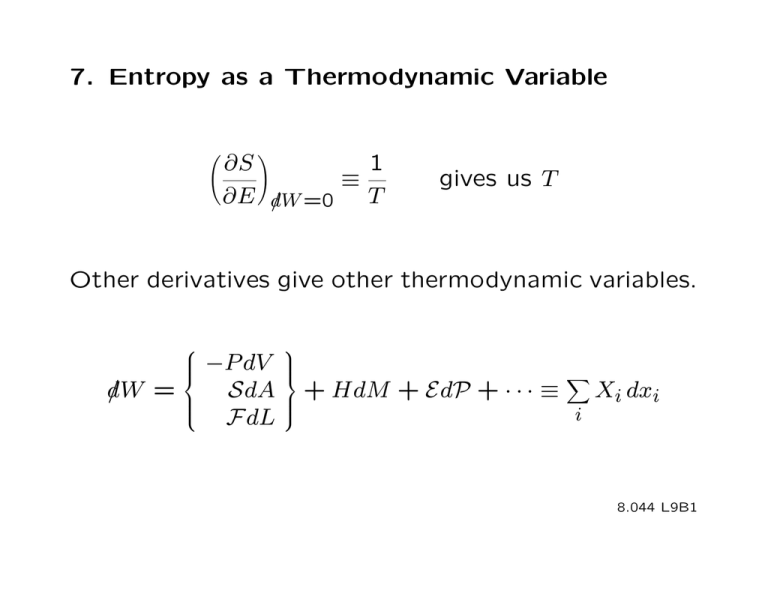
7. Entropy as a Thermodynamic Variable
∂S
1
≡
∂E d/W =0 T
�
�
gives us T
Other derivatives give other thermodynamic variables.
⎧
⎪
⎪
⎨
−P dV
d
/W
=
⎪
⎪
⎩
⎫
⎪
⎪
⎬
SdA ⎪ + HdM + EdP + · · · ≡
Xi dxi
⎪
i
FdL
⎭
8.044 L9B1
We chose to use the extensive external variables (a
complete set) as the constraints on Ω. Thus
S ≡ k ln Ω = S( E, V, M, · · · )
Now solve for E.
S(E, V, M, · · ·) ↔ E(S, V, M, · · ·)
We know
/Q
dE|d/W =0 = d
from the 1ST law
dE|d/W =0 ≤ T dS
utilizing the 2N D law
8.044 L9B2
Now include the work.
dE = d
/Q + d
/W
dE ≤ T dS + d
/W
⎧
⎪
⎪
⎨
−P dV
dE
≤
T dS +
⎪
⎪
⎩
⎫
⎪
⎪
⎬
SdA ⎪ + HdM + EdP + ·
· ·
⎪
FdL
⎭
The last line expresses the combined
1ST and 2N D laws of thermodynamics.
8.044 L9B3
Solve for dS.
1
P
H
E
dS = dE + dV − dM − dP + · · ·
T
T
T
T
Examine the partial derivatives of S.
∂S
1
=
T
∂E V,M,P
∂S
P
=
∂V E,M,P
T
∂S
H
=−
∂M E,V,P
T
⎛
⎝
⎞
Xj
∂S ⎠
=−
∂xj E,x =x
T
i
j
8.044 L9B4
INTERPRETATION
S(E,V)
�
dS =
V
∂S
∂S
dE +
dV
∂E V
∂V E
�
�
�
1
P
=
dE + dV
T
T
E
8.044 L9B5
UTILITY
Internal Energy
⎛
⎝
⎞
∂S(E, V, N ) ⎠
1
=
∂E
T
V
→ T (E, V, N ) ↔ E(T, V, N )
Equation of State
⎛
⎝
⎞
∂S(E, V, N ) ⎠
P
=
∂V
T
E
→ P (E, T, V, N ) → P (T, V, N )
8.044 L9B6
Example Ideal Gas
⎧
⎪
⎨
S(E, N, V ) = k ln Φ = kN ln
⎪V
⎩
4
E
πem
3
N
⎫
3/2
⎪
⎬
⎪
⎭
∂S
kN {}
kN
P
=
=
=
∂V E,N
{} V
V
T
P V = N kT
8.044 L9B7
COMBINATORIAL FACTS
# different orderings (permutations) of K distin­
guishable objects = K!
# of ways of choosing L from a set of K:
K!
(K − L)!
if order matters
K!
L!(K − L)!
if order does not matter
8.044 L9B8a
EXAMPLE Dinner Table, 5 Chairs (places)
Seating, 5 people
5·4·3·2·1 = 5! = 120
Seating, 3 people
5 · 4 · 3 = 5!
2! = 60
Place settings, 3 people
1 = 10
5· 4 · 3/6 = 5!
2! 3!
8.044 L9B8b
EXAMPLE
2 Level System
Ensemble of N "independent" systems
ENERGY
|1
>
ε
N = N0 + N1
E = ε N1
|0
>
0
8.044 L9B9
8.044 L16B1
SURFACE MOLECULES
IONS IN A CRYSTAL
LOWEST LYING STATES
ENERGY
0
ε
0
ε
ε
0
E
N1
NO WORK POSSIBLE (JUST HEAT FLOW)
8.044 L9B10
8.044 L16B2
Ω(E) =
1 when N1 = 0 or N
N!
N1!(N −N1)!
Maximum when N1 = N/2
T=
S(E)
S(E) = k ln Ω(E)
E=
T= 0
ε N/2
(or -
)
E=
εN
E
T> 0
T< 0
8.044 L9B11
ln N ! ≈ N ln N − N
S(E) = k[N ln N − N1 ln N1 − (N − N1) ln(N − N1)
− N + N1 + N − N1]
1
=
T
∂S
∂S ∂N1
k
=
= [−1 − ln N1 + 1 + ln(N − N1)]
∂E N
∂N1 ,∂E
E
af �
1/E
⎛
⎞
⎛
⎞
k ⎝ N − N1 ⎠
k ⎝N
=
ln
= ln
− 1⎠
E
N1
E
N1
8.044 L9B12
N
− 1 = eE/kT
N1
N
→ N1 = E/kT
e
+1
EN
E = EN1 = E/kT
e
+1
1.0
N1 /N or E/ εN
~ e−ε/ k T
0.5
1
2
3
kT/ε
4
8.044 L9B13
∂E
E
C≡
= Nk
∂T
kT
�
E
→ Nk
kT
�
�2
�2
e−E/kT
eE/kT
(eE/kT + 1)2
Nk E
→
4 kT
�
low T ,
�2
high T
0.5
C/Nk
0.4
0.3
0.2
0.1
1
2
3
kT/ε
4
8.044 L9B14
Ω'
p(n) =
Ω
p(n) =? n = 0, 1
In Ω'
N →N −1
p(n) =
and
N1 → N1 − n
(N −1)!
(N1−n)!(N −1−N1+n)!
N!
N1!(N −N1)!
8.044 L9B15
(N − 1)!
N1!
(N − N1)!
p(n) =
N
− n)!� �(N − N1 ��− 1 + n)!�
�
��!
� �(N1 ��
1/N
p(0) =
N −N1
N
1 n=0
N − N1 n = 0
N1 n = 1
1 n=1
=1−
N1
N
1 = [eE/kT + 1]−1
p(1) = N
N
⎫
⎪
⎪
⎪
⎬
p(0) + p(1) = 1
⎪
⎪
⎪
⎭
8.044 L9B16
1
p(n)
p(0)
0.5
p(1)
0
1
n
1
2
3
kT/ε
4
EN
E = (0)N p(0) + (E)N p(1) = E/kT
e
+1
But we knew E, so we could have worked back­
wards to find p(1).
8.044 L9B17
MICROCANONICAL ENSEMBLE
MODEL THE SYSTEM
FIND
THERMODYNAMIC RESULTS
FIND S(E,N,V ....)
S
E
Ω(E,N,V ....)
MICROSCOPIC INFORMATION
P(~~~) =
Ω'/Ω
= 1
T
N,V
S
= P
T
V E,N
etc.
8.044
L9B18
8.044 L16B10
The microcanonical ensemble is the starting point for
Statistical Mechanics.
• We will no longer use it to solve problems.
• We will develop our understanding of the 2N D law.
• We will derive the canonical ensemble, the real
workhorse of S.M.
8.044 L9B19
MIT OpenCourseWare
http://ocw.mit.edu
8.044 Statistical Physics I
Spring 2013
For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms.